Abstract
Numerous studies published in the past two decades have identified the viral protein R (Vpr) as one of the most versatile proteins in the life cycle of human immunodeficiency virus type 1 (HIV-1). In this regard, more than a thousand Vpr molecules are present in extracellular viral particles. Subsequent to viral entry, Vpr participates in early replicative events by assisting in viral genome nuclear import and, during the viral life cycle, by shuttling between the nucleus and the cytoplasm to accomplish its functions within the context of other replicative functions. Additionally, several studies have implicated Vpr as a proapoptotic protein because it promotes formation of permeability transition pores in mitochondria, which in turn affects transmembrane potential and adenosine triphosphate synthesis. Recent studies have identified Vpr as a virion-free protein in the serum and cerebrospinal fluid of patients infected with HIV-1 whose plasma viremia directly correlates with the extracellular concentration of Vpr. These observations pointed to a new role for Vpr as an additional weapon in the HIV-1 arsenal, involving the use of an extracellular protein to target and possibly inhibit HIV-1-uninfected bystander cells to enable them to escape immune surveillance. In addition, extracellular Vpr decreases aden-osine triphosphate levels and affects the intracellular redox balance in neurons, ultimately causing their apoptosis. Herein, we review the role of Vpr as an extracellular protein and its downstream effects on cellular metabolism, functionality, and survival, with particular emphasis on how extracellular Vpr-induced oxidative stress might aggravate HIV-1-induced symptoms, thus affecting pathogenesis and disease progression.
I. INTRODUCTION
From the discovery and early structural and functional studies (Cohen et al., 1990a,b; Ogawa et al., 1989) to the present, the virion-associated viral regulatory protein, viral protein R (Vpr), has been assigned a number of roles throughout the viral life cycle. Numerous reviews (Ayyavoo et al., 1997a; Bukrinsky and Adzhubei, 1999; Majumder et al., 2009; Morellet et al., 2009; Romani and Engelbrecht, 2009) have elegantly analyzed and summarized the body of literature regarding the functional properties of Vpr. Because Vpr is a virion structural protein packaged during viral budding from infected cells (Cohen et al., 1990a; Yuan et al., 1990), its journey through numerous intra- and extracellular pathways and environments remains of great interest to those studying the human immunodeficiency virus type 1 (HIV-1) pathogenic process and engaging in the quest to prevent and treat diseases associated with viral infection. In this regard, Vpr is delivered into the newly infected cell along with the viral genome and a number of other virion proteins subsequent to fusion of the viral envelope with the plasma membrane. Within this intracellular cytoplasmic context, Vpr acts as an early protein by interfacing with the preintegration complex (Hrimech et al., 1999), which is composed of the viral genome and other viral proteins, to facilitate continued reverse transcription and genomic access to the nucleus. In addition to its immediate-early role in the cytoplasm, Vpr has been shown to localize to the nucleus (Di Marzio et al., 1995; Lu et al., 1993), where it may function as a transcriptional regulatory protein participating in the production of early viral transcripts following integration of the proviral genome (Agostini et al., 1996; Felzien et al., 1998; Sawaya et al., 1998, 2000; Subbramanian et al., 1998; Wang et al., 1995). As a shuttling protein (Sherman et al., 2001), Vpr is also able to exit the nucleus to participate in other aspects of the viral life cycle (Sherman et al., 2003). In this regard, Vpr interacts with the p6 Gag protein product during the process of incorporation into newly formed and budding virions (Bachand et al., 1999; Huang et al., 1995; Kondo et al., 1995; Paxton et al., 1993). Vpr also associates with a number of different cellular proteins, thereby halting the cell cycle at the G2/M phase (Chowdhury et al., 2003; He et al., 1995; Mahalingam et al., 1998), a function that has been proposed to occur independently of (Ayyavoo et al., 1997a; Goh et al., 1998) or as an event leading to apoptosis (Fukumori et al., 2000). The Vpr-induced proapoptotic phenotype may also play an important role in immune escape (Ayyavoo et al., 1997b). Indeed, cell-cycle arrest coincides with the peak of viral transcription (Goh et al., 1998), and Vpr has been shown to induce apoptosis of the infected cell. These two events promote increased viral production and release, thus facilitating the production of infectious extracellular virus, the demise of the infected cell, and evasion of immune surveillance. Additionally, within the context of cell-cycle arrest and apoptosis, Vpr is able to either actively or passively gain access to the extracellular compartment, thereby functioning as an extracellular soluble protein (Fig. 1). This role is particularly significant because cell- and virion-free extracellular Vpr causes detrimental effects to uninfected bystander cells. Further, patients infected with HIV-1 develop antibodies against Vpr-immunodominant peptides (Herzenberg et al., 1997; Reiss et al., 1990; Richardson et al., 2003), suggesting that Vpr either is recognized in the extracellular environment by the immune response of the host or is presented as a processed peptide to the immune system within the context of the viral life cycle. Nonetheless, despite recent evidence concerning the role of Vpr as a secreted protein, its role in the extracellular milieu is not fully understood.
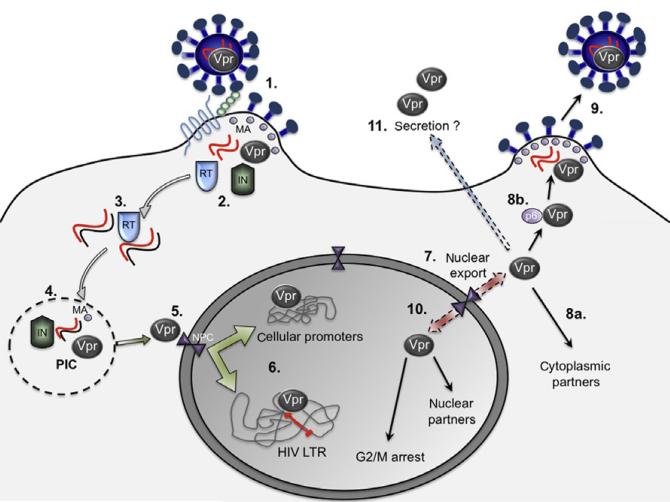
FIGURE 1. Role of Vpr in the viral life cycle.
Vpr, located within HIV-1 virions (1), enters cells and is released into the cytoplasm when the virion is uncoated (2). After reverse transcription of the viral RNA genome by the viral reverse transcriptase (RT) (3), Vpr forms the preintegration complex (PIC) (4) along with other viral proteins (primarily integrase [IN] and matrix [MA]). Vpr subsequently facilitates the transport of the PIC to the nucleus through the nuclear pore complex in the nuclear membrane (5). Once the PIC gains access to the nucleus, the reverse-transcribed HIV proviral DNA serves as a substrate for the viral-encoded integrase and is incorporated into cellular chromosomes. Subsequently, Vpr has been shown to act as a transcription factor by binding to the HIV-1 promoter or long terminal repeat (LTR) of the newly integrated proviral genome as well as to promoters of other cellular genes, driving synthesis of both viral and cellular gene transcripts (6). Due to the presence of a nuclear export signal, Vpr can also be exported from the nucleus to the cytoplasmic environment (7). Within the cytoplasm, Vpr interacts with cellular proteins (8a) and with the Gag C-terminus protein (p6)/Gag (8b), thereby being incorporated into nascent virions, which then bud out of the surface of the infected cell (9). During later stages, Vpr continues to function as a nucleocytoplasmic shuttling protein within the viral life cycle (10) and halts cell-cycle progression. This activity is believed to increase viral transcription to guarantee a large number of viral RNA genomes in order to drive the particle assembly process. Represented in light green and light blue at the cell surface are CD4 and CXCR4/CCR5, the receptor and coreceptors, respectively.
II. EXTRACELLULAR VPR: ACTIVE SECRETION OR NONSPECIFIC RELEASE?
Although numerous studies over the years have outlined the detrimental effects of Vpr as a virus- and cell-free protein in the extracellular milieu, the process of Vpr secretion from specific producer cell populations has only recently been investigated (Xiao et al., 2008). In this study, which represents the first evidence of Vpr secretion from cells, the authors evaluated the Vpr phenotypes from three different cell types: human endothelial kidney (HEK) 293T cells, Jurkat T cells (a proleukocytic cell lineage), and peripheral blood mononuclear cells (PBMCs). All of these cell populations were assayed for the presence of Vpr in the virus- and cell-free extracellular compartment 2 days after transfection, which is thought to be similar to the time required in an infected cell for Vpr to be transcribed or translated from the integrated provirus, thereby modeling acute in vivo viral replication. However, when the results from these studies are analyzed, a number of caveats require consideration. First, cells were transfected, not infected, so distinctive pathways and proteins could play intracellular roles that would differ from those played in infected target cells. Second, because Vpr is known to confer a proapoptotic phenotype in both infected and transfected cells, the secretory process might be the result of excessive cell death, which in turn would be responsible for the release of detectable amounts of Vpr into the extracellular space. In addition, secretion was evaluated at about 40 h after transfection, which corresponds to the peak of viral particle production; therefore, secretion of Vpr could be driven by high concentrations of Vpr in the cytoplasm late in the transfection process. Indeed, Vpr is known to shuttle between the nucleus and the cytoplasm; thus, excessive transcription and translation of intracellular Vpr could overload or hijack the secretory (classical or nonclassical) pathway for its own use, thereby leading to a passive nonspecific secretory process. Because Vpr is found in the extracellular environment, its ability to hijack the secretory process could represent an additional weapon in the viral arsenal to induce detrimental effects during specific stages of disease progression. Further, transfection of cells could mimic a scenario of active infection with abundant production of virions and proteins as transcription driven by the HIV-1 promoter is increased. Because Vpr has been shown to play a role in halting the cell cycle at the G2/M phase, a time at which viral production is maximal, it would be interesting to define the timing of these events with respect to Vpr secretion. In other words, Vpr-induced arrest at the G2/M phase could facilitate enhanced viral production, which in turn could augment Vpr secretion. However, these studies were performed in HEK 293T cells, which have little relevance to the pathogenesis of HIV-1 infection.
Previous intracellular fractionation studies (Lu et al., 1993) have also identified Vpr in cytosolic as well as plasma membrane fractions derived from PBMCs, which suggests that Vpr may function in more than just the nuclear and cytosolic compartments. Vpr may function within cellular membranes or be exported to the extracellular environment where it may impact other extracellular signaling pathways or other secondary bystander target cells. In this regard, the kinetics of Vpr secretion has not yet been defined. This information could have great impact on our understanding of the functional properties of Vpr. Relevant to these observations, studies have shown that Vpr needs most of the other viral proteins in order to be secreted. Recent studies have also shown that Vpr was secreted as a dimer (Zhao et al., 1994), with additional studies reevaluating its role in viral pathogenesis (Fritz et al., 2008; Venkatachari et al., 2010). Nevertheless, none of the studies published thus far regarding Vpr secretion has examined the functional properties of the secreted form of the protein. On the basis of results from studies obtained with immunoprecipitated extracellular media performed in immunoblot assays, it is not possible to determine whether the secreted Vpr protein is in its native, misfolded, or denatured form. Thus, conclusions concerning the functional properties of extracellular Vpr must remain conservative when one evaluates the downstream effects of extracellular Vpr on target cells because no studies have actually investigated the structural nature of extracellular Vpr. Nonetheless, a recent report has evaluated the difference between the consequences of native and denatured extracellular Vpr on target cells (Sherman et al., 2002). This study concluded that Vpr requires proper folding in order for the extracellular protein to cause detrimental effects on secondary target cells. Collectively, the studies concerning how Vpr affects cellular viability suggest that, if extracellular Vpr is the cause of the observed effects in the periphery and central nervous system (CNS), then the protein is most likely properly folded. Nevertheless, a well-designed study clearly establishing a direct correlation between proper folding of extracellular Vpr and its induction of downstream effects on target cells has yet to be performed in a convincing manner.
