Abstract
Purpose
Despite limited evidence on the association of vitamin D with outcomes in breast cancer survivors, some clinicians advise breast cancer patients to use vitamin D supplements. More evidence is needed to inform these recommendations.
Methods
In the Health, Eating, Activity and Lifestyle Study, we examined associations of post-treatment serum concentrations of 25-hydroxyvitamin D (25(OH)D) on overall and breast-cancer-specific mortality in 585 breast cancer survivors from western Washington State, New Mexico, and Los Angeles County. 25(OH)D was measured in stored blood collected 2 years post-enrollment. Outcomes were ascertained from the Surveillance, Epidemiology, and End Results registries and medical records. Cox proportional hazards models were fit to assess associations of serum 25(OH)D with overall and breast-cancer-specific mortality.
Results
After a median follow-up of 9.2 years; 110 women died, including 48 from breast cancer. Standard cut points classified 211 (31.6%) women as serum 25(OH)D deficient (<20 ng/mL), 189 (32.2%) as insufficient (20–30 ng/mL), and 185 (36.2%) as sufficient (>30 ng/mL). Compared to women with deficient 25(OH)D, those in the sufficient ranges had a decreased risk of overall mortality (age-adjusted HR=0.58; 95%CI 0.36–0.96); however multivariate adjustments attenuated the association (HR=0.90; 95%CI 0.50–1.61). No association was found between serum 25(OH)D and breast-cancer-specific mortality (sufficient: HR=1.21; 95%CI 0.52–2.80) in multivariate models.
Conclusion
In this breast cancer cohort, higher serum 25(OH)D may be associated with improved survival, but results were not statistically significant and must be interpreted with caution. The potential prognostic effect of vitamin D from diet, supplements or both should be evaluated in future larger studies with additional endpoints from breast cancer patients.
Keywords: 25-hydroxyvitamin D, overall mortality, breast-cancer-specific mortality, vitamin D
INTRODUCTION
Vitamin D has several biological functions that could lower the risk for mortality of several cancers, including breast cancer [1–4]. Vitamin D alters cellular proliferation, as demonstrated among in vivo and in vitro studies, by modulating gene activity, apoptosis, and cell signaling pathways.[5, 6] Moreover, the vitamin D receptor (VDR) and 1α-hydroxylase, an enzyme that converts vitamin D pro-hormone (25(OH)D) to vitamin D hormone (1, 25-dihydroxyvitamin D), are ubiquitously expressed throughout the body, including in breast tissue [7]. In cross-sectional analyses, lower versus higher serum 25(OH)D concentrations were strongly associated with more advanced stage in breast cancer diagnosis [8–10], which has led to speculation that 25(OH)D deficiency may contribute to tumor progression.
Clinical practice guidelines have been published suggesting that most individuals need to maintain serum vitamin D concentrations ≥30 ng/mL for optimal health [11, 12]. We recently reported a high prevalence of serum concentrations of vitamin D <30 ng/mL in a cohort of breast cancer survivors [9]. However, whether this widespread deficiency is associated with survival is unknown, because evidence linking vitamin D to breast cancer prognosis is sparse and elusive. Published data are available from four studies which examined the relationship between circulating concentrations of 25(OH)D and prognosis among women diagnosed with breast cancer [10, 13–15]. In a Norwegian population-based study by Tretli et al., women diagnosed with breast cancer with serum concentrations of 25(OH)D >35 ng/mL had a significantly decreased risk of breast cancer-specific mortality compared to women with 25(OH)D <20 ng/mL (Hazards ratio [HR]= 0.42; 95% confidence interval [CI], 0.1 to 0.82) [14]. Within a clinic-based cohort of 512 non-Hispanic white women diagnosed with breast cancer, Goodwin et al. reported that women with low concentrations of serum 25(OH)D (<20 ng/mL) had poorer prognostic outcomes compared to those with “sufficient” 25(OH)D (≥30 ng/mL) (HR = 1.71; 95% CI, 1.02 to 2.86 for distant recurrence; HR = 1.60; 95% CI, 0.96 to 2.64 for death) [10]. Similarly, Vrieling et al. reported German postmenopausal women diagnosed with breast cancer (in situ, stage I to IV) and the lowest tertile of serum 25(OH)D (<14 ng/mL) had an increased risk of overall (HR=1.55; 95% CI 1.00 to 2.39) and distant disease (HR=2.09; 95% CI 1.29 to 3,41), in relation to highest tertile (≥22 ng/mL)[15]. However, in a matched nested case-control design of 500 multi-ethnic breast cancer survivors, no association was reported between serum 25(OH)D and cancer prognosis. Compared to women with serum 25(OH)D ≥20 ng/mL, women with serum 25(OH)D <20 ng/mL had an odds ratio (OR) of 1.00 (95% CI, 0.68 to 1.48) for distant recurrence and an OR of 1.13 (95% CI, 0.72 to 1.79) for death [13].
We examined the association of vitamin D status based on the aforementioned clinical practice guidelines, at approximately 36 months post-diagnosis, with overall and breast cancer-specific mortality in a multi-ethnic cohort of early stage breast cancer survivors. 25(OH)D is the primary circulating form of vitamin D in the body and the substrate for production of active vitamin D hormone, 1,25-dihydroxyvitamin D. The amount of circulating serum 25(OH)D is a rate limiting step in the synthesis of the active hormone: individuals with vitamin D <30 ng/mL may also have decreased vitamin D activity. The maintenance and management of sufficient 25(OH)D may therefore reveal a potentially modifiable factor to mediate morbidity and mortality among breast cancer patients.
