Abstract
Intracerebral hemorrhage (ICH) is the second most common and deadliest form of stroke. Currently, no pharmacologic treatment strategies exist for this devastating disease. Following the initial mechanical injury suffered at hemorrhage onset, secondary brain injury proceeds through both direct cellular injury and inflammatory cascades, which trigger infiltration of granulocytes and monocytes, activation of microglia, and disruption of the blood–brain barrier with resulting cerebral edema. The complement cascade has been shown to play a central role in the pathogenesis of secondary injury following ICH, although the specific mechanisms responsible for the proximal activation of complement remain incompletely understood. Cerebral injury following cleavage of complement component (C3) proceeds through parallel but interrelated pathways of anaphylatoxin-mediated inflammation and direct toxicity secondary to membrane attack complex-driven erythrocyte lysis. Complement activation also likely plays an important physiologic role in recovery following ICH. As such, a detailed understanding of the variation in functional effects of complement activation over time is critical to exploiting this target as an exciting translational strategy for intracerebral hemorrhage.
Keywords: Complement cascade, Edema, Hemoglobin, Intracerebral hemorrhage, Mechanism
Intracerebral hemorrhage and inflammation
Intracerebral hemorrhage (ICH) is the second most common and deadliest form of stroke, annually affecting 12–15 persons per 100,000 in the United States and carrying a mortality rate of 30–50% which has not improved over the last two decades (Aronowski and Hall, 2005). Moreover, ICH imparts some form of disability in close to 90% of its survivors (Taylor et al., 1997; Zhang et al., 2006). The most common etiology of ICH is hypertension, placing a large proportion of the population at risk, although other causes include cerebrovascular malformations, amyloid angiopathy, neoplasms, thrombocytopenia, and coagulation disorders. The prognosis for patients with ICH varies widely and depends on several factors, including the location, volume, and degree of expansion of the hematoma. The volume of the initial hematoma is known to correlate with morbidity and mortality following ICH, and hematoma expansion has been shown to be associated with a worse clinical outcome (Fujii et al., 1994).
At the onset of ICH, an accumulated mass of extravasated blood products immediately compresses surrounding tissue, followed rapidly by the initiation of early cellular and molecular pathologic processes. This cellular and inflammatory activation plays an important role in secondary brain injury by triggering polymorpho-nuclear leukocyte and monocyte infiltration, microglial activation, disruption of the blood–brain barrier, and development of cerebral edema. On the other hand, this same response is critical for the clearance of erythrocytes and other post-hemorrhagic cellular debris. In the latter stages of ICH, reparative processes predominate, with the release of trophic factors and adjustment of the brain to a post-hemorrhagic milieu (Aronowski and Hall, 2005; Wang and Dore, 2007).
Current therapy for ICH generally includes supportive care, as well as management of increased intracranial pressure via hyperosmolar therapy and external ventricular drainage of cerebrospinal fluid (CSF; Broderick et al., 2007; Morgenstern et al., 1998). With multiple randomized trials failing to show a clear benefit of surgical evacuation for ICH, and several proven methods (including blood pressure control and reversal of coagulopathy) for the prevention of secondary hematoma expansion, recent investigations have focused on the pathophysiology of ICH-induced inflammation and cerebral edema as potential therapeutic targets. In the setting of ICH, significant morbidity and mortality occurs secondary to the development of peri-hematomal inflammation and vasogenic cerebral edema within 72–96 h post-ictus. This edema compounds the mass effect of the initial hematoma, exacerbating compression of adjacent structures causing cerebral ischemia and infarction, and at its most severe, herniation and death. Following ICH, cerebral edema has been shown to develop in three distinct phases: (1) an early phase occurring within the first few hours after the initial hemorrhage involving hydrostatic pressure and clot retraction, (2) a second phase developing within the next 48 h wherein the coagulation cascade is activated and thrombin produced, and (3) a final phase characterized by erythrocyte lysis and hemoglobin-mediated neuronal toxicity (Xi et al., 2002a, b). As yet, however, there are no effective pharmacological treatments targeting cerebral edema following ICH in humans.
Recently, animal models employing either autologous blood or collagenase infusion have highlighted multiple distinct, yet interconnected, pathways that mediate inflammation and edema following ICH. Previous reviews have highlighted the roles of cytokines, matrix metalloproteinases, and reactive oxygen species in injury after ICH (Aronowski and Hall, 2005; Wang and Dore, 2007; Xi et al., 2006), but relatively little attention has been paid to the complement cascade. A phylogenetically conserved component of the innate immune system, the complement cascade has been shown to play a central role in the pathogenesis of cerebral edema formation, inflammation, and cell death following ICH (Aronowski and Hall, 2005; Xi et al., 2001, 2002a, b; Yang et al., 2006a, b). This review highlights the role of complement activation in the pathophysiology of ICH and frames a paradigm for future translational efforts of therapeutic complement inhibition.