The presence of extracellular Vpr in both the serum and cerebrospinal fluid (CSF) of HIV-1-infected patients was initially reported by two studies (Levy et al., 1994, 1995); the concentration of extracellular Vpr was found to increase with disease progression and to augment viral particle release from infected PBMCs. Additionally, latent HIV-1-infected cell lines exposed to extracellular Vpr reactivated viral transcription from the latent state and subsequently induced the release of newly synthesized infectious viral particles (Levy et al., 1995). Evidence suggesting the secretory nature of Vpr also comes from two recent studies (Hoshino et al., 2007; Jones et al., 2007), wherein Vpr was found as a free extracellular protein in the serum of patients infected with HIV-1. In one of these reports, the concentration of extracellular Vpr in the plasma of a number of patients infected with HIV-1 (with different viral loads) was estimated by a qualitative immunoblotting assay to be 5–10 ng/ml (Hoshino et al., 2007). Although a better and more quantitative assessment is needed, this effort represents the first to determine the extracellular Vpr concentration in circulation. Further, this study showed the extracellular Vpr concentration in the serum to be directly proportional to HIV-1 viral load in the plasma, which validates the concept that increased secretion of Vpr occurs in the context of higher viral replication. Consistent with these observations, Vpr has been detected by enzyme-linked immunosorbent assay (ELISA) in the plasma of Vpr-transgenic mice at concentrations between 10 and 22 pg/ml (Balasubramanyam et al., 2007). These data suggest that Vpr is released in at least two different host species, which may indicate that the cellular processes required for Vpr secretion are conserved across species as a result of critical roles they play in cellular physiological processes. Nevertheless, transgenic mice carrying a Vpr gene driven by a cytomegalovirus promoter with no other viral genes present were used to demonstrate that, in contrast to results derived from in vitro studies (Xiao et al., 2008), Vpr was released only when most of the other viral proteins were present (except for the envelope and Nef proteins). The discovery of extracellular Vpr in the plasma of Vpr-transgenic mice, as compared to the situation in patients infected with HIV-1, suggests either that, in mice, Vpr is secreted without the aid of any other viral proteins or that the process of Vpr secretion is forced by overabundant production of the viral protein that saturates the intracellular compartment, thereby resulting in enhanced secretion. However, the concentration of HIV-1 Vpr in the blood of Vpr-transgenic mice is lower than that in patients infected with HIV-1 by at least two orders of magnitude, which could suggest that the presence of other components of the viral genome is required for more robust secretion. In another study (Jones et al., 2007), extracellular Vpr was detected in a different type of transgenic mice expressing Vpr driven by the c-fms (M-CSF receptor) promoter, which drives the expression of the Vpr gene only in cells of the monocytoid lineage. One might conclude that peripheral promonocytic cells or CNS microglia, perivascular cells, or parenchymal cells, all of which support transcription from the c-fms promoter, may be completely or partially responsible for the observed in vivo release of extracellular Vpr. Despite measurements in the serum of both humans and mice, the extracellular concentration of Vpr in the CNS compartment has not yet been quantitated (Levy et al., 1994).
III. EXTRACELLULAR PRESENCE OF OTHER HIV-1 PROTEINS
Other HIV-1 proteins are also secreted or released from either infected or transfected cells after exposure to the relative expression plasmids. This could either represent an active process that would methodically increase the pathogenic potential of the virus, leading to spread of infectivity to a wide variety of tissues or a passive release from HIV-1-infected cells, which are constitutively expressing viral proteins. One of the first proteins found to be secreted was the envelope glycoprotein gp120 (Hallenberger et al., 1993), along with the larger gp160 protein, especially within the CNS (Kanmogne et al., 2002). As a secreted extracellular protein, gp120 has been shown to stimulate the release of neurotoxic factors (Giulian et al., 1993) and impair astrocytic uptake of glutamate by reducing expression of its transporter (Vesce et al., 1997; Wang et al., 2003).
HIV-1 Tat, primarily known as the major transactivator protein of viral gene expression, is also secreted from cells. Initial studies have shown that Tat is secreted both after transfection with a Tat-expressing plasmid and during acute infection when viral production is maximal and the rate of cell death is minimal (Ensoli et al., 1993), which suggests a mechanism different from cell death based on the timing of Tat secretion. Indeed, Tat exploits the secretory pathway to reach the extracellular environment (Chang et al., 1997) due to its intracellular shuttling ability (Stauber and Pavlakis, 1998). As an extracellular protein, Tat may be taken up by specific receptor-bearing cells, thus being able to promote cell growth and HIV-1 gene expression (Ensoli et al., 1993). In addition, not only are T cells (unlike monoblastoid cells) capable of taking up extracellular Tat, but also is the viral transactivator protein able to induce programmed cell death (Vendeville et al., 2004; Yang et al., 2003).
HIV-1 Nef, known predominantly for its role in viral replication and pathogenesis, has also been shown to be secreted, both by infected and transfected cells, into vesicles (Campbell et al., 2008) that may fuse with virions or uninfected bystander cells, thereby entering the cellular cytoplasm to cause downstream cellular signaling (Macreadie et al., 1998). Exposure to extracellular Nef causes apoptosis in different cell types, especially HIV-1-infected CD4+ T cells (James et al., 2004).
IV. SOURCE OF EXTRACELLULAR VPR IN THE BRAIN
The presence of a soluble form of Vpr in the extracellular milieu of patients infected with HIV-1 raises several questions: (1) Is Vpr secreted through an active classical or nonclassical secretory pathway? (2) Is extracellular Vpr passively released from apoptotic cells? (3) Is extracellular Vpr the result of a necrotic process involving some HIV-1-infected cells? (4) Do decaying virions contribute to seeding Vpr in the extracellular compartment? (5) Does Vpr diffuse out nonspecifically as a result of a “slippery process” based on a lack of an interaction with the Gag p6 protein, which is known to be fundamental for incorporation of Vpr into budding virions? (6) Could Vpr be transmitted from cell to cell through the formation of virological synapses, which have been shown to facilitate virus transmission?
In general, the detection of Vpr in the extracellular milieu results from either active secretion or “passive diffusion” from cells undergoing programmed cell death. Additionally, the detection of Vpr in the plasma and in the CSF of patients infected with HIV-1 certainly suggests that Vpr could go through a secretory process in the CNS similar to that demonstrated in the peripheral circulation. It is of interest to speculate which cells within the CNS might secrete Vpr during the course of HIV-1 disease; likely candidates include infected microglia, one of the brain-resident cells most productively infected with HIV-1, and perivascular macrophages at the lining of the blood–brain barrier (BBB) because they might seed infection directly within the CNS and contribute to the release of Vpr during and after brain entry. In addition, infected cells in the process of crossing the BBB are a possible source of Vpr. Under physiological conditions, only a limited number of activated T cells, regardless of their antigen specificity, gain access to the CNS during the “immune surveillance” that functions to clear foreign pathogens and nonself molecules (Hickey, 1999). Under normal conditions, blood-borne monocytes travel to healthy CNS tissues in low numbers (Ransohoff, 2003), but under inflammatory conditions, leukocytes, through a process referred to as diapedesis, cross the BBB and accumulate in the perivascular area, a hallmark of CNS immune response during severe forms of HIV-1-associated neurological disease (reviewed by Williams and Hickey, 1996). Although other blood-derived cell types might migrate to the CNS (B cells, neutrophils, and dendritic cells) under inflammatory conditions, the majority of infiltrating cells are monocyte-derived macrophages (MDMs) with some infiltration of CD4+ T cells. Although the former are known to extravasate, especially during periods of increased inflammation, infected CD4+ T cells are far less prone to cross the BBB, making them a less likely candidate for intra-CNS Vpr secretion. In either case, infected blood-borne cells are likely involved in the secretory process. Moreover, MDMs or CD4+ T cells could also be responsible for secretion of other viral proteins including Tat (Chang et al., 1997; Ensoli et al., 1993) and Nef (Ali et al., 2010; Campbell et al., 2008; Lenassi et al., 2010) along with the shedding of the envelope protein gp120 (Hallenberger et al., 1993; Hart et al., 1991; Li et al., 1994) and a complex network of inflammatory cytokines and chemokines after traversing the BBB. Among brain-resident cells, in addition to microglia, astrocytes must also be considered, although their loss has not been directly associated with disease progression (Fig. 2). Indeed, recent reports (Churchill et al., 2006, 2009) have reevaluated the role astrocytes play in neuropathogenesis. The facts that they represent the most abundant cell type within the CNS and are susceptible to HIV-1 infection make them a potential source of secreted Vpr with the CNS. Although the loss of neurons represents one of the pathological hallmarks of the severe forms of HIV-associated neurocognitive impairment, they are not considered susceptible to HIV-1 infection and therefore are an unlikely source of Vpr within the CNS compartment. Another potential source of soluble Vpr within the CNS is represented by molecules released in the peripheral lymph nodes or circulation with intra-CNS activity achieved after crossing the BBB. Although this possibility would seem less likely on the basis of pharmacological considerations alone, it cannot be ruled out. During the course of HIV-1 disease, viral infection has been associated with functional and physical alterations in the BBB (Andersson et al., 2001; Dallasta et al., 1999; Eugenin et al., 2006; Liu et al., 2002; Wang et al., 2008) that compromise its integrity, thereby possibly allowing otherwise impenetrable compounds to gain access to the finely controlled CNS environment.
FIGURE 2.
Possible routes of entry of soluble Vpr from the periphery to the central nervous system. Within the periphery, lymphocytes (green) and cells of the monocyte-macrophage (blue) lineage are the major cellular carriers of HIV-1, although the number of infected undifferentiated monocytes is low. After traversing the blood–brain barrier (BBB), monocytes differentiate into short-lived MDMs that are capable of seeding infection along with releasing Vpr throughout the CNS. Lymphocytes are also able to traverse the BBB after antigen presentation by local infected perivascular phagocytic cells, thus representing a source for Vpr release once activated. Among brain-resident cells, microglial cells (red/maroon) represent the primary susceptible and permissive cellular target for HIV-1, possibly capable of releasing/secreting newly synthesized Vpr protein on activation in response to the presence of pathogens within the CNS. Resting microglia are only capable of supporting low levels of viral transcription and are therefore not a main source of Vpr. Brain microvascular endothelial cells (BMVEC) (orange) are also infected by HIV-1 and therefore may be responsible for disseminating Vpr into the CNS. Astrocytes (yellow) are the most abundant cells in the CNS; however, their frequency of infection and level of permissivity are likely lower than cells of the monocyte-macrophage lineage. During late stages of HIV-1 disease, the number of HIV-infected astroglial cells increases, thus seeding infection and participating in Vpr release. Neurons (pink) are thus far not known to be infected by HIV-1.
Although several researchers have investigated the effects of extracellular Vpr on native cell populations within the brain, primarily neurons, we may only speculate as to the source of Vpr. It is likely that a combination of different sources could participate in Vpr release to the extracellular space (whether active or passive), which represents one of the several functions accomplished by this pleiotropic viral protein. It has also been proposed that soluble virus- and cell-free extracellular Vpr could be more active than endogenously expressed Vpr in increasing transcriptional activity (Levy et al., 1994). This hypothesis, if proven in in vivo studies, could explain why Vpr has evolved to gain access to the extracellular environment. It might represent a trigger to reactivate viral expression from latently HIV-1-infected cells, especially cells of the monocytoid lineage (Varin et al., 2005), or from persistently infected cells, such as astrocytes. Indeed, several different cell types are susceptible to increased viral production when exposed to extracellular Vpr. Cells acutely infected with HIV-1 show a higher peak in release of viral particles when cocultured with extracellular Vpr; conversely, cells chronically infected with HIV-1, which under normal conditions show undetectable levels of viral synthesis, display a characteristic bell-shaped curve in virion production when exposed to extracellular Vpr (Levy et al., 1995). This finding clearly underscores the importance of extracellular Vpr, especially in the milieu of chronically infected cells, because it might potentially trigger reactivation of viral production. Another important relevant observation is the corresponding detection of increased levels of extracellular Vpr with disease progression, especially in patients with AIDS (Hoshino et al., 2007), which could represent an important component of the pathological process associated with viral replication and reactivation during the late stages of disease.