MATERIALS AND METHODS
Study Participants
The methods for this prospective cohort study have been described elsewhere [9, 16]. Briefly, we collected data from 1,183 women, between 18–64 years, enrolled in the Health, Eating, Activity and Lifestyle (HEAL) Study, a multicenter, multiethnic cohort of female breast cancer patients. Using the Surveillance Epidemiology and End Results (SEER) registry in one of three regions in the western United States: north-central New Mexico, Los Angeles County (CA), and Washington State, we recruited women with in situ or Stage I–IIIA disease, within 12 months of diagnosis, between July 1996 and March 1999. Participants completed a self-report questionnaire and an in-person interview at study inception. In addition, a 24 month post-enrollment assessment was conducted and consisted of an in-person interview, a self-administered food frequency questionnaire (FFQ), anthropometry, and a 12-hr fasting blood draw. Annual data collected from SEER registries and abstracted medical records were used to follow each participant and determine health related outcomes, including mortality due to breast cancer-specific cause or any other cause.
Of the 1,183 women enrolled in the study, 935 women had invasive breast cancer. Of these, 806 had serum 25-hydroxyvitamin D [25(OH)D] measured using the 2nd assessment blood draw (mean 30-months post-diagnosis). In the end, 585 women diagnosed with invasive breast cancer, who had successfully measured serum 25(OH)D, and who were alive but had not experienced either a disease recurrence or new primary cancer before the 2nd assessment blood draw were used in the final analysis. Written informed consent was obtained from each participant. The study was performed with the approval of the Institutional Review Boards at participating institutions in accordance with an assurance filed with and approved by the U.S. Department of Health and Human Services.
Serum 25(OH)D assay
The circulating concentration of vitamin D hormone, 1,25-dihydoxyvitamin D, is under tight homeostatic control, has a short half-life (i.e., 4–7 hours), and does not give a reliable estimate of an individual’s longer-term vitamin D status [17]. Therefore, serum 25(OH)D is a standard biomarker used to determine vitamin D status and is proportionally associated with dietary or supplemental intake of vitamin D as well as cutaneous synthesis [18, 19]. Using stored fasting blood specimens, drawn, on average, 36 months post-diagnosis, we measured serum 25(OH)D using a radioimmunoabsorbant assay (DiaSorin Inc., Sillwater, MN). For each assay, we included blinded duplicates for quality control assessment. The intra-assay coefficient of variation was 3.7%.
Outcomes ascertainment
Women were followed for vital status from the 24-month post-enrollment blood draw until December 31, 2009. Outcomes were obtained from the SEER registries and abstracted medical records. Causes of death were coded using International Classification of Diseases, 10th Revision (ICD-10) codes [20]. The time frame from the primary end point of interest, overall mortality, was initiated on the date of the 24-month post-enrollment blood draw and ended on the date of death. Non-deceased participants were censored on December 31, 2009. The secondary end point of interest, breast cancer-specific mortality, was defined using ICD-10 code C50 [20]. In analysis of breast cancer-specific deaths, women dying from other causes were censored on their dates of death.
Covariates
We collected standardized information on covariates known to be associated with both serum 25(OH)D and the specified endpoints using data on demographics, anthropometry, medical history, medication use, physical activity (including outdoor activity), diet and dietary supplement intake, and disease staging and treatment used [9, 10, 21]. Information on demographic characteristics and dietary supplement use was obtained using standardized questionnaires; dietary intake was obtained using a food frequency questionnaire (FFQ) collected at the 24 month post-enrollment assessment. Breast cancer stage at diagnosis was obtained from the SEER registry records, and breast cancer treatment data were obtained from participants’ medical records and SEER. Hours of outdoor physical activity per week and geographic locale served as surrogates of ultraviolet B exposure [22].
Statistical Analysis
This study’s objective was to examine the relationship of serum 25(OH)D with overall and breast cancer-specific mortality. Serum 25(OH)D status was modeled using the following three categories of vitamin D status: deficient (<20 ng/mL), insufficient (20 to 30 ng/mL) and sufficient (>30 ng/mL) [23]. Analyses of variance (ANOVA) and chi-square tests were used to test for differences in continuous and categorical variables, respectively by the three vitamin D categories. We used the Kaplan-Meier technique to construct survival curves and to calculate unadjusted 5-year and 10-year survival rates from death due to any cause and death from breast cancer [24]. We next employed multivariable Cox proportional hazards models to estimate the HR and 95% CI for death due to any cause and due to breast cancer associated with serum 25(OH)D (per 10 ng/mL increments) and vitamin D status (deficient 25(OH)D as referent). Age at diagnosis (years) was used as the time metric for all regression analysis. We tested and confirmed non-violation of the proportionality assumption based on a graphical approach (i.e., log(-log) plots) and the goodness-of-fit test using Schoenfeld residuals [25, 26].
Covariates were examined for inclusion in the multivariable model based on a list of traditional breast cancer prognostic factors, including age at diagnosis (years, continuous), body mass index (BMI, <18.5 kg/m2, 18.5 to 24.9 kg/m2, 25.0–29.9 kg/m2 and ≥30 kg/m2) [27], menopausal status (postmenopausal, yes/no), hormone receptor status (ER+, yes/no), disease stage (“localized” was defined as stage I and II vs. “regional” was defined as stage III), adjuvant hormone therapy (tamoxifen use, yes/no), treatment type (surgery only; surgery and radiation; surgery and chemotherapy; and surgery, radiation and chemotherapy) diagnosis of osteoporosis (yes/no), diagnosis of kidney, liver or thyroid disease (yes/no) and lifestyle-related covariates, including calcium supplement use (yes/no) and alcohol use (>1 drink/week, yes/no), duration of outdoor physical activity (hours per week, continuous) and current smoking status (smoker/non-smoker).