Putative mechanisms of proximal pathway complement activation following ICH
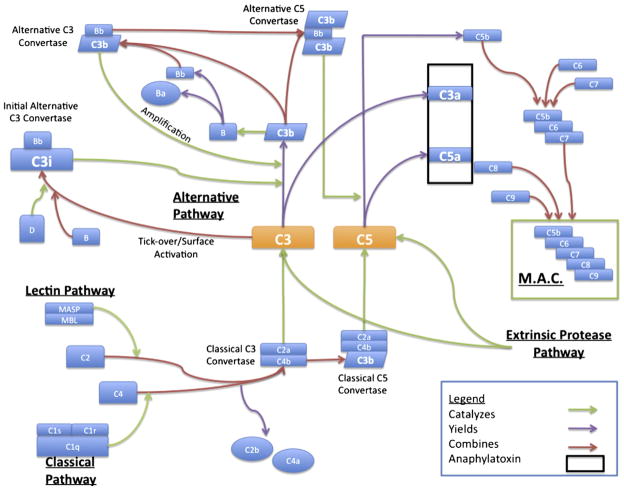
The complement cascade involves more than 30 serum and membrane-bound proteins and is involved in inflammation, opsonization, and cytolysis in a wide range of diseases (Chavez-Cartaya et al., 1995; Foreman et al., 1996; Hill et al., 1992; Hill and Ward, 1971; Ikai et al., 1996; Pemberton et al., 1993; Zamora et al., 1999). Activation of the complement cascade traditionally occurs via one of three proximal pathways – the classical, mannose-binding lectin, or alternative pathway – all of which converge on complement component 3 (C3; Fig. 1). Cleavage of C3 to form C3a and C3b leads to C5a production and ultimately to the assembly of the membrane attack complex (MAC, C5b-9). The three aforementioned proximal pathways rely on differing inciting stimuli. For example, the classical pathway of activation occurs through C1q binding to immune complexes (Mollnes et al., 2002). C1q then associates with the serine proteases C1r and C1s in a large enzymatic complex which serves to cleave C4 and C2, producing C4b and C2a, which assemble to form the classical C3 convertase. The alternative pathway, which has been shown to be functionally important in a variety of disease processes including experimental head trauma (Leinhase et al., 2007), is activated spontaneously, albeit slowly, by hydrolysis of the internal thioester bond of C3 and further triggered by contact with various exposed factors found on microorganisms and other foreign surfaces. This spontaneous “tickover” generates C3(H2O), which then binds factor B, allowing it to be cleaved to Ba and Bb by the protease factor D. This process generates a C3 convertase, C3(H2O)Bb, which is able to cleave additional C3 molecules. Although this spontaneous process occurs at a low rate, C3b generated by any of the three proximal pathway C3 convertases may bind factor B and propagate further C3 activation through an ‘amplification loop’ (Holers, 2008). Finally, the mannose binding lectin (MBL) pathway is triggered by the binding of MBL to repeating carbohydrate residues on the surface of target cells (Holers, 2008). Activation of this pathway has been described in the setting of ischemia and reperfusion of systemic organ systems, where it is believed that ischemia leads to exposure of self-antigens, which are subsequently bound by circulating natural antibody or directly by MBL (Thrane et al., 2007). MBL, assisted by mannose-associated serine protease 2 (MASP-2), then proceeds to cleave C4 and C2 leading to the assembly of a C3 convertase. The specific mechanisms triggering activation of these three different proximal complement pathways have not been explored following experimental ICH.
Fig. 1.
Schematic depicting the three proximal pathways of complement activation converging on the central component C3, with subsequent production of the anaphylatoxins C3a and C5a as well as the more distal assembly of the membrane attack complex (MAC).
While proximal activation of the complement cascade may occur through traditional routes, there have been several additional proteases described that are able to serve as independent or parallel pathway C3 and/or C5 convertases both in vitro and in vivo (Amara et al., 2008; Clark et al., 2008). For example, thrombin, a serine protease and an essential component of the coagulation cascade, is produced immediately following intracerebral hemorrhage and may be responsible for early brain edema formation and neuronal injury (Hua et al., 2007). Thrombin-induced cerebral injury following ICH may be mediated in large part by complement activation, as inhibition of complement using N-acetylheparin attenuates thrombin-induced edema, complement deposition, and improves neurologic function (Gong et al., 2005).
Complement-mediated injury cascades in ICH
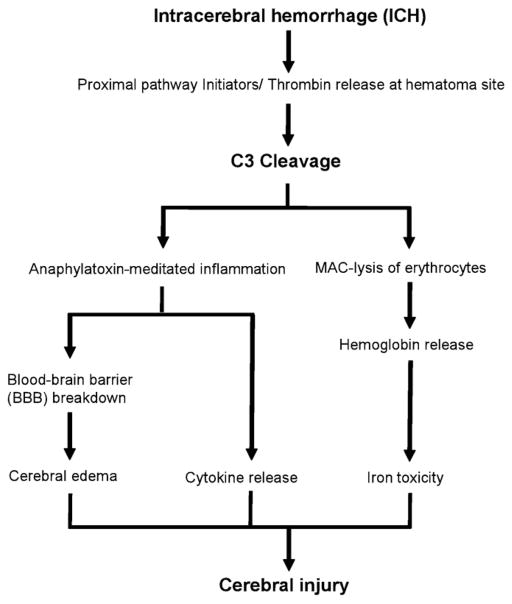
In the setting of ICH, complement activation results in the rapid induction of multiple parallel downstream pathways of cerebral injury (Fig. 2). Cleavage of C3 results in the release of the C3a anaphylatoxin, as well as the association of C3b with C4b2a, producing the C5 convertase. This enzymatic complex cleaves C5, releasing the C5a anaphylatoxin, as well as resulting in the deposition of C5b and subsequent assembly of the pore-forming membrane attack complex (MAC, C5b-9) on the target cell surface. In the setting of ICH, a diffusible anaphylatoxin-mediated response, as well as MAC-mediated lysis of erythrocytes, subserve distinct but parallel roles in exacerbating perihematomal cerebral injury.