V. IMPACT OF EXTRACELLULAR VPR ON CELLS IN THE PERIPHERY
Many recent studies have examined the impact of purified recombinant preparations of Vpr on different target cell populations to evaluate the downstream effects of this viral protein on functional properties and viability. One of the first reports of Vpr as an extracellular protein (Levy et al., 1994) was unique in that it demonstrated the ability of this viral accessory protein to increase viral replication and viral particle release, thus allowing increased production of viral progeny and the spread of infection to other organ systems (Levy et al., 1994, 1995; Nakamura et al., 2002; Sherman et al., 2002). In addition, one of the first studies to use synthetic Vpr showed that the viral protein can be internalized and localized both to the cytoplasm and the nucleus (Henklein et al., 2000). Because endogenously expressed Vpr is known to localize to the nucleus and nuclear envelope (Kamata and Aida, 2000; Sherman et al., 2001; Waldhuber et al., 2003), the ability of endocytosed extracellular Vpr to display a similar localization pattern might be the result of interaction with intracellular protein partners. Comprehensive reviews of these experimental observations have indicated that Vpr localizes to specific intracellular organelles (Sherman et al., 2002). However, despite these interesting investigations, the phenotype resulting from extracellular Vpr transduction is not fully understood. Neither the process that drives Vpr internalization (either by receptor-mediated invagination or receptor-independent endocytosis) nor the cause of this event has been investigated, leaving a multitude of unresolved questions: (1) Is Vpr actually endocytosed or is Vpr internalized through a process caused by the excessive presence of Vpr in conditions reproduced in vitro? (2) Is Vpr internalized by a receptor-mediated mechanism? (3) Why is Vpr internalized? (4) Is Vpr internalization part of the “viral plan” to escape immune surveillance or is it a completely “nonspecific event”? (5) Are there differences in function between internalized Vpr and endogenously expressed Vpr? (6) Is Vpr taken up by any cell type, or is the uptake cell type dependent? (7) Are there cells resistant to Vpr uptake? (8) Does Vpr signal through a yet unknown surface receptor(s)?
Vpr enters a number of different cell types, including PBMCs (Sherman et al., 2002). This biological activity likely involves the hydrophobic structure of Vpr within its three alpha-helices centered in the middle of the protein (Morellet et al., 2003), which could potentially confer the Vpr transducing property. Indeed, studies have identified the third helix as being responsible for the Vpr transducing activity (Taguchi et al., 2004). Moreover, it was shown in several human and nonhuman cell lines that a chimeric protein containing the C-terminal half of Vpr possesses higher transfection efficiency than its native counterpart. This experimental approach facilitates delivery of any desired DNA to the nucleus through a yet unknown pH-independent pathway (Heinzinger et al., 1994; Kichler et al., 2000). Therefore, the Vpr C-terminal moiety, which includes the third alpha-helix, has the ability to transduce the plasma membrane, likely based on high-affinity interactions with plasma membrane-bound proteins or phospholipids (Coeytaux et al., 2003). Consequently, Vpr not only displays a high affinity for nucleic acids (Zhang et al., 1998), but it is also capable of carrying DNA molecules to the nucleus, where plasmids could then be transcribed in order to express any desired gene in transfection-based experimentation (Mizoguchi et al., 2005).
The ability of Vpr to cause internalization and cell death when added externally was initially proven in a variety of yeast strains and attributed to a peptide (HFRIGCRHSRIG) located at the end of the third alpha-helix (Macreadie et al., 1995, 1996). The transducing ability was then demonstrated using CD4+ T cells, the primary cellular target of HIV-1 (Sattentau et al., 1986). Indeed, extracellular Vpr gains access to the cellular cytoplasm through a CD4-independent mechanism and penetrates lymphocytes, with ensuing rapid reduction in mitochondrial transmembrane potential, formation of apoptotic bodies, and DNA fragmentation, with cell death realized as an end point (Arunagiri et al., 1997). In addition, extracellular Vpr impairs T-cell activation and proliferation after antigen-specific priming because it inhibits nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-mediated activation, which blocks production of cytokines indispensable for suppression of viral replication, compromising the host immune response. Extracellular Vpr has also been shown to induce apoptosis in the absence of T-cell receptor (TCR)-mediated activation, thus promoting apoptosis of uninfected bystander T cells. In contrast, in the presence of TCR-mediated activation, extracellular Vpr halts the expected induction of apoptosis, hence sparing HIV-1-infected cells, which could function as long-lived viral reservoirs (Ayyavoo et al., 1997b). In addition, endogenously synthesized Vpr (Andersen et al., 2005, 2006; Jian and Zhao, 2003; Nonaka et al., 2009; Sabbah et al., 2006; Stewart et al., 1997, 2000), virion-associated Vpr (Arokium et al., 2009), and soluble extracellular Vpr (Goh et al., 2004; Hoshino et al., 2007; Jones et al., 2007; Mishra et al., 2007) have been shown to induce cell-cycle arrest and apoptosis, which may point to a conserved proapoptogenic property of Vpr regardless of the origin of or delivery source into the cellular environment. However, researchers have recently reported an antiapoptotic property specifically for endogenously expressed Vpr (Conti et al., 1998, 2000; Fukumori et al., 1998; Matarrese et al., 2000; Zhu et al., 2003). This apparent controversy raises the possibility that endogenously expressed Vpr acts either as a promoter or an inhibitor of apoptosis on the basis of its intracellular concentration or location. Indeed, during acute infection (modeled in vitro by transient transfection experimentation), productive synthesis of Vpr is obtained, which induces cell-cycle arrest and apoptosis. In contrast, during chronic or latent infection (simulated in vitro by low Vpr-expressing stably transfected clones), a lower quantity of intra-cellular Vpr is produced, which does not seem to be sufficient to induce apoptosis but instead promotes cell survival through a regulated balance of pro- and antiapoptotic factors (bcl-2 and bax; Cheng et al., 1996). This observation represents an interesting hypothesis to explain the differential cellular fate observed during the progression of HIV-1 disease. During acute infection (when synthesis of viral proteins is high and the concentration of Vpr is therefore elevated), cells (especially CD4+ T lymphocytes) undergo extensive apoptosis, which is followed by a long period of cell survival, limited cell turnover, and limited viral gene expression and synthesis (Conti et al., 1998). Although the intracellular levels of Vpr may explain why cells are either prone or resistant to apoptosis, the intrinsic molecular switch mechanism is not understood. Similar to Vpr expressed endogenously, extracellular Vpr concentrations likely regulate cellular fate toward either programmed cell death or survival. This idea is in line with studies reporting that increased extracellular Vpr concentration is correlated with HIV-1 disease progression (Levy et al., 1994, 1995).
Proapoptotic biological activity of Vpr is particularly important when considering antigen-presenting cells, primarily dendritic cells, and macrophages, which could potentially be shut down on engulfment of extracellular Vpr in order to eliminate it from the extracellular environment. Therefore, the cells responsible for Vpr clearance could undergo the damaging downstream effects caused by Vpr, thus becoming detrimental to the host. In this regard, in a recent study, dendritic cells infected with a Vpr-containing virus demonstrated a reduced ability with respect to antigen presentation by inhibiting transcription of essential costimulatory molecules and impairing expression of cytokines indispensable for immune activation (Majumder et al., 2005). A similar study performed by treating predifferentiated cells of monocytic origin with recombinant Vpr showed impairment of the differentiation process even in the presence of maturation signals, because the costimulatory receptors (CD80 and CD86) were not expressed in a high enough concentration on the plasma membrane (Muthumani et al., 2005). This event, in turn, abolishes maturation of these cells into professional antigen-presenting cells. A similar study showed that extracellular Vpr-treated dendritic cells not only promote excessive production of the inflammatory cytokine tumor necrosis factor-α (TNF-α) but also dysregulate the CD8+ T-cell proliferation pathway, leading to their death, thus diminishing the reservoir of cytotoxic cells capable of mounting an adequate immune response to the virus (Majumder et al., 2007). This study shows how the effects of extracellular Vpr are important not only to the cells directly exposed to the viral protein but also to the downstream cell populations, whose interactions are finely tuned by the immune system. In addition, activation of CD8+ T cells has been shown to be directly impaired after treatment with extracellular Vpr, thereby profoundly suppressing their cytotoxic activity and compromising T-cell-mediated immunity (Muthumani et al., 2002).
Extracellular Vpr also impacts cells of the monocytic lineage, another cell population targeted by HIV-1. Vpr activates the three branches of the mitogen-activated protein kinase (MAPK) pathway through phosphorylation of p38, extracellular-signal-regulated kinases (ERK; Re et al., 2006), and c-Jun N-terminal kinases (JNK), with subsequent downregulation of the antiapoptotic proteins Bcl-2 and c-IAP1. These events, in turn, induce mitochondrial permeabilization and cytochrome c release through a caspase cascade process, which leads to the cleavage of poly ADP ribose polymerase (PARP) in the nucleus and subsequent apoptosis (Mishra et al., 2007). Studies have demonstrated how extracellular Vpr activates the MAPK pathway and promotes viral transcription in both promonocytic cells and fully differentiated macrophages (Varin et al., 2005). Recently, the same cells have been shown to actively produce interleukin-6 (IL-6), a proinflammatory cytokine, by signaling through the Toll-like receptor (TLR)-4 and the MyD88 adaptor, which in turn requires the NF-κB pathway (Hoshino et al., 2010) and phosphorylation of CCAAT-enhancer-binding protein β (C/EBPβ). Additionally, extracellular Vpr induces oxidation of phospholipids, thus having negative effects on the intracellular redox balance. Considering the fact that MDMs are antigen-presenting cells, the effects caused by extracellular Vpr are particularly abrupt because the immune system begins to lose the capability of defending the host against pathogenic agents. Moreover, if HIV-1-infected MDMs are taken into account, the effects are amplified because viral particles are synthesized intracellularly, thereby increasing viral load, and extracellular Vpr arrests their differentiation (Levy et al., 1993), halting their ability to present antigens. Although the aforementioned studies clearly identify Vpr as being involved in antigen presentation by dendritic cells, further studies are needed to determine how extracellular Vpr influences antigen presentation by MDMs and corrects antigen uptake and processing by both dendritic cells and MDMs. Further, natural killer (NK) cells (important in the lysis and removal of HIV-1-infected cells) exposed to recombinant Vpr show impaired function, with reduced target cell killing activity and inter-feron-γ production (Majumder et al., 2008). The plethora of effects that extracellular Vpr displays on a multitude of cells in the peripheral circulation is of particular importance not only in lymph nodes, where antigens are presented to effector cells, but also within the context of HIV-1-associated neuropathogenesis in the brain, because antigen-presenting cells are able to traverse the BBB where they localize and process pathogens present within the CNS. Because Vpr is found in the serum of patients infected with HIV-1 (Hoshino et al., 2007; Levy et al., 1994), these studies could explain the inability of the immune system to maintain an adequate response against the virus during the course of the disease.
VI. EFFECTS OF EXTRACELLULAR VPR ON NEURONS
One of the first lines of evidence demonstrating the ability of extracellular Vpr to alter neuronal function was published over a decade ago. This study demonstrated that extracellular Vpr induced formation of cation-selective pores in lipid bilayers in vitro (Piller et al., 1996). The authors proposed that the positively charged Vpr C-terminus interacted with the physiological negatively charged intracellular environment to promote formation of channels. This process, in turn, altered physiological ionic currents and gradients across the plasma membrane, which is critically important in excitable cells like neurons. Additional studies using patch-clamp experimentation demonstrated the “association” of Vpr with the plasma membrane, thus impairing transmembrane potential by induction of a large influx of sodium (Piller et al., 1998). Recent studies have also proposed that neurons are capable of internalizing Vpr (Henklein et al., 2000; Rom et al., 2009). These two observations have pointed to the roles that extracellular Vpr plays as it enters neurons through a yet unknown mechanism. Vpr entry into cells has been shown to cause ionic imbalance between the intraand extracellular environment subsequent to transduction of the plasma membrane. Vpr-induced inward current is especially deleterious to neurons because it alters ionic concentration and electrochemical balance, which leads to their dysfunction and consequently their death (Jones et al., 2007; Kitayama et al., 2008; Piller et al., 1996, 1998). Extracellular Vpr clearly interferes with neuronal physiological function by entering the neurons it affects and subsequently altering metabolism and axonal function and growth (Kitayama et al., 2008). This process is of great importance in neuronal plasticity and memory loss, which could explain and correlate with the increased detection of extracellular Vpr in patients with AIDS and neurocognitive impairment as a direct effect of extracellular Vpr on neurons. However, all extracellular Vpr-induced depolarizing effects were both time and dose dependent and irreversibly compromised neuronal viability. On the basis of these observations, we hypothesize that Vpr may influence neuronal ability to conduct action potentials over long distances, which could explain the observed in vivo alterations in neuronal functionality. Indeed, because the presence of an excessive amount of extracellular Vpr in the CSF correlates positively with disease progression (Levy et al., 1994, 1995), we speculate that Vpr might play a fundamental role either in causing neurocognitive impairment or in accelerating the symptoms already present in patients with late-stage HIV-1 disease. Nevertheless, despite in vitro evidence of the effects of extracellular Vpr on neurons, the outcomes that the presence of this protein in the extracellular compartment induces in vivo remain to be determined because only mouse models have been used to examine this question.