Variables were retained in the final model if they were associated with vitamin D status, associated with overall or breast cancer-specific mortality in survivors with ≥ 30ng/mL 25(OH)D and had altered the risk estimate of the model containing vitamin D status plus age by at least 10%. Because each study site had distinct race/ethnic composition, we generated a composite adjustment variable for race-ethnicity/study site [28]. Serum 25(OH)D varies by race/ethnicity, geographic location and season of blood draw (winter/spring vs. summer/fall) [29, 30]. For this reason, both the composite race/study site and blood draw season variables were included in all multivariable analyses. We reclassified missing data for categorical variables with its own term and for continuous variables we replaced the missing value with the variable median. All multivariable models included age, race-ethnicity/study site, season of blood draw, BMI, disease stage, adjuvant hormone therapy and treatment type. For overall mortality, models also included duration of outdoor physical activity and smoking status. Statistical analyses were performed using Stata (version 11.1; StataCorp LP, College Station, TX) software. All statistical tests were two-sided and statistical significance was set at p < 0.05.
RESULTS
Descriptive characteristics of the study participants are provided in Table 1. Mean age at diagnosis was 55.8 ± 10.8 years. The mean serum 25(OH)D was 24.8 ± 10.3 ng/mL (range 3.9 to 71.7 ng/mL); and the mean serum 25(OH)D for each clinical category of vitamin D status was 13.9 ± 3.7 ng/mL for n=211 women classified as vitamin D deficient (<20 ng/mL), 25.5 ± 2.9 ng/mL for n=189 vitamin D insufficient women, and 36.6 ± 6.1 ng/mL among n=185 vitamin D sufficient women. The distribution of traditional prognostic risk factors such as age, BMI, race-ethnicity/study site, calcium supplementation, physical activity, kidney/liver/thyroid disease occurrence, disease characteristics (i.e., tumor stage, nodal involvement, tumor hormone receptor status) and treatment used (i.e., adjuvant treatment following surgery and adjuvant hormone treatment) differed by vitamin D status (Table 1). Lower serum 25(OH)D was associated with younger age at diagnosis, greater BMI, race-ethnicity/study site for both African American/Los Angeles and Hispanic/New Mexico, no report of calcium supplementation use, specific treatment types (i.e., surgery only or surgery and chemotherapy), aggressive disease characteristics (i.e., regional tumor stage, ≥1 nodal involved, and negative tumor hormone receptor status), no report of Tamoxifen use, history of metabolic disease, and less physical activity. Further details on the associations between HEAL participant characteristics and serum 25(OH)D have been published elsewhere [9].
TABLE 1.
Circulating Concentrations of 25(OH)D By Participant Characteristics In Breast Cancer Survivors
| N | % | Serum 25(OH)D (ng/mL) | Distribution of Vitamin D Status
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deficient <20 ng/mL
|
Insufficient20–30 ng/mL
|
Sufficient >30 ng/mL
|
|||||||||
| Mean | SD | No. | % | No. | % | No. | % | P | |||
| All Participants | 585 | 100 | 24.8 | 10.3 | 211 | 36.1 | 189 | 32.3 | 185 | 31.6 | |
| Age at diagnosis, yrs | <0.001 | ||||||||||
| <50 | 150 | 25.6 | 22.8 | 11.1 | 71 | 33.6 | 42 | 22.2 | 37 | 20.0 | |
| ≥50 | 435 | 74.4 | 25.5 | 9.9 | 140 | 66.4 | 147 | 77.8 | 148 | 80.0 | |
| Body Mass Index, kg/m^2 | <0.001 | ||||||||||
| < 18.0 | 10 | 1.7 | 26.1 | 7.5 | 2 | 0.9 | 5 | 2.6 | 3 | 1.6 | |
| 18.0–24.9 | 218 | 37.3 | 27.8 | 9.6 | 49 | 23.2 | 73 | 38.6 | 96 | 51.9 | |
| 25.0–29.9 | 170 | 29.1 | 25.4 | 10.8 | 60 | 28.4 | 55 | 29.1 | 55 | 29.7 | |
| ≥ 30.0 | 172 | 29.4 | 20.3 | 9.4 | 96 | 45.5 | 50 | 26.5 | 26 | 14.1 | |