Further studies have also attributed the apoptotic-inducing phenotype to the C-terminal domain of Vpr (Sabbah and Roques, 2005). This truncated form of Vpr possesses higher apoptotic ability than the full-length Vpr, because the N-terminus domain of Vpr did not show any induction of apoptosis. In another study, the effects of recombinant Vpr on a neuronal cell line and on primary neurons of both rat and human origin were investigated (Jones et al., 2007). At a lower concentration of Vpr, only human fetal neurons were vulnerable, although to a lesser extent. At increased concentrations of extracellular Vpr, all neurons examined were susceptible to cell death. The proapoptotic phenotype of Vpr was evident as early as 3 h after treatment and was caused by release of cytochrome c into the cytoplasm. Excessive accumulation of cytoplasmic cytochrome c then promoted increased expression of caspase 3 and p53 and a decrease in phosphorylation of Akt. Additionally, the incomplete reversal of Vpr effects by cotreatment with anti-Vpr antibodies suggests the existence of other non-Vpr-induced indirect effects. In fact, in the same study, a more relevant in vivo model was used by treating astrocytes with recombinant Vpr and then exposing neurons to the resultant astrocytic-conditioned media. Although high concentrations of Vpr were used to observe any significant effect on neuronal viability, only a modest and not statistically significant reversal of neuronal loss was obtained when neurons were cotreated with anti-Vpr antibody. This observation suggested the possibility that a Vpr-induced effect on astrocytes may indirectly affect the functional properties of neighboring neurons. In this regard, increased IL-6 transcript levels were found in Vpr-treated astrocytes. If the corresponding proteins were secreted into the extracellular environment, the resultant inflammatory cytokine storm would likely impair the survival of the neighboring neuronal population. Interestingly, conditioned media from Vpr-treated monocytic cells induced significant neuronal death at low concentrations of Vpr compared with astrocytic-conditioned media. This observation might provide an explanation for the in vivo symptoms observed in patients infected with HIV-1 in that monocytes exposed to a low concentration of extracellular Vpr could represent a harmful source of inflammatory molecules that could cause neuronal damage after traversing the BBB. Although this study was conducted using a monocytoid cell line (U-937 cells) and would thus need further confirmation with primary monocytes, the results represent the first evidence suggesting that some of the damage observed in the CNS of patients infected with HIV-1 might be caused by an indirect mechanism induced by Vpr. Additional studies are required to address the following questions: (1) Are the inflammatory molecules secreted by monocytes after traversing the BBB the result of exposure to Vpr in the peripheral blood or in the CNS? (2) Are the production and secretion of these cytokines/chemokines affected by the differentiation process monocytes undergo after crossing the BBB? (3) Because Vpr is known to affect the differentiation process of monocytic cells, would both uninfected and HIV-1-infected monocytes be able to cross the BBB in the presence of pathogens within the CNS? In this regard, soluble Vpr present in serum of HIV-1-infected individuals could arrest the differentiation process by decreasing surface expression of costimulatory molecules, possibly halting transmigration of additional monocytic cells. This process could represent an additional viral mechanism to enhance active replication of the virus within the CNS, thereby minimizing immune surveillance such that viral infection could spread to new areas of the CNS. In this scenario, the effects of extracellular Vpr in the peripheral circulation would impact the CNS, which could explain why the virus needs a sufficient buildup of extracellular Vpr in the serum during late-stage HIV-1 disease to promote any detrimental effect in the CNS, providing a reason why severe neurocognitive impairment is mostly observed during the later stages of disease.
A number of experimental observations could be combined to develop a more relevant model of the BBB, wherein all cell types (MDMs, astrocytes, and neurons) would be included to model more accurately CNS damage caused by Vpr toxicity and by other viral proteins as well as the accumulation of other cellular neurotoxic mediators. This model would also include interactions involving brain microvascular endothelial cells, which are found at the lining between the capillaries and the brain environment and are negatively affected by extracellular Vpr, which induces excessive production of TNF-α and eventually leads to apoptosis of these cells (Acheampong et al., 2002).
Recently, exogenous Vpr has been shown to induce formation of reactive oxygen species (ROS) in neurons (Rom et al., 2009; Sabbah and Roques, 2005) and in other cell types, including MDMs and microglia (Hoshino et al., 2010; Rom et al., 2009). This observation underscores either the cell-independent induction of oxidative stress by Vpr or the presence of membrane-bound receptors widely expressed in several cell types that are capable of inducing a cascade of events leading to intracellular oxidation. ROS are chemically reactive oxygen-containing molecules produced under physiological conditions as a consequence of numerous intracellular biochemical and metabolic processes that are promptly disposed of to maintain a reduced intracellular environment. However, excessive accumulation of intracellular ROS has been shown to activate the oxidative stress pathway because the cellular antioxidant machinery may not readily detoxify them. This situation generally results in the production of abundant quantities of peroxides and free radicals, which affect neuronal function, and, when the oxidative-inducing stimulus is kept for an extended period, ultimately leads to cell death through DNA damage. Extracellular Vpr-induced damage has also been proven to occur in microglial cells, the most common HIV-1-infected cells within the CNS (Michaels et al., 1988), wherein the enhanced expression of hypoxia-inducible factor-1 and the subsequent increase in viral transcription and virion production have been observed (Deshmane et al., 2009). In addition, promotion of oxidative stress causes viral activation in microglial cells, which leads to increased viral particle release and possibly Vpr. Therefore, extracellular Vpr may induce a vicious cycle: Not only does it directly affect neurons, but it also induces viral production and release of Vpr in microglia, which also impacts neuronal function.
Another feature attributed to extracellular Vpr centers on the induction of the loss of adenosine triphosphate (ATP), the major source of intracellular energy (Rom et al., 2009) in neurons. This observation, in conjunction with the aforementioned Vpr-promoted accumulation of ROS species, points to a direct effect of Vpr on mitochondria, the intracellular organelles involved in disposal of ROS and exchange of ATP/ADP with the cytoplasm. Indeed, previous studies have demonstrated that Vpr can rapidly decrease mitochondrial membrane potential when added exogenously to different cell types or to isolated mitochondria (Jacotot et al., 2000); this process, in turn, was shown to induce formation of the permeability transition pore complex (PTPC; Vieira et al., 2000), which comprises the voltage-dependent anion channel (VDAC) on the outer mitochondrial membrane and the adenine nucleotide translocator (ANT) on the inner mitochondrial membrane. These effects suggest a direct interaction between Vpr and domains of the PTPC including both the ANT and VDAC proteins (Jacotot et al., 2001; Sabbah et al., 2006). Formation of PTPC induced by Vpr promotes the formation of mitochondrial conductance channels, with subsequent release of cytochrome c and apoptosis-inducing factor into the cytoplasm (Roumier et al., 2002), which translocates to the nucleus, thus promoting DNA fragmentation and programmed cell death.
Neurons accumulate an excessive amount of calcium ions under certain physiological circumstances, which affects calcium secretion and interneuronal communication (Rom et al., 2009). This response has been directly attributed to Vpr-induced downregulation of a plasma membrane-bound calcium transporter (plasma membrane calcium ATPase), responsible for the removal of calcium ions from cells. This result, which needs confirmation in primary neurons, is of particular interest because calcium plays a role as an intracellular messenger (in all cell types) and regulates several different neuronal developmental processes, such as migration into the CNS, plasticity of dendrites and filopodia, membrane excitability, and neurotransmitter release (Ryglewski et al., 2007). Alteration of any of the aforementioned activities by Vpr has potential deleterious downstream effects on neuronal survival, which impacts processes such as memory loss, thus leading to neurocognitive impairment.
Studies of neurons exposed to extracellular Vpr demonstrated the complete spectrum of effects that this viral protein has on this cell population, from intracellular transduction to impairment of calcium signaling and decreased levels of ATP storage. This latter observation needs further examination in primary neurons because a reduction in intracellular ATP levels could be due either to abundant energy consumption or to excessive neuronal release. In this regard, neuronal axons firing action potentials are known to secrete ATP, which functions as a neurotransmitter (Ishibashi et al., 2006), and alteration of its extracellular concentration and associated gradient may disrupt interneuronal signaling.
VII. EFFECTS OF EXTRACELLULAR VPR ON ASTROCYTES
As outlined in the previous section, numerous studies have evaluated the detrimental effects that soluble Vpr exerts on neurons of both mouse and human origin, thereby affecting neuronal survival in a dose-dependent manner, starting at concentrations similar to those found in vivo in patients infected with HIV-1 (of the order of pM). Recently, cells of the astrocytic lineage have been used to evaluate the effects of exogenous Vpr in order to make correlations with the in vivo neurodegenerative symptoms observed in patients with late-stage HIV-1 infection (Huang et al., 2000; Jones et al., 2007; Noorbakhsh et al., 2010). Astrocytes have been selected as an experimental focal point because they are the most abundant cell population, because of their proximity to the BBB and to the neurons within the CNS, and because their plasticity allows them to change shape in response to nearby neuronal activity (affecting complex neuronal connections) and enwrap several different neuronal synapses. The synaptic cleft becomes compartmentalized, resulting in more rapid uptake of ions and neurotransmitters, especially glutamate, ATP, and d-serine, thereby controlling homeostasis and eliminating otherwise neurotoxic molecules. Astrocytes are glial fibrillary acidic protein-positive cells, important in transmission and modulation of action potentials because they control calcium concentration in the neuronal synaptic cleft and facilitate release of neurotransmitters from presynaptic termini. Because glutamate uptake by astrocytes (the major cell type responsible for this process) is finely regulated by a balanced transport of ions (mainly Na+, K+, and H+), this neurotoxic amino acid is quickly taken up and able to trigger aerobic glycolysis (Pellerin and Magistretti, 1994) as well as release of neuroprotective factors such as lactate, glutathione, and cysteine (Garg et al., 2008). These molecules are critically important for neuronal survival, with lactate taken up and converted into pyruvate for use in the tricarboxylic acid cycle, whereas glutathione is extracellularly cleaved into cysteine, which is subsequently taken up and used as a fuel molecule to resynthesize glutathione within neurons, thus promoting a reduced intracellular environment. Given the fundamental role astrocytes play in providing precursors to neurons (Dringen et al., 1999), any toxic compound or molecule present within the CNS first directly affects astrocytic functions, which in turn impacts neuronal metabolism and fate. A number of investigative groups including our own have recently initiated studies to determine how extracellular Vpr affects astrocytic functions. One of the main areas of exploration centers on how Vpr impairs calcium signaling in astrocytes (Noorbakhsh et al., 2010), as previously proven in neurons (Rom et al., 2009), which suggests another conserved feature between the two different cell types. Calcium is fundamental in intercell communication, particularly among excitable (neurons) and nonexcitable (astrocytes) cells within the CNS (reviewed by Scemes and Giaume, 2006). Disruption of calcium concentrations and influx affects not only calcium-dependent intracellular pathways (such as the CREB pathway) but also intercellular calcium waves, which are used by astrocytes to transmit signals to adjacent nonstimulated cells, thus creating an astrocytic network capable of reflecting local changes to a more macroscopic level. Although recent reports have demonstrated the ability of recombinant Vpr to transduce different cell types, there is no evidence yet for Vpr uptake by astrocytic cells. Whether Vpr enters cells or exerts its effects by signaling through yet unidentified receptors on the plasma membrane, this toxic viral protein has been shown to activate p53, leading to apoptosis through induction of the caspase cascade (Noorbakhsh et al., 2010). The same study showed that transgenic mice expressing Vpr in cells of the monocytoid lineage displayed decreased glial fibrillary acidic protein immunostaining within both the cortex and the basal ganglia, symptoms of increased astrocytic cell death. This experimental result suggested a mechanism whereby MDMs traverse the BBB or microglial cells, through direct secretion of Vpr or release of Vpr-induced cytokines, promoting programmed cell death in astrocytes. Additionally, the increased presence of excitatory molecules (including glutamate and l- and d-serine) was detected within the brain along with decreased production of transcripts of the excitatory amino acid transporters (EAAT1 and EAAT2), the major glutamate transporters expressed primarily within astrocytes (Noorbakhsh et al., 2010). The evidence provided therein could point to an association between the increased levels of glutamate found within the brain and the late-stage symptoms of HIV-associated neurocognitive impairment as an effect of exogenous Vpr. In this regard, extracellular glutamate may not be efficiently cleared out by astrocytes, either because of diminished expression of EAAT transporters or their decreased activity, as a result of a saturation event (for instance, direct formation of an EAAT/Vpr complex) or possibly steric hindrance factors. In either scenario, the net local effect is an excessive buildup of extracellular excitatory glutamate, which affects neuronal activity, both directly and indirectly through astrocytic dysfunction. Extracellular glutamate is indeed taken up by astrocytes and subsequently converted into the less neurotoxic glutamine. Glutamine in turn is released and taken up by presynaptic neurons, where it is reconverted into glutamate, which is used as a neurotransmitter to propagate excitatory stimuli, thus completing the cycle. An excessive concentration of extracellular glutamate generates a steeper gradient, not only becoming neurotoxic but also generating more rapid and dramatic influx and efflux waves. Because glutamate uptake is finely regulated through both the cysteine–glutamate exchanger and the sodium–potassium ATPase pump (Garg et al., 2008), a glutamate imbalance also impairs cysteine and ionic gradients. Nonetheless, blood-borne monocytes traffic to the brain following brain injury and, on differentiation into tissue macrophages, express EAAT transporters, thus becoming competent in clearing extracellular glutamate (Rimaniol et al., 2000). This finding may represent a double-edged sword because it could provide an adaptive mechanism that MDMs develop as the disease progresses to promptly eliminate abundant extracellular glutamate while undermining the survival of CNS-resident cells such as HIV-1-infected monocytes that might also cross the BBB, thus reseeding viral infection in the brain.