| Missing | 15 | 2.6 | 26.4 | 7.4 | 4 | 1.9 | 6 | 3.2 | 5 | 2.7 | |
| Race-ethnicity/study site | <0.001 | ||||||||||
| Non-Hispanic white/Washington | 94 | 16.1 | 25.9 | 9.3 | 28 | 13.3 | 35 | 18.5 | 31 | 16.8 | |
| Non-Hispanic white/New Mexico | 256 | 43.8 | 29.7 | 9.6 | 40 | 19.0 | 91 | 48.1 | 125 | 67.6 | |
| Hispanic/New Mexico | 65 | 11.1 | 21.8 | 8.6 | 33 | 15.6 | 21 | 11.1 | 11 | 5.9 | |
| African American/Los Angeles | 153 | 26.2 | 17.6 | 8.0 | 104 | 49.3 | 36 | 19 | 13 | 7.0 | |
| Other/Washington & New Mexico | 17 | 2.9 | 23.9 | 8.0 | 6 | 2.8 | 6 | 3.2 | 5 | 2.7 | |
| Season of blood draw | 0.09 | ||||||||||
| Summer/Fall, April-September | 327 | 55.9 | 25.7 | 10.1 | 107 | 50.7 | 106 | 56.1 | 114 | 61.6 | |
| Winter/Spring, October-March | 258 | 44.1 | 23.8 | 10.5 | 104 | 49.3 | 83 | 43.9 | 71 | 38.4 | |
| Menopausal status | 0.08 | ||||||||||
| Pre | 90 | 15.4 | 24.4 | 11.4 | 35 | 16.6 | 30 | 15.9 | 25 | 13.5 | |
| Post | 460 | 78.6 | 25.3 | 10.0 | 156 | 73.9 | 152 | 80.4 | 152 | 82.2 | |
| Unknown | 35 | 6.0 | 20.4 | 9.8 | 20 | 9.5 | 7 | 3.7 | 8 | 4.3 | |
| Vitamin D supplement use | 0.64 | ||||||||||
| Yes | 160 | 27.4 | 25.9 | 10.4 | 53 | 25.1 | 53 | 28 | 54 | 29.2 | |
| No | 425 | 72.6 | 24.4 | 10.2 | 158 | 74.9 | 136 | 72 | 131 | 70.8 | |
| Calcium supplement use | <0.001 | ||||||||||
| Yes | 501 | 85.6 | 26.0 | 9.9 | 156 | 73.9 | 168 | 88.9 | 177 | 95.7 | |
| No | 55 | 9.4 | 19.5 | 9.9 | 33 | 15.6 | 16 | 8.5 | 6 | 3.2 | |
| Don’t know | 29 | 5.0 | 15.7 | 8.7 | 22 | 10.4 | 5 | 2.6 | 2 | 1.1 | |
| Treatment Type | 0.04 | ||||||||||
| Surgery only | 137 | 23.4 | 23.7 | 10.5 | 56 | 26.5 | 40 | 21.2 | 41 | 22.2 | |
| Surgery and radiation | 216 | 36.9 | 26.0 | 9.4 | 59 | 28.0 | 83 | 43.9 | 74 | 40.0 | |
| Surgery and chemotherapy | 73 | 12.5 | 23.3 | 11.2 | 30 | 14.2 | 24 | 12.7 | 19 | 10.3 | |
| Surgery, chemotherapy and radiation | 159 | 27.2 | 24.9 | 10.8 | 66 | 31.3 | 42 | 22.2 | 51 | 27.6 | |
| Tumor stage | 0.02 | ||||||||||
| Localized | 414 | 70.8 | 25.4 | 10.2 | 135 | 64.0 | 138 | 73 | 141 | 76.2 | |
| Regional | 171 | 29.2 | 23.5 | 10.4 | 76 | 36.0 | 51 | 27 | 44 | 23.8 | |
| Nodal involvement | <0.001 | ||||||||||
| None/Unknown | 362 | 61.9 | 25.4 | 10.3 | 119 | 56.4 | 115 | 60.8 | 128 | 69.2 | |
| ≥1 | 162 | 27.7 | 23.8 | 10.7 | 72 | 34.1 | 45 | 23.8 | 45 | 24.3 | |
| None examined | 61 | 10.4 | 24.1 | 8.7 | 20 | 9.5 | 29 | 15.3 | 12 | 6.5 | |
| Tumor hormone receptor status | <0.001 | ||||||||||
| ER+ and/or PR+ | 418 | 71.5 | 25.6 | 10.0 | 135 | 64.0 | 141 | 74.6 | 142 | 76.8 | |
| ER−/PR− | 113 | 19.3 | 21.5 | 10.7 | 60 | 28.4 | 28 | 14.8 | 25 | 13.5 | |
| Unknown/Missing | 54 | 9.2 | 25.7 | 10.9 | 16 | 7.6 | 20 | 10.6 | 18 | 9.7 | |
| Tamoxifen use | 0.03 | ||||||||||
| Yes | 305 | 52.1 | 26.1 | 10.2 | 98 | 46.4 | 97 | 51.3 | 110 | 59.5 | |
| No | 280 | 47.9 | 23.5 | 10.2 | 113 | 53.6 | 92 | 48.7 | 75 | 40.5 | |
| Kidney/Liver/Thyroid Disease | <0.01 | ||||||||||
| Yes | 144 | 24.6 | 27.1 | 10.2 | 37 | 17.5 | 51 | 27 | 56 | 30.3 | |
| No | 441 | 75.4 | 24.1 | 10.2 | 174 | 82.5 | 138 | 73 | 129 | 69.7 | |
| Osteoporosis | |||||||||||
| Yes | 72 | 12.3 | 27.1 | 9.4 | 19 | 9.0 | 25 | 13.2 | 28 | 15.1 | |
| No | 512 | 87.5 | 24.5 | 10.4 | 191 | 90.5 | 164 | 86.8 | 157 | 84.9 | |
| Don’t know | 1 | 0.2 | 15.8 | 0.0 | 1 | 0.5 | 0 | 0 | 0 | 0.0 | |
| Charlson Comorbidity Score | 0.45 | ||||||||||
| 0 | 299 | 51.1 | 23.3 | 9.8 | 122 | 57.8 | 97 | 51.3 | 80 | 43.2 | |
| 1 | 231 | 39.5 | 26.2 | 10.9 | 75 | 35.5 | 71 | 37.6 | 85 | 45.9 | |
| 2 | 55 | 9.4 | 27.5 | 8.7 | 14 | 6.6 | 21 | 11.1 | 20 | 10.8 | |
| Dietary intake of alcohol, drink/day | 0.05 | ||||||||||
| None | 300 | 51.3 | 23.3 | 9.8 | 123 | 58.3 | 97 | 51.3 | 80 | 43.2 | |
| <1 | 231 | 39.5 | 26.2 | 10.9 | 75 | 35.5 | 71 | 37.6 | 85 | 45.9 | |