A recent study showed that neurons treated with conditioned media from Vpr-stimulated U-373 MG cells (an astrocytic cell line) underwent cell death and that they could not be rescued by the addition of anti-Vpr antibody to the extracellular medium (Jones et al., 2007). This observation points again to an indirect effect induced by Vpr because Vpr-treated astrocytes might take up extracellular Vpr and release proinflammatory cytokines, leading to a reduction in neuronal viability. This study shows how any toxic effect observed in astrocytes, although not performed under cocultivation conditions, is transmitted to neurons.
From studies published in the literature and some of our recent unpublished observations, we have presented a model of HIV-1 extracellular Vpr action on astrocytic functions (Fig. 3). A decrease in Vpr-induced intracellular glutathione might be a direct consequence of reduced ATP availability due to Vpr binding to the ANT on the inner mitochondrial membrane. By binding to ANT, Vpr may halt the physiological transport of ATP molecules produced through the tricarboxylic acid cycle, lowering cytosolic levels of ATP, with natural consequences for all cellular ATP-driven reactions. This process, in turn, could be reflected by a decrease in reduced glutathione, which may also cause a shift toward production of oxidized glutathione, no longer capable of acting as an electron donor, thus inducing accumulation of intracellular ROS. The increase of ROS in astrocytes then would lead to increased viral transactivation from the integrated proviral genome, which may restore viral gene expression or particle production from persistently infected astrocytes. Conversely, decreased synthesis of GSH may be caused by impaired glutamate uptake from firing neurons, thus inducing excitotoxi-city. In addition, intracellular decrease in ATP levels has detrimental effects on the finely regulated ATP-dependent uptake of glutamate by astrocytes, thus causing excessive extracellular accumulation and neurotoxicity. We propose that these effects are mediated directly by soluble extracellular Vpr and possibly by the secretion of Vpr-induced cytokines, such as IL-6 as previously documented (Hoshino et al., 2010). Impairment of glutathione metabolism in an oxidizing astrocytic intracellular milieu reduces secretion of neuroprotective factors (lactate and cysteine), on which neurons rely for their metabolism, thus promoting their dysfunction and death. Additionally, reduced levels of ATP in astrocytes have potentially detrimental effects not only in all metabolic processes, but also in ATP release, where it functions as a gliotransmitter, and in intercellular calcium wave propagation, which leads to a cumulative detrimental impact on neurons.
FIGURE 3.
Model of HIV-1 extracellular Vpr action on astrocytic functions. A decrease in Vpr-induced intracellular ATP might be a direct consequence of Vpr binding to the adenine nucleotide translocator (ANT) on the inner mitochondrial membrane. Indeed, Vpr might halt the physiological transport of ATP molecules produced during the tricarboxylic acid (TCA) cycle, lowering the cytosolic availability of ATP, with consequences for all cellular ATP-driven reactions. One such reaction is characterized by a decrease in reduced glutathione (GSH), the main cellular antioxidant, which is synthesized in two ATP-dependent reactions by the initial formation of cysteine–glutamate (Cys–Glu) (through the action of glutamyl-cysteine synthase [γ-GCS]) and the subsequent formation of GSH on addition of glycine (Gly) (by glutathione synthetase [GSH-S]). GSH functions as an electron donor in all cellular redox reactions by detoxifying ROS produced as byproducts of physiological cellular metabolism. Decreased concentration of reduced GSH may also cause a shift toward production of oxidized glutathione (GSSG), which then no longer acts as an electron donor and hence induces the intracellular accumulation of ROS. ROS buildup in astrocytes then leads to activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway, which induces transcription of NF-κB-dependent promoters, including the HIV long terminal repeat (LTR), hence increasing viral transactivation and viral particle production from persistently infected astrocytes. Conversely, decreased GSH synthesis may also be caused by impaired glutamate uptake from firing neurons, thus inducing excitotoxicity, and may affect the release of neuroprotective factors (lactate and GSH) on which neurons rely for their metabolism. This cascade of events, in turn, is predicted to be indicative of disease progression because patients in late-stage disease show increased levels of extracellular Vpr in the serum and the cerebrospinal fluid, which may ultimately escalate and induce neurocognitive impairment. ARE, antioxidant responsive element; EAAT, excitatory amino acid transporter; GPx, glutathione peroxidase; GR, glutathione reductase; PBR, peripheral-type benzodiazepine receptor; PKC, protein kinase C; VDAC, voltage-dependent anion channel.
VIII. CONCLUSION
This review had several objectives. We focused on an analysis of all published studies that provided evidence of secretion or nonspecific release of HIV-1 Vpr. We have critically reviewed those studies that have provided evidence supporting a role for Vpr as an exogenous protein as part of a plan to evade immune surveillance. We subsequently evaluated the cellular sources of exogenous Vpr within the periphery and the possible route(s) of entry and causes of soluble Vpr into the CNS. Once Vpr enters the CNS, its detrimental effects could be implemented on all resident cells, similar to those observed in the periphery (Table I). Future studies will need to provide convincing evidence with respect to the secretion or nonspecific release of HIV-1 Vpr. Additional studies are also required to delineate specific cell types involved in this “secretory process” and the important mechanisms of the viral life cycle that are necessary for the process of secretion to occur, as these studies might prove useful in drug design aimed at blocking Vpr intracellular shuttling.
TABLE I.
Effects of extracellular Vpr on cells in the periphery and the CNS
ATP, adenosine triphosphate; BMVEC, brain microvascular endothelial cell; CNS, central nervous system; DC, dendritic cell; EAAT, excitatory amino acid transporter; GFAP, glial fibrillary acidic protein; Glu, Glutamate; IFN-γ, interferon-γ; IL-6, interleukin-6; MAPK, mitogen-activated protein kinase; MMP, mitochondrial membrane potential; NK, natural killer cell; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-α.
We also focused our attention primarily on astrocytes and neurons, because the intricate communication between these cell types is ultimately responsible for the fate of neurons, which has consequences for the health of patients with late-stage HIV-1 infection, by inducing neurocognitive impairment. Nonetheless, the mechanisms used during HIV-induced neurovirulence are not understood and need further investigation. We also reported a model of action by exogenous Vpr. The hypothesis summarizes a possible correlation between increased ROS formation and viral load burden in the CNS. We propose that the in vivo manifestations of intracellular oxidative stress observed in patients with late-stage HIV-1 infection correlate with increased Vpr secretion as the disease progresses to include minor forms of neurocognitive impairment or more severe forms of neurological damage such as HIV-1-associated dementia. We have suggested that HIV-1 Vpr within the CNS either is actively secreted or passively diffuses out of infected cells (T cells, MDMs, and microglia) or from apoptotic/necrotic cells and is taken up by uninfected bystander cells or persistently infected astrocytes, affecting the biosynthesis of glutathione and diminishing the intracellular ATP pool.
The causes of HIV-1 disease progression are multifaceted as multiple events occur leading to a cascade of pathological consequences. It is an attractive objective to correlate the observed increase in ROS formation with the augmented viral load burden within the CNS, although the leading causes might be various. Additionally, patients with late-stage HIV-1 infection have been shown to benefit from treatment with antioxidant compounds, as these remedies lengthen life expectancy but do not halt the ongoing oxidation phenomena, either because the process is irreversible or because of an excessive accumulation of ROS, which may no longer be scavenged by the weakened antioxidant cellular defense mechanisms. Additionally, because extracellular Vpr concentrations are very low in the early phases of disease compared with late stages, it is appealing, although speculative, to hypothesize that in early disease stages exogenous levels of Vpr are too low to cause immediate detrimental effects, and the observed downstream effects are generated only later during disease progression when Vpr levels rise above this “threshold.” It is possible, however, that in early stages, astrocytes, although exposed to subthreshold concentrations of Vpr, might become sensitized to subsequent exposure to Vpr so that lower amounts of Vpr are necessary to impair astrocytic functionality. Studies in our laboratory are under way to establish the intimate, intrinsic connections among decreased GSH and ATP concentrations, formation of ROS, DNA damage, and viral activation as an outcome of late-stage HIV-1 infection.
ACKNOWLEDGMENTS
This work was supported in part by funds from the Public Health Service, National Institutes of Health, and through grants from the National Institute of Neurological Disorders and Stroke (NS32092 to B. W.) and the National Institute of Drug Abuse (DA19807 to B. W.). Dr. Michael Nonnemacher was also supported by faculty development funds provided by the Department of Microbiology and Immunology and the Institute for Molecular Medicine and Infectious Disease.
REFERENCES
- Acheampong E, Mukhtar M, Parveen Z, Ngoubilly N, Ahmad N, Patel C, Pomerantz RJ. Ethanol strongly potentiates apoptosis induced by HIV-1 proteins in primary human brain microvascular endothelial cells. Virology. 2002;304(2):222–234. doi: 10.1006/viro.2002.1666. [DOI] [PubMed] [Google Scholar]
- Agostini I, Navarro JM, Rey F, Bouhamdan M, Spire B, Vigne R, Sire J. The human immunodeficiency virus type 1 Vpr transactivator: Cooperation with promoter-bound activator domains and binding to TFIIB. J. Mol. Biol. 1996;261(5):599–606. doi: 10.1006/jmbi.1996.0485. [DOI] [PubMed] [Google Scholar]
- Ali SA, Huang MB, Campbell PE, Roth WW, Campbell T, Khan M, Newman G, Villinger F, Powell MD, Bond VC. Genetic characterization of HIV type 1 Nef-induced vesicle secretion. AIDS Res. Hum. Retroviruses. 2010;26(2):173–192. doi: 10.1089/aid.2009.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JL, Zimmerman ES, DeHart JL, Murala S, Ardon O, Blackett J, Chen J, Planelles V. ATR and GADD45alpha mediate HIV-1 Vpr-induced apoptosis. Cell Death Differ. 2005;12(4):326–334. doi: 10.1038/sj.cdd.4401565. [DOI] [PubMed] [Google Scholar]
- Andersen JL, DeHart JL, Zimmerman ES, Ardon O, Kim B, Jacquot G, Benichou S, Planelles V. HIV-1 Vpr-induced apoptosis is cell cycle dependent and requires Bax but not ANT. PLoS Pathog. 2006;2(12):e127. doi: 10.1371/journal.ppat.0020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson LM, Hagberg L, Fuchs D, Svennerholm B, Gisslen M. Increased blood–brain barrier permeability in neuro-asymptomatic HIV-1-infected individuals–correlation with cerebrospinal fluid HIV-1 RNA and neopterin levels. J. Neurovirol. 2001;7(6):542–547. doi: 10.1080/135502801753248123. [DOI] [PubMed] [Google Scholar]
- Arokium H, Kamata M, Chen I. Virion-associated Vpr of human immunodeficiency virus type 1 triggers activation of apoptotic events and enhances fas-induced apoptosis in human T cells. J. Virol. 2009;83(21):11283–11297. doi: 10.1128/JVI.00756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunagiri C, Macreadie I, Hewish D, Azad A. A C-terminal domain of HIV-1 accessory protein Vpr is involved in penetration, mitochondrial dysfunction and apoptosis of human CD4+ lymphocytes. Apoptosis. 1997;2(1):69–76. doi: 10.1023/a:1026487609215. [DOI] [PubMed] [Google Scholar]
- Ayyavoo V, Mahalingam S, Rafaeli Y, Kudchodkar S, Chang D, Nagashunmugam T, Williams WV, Weiner DB. HIV-1 viral protein R (Vpr) regulates viral replication and cellular proliferation in T cells and monocytoid cells in vitro. J. Leukoc. Biol. 1997a;62(1):93–99. doi: 10.1002/jlb.62.1.93. [DOI] [PubMed] [Google Scholar]
- Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams WV, Green DR, Weiner DB. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat. Med. 1997b;3(10):1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- Bachand F, Yao XJ, Hrimech M, Rougeau N, Cohen EA. Incorporation of Vpr into human immunodeficiency virus type 1 requires a direct interaction with the p6 domain of the p55 gag precursor. J. Biol. Chem. 1999;274(13):9083–9091. doi: 10.1074/jbc.274.13.9083. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam A, Mersmann H, Jahoor F, Phillips TM, Sekhar RV, Schubert U, Brar B, Iyer D, Smith EO, Takahashi H, Lu H, Anderson P, et al. Effects of transgenic expression of HIV-1 Vpr on lipid and energy metabolism in mice. Am. J. Physiol. Endocrinol. Metab. 2007;292(1):E40–E48. doi: 10.1152/ajpendo.00163.2006. [DOI] [PubMed] [Google Scholar]
- Bukrinsky M, Adzhubei A. Viral protein R of HIV-1. Rev. Med. Virol. 1999;9(1):39–49. doi: 10.1002/(sici)1099-1654(199901/03)9:1<39::aid-rmv235>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Campbell TD, Khan M, Huang MB, Bond VC, Powell MD. HIV-1 Nef protein is secreted into vesicles that can fuse with target cells and virions. Ethn. Dis. 2008;18(2 Suppl. 2):S2-14–S2-19. [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997;11(12):1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Levine B, Boise LH, Thompson CB, Hardwick JM. Bax-independent inhibition of apoptosis by Bcl-XL. Nature. 1996;379(6565):554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- Chowdhury IH, Wang XF, Landau NR, Robb ML, Polonis VR, Birx DL, Kim JH. HIV-1 Vpr activates cell cycle inhibitor p21/Waf1/Cip1: A potential mechanism of G2/M cell cycle arrest. Virology. 2003;305(2):371–377. doi: 10.1006/viro.2002.1777. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DF, Thompson KA, Gabuzda D, McArthur JC, Pardo CA, Wesselingh SL. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J. Neurovirol. 2006;12(2):146–152. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann. Neurol. 2009;66(2):253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- Coeytaux E, Coulaud D, Le Cam E, Danos O, Kichler A. The cationic amphipathic alpha-helix of HIV-1 viral protein R (Vpr) binds to nucleic acids, permeabilizes membranes, and efficiently transfects cells. J. Biol. Chem. 2003;278(20):18110–18116. doi: 10.1074/jbc.M300248200. [DOI] [PubMed] [Google Scholar]
- Cohen EA, Dehni G, Sodroski JG, Haseltine WA. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J. Virol. 1990a;64(6):3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EA, Terwilliger EF, Jalinoos Y, Proulx J, Sodroski JG, Haseltine WA. Identification of HIV-1 vpr product and function. J. Acquir. Immune Defic. Syndr. 1990b;3(1):11–18. [PubMed] [Google Scholar]
- Conti L, Rainaldi G, Matarrese P, Varano B, Rivabene R, Columba S, Sato A, Belardelli F, Malorni W, Gessani S. The HIV-1 vpr protein acts as a negative regulator of apoptosis in a human lymphoblastoid T cell line: Possible implications for the pathogenesis of AIDS. J. Exp. Med. 1998;187(3):403–413. doi: 10.1084/jem.187.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti L, Matarrese P, Varano B, Gauzzi MC, Sato A, Malorni W, Belardelli F, Gessani S. Dual role of the HIV-1 vpr protein in the modulation of the apoptotic response of T cells. J. Immunol. 2000;165(6):3293–3300. doi: 10.4049/jimmunol.165.6.3293. [DOI] [PubMed] [Google Scholar]
- Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, Achim CL. Blood–brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am. J. Pathol. 1999;155(6):1915–1927. doi: 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane SL, Mukerjee R, Fan S, Del Valle L, Michiels C, Sweet T, Rom I, Khalili K, Rappaport J, Amini S, Sawaya BE. Activation of the oxidative stress pathway by HIV-1 Vpr leads to induction of hypoxia-inducible factor 1alpha expression. J. Biol. Chem. 2009;284(17):11364–11373. doi: 10.1074/jbc.M809266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzio P, Choe S, Ebright M, Knoblauch R, Landau NR. Mutational analysis of cell cycle arrest, nuclear localization and virion packaging of human immunodeficiency virus type 1 Vpr. J. Virol. 1995;69(12):7909–7916. doi: 10.1128/jvi.69.12.7909-7916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Kussmaul L, Gutterer JM, Hirrlinger J, Hamprecht B. The glutathione system of peroxide detoxification is less efficient in neurons than in astroglial cells. J. Neurochem. 1999;72(6):2523–2530. doi: 10.1046/j.1471-4159.1999.0722523.x. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 1993;67(1):277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood–brain barrier: A potential mechanism of HIV-CNS invasion and neuroAIDS. J. Neurosci. 2006;26(4):1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felzien LK, Woffendin C, Hottiger MO, Subbramanian RA, Cohen EA, Nabel GJ. HIV transcriptional activation by the accessory protein, VPR, is mediated by the p300 co-activator. Proc. Natl. Acad. Sci. USA. 1998;95(9):5281–5286. doi: 10.1073/pnas.95.9.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz JV, Didier P, Clamme JP, Schaub E, Muriaux D, Cabanne C, Morellet N, Bouaziz S, Darlix JL, Mely Y, de Rocquigny H. Direct Vpr–Vpr interaction in cells monitored by two photon fluorescence correlation spectroscopy and fluorescence lifetime imaging. Retrovirology. 2008;5:87. doi: 10.1186/1742-4690-5-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumori T, Akari H, Iida S, Hata S, Kagawa S, Aida Y, Koyama AH, Adachi A. The HIV-1 Vpr displays strong anti-apoptotic activity. FEBS Lett. 1998;432(1–2):17–20. doi: 10.1016/s0014-5793(98)00824-2. [DOI] [PubMed] [Google Scholar]
- Fukumori T, Akari H, Yoshida A, Fujita M, Koyama AH, Kagawa S, Adachi A. Regulation of cell cycle and apoptosis by human immunodeficiency virus type 1 Vpr. Microbes Infect. 2000;2(9):1011–1017. doi: 10.1016/s1286-4579(00)01255-7. [DOI] [PubMed] [Google Scholar]
- Garg SK, Banerjee R, Kipnis J. Neuroprotective immunity: T cell-derived glutamate endows astrocytes with a neuroprotective phenotype. J. Immunol. 2008;180(6):3866–3873. doi: 10.4049/jimmunol.180.6.3866. [DOI] [PubMed] [Google Scholar]
- Giulian D, Wendt E, Vaca K, Noonan CA. The envelope glycoprotein of human immunodeficiency virus type 1 stimulates release of neurotoxins from monocytes. Proc. Natl. Acad. Sci. USA. 1993;90(7):2769–2773. doi: 10.1073/pnas.90.7.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh WC, Rogel ME, Kinsey CM, Michael SF, Fultz PN, Nowak MA, Hahn BH, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: A mechanism for selection of Vpr in vivo. Nat. Med. 1998;4(1):65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- Goh WC, Manel N, Emerman M. The human immunodeficiency virus Vpr protein binds Cdc25C: Implications for G2 arrest. Virology. 2004;318(1):337–349. doi: 10.1016/j.virol.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Hallenberger S, Tucker SP, Owens RJ, Bernstein HB, Compans RW. Secretion of a truncated form of the human immunodeficiency virus type 1 envelope glycoprotein. Virology. 1993;193(1):510–514. doi: 10.1006/viro.1993.1156. [DOI] [PubMed] [Google Scholar]
- Hart TK, Kirsh R, Ellens H, Sweet RW, Lambert DM, Petteway SR, Jr., Leary J, Bugelski PJ. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA. 1991;88(6):2189–2193. doi: 10.1073/pnas.88.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 1995;69(11):6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzinger NK, Bukinsky MI, Haggerty SA, Ragland AM, Kewalramani V, Lee MA, Gendelman HE, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA. 1994;91(15):7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henklein P, Bruns K, Sherman MP, Tessmer U, Licha K, Kopp J, de Noronha CM, Greene WC, Wray V, Schubert U. Functional and structural characterization of synthetic HIV-1 Vpr that transduces cells, localizes to the nucleus, and induces G2 cell cycle arrest. J. Biol. Chem. 2000;275(41):32016–32026. doi: 10.1074/jbc.M004044200. [DOI] [PubMed] [Google Scholar]
- Herzenberg LA, De Rosa SC, Dubs JG, Roederer M, Anderson MT, Ela SW, Deresinski SC. Glutathione deficiency is associated with impaired survival in HIV disease. Proc. Natl. Acad. Sci. USA. 1997;94(5):1967–1972. doi: 10.1073/pnas.94.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WF. Leukocyte traffic in the central nervous system: The participants and their roles. Semin. Immunol. 1999;11(2):125–137. doi: 10.1006/smim.1999.0168. [DOI] [PubMed] [Google Scholar]
- Hoshino S, Sun B, Konishi M, Shimura M, Segawa T, Hagiwara Y, Koyanagi Y, Iwamoto A, Mimaya J, Terunuma H, Kano S, Ishizaka Y. Vpr in plasma of HIV type 1-positive patients is correlated with the HIV type 1 RNA titers. AIDS Res. Hum. Retroviruses. 2007;23(3):391–397. doi: 10.1089/aid.2006.0124. [DOI] [PubMed] [Google Scholar]