| 1+ | 55 | 9.4 | 27.5 | 8.7 | 14 | 6.6 | 21 | 11.1 | 20 | 10.8 | |
| Met physical activity recommendation (>150 min/wk) | <0.001 | ||||||||||
| Yes | 439 | 75.0 | 25.7 | 10.1 | 142 | 67.3 | 142 | 75.1 | 155 | 83.8 | |
| No | 146 | 25.0 | 22.2 | 10.5 | 69 | 32.7 | 47 | 24.9 | 30 | 16.2 | |
| Current Smoker (%) | 0.39 | ||||||||||
| Yes | 21 | 3.6 | 23.0 | 10.5 | 10 | 4.7 | 7 | 3.7 | 4 | 2.2 | |
| No | 564 | 96.4 | 24.9 | 10.3 | 201 | 95.3 | 182 | 96.3 | 181 | 97.8 | |
Abbreviations: SD, standard deviation
Note: Not all sum to 100% due to missing values
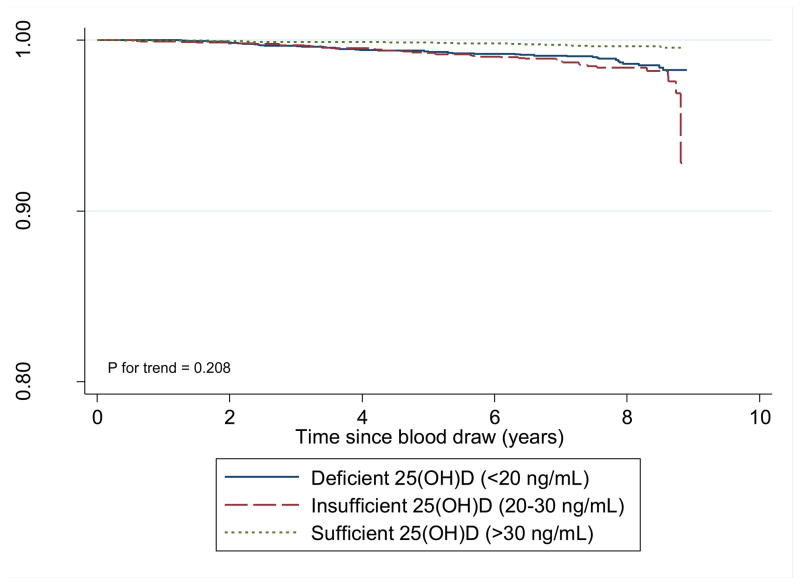
After a median follow-up of 9.2 years, among the 585 breast cancer survivors, 110 women died including 48 women whose deaths were due to breast cancer. Serum vitamin D was associated with reduced overall mortality. The unadjusted associations of serum vitamin D with overall survival suggests a linear dose-response pattern (p for trend = 0.21, Figure 1). The 5-year overall survival rates in women with sufficient, insufficient and deficient serum 25(OH)D were 98.4%, 97.4% and 97.6%, respectively; and the comparable 10-year figures were 88.0%, 83.1% and 84.8%, respectively (log rank p = 0.19, data not shown).
Figure 1.
Overall Survival by Vitamin D Status
Table 2 provides results for the association of vitamin D and overall mortality. A trend towards decreased risk of death was suggested, with a multivariate-adjusted HR of 0.85 (95% CI, 0.68–1.09; p for trend = 0.20) per 10 ng/mL increment in serum 25(OH)D. Compared to participants with deficient serum 25(OH)D, the age-adjusted association for those with sufficient serum 25(OH)D was 48% lower for overall mortality. Adjustment for confounders attenuated the association between serum vitamin D and overall mortality; women with sufficient 25(OH)D had a non-significant 10% reduced risk of mortality. The results for women with insufficient serum 25(OH)D were similar and not statistically significant.
TABLE 2.
Overall and Breast Cancer-Specific Mortality Associations of Vitamin D Status in Breast Cancer Survivors
| Models of association of vitamin D status and death from any cause | Serum 25(OH)D | |||||||
|---|---|---|---|---|---|---|---|---|
| Continuous per 10 ng/mL (N=585)
|
Deficient <20 ng/mL (N=211)
|
Insufficient 20–30 ng/mL (N=189)
|
Sufficient >30 ng/mL (N=185)
|
|||||
| HR (95% CI) | Ptrend | HR | (95%CI) | HR | (95% CI) | HR | (95% CI) | |
| Death, any cause | 43 deaths | 41 deaths | 26 deaths | |||||
| Unadjusted | 0.74 (0.60 – 0.91) | <0.01 | 1.00 | - | 0.83 | (0.53 – 1.29) | 0.58 | (0.35 – 0.95) |
| Age-adjusteda | 0.74 (0.60 – 0.92) | <0.01 | 1.00 | - | 0.84 | (0.54 – 1.31) | 0.58 | (0.36 – 0.96) |
| Multivariateb,c | 0.85 (0.68 – 1.09) | 0.20 | 1.00 | - | 1.07 | (0.66 – 1.75) | 0.90 | (0.50 – 1.61) |
| Death, breast cancer-specific cause | 22 events | 14 events | 12 events | |||||
| Unadjusted | 0.80 (0.59 – 1.09) | 0.15 | 1.00 | - | 0.75 | (0.38 –1.48) | 0.62 | (0.31 – 1.27) |
| Age-adjusteda | 0.80 (0.59 – 1.09) | 0.15 | 1.00 | - | 0.75 | (0.38 – 1.48) | 0.62 | (0.31 –1.27) |
| Multivariateb | 1.08 (0.75 – 1.54) | 0.68 | 1.00 | - | 1.12 | (0.54 – 2.33) | 1.21 | (0.52 – 2.80) |
Abbreviation: HR, hazard ratio; 95% CI, 95 percent confidence interval
Ptrend was calculated by using serum 25(OH)D as a continuous variable
Model adjusted solely for age at diagnosis
Models adjusted for age at diagnosis, tumor stage, body mass index categories, race-ethnicity/study site, Tamoxifen use, season of blood draw and treatment used
Additional adjustment for physical activity and smoking status
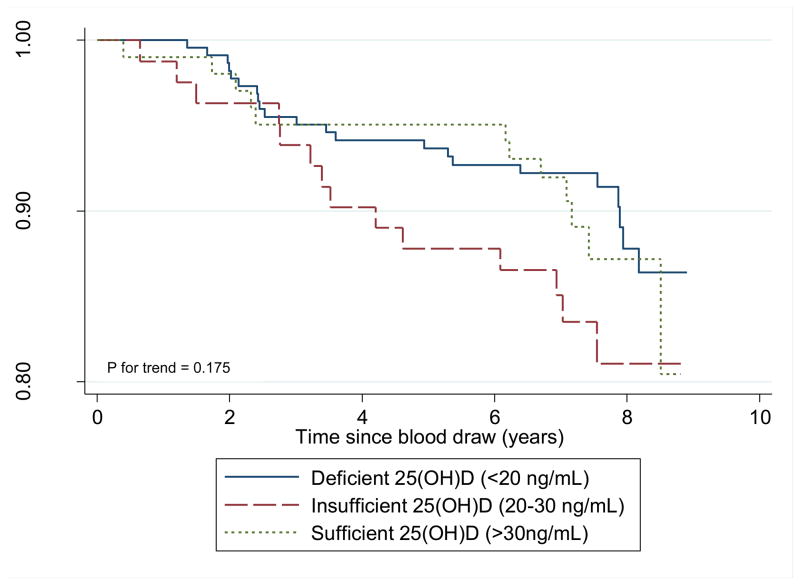
In relation to breast cancer-specific survival, we found no association between serum vitamin D and risk of breast cancer-specific mortality (Table 2). The unadjusted association of serum vitamin D with breast cancer-specific survival does not indicate a linear dose-response pattern (p for trend = 0.18, Figure 2). The breast cancer-specific 5-year survival rates were approximately 98%, regardless of serum 25(OH)D status, and the 10-year rates ranged from 91.7% (deficient) to 95.5% (sufficient) (log rank p = 0.38, data not shown).
Figure 2.
Breast Cancer-specific Survival by Vitamin D Status
DISCUSSION
In this cohort of 585 breast cancer survivors, serum 25(OH)D was associated with reduced overall mortality. Compared to women with lowest values of 25(OH)D (<20 ng/mL), those with “sufficient” 25(OH)D values (≥ 30 ng/mL) had a 42% reduced risk of overall mortality in age-adjusted models which were attenuated and lost statistical significance with adjustment for other potential confounders. These results, including the size of the HRs and the lack of statistical significance on adjustment for confounders, are comparable to reports from previously published studies of women diagnosed with breast cancer. In 2009, Goodwin et al. reported that 512 non-Hispanic white women with the lowest serum 25(OH)D (<20 ng/mL) had a statistically non-significant 71% increased risk of overall mortality, compared to those with 25(OH)D concentrations ≥32 ng/mL [10]. In a nested case-control study with 250 matched pairs, Jacobs et al. reported a non-significant 13% increased odds of overall mortality in breast cancer survivors with 25(OH)D <20 ng/mL, compared to those with serum 25(OH)D >20 ng/mL [13]. In a cohort of 1,295 postmenopausal women, Vieling et al. (2011) reported with those with 25(OH)D <14 ng/mL had a 55% increased risk of overall mortality compared to those with 25(OH)D ≥22 ng/mL[15]. Most recently (2012), in a Norwegian population-based study among 251 breast cancer patients, Tretli et al. report women with 25(OH)D >33 ng/mL had a decreased risk of overall mortality, compared to women with 25(OH)D <18.4 ng/mL (HR=0.37; 95% CI 0.21 to 0.67) [14].
We reported an age-adjusted non-significant 38% decreased risk of breast cancer-specific mortality between survivors with 25(OH)D >30 ng/mL compared to 25(OH)D <20 ng/mL. The magnitude of this association is similar to the results reported by both Goodwin [10] and Tretli [14], but these two studies only adjusted for age at diagnosis, tumor characteristics, and/or season of blood draw. Upon adjustment for potential confounders, including race-ethnicity/study site, we reported a non-statistically significant association between serum 25(OH)D and breast cancer-specific mortality. These results are somewhat similar to related results (i.e. disease-free survival) reported by Vrieling [15] and Jacobs [13]. The association between serum 25(OH)D and breast cancer-specific mortality was not specifically examined in the cohort of German postmenopausal women or the Women’s Healthy Eating and Living (WHEL) intervention study. However, Vrieling reported no association between serum 25(OH)D, measured after completing chemotherapy, and risk of distant disease (i.e., distant recurrence, death, second primary invasive non-breast cancer) for German women with serum 25(OH)D < 14 ng/mL, compared to those with serum 25(OH)D > 22 ng/mL. In a subset of the WHEL participants, serum 25(OH)D was assessed and examined with breast cancer recurrence or new breast cancer primary and women with 25(OH)D <20 ng/mL had a statistically non-significant increased odds of local recurrence (OR, 1.48; 95% CI 0.47, 4.65) and regional recurrence (OR,1.13; 95% CI 0.20, 6.44) compared to 25(OH)D >20 ng/mL [13]. Our data, which reveals significant attenuation of the association in multivariate-adjusted model, may be in part due to confounding from race-ethnicity/study site variable.