- Hoshino S, Konishi M, Mori M, Shimura M, Nishitani C, Kuroki Y, Koyanagi Y, Kano S, Itabe H, Ishizaka Y. HIV-1 Vpr induces TLR4/MyD88-mediated IL-6 production and reactivates viral production from latency. J. Leukoc. Biol. 2010;87(6):1133–1143. doi: 10.1189/jlb.0809547. [DOI] [PubMed] [Google Scholar]
- Hrimech M, Yao XJ, Bachand F, Rougeau N, Cohen EA. Human immunodeficiency virus type 1 (HIV-1) Vpr functions as an immediate-early protein during HIV-1 infection. J. Virol. 1999;73(5):4101–4109. doi: 10.1128/jvi.73.5.4101-4109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Orenstein JM, Martin MA, Freed EO. p6Gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J. Virol. 1995;69(11):6810–6818. doi: 10.1128/jvi.69.11.6810-6818.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MB, Weeks O, Zhao LJ, Saltarelli M, Bond VC. Effects of extracellular human immunodeficiency virus type 1 vpr protein in primary rat cortical cell cultures. J. Neurovirol. 2000;6(3):202–220. doi: 10.3109/13550280009015823. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49(6):823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacotot E, Ravagnan L, Loeffler M, Ferri KF, Vieira HL, Zamzami N, Costantini P, Druillennec S, Hoebeke J, Briand JP, Irinopoulou T, Daugas E, et al. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med. 2000;191(1):33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacotot E, Ferri KF, El Hamel C, Brenner C, Druillennec S, Hoebeke J, Rustin P, Metivier D, Lenoir C, Geuskens M, Vieira HL, Loeffler M, et al. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein rR and Bcl-2. J. Exp. Med. 2001;193(4):509–519. doi: 10.1084/jem.193.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CO, Huang MB, Khan M, Garcia-Barrio M, Powell MD, Bond VC. Extracellular Nef protein targets CD4+ T cells for apoptosis by interacting with CXCR4 surface receptors. J. Virol. 2004;78(6):3099–3109. doi: 10.1128/JVI.78.6.3099-3109.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian H, Zhao LJ. Pro-apoptotic activity of HIV-1 auxiliary regulatory protein Vpr is subtype-dependent and potently enhanced by nonconservative changes of the leucine residue at position 64. J. Biol. Chem. 2003;278(45):44326–44330. doi: 10.1074/jbc.C300378200. [DOI] [PubMed] [Google Scholar]
- Jones GJ, Barsby NL, Cohen EA, Holden J, Harris K, Dickie P, Jhamandas J, Power C. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J. Neurosci. 2007;27(14):3703–3711. doi: 10.1523/JNEUROSCI.5522-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata M, Aida Y. Two putative alpha-helical domains of human immunodeficiency virus type 1 Vpr mediate nuclear localization by at least two mechanisms. J. Virol. 2000;74(15):7179–7186. doi: 10.1128/jvi.74.15.7179-7186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmogne GD, Kennedy RC, Grammas P. HIV-1 gp120 proteins and gp160 peptides are toxic to brain endothelial cells and neurons: Possible pathway for HIV entry into the brain and HIV-associated dementia. J. Neuropathol. Exp. Neurol. 2002;61(11):992–1000. doi: 10.1093/jnen/61.11.992. [DOI] [PubMed] [Google Scholar]
- Kichler A, Pages JC, Leborgne C, Druillennec S, Lenoir C, Coulaud D, Delain E, Le Cam E, Roques BP, Danos O. Efficient DNA transfection mediated by the C-terminal domain of human immunodeficiency virus type 1 viral protein R. J. Virol. 2000;74(12):5424–5431. doi: 10.1128/jvi.74.12.5424-5431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama H, Miura Y, Ando Y, Hoshino S, Ishizaka Y, Koyanagi Y. Human immunodeficiency virus type 1 Vpr inhibits axonal outgrowth through induction of mitochondrial dysfunction. J. Virol. 2008;82(5):2528–2542. doi: 10.1128/JVI.02094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo E, Mammano F, Cohen EA, Gottlinger HG. The p6gag domain of human immunodeficiency virus type 1 is sufficient for the incorporation of Vpr into heterologous viral particles. J. Virol. 1995;69(5):2759–2764. doi: 10.1128/jvi.69.5.2759-2764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, Cheng Y, Krogan NJ, Plemenitas A, Peterlin BM. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11(1):110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DN, Fernandes LS, Williams WV, Weiner DB. Induction of cell differentiation by human immunodeficiency virus 1 vpr. Cell. 1993;72(4):541–550. doi: 10.1016/0092-8674(93)90073-y. [DOI] [PubMed] [Google Scholar]
- Levy DN, Refaeli Y, MacGregor RR, Weiner DB. Serum Vpr regulates productive infection and latency of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA. 1994;91(23):10873–10877. doi: 10.1073/pnas.91.23.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DN, Refaeli Y, Weiner DB. Extracellular Vpr protein increases cellular permissiveness to human immunodeficiency virus replication and reactivates virus from latency. J. Virol. 1995;69(2):1243–1252. doi: 10.1128/jvi.69.2.1243-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Luo L, Thomas DY, Kang CY. Control of expression, glycosylation, and secretion of HIV-1 gp120 by homologous and heterologous signal sequences. Virology. 1994;204(1):266–278. doi: 10.1006/viro.1994.1531. [DOI] [PubMed] [Google Scholar]
- Liu NQ, Lossinsky AS, Popik W, Li X, Gujuluva C, Kriederman B, Roberts J, Pushkarsky T, Bukrinsky M, Witte M, Weinand M, Fiala M. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J. Virol. 2002;76(13):6689–6700. doi: 10.1128/JVI.76.13.6689-6700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YL, Spearman P, Ratner L. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J. Virol. 1993;67(11):6542–6550. doi: 10.1128/jvi.67.11.6542-6550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macreadie IG, Castelli LA, Hewish DR, Kirkpatrick A, Ward AC, Azad AA. A domain of human immunodeficiency virus type 1 Vpr containing repeated H (S/F)RIG amino acid motifs causes cell growth arrest and structural defects. Proc. Natl. Acad. Sci. USA. 1995;92(7):2770–2774. doi: 10.1073/pnas.92.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macreadie IG, Arunagiri CK, Hewish DR, White JF, Azad AA. Extracellular addition of a domain of HIV-1 Vpr containing the amino acid sequence motif H(S/F)RIG causes cell membrane permeabilization and death. Mol. Microbiol. 1996;19(6):1185–1192. doi: 10.1111/j.1365-2958.1996.tb02464.x. [DOI] [PubMed] [Google Scholar]
- Macreadie IG, Fernley R, Castelli LA, Lucantoni A, White J, Azad A. Expression of HIV-1 nef in yeast causes membrane perturbation and release of the myristylated Nef protein. J. Biomed. Sci. 1998;5(3):203–210. doi: 10.1007/BF02253470. [DOI] [PubMed] [Google Scholar]
- Mahalingam S, Ayyavoo V, Patel M, Kieber-Emmons T, Kao GD, Muschel RJ, Weiner DB. HIV-1 Vpr interacts with a human 34-kDa mov34 homologue, a cellular factor linked to the G2/M phase transition of the mammalian cell cycle. Proc. Natl. Acad. Sci. USA. 1998;95(7):3419–3424. doi: 10.1073/pnas.95.7.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder B, Janket ML, Schafer EA, Schaubert K, Huang XL, Kan-Mitchell J, Rinaldo CR, Jr., Ayyavoo V. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: Implications for viral immune escape. J. Virol. 2005;79(13):7990–8003. doi: 10.1128/JVI.79.13.7990-8003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder B, Venkatachari NJ, Schafer EA, Janket ML, Ayyavoo V. Dendritic cells infected with vpr-positive human immunodeficiency virus type 1 induce CD8+ T-cell apoptosis via upregulation of tumor necrosis factor alpha. J. Virol. 2007;81(14):7388–7399. doi: 10.1128/JVI.00893-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder B, Venkatachari NJ, O'Leary S, Ayyavoo V. Infection with Vpr-positive human immunodeficiency virus type 1 impairs NK cell function indirectly through cytokine dysregulation of infected target cells. J. Virol. 2008;82(14):7189–7200. doi: 10.1128/JVI.01979-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder B, Venkatachari NJ, Srinivasan A, Ayyavoo V. HIV-1 mediated immune pathogenesis: Spotlight on the role of viral protein R (Vpr). Curr. HIV Res. 2009;7(2):169–177. doi: 10.2174/157016209787581445. [DOI] [PubMed] [Google Scholar]
- Matarrese P, Conti L, Varano B, Gauzzi MC, Belardelli F, Gessani S, Malorni W. The HIV-1 vpr protein induces anoikis-resistance by modulating cell adhesion process and microfilament system assembly. Cell Death Differ. 2000;7(1):25–36. doi: 10.1038/sj.cdd.4400616. [DOI] [PubMed] [Google Scholar]
- Michaels J, Price RW, Rosenblum MK. Microglia in the giant cell encephalitis of acquired immune deficiency syndrome: Proliferation, infection and fusion. Acta Neuropathol. 1988;76(4):373–379. doi: 10.1007/BF00686974. [DOI] [PubMed] [Google Scholar]
- Mishra S, Mishra JP, Kumar A. Activation of JNK-dependent pathway is required for HIV viral protein R-induced apoptosis in human monocytic cells: Involvement of antiapoptotic BCL2 and c-IAP1 genes. J. Biol. Chem. 2007;282(7):4288–4300. doi: 10.1074/jbc.M608307200. [DOI] [PubMed] [Google Scholar]
- Mizoguchi I, Ooe Y, Hoshino S, Shimura M, Kasahara T, Kano S, Ohta T, Takaku F, Nakayama Y, Ishizaka Y. Improved gene expression in resting macrophages using an oligopeptide derived from Vpr of human immunodeficiency virus type-1. Biochem. Biophys. Res. Commun. 2005;338(3):1499–1506. doi: 10.1016/j.bbrc.2005.10.112. [DOI] [PubMed] [Google Scholar]
- Morellet N, Bouaziz S, Petitjean P, Roques BP. NMR structure of the HIV-1 regulatory protein VPR. J. Mol. Biol. 2003;327(1):215–227. doi: 10.1016/s0022-2836(03)00060-3. [DOI] [PubMed] [Google Scholar]
- Morellet N, Roques BP, Bouaziz S. Structure-function relationship of Vpr: Biological implications. Curr. HIV Res. 2009;7(2):184–210. doi: 10.2174/157016209787581490. [DOI] [PubMed] [Google Scholar]
- Muthumani K, Hwang DS, Dayes NS, Kim JJ, Weiner DB. The HIV-1 accessory gene vpr can inhibit antigen-specific immune function. DNA Cell Biol. 2002;21(9):689–695. doi: 10.1089/104454902760330237. [DOI] [PubMed] [Google Scholar]
- Muthumani K, Hwang DS, Choo AY, Mayilvahanan S, Dayes NS, Thieu KP, Weiner DB. HIV-1 Vpr inhibits the maturation and activation of macrophages and dendritic cells in vitro. Int. Immunol. 2005;17(2):103–116. doi: 10.1093/intimm/dxh190. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Suzuki H, Okamoto T, Kotani S, Atsuji Y, Tanaka T, Ito Y. Recombinant Vpr (rVpr) causes augmentation of HIV-1 p24 Ag level in U1 cells through its ability to induce the secretion of TNF. Virus Res. 2002;90(1–2):263–268. doi: 10.1016/s0168-1702(02)00230-7. [DOI] [PubMed] [Google Scholar]