Vitamin D may influence survival following a diagnosis of breast cancer via several mechanisms. The binding of 1,25(OH)2D to the vitamin D receptor (VDR) results in the enhancement or suppression of gene transcription to modulate the inhibition of cell proliferation and angiogenesis, promotion of cell differentiation, and induction of apoptosis in both normal and malignant cells [1–4, 18, 31]. In vitro and in vivo studies report direct effects on breast cancer cell lines, such as growth inhibition, cellular differentiation and apoptosis when both 25(OH)D and 1,25(OH)D are applied directly to MCF-7 cell lines. [32–35] Additional data from animal models suggest that vitamin D deficiency may play an important role in cancer progression by promoting metastasis in bone, via vitamin D endocrine pathways [36]. While it is not possible to test these specific mechanisms in humans, these data from in vitro and animal model studies provide support for the associations observed here as well as elsewhere [10, 13].
The strengths of our study include the prospective design and participation by non-Hispanic White, non-Hispanic African-American and Hispanic women. We used well-annotated tumor-, treatment-, and traditional prognostic-related data to adjust for confounding and have approximately 10 years of mortality outcomes from annual SEER registry updates. We used a reliable biomarker for vitamin D status, serum concentration of 25(OH)D, which was measured after the completion of breast cancer treatment so that measures would not be confounded by concurrent exposure to chemotherapy or radiation treatments.
Limitations include the observational design, which limits the ability to infer cause and effect. While we adjusted our models for clinical and lifestyle-related covariates, as with all observational studies, residual confounding may still exist. Other limitations are that we were unable to adjust for completion of primary or hormone therapy, quantitative UV exposure or serum PTH concentration, the latter is directly linked with vitamin D metabolism and vitamin D activity [35]. However, following adjustment for hours of outdoor physical activity (a surrogate variable for direct UV exposure), we observed no appreciable change in the HR for vitamin D status. We carefully considered examining the effect of serum 25(OH)D on disease recurrence. The pathological diagnosis to differentiate between recurrence and the new occurrence of a breast cancer is sometimes difficult [37]. Rather than risk misclassification, we have chosen to report only data with clear outcomes, which is breast-cancer specific and total mortality. In addition, few breast cancer-specific deaths occurred in our cohort, resulting in limited power to examine breast cancer-specific mortality by vitamin D status or examine the effects of confounding or effect modification. Further, there is a possibility that the association of low serum 25(OH)D with high BMI, African-American and Hispanic race-ethnicity, less physical activity, and other known prognostic factors for breast cancer may explain the significant association observed in the unadjusted analysis. Finally, as with all observational studies there may be residual confounding.
In conclusion, in this multi-ethnic cohort of breast cancer survivors, higher vs. lower serum 25(OH)D suggest an association with improved overall survival, but results were not statistically significant and must be interpreted with caution. However, lacking clinical trial evidence, we cannot confirm that supplementation with vitamin D will improve survival, nor can we speculate on the dosage necessary to achieve optimal ranges in this population. We note that there is ongoing debate on these cut-off values with others suggesting lower cut-off levels for vitamin D deficiency (e.g. 12 ng/mL) or believe that levels of 20 ng/mL are sufficient [38–41]. Of interest, the Institute of Medicine (2010) and the Endocrine Society (2011) recommend that in order to maintain bone health and normal calcium metabolism, normal weight healthy women aged 19–50 consume 600 IU/day of vitamin D3 and normal weight healthy women older than 50 years consume 800 IU/day [42, 43]. Additional research is needed to determine whether breast cancer patients should be screened for vitamin D deficiency and to determine the optimal practice guidelines for treatment.
Acknowledgments
The authors would like to thank the HEAL participants for their ongoing dedication to this study. This study was supported through National Cancer Institute contracts NO1-CN-75036-20, NO1-CN-05228, NO1-PC-67010, U54-CA116847 and training grant R25-CA094880. A portion of this work was conducted through support by the National Institutes of Health grant MO1-RR-0037, and University of New Mexico grant, NCRR MO1-RR-0997. Data collection for the Women’s CARE Study at the University of Southern California was supported by contract N01-HD-3-3175 from the National Institute of Child Health and Human Development and patient identification was supported in part by contract 050Q-8709-S1528 from the California Department of Health Services.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
References
- 1.Schwartz GG, Blot WJ. Vitamin D status and cancer incidence and mortality: something new under the sun. Journal of the National Cancer Institute. 2006;98:428–30. doi: 10.1093/jnci/djj127. [DOI] [PubMed] [Google Scholar]
- 2.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nature reviews. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. The American journal of clinical nutrition. 2004;79:362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 4.Tuohimaa P. Vitamin D, aging, and cancer. Nutrition reviews. 2008;66:S147–52. doi: 10.1111/j.1753-4887.2008.00095.x. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Annals of epidemiology. 2009;19:73–8. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D: its role in cancer prevention and treatment. Progress in biophysics and molecular biology. 2006;92:49–59. doi: 10.1016/j.pbiomolbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Hewison M, Zehnder D, Chakraverty R, Adams JS. Vitamin D and barrier function: a novel role for extra-renal 1 alpha-hydroxylase. Molecular and cellular endocrinology. 2004;215:31–8. doi: 10.1016/j.mce.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Palmieri C, MacGregor T, Girgis S, Vigushin D. Serum 25-hydroxyvitamin D levels in early and advanced breast cancer. Journal of clinical pathology. 2006;59:1334–6. doi: 10.1136/jcp.2006.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuhouser ML, Sorensen B, Hollis BW, et al. Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. The American journal of clinical nutrition. 2008;88:133–9. doi: 10.1093/ajcn/88.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757–63. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- 11.Bolanowski M. Commentary on an Endocrine Society clinical practice guidelines published in Journal of Clinical Endocrinology and Metabolism 2011; 96: 273–288. Endokrynologia Polska. 2011;62(Suppl 3):23–4. [PubMed] [Google Scholar]
- 12.IoM IoM. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: Insitiute of Medicine of the National Academies; 2010. [Google Scholar]
- 13.Jacobs ET, Thomson CA, Flatt SW, et al. Vitamin D and breast cancer recurrence in the Women’s Healthy Eating and Living (WHEL) Study. The American journal of clinical nutrition. 2011;93:108–17. doi: 10.3945/ajcn.2010.30009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tretli S, Schwartz GG, Torjesen PA, Robsahm TE. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: a population-based study. Cancer Causes Control. 2012;23:363–70. doi: 10.1007/s10552-011-9885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vrieling A, Hein R, Abbas S, Schneeweiss A, Flesch-Janys D, Chang-Claude J. Serum 25-hydroxyvitamin D and postmenopausal breast cancer survival: a prospective patient cohort study. Breast Cancer Res. 2011;13:R74. doi: 10.1186/bcr2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McTiernan A, Rajan KB, Tworoger SS, et al. Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol. 2003;21:1961–6. doi: 10.1200/JCO.2003.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rojas-Rivera J, De La Piedra C, Ramos A, Ortiz A, Egido J. The expanding spectrum of biological actions of vitamin D. Nephrol Dial Transplant. 2010;25:2850–65. doi: 10.1093/ndt/gfq313. [DOI] [PubMed] [Google Scholar]
- 18.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 19.Heaney RP. Vitamin D: how much do we need, and how much is too much? Osteoporos Int. 2000;11:553–5. doi: 10.1007/s001980070074. [DOI] [PubMed] [Google Scholar]
- 20.The International Classification of Diseases, 10th Edition (ICD-10) changeover is coming. Optometry. 2010;81:551–3. [PubMed] [Google Scholar]
- 21.Ng K, Wolpin BM, Meyerhardt JA, et al. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. British journal of cancer. 2009;101:916–23. doi: 10.1038/sj.bjc.6605262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the lunar DPX-L densitometer. J Clin Densitom. 2000;3:35–41. doi: 10.1385/jcd:3:1:035. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF. Vitamin D deficiency. The New England journal of medicine. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 24.Therneau TM, Grambsch PM. Modeling survival data : extending the Cox model. New York: Springer; 2000. [Google Scholar]
- 25.Kleinbaum DG, Klein M. Survial Analysis A Self-Learning Text. 2. New York: Springer; 2005. Evaluating the Proportional Hazards Assumption; pp. 131–71. [Google Scholar]
- 26.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.WHO. Obesity: preventing and managing the global epidemic. Geneva, Switzerland: World Health Organization; 2000. [PubMed] [Google Scholar]
- 28.Wener MH, Daum PR, McQuillan GM. The influence of age, sex, and race on the upper reference limit of serum C-reactive protein concentration. Journal of Rheumatology. 2000;27:2351–9. [PubMed] [Google Scholar]
- 29.Bodnar LM, Catov JM, Wisner KL, Klebanoff MA. Racial and seasonal differences in 25-hydroxyvitamin D detected in maternal sera frozen for over 40 years. British Journal of Nutrition. 2009;101:278–84. doi: 10.1017/S0007114508981460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman D, Pike JW, Glorieux FH. Vitamin D. 2. Burlington, MA: Elsevier Academic Press; 2005. [Google Scholar]
- 31.Brown AJ, Dusso A, Slatopolsky E. Vitamin D. The American journal of physiology. 1999;277:F157–75. doi: 10.1152/ajprenal.1999.277.2.F157. [DOI] [PubMed] [Google Scholar]
- 32.Honda H, Qureshi AR, Axelsson J, et al. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. The American journal of clinical nutrition. 2007;86:633–8. doi: 10.1093/ajcn/86.3.633. [DOI] [PubMed] [Google Scholar]
- 33.Mathiasen IS, Lademann U, Jaattela M. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res. 1999;59:4848–56. [PubMed] [Google Scholar]
- 34.Eisman JA, Barkla DH, Tutton PJ. Suppression of in vivo growth of human cancer solid tumor xenografts by 1,25-dihydroxyvitamin D3. Cancer Res. 1987;47:21–5. [PubMed] [Google Scholar]
- 35.Ooi LL, Zhou H, Kalak R, et al. Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer Res. 2010;70:1835–44. doi: 10.1158/0008-5472.CAN-09-3194. [DOI] [PubMed] [Google Scholar]
- 36.Ooi LL, Zheng Y, Zhou H, et al. Vitamin D deficiency promotes growth of MCF-7 human breast cancer in a rodent model of osteosclerotic bone metastasis. Bone. 2010;47:795–803. doi: 10.1016/j.bone.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Ostrovnaya I, Olshen AB, Seshan VE, Orlow I, Albertson DG, Begg CB. A metastasis or a second independent cancer? Evaluating the clonal origin of tumors using array copy number data. Stat Med. 2010;29:1608–21. doi: 10.1002/sim.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holick MF, Garabedian M. Vitamin D: photobiology, metabolism, mechasnism of action, and clinical applications. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. Washington, DC: American Society for Bone and Mineral Research; 2006. pp. 129–37. [Google Scholar]
- 39.IoM IoM. Dietary reference intakes: calcium, phosphorus, magnesium, vitamin D, fluoride. Washington DC, USA: National Academy Press; 1997. [PubMed] [Google Scholar]
- 40.Alpert PT, Shaikh U. The effects of vitamin D deficiency and insufficiency on the endocrine and paracrine systems. Biol Res Nurs. 2007;9:117–29. doi: 10.1177/1099800407308057. [DOI] [PubMed] [Google Scholar]
- 41.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. The American journal of clinical nutrition. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 42.Medicine) IIo. Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Press; Washington, DC: 2010. [PubMed] [Google Scholar]
- 43.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]