- Nonaka M, Hashimoto Y, Takeshima SN, Aida Y. The human immunodeficiency virus type 1 Vpr protein and its carboxy-terminally truncated form induce apoptosis in tumor cells. Cancer Cell Int. 2009;9:20. doi: 10.1186/1475-2867-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorbakhsh F, Ramachandran R, Barsby N, Ellestad KK, LeBlanc A, Dickie P, Baker G, Hollenberg MD, Cohen EA, Power C. MicroRNA profiling reveals new aspects of HIV neurodegeneration: Caspase-6 regulates astrocyte survival. FASEB J. 2010;24(6):1799–1812. doi: 10.1096/fj.09-147819. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Shibata R, Kiyomasu T, Higuchi I, Kishida Y, Ishimoto A, Adachi A. Mutational analysis of the human immunodeficiency virus vpr open reading frame. J. Virol. 1989;63(9):4110–4114. doi: 10.1128/jvi.63.9.4110-4114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton W, Connor RI, Landau NR. Incorporation of Vpr into human immunodeficiency virus type 1 virions: Requirement for the p6 region of gag and mutational analysis. J. Virol. 1993;67(12):7229–7237. doi: 10.1128/jvi.67.12.7229-7237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA. 1994;91(22):10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piller SC, Ewart GD, Premkumar A, Cox GB, Gage PW. Vpr protein of human immunodeficiency virus type 1 forms cation-selective channels in planar lipid bilayers. Proc. Natl. Acad. Sci. USA. 1996;93(1):111–115. doi: 10.1073/pnas.93.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piller SC, Jans P, Gage PW, Jans DA. Extracellular HIV-1 virus protein R causes a large inward current and cell death in cultured hippocampal neurons: Implications for AIDS pathology. Proc. Natl. Acad. Sci. USA. 1998;95(8):4595–4600. doi: 10.1073/pnas.95.8.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. Snip-snip, kill-kill: Truncated SDF-1 and HIV-associated neurode-generation. Nat. Neurosci. 2003;6(10):1009–1011. doi: 10.1038/nn1003-1009. [DOI] [PubMed] [Google Scholar]
- Re DB, Nafia I, Melon C, Shimamoto K, Kerkerian-Le Goff L, Had-Aissouni L. Glutamate leakage from a compartmentalized intracellular metabolic pool and activation of the lipoxygenase pathway mediate oxidative astrocyte death by reversed glutamate transport. Glia. 2006;54(1):47–57. doi: 10.1002/glia.20353. [DOI] [PubMed] [Google Scholar]
- Reiss P, Lange JM, de Ronde A, de Wolf F, Dekker J, Danner SA, Debouck C, Goudsmit J. Antibody response to viral proteins U (vpu) and R (vpr) in HIV-1-infected individuals. J. Acquir. Immune Defic. Syndr. 1990;3(2):115–122. [PubMed] [Google Scholar]
- Richardson MW, Mirchandani J, Duong J, Grimaldo S, Kocieda V, Hendel H, Khalili K, Zagury JF, Rappaport J. Antibodies to Tat and Vpr in the GRIV cohort: Differential association with maintenance of long-term non-progression status in HIV-1 infection. Biomed. Pharmacother. 2003;57(1):4–14. doi: 10.1016/s0753-3322(02)00327-x. [DOI] [PubMed] [Google Scholar]
- Rimaniol AC, Haik S, Martin M, Le Grand R, Boussin FD, Dereuddre-Bosquet N, Gras G, Dormont D. Na+-dependent high-affinity glutamate transport in macrophages. J. Immunol. 2000;164(10):5430–5438. doi: 10.4049/jimmunol.164.10.5430. [DOI] [PubMed] [Google Scholar]
- Rom I, Deshmane SL, Mukerjee R, Khalili K, Amini S, Sawaya BE. HIV-1 Vpr deregulates calcium secretion in neural cells. Brain Res. 2009;1275:81–86. doi: 10.1016/j.brainres.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani B, Engelbrecht S. Human immunodeficiency virus type 1 Vpr: Functions and molecular interactions. J. Gen. Virol. 2009;90(Pt 8):1795–1805. doi: 10.1099/vir.0.011726-0. [DOI] [PubMed] [Google Scholar]
- Roumier T, Vieira HL, Castedo M, Ferri KF, Boya P, Andreau K, Druillennec S, Joza N, Penninger JM, Roques B, Kroemer G. The C-terminal moiety of HIV-1 Vpr induces cell death via a caspase-independent mitochondrial pathway. Cell Death Differ. 2002;9(11):1212–1219. doi: 10.1038/sj.cdd.4401089. [DOI] [PubMed] [Google Scholar]
- Ryglewski S, Pflueger HJ, Duch C. Expanding the neuron's calcium signaling repertoire: Intracellular calcium release via voltage-induced PLC and IP3R activation. PLoS Biol. 2007;5(4):e66. doi: 10.1371/journal.pbio.0050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah EN, Roques BP. Critical implication of the (70–96) domain of human immunodeficiency virus type 1 Vpr protein in apoptosis of primary rat cortical and striatal neurons. J. Neurovirol. 2005;11(6):489–502. doi: 10.1080/13550280500384941. [DOI] [PubMed] [Google Scholar]
- Sabbah EN, Druillennec S, Morellet N, Bouaziz S, Kroemer G, Roques BP. Interaction between the HIV-1 protein Vpr and the adenine nucleotide translocator. Chem. Biol. Drug Des. 2006;67(2):145–154. doi: 10.1111/j.1747-0285.2006.00340.x. [DOI] [PubMed] [Google Scholar]
- Sattentau QJ, Dalgleish AG, Weiss RA, Beverley PC. Epitopes of the CD4 antigen and HIV infection. Science. 1986;234(4780):1120–1123. doi: 10.1126/science.2430333. [DOI] [PubMed] [Google Scholar]
- Sawaya BE, Khalili K, Mercer WE, Denisova L, Amini S. Cooperative actions of HIV-1 Vpr and p53 modulate viral gene transcription. J. Biol. Chem. 1998;273(32):20052–20057. doi: 10.1074/jbc.273.32.20052. [DOI] [PubMed] [Google Scholar]
- Sawaya BE, Khalili K, Gordon J, Taube R, Amini S. Cooperative interaction between HIV-1 regulatory proteins Tat and Vpr modulates transcription of the viral genome. J. Biol. Chem. 2000;275(45):35209–35214. doi: 10.1074/jbc.M005197200. [DOI] [PubMed] [Google Scholar]
- Scemes E, Giaume C. Astrocyte calcium waves: What they are and what they do. Glia. 2006;54(7):716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MP, de Noronha CM, Heusch MI, Greene S, Greene WC. Nucleocytoplasmic shuttling by human immunodeficiency virus type 1 Vpr. J. Virol. 2001;75(3):1522–1532. doi: 10.1128/JVI.75.3.1522-1532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MP, Schubert U, Williams SA, de Noronha CM, Kreisberg JF, Henklein P, Greene WC. HIV-1 Vpr displays natural protein-transducing properties: Implications for viral pathogenesis. Virology. 2002;302(1):95–105. doi: 10.1006/viro.2002.1576. [DOI] [PubMed] [Google Scholar]
- Sherman MP, de Noronha CM, Eckstein LA, Hataye J, Mundt P, Williams SA, Neidleman JA, Goldsmith MA, Greene WC. Nuclear export of Vpr is required for efficient replication of human immunodeficiency virus type 1 in tissue macrophages. J. Virol. 2003;77(13):7582–7589. doi: 10.1128/JVI.77.13.7582-7589.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber RH, Pavlakis GN. Intracellular trafficking and interactions of the HIV-1 Tat protein. Virology. 1998;252(1):126–136. doi: 10.1006/viro.1998.9400. [DOI] [PubMed] [Google Scholar]
- Stewart SA, Poon B, Jowett JB, Chen IS. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J. Virol. 1997;71(7):5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SA, Poon B, Song JY, Chen IS. Human immunodeficiency virus type 1 vpr induces apoptosis through caspase activation. J. Virol. 2000;74(7):3105–3111. doi: 10.1128/jvi.74.7.3105-3111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbramanian RA, Kessous-Elbaz A, Lodge R, Forget J, Yao XJ, Bergeron D, Cohen EA. Human immunodeficiency virus type 1 Vpr is a positive regulator of viral transcription and infectivity in primary human macrophages. J. Exp. Med. 1998;187(7):1103–1111. doi: 10.1084/jem.187.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi T, Shimura M, Osawa Y, Suzuki Y, Mizoguchi I, Niino K, Takaku F, Ishizaka Y. Nuclear trafficking of macromolecules by an oligopeptide derived from Vpr of human immunodeficiency virus type-1. Biochem. Biophys. Res. Commun. 2004;320(1):18–26. doi: 10.1016/j.bbrc.2004.05.126. [DOI] [PubMed] [Google Scholar]
- Varin A, Decrion AZ, Sabbah E, Quivy V, Sire J, Van Lint C, Roques BP, Aggarwal BB, Herbein G. Synthetic Vpr protein activates activator protein-1, c-Jun N-terminal kinase, and NF-kappaB and stimulates HIV-1 transcription in promonocytic cells and primary macrophages. J. Biol. Chem. 2005;280(52):42557–42567. doi: 10.1074/jbc.M502211200. [DOI] [PubMed] [Google Scholar]
- Vendeville A, Rayne F, Bonhoure A, Bettache N, Montcourrier P, Beaumelle B. HIV-1 Tat enters T cells using coated pits before translocating from acidified endosomes and eliciting biological responses. Mol. Biol. Cell. 2004;15(5):2347–2360. doi: 10.1091/mbc.E03-12-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachari NJ, Walker LA, Tastan O, Le T, Dempsey TM, Li Y, Yanamala N, Srinivasan A, Klein-Seetharaman J, Montelaro RC, Ayyavoo V. Human immunodeficiency virus type 1 Vpr: Oligomerization is an essential feature for its incorporation into virus particles. Virol. J. 2010;7(1):119. doi: 10.1186/1743-422X-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesce S, Bezzi P, Rossi D, Meldolesi J, Volterra A. HIV-1 gp120 glycoprotein affects the astrocyte control of extracellular glutamate by both inhibiting the uptake and stimulating the release of the amino acid. FEBS Lett. 1997;411(1):107–109. doi: 10.1016/s0014-5793(97)00674-1. [DOI] [PubMed] [Google Scholar]
- Vieira HL, Haouzi D, El Hamel C, Jacotot E, Belzacq AS, Brenner C, Kroemer G. Permeabilization of the mitochondrial inner membrane during apoptosis: Impact of the adenine nucleotide translocator. Cell Death Differ. 2000;7(12):1146–1154. doi: 10.1038/sj.cdd.4400778. [DOI] [PubMed] [Google Scholar]
- Waldhuber MG, Bateson M, Tan J, Greenway AL, McPhee DA. Studies with GFP-Vpr fusion proteins: Induction of apoptosis but ablation of cell-cycle arrest despite nuclear membrane or nuclear localization. Virology. 2003;313(1):91–104. doi: 10.1016/s0042-6822(03)00258-7. [DOI] [PubMed] [Google Scholar]
- Wang L, Mukherjee S, Jia F, Narayan O, Zhao LJ. Interaction of virion protein Vpr of human immunodeficiency virus type 1 with cellular transcription factor Sp1 and trans-activation of viral long terminal repeat. J. Biol. Chem. 1995;270(43):25564–25569. doi: 10.1074/jbc.270.43.25564. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312(1):60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun J, Goldstein H. Human immunodeficiency virus type 1 infection increases the in vivo capacity of peripheral monocytes to cross the blood–brain barrier into the brain and the in vivo sensitivity of the blood–brain barrier to disruption by lipopolysaccharide. J. Virol. 2008;82(15):7591–7600. doi: 10.1128/JVI.00768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Hickey WF. Traffic of lymphocytes into the CNS during inflammation and HIV infection. J. NeuroAIDS. 1996;1(1):31–55. doi: 10.1300/j128v01n01_02. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Chen G, Richard J, Rougeau N, Li H, Seidah NG, Cohen EA. Cell-surface processing of extracellular human immunodeficiency virus type 1 Vpr by proprotein convertases. Virology. 2008;372(2):384–397. doi: 10.1016/j.virol.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Tikhonov I, Ruckwardt TJ, Djavani M, Zapata JC, Pauza CD, Salvato MS. Monocytes treated with human immunodeficiency virus Tat kill uninfected CD4(+) cells by a tumor necrosis factor-related apoptosis-induced ligand- mediated mechanism. J. Virol. 2003;77(12):6700–6708. doi: 10.1128/JVI.77.12.6700-6708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Matsuda Z, Matsuda M, Essex M, Lee TH. Human immunodeficiency virus vpr gene encodes a virion-associated protein. AIDS Res. Hum. Retroviruses. 1990;6(11):1265–1271. doi: 10.1089/aid.1990.6.1265. [DOI] [PubMed] [Google Scholar]
- Zhang S, Pointer D, Singer G, Feng Y, Park K, Zhao LJ. Direct binding to nucleic acids by Vpr of human immunodeficiency virus type 1. Gene. 1998;212(2):157–166. doi: 10.1016/s0378-1119(98)00178-4. [DOI] [PubMed] [Google Scholar]
- Zhao LJ, Wang L, Mukherjee S, Narayan O. Biochemical mechanism of HIV-1 Vpr function. Oligomerization mediated by the N-terminal domain. J. Biol. Chem. 1994;269(51):32131–32137. [PubMed] [Google Scholar]
- Zhu Y, Roshal M, Li F, Blackett J, Planelles V. Upregulation of survivin by HIV-1 Vpr. Apoptosis. 2003;8(1):71–79. doi: 10.1023/a:1021653119934. [DOI] [PubMed] [Google Scholar]