Fig. 2.
The complement system plays a crucial role in the pathophysiology of cerebral injury following intracerebral hemorrhage.
The anaphylatoxins C3a and C5a serve as powerful chemoat-tractants for leukocytes and may damage the blood–brain barrier following ICH. Anaphylatoxin induction induces rapid activation of endothelial cells and resident microglia, as well as infiltration of granulocytes, in the perihematomal region (Rynkowski et al., 2008, 2009). Activated microglia secrete inflammatory cytokines such as TNF-α and IL-1β, thereby amplifying the inflammatory response (Aronowski and Hall, 2005). Inflammatory cells also release myeloperoxidase and other toxic products when activated, producing reactive oxygen species which cause direct cerebral injury. These coordinated responses culminate in an exacerbation of brain edema, leading to secondary cerebral injury (Ganz and Faustmann, 1994; Okusawa et al., 1988; Rynkowski et al., 2008, 2009; Takabayashi et al., 1996; Xi et al., 2002a, b).
The formation of the MAC promotes erythrocyte lysis is a parallel injury pathway following ICH. This pathway enhances brain edema in both the acute and delayed phases and functions through a mechanism relying on the breakdown of hemoglobin, which is degraded by heme oxygenase into iron, carbon monoxide, and biliverdin (Wu et al., 2003; Xi et al., 1998). High concentrations of iron lead to increases in brain edema and have also been demonstrated to result in direct cerebral injury mediated through oxidative stress (Wang et al., 2002). Further support for this mechanism is derived from the observation that deferoxamine, an iron chelator, reduces hematoma and hemoglobin-induced edema, and antioxidants block neuronal toxicity induced by hemoglobin (Hua et al., 2007; Wagner et al., 2003). Additionally, direct cellular injury via this mechanism also triggers additional inflammation, serving to amplify the injury response.
Experimental support for a pathophysiologic role for complement activation following ICH
In the central nervous system, complement activation has been implicated in the pathophysiology of multiple disease processes including multiple sclerosis (Gasque et al., 1998), stroke (Ducruet et al., 2008; Mocco et al., 2006a, b), aneurysmal subarachnoid hemorrhage (Kasuya and Shimizu, 1989; Mack et al., 2007), and age-related macular degeneration (Yates et al., 2007). The role of the complement cascade in the pathophysiology of ICH has only recently been explored, and initial work was performed in a rat model employing nonspecific inhibitors of complement activation. Hua and colleagues provided the first direct evidence for complement activation in ICH and its involvement in cerebral edema formation (Hua et al., 1999, 2000). Increased perihematomal C9 was detected 24 h following the injection of autologous blood into rat basal ganglia and MAC formation was evident by 72 h. The addition of N-acetylheparin (a complement inhibitor) was found to significantly reduce cerebral edema at 24 and 72 h post-ICH in the same model. Subsequent studies have confirmed a central role for complement activation in the pathogenesis of ICH. Systemic depletion of complement using cobra venom factor (CVF) in rats resulted in significant reductions in perihematomal C9 deposition, C5a, TNF-α, myeloperoxidase (MPO)-positive cells, and C3d production, following ICH (Xi et al., 2001, 2002a, b). Additionally, brain water measurements revealed a marked decrease in perihematomal edema (Xi et al., 2001). A study utilizing a similar nonspecific complement inhibitor, venom defibrase DF-521 batroxobin, confirmed these findings (Wu and Huang, 2005). This early work focused primarily on the contribution of MAC-mediated erythrocyte lysis in the pathogenesis of brain edema. Evidence also suggests that MAC may be directly injurious to neurons, astrocytes, and endothelial cells leading to blood–brain barrier disruption (Bellander et al., 2001). However, future studies aiming to elucidate the role of the distal pathway in the pathogenesis of secondary injury following ICH must employ techniques which specifically target the MAC without impairing anaphylatoxin production.
A potential role for the potent anaphylatoxin C5a in early edema formation and cerebral injury has also been suggested. Early studies once again utilized non-specific complement inhibitors and were therefore not able to precisely define the contribution of specific subcomponents of the complement cascade to injury after ICH. The recent advent of complement-deficient mice engendered a new phase of research in the pathogenesis of a wide variety of diseases. The first study employed C5-deficient mice in the setting of ICH and yielded surprising results. Relative to wild type C5-sufficent mice, C5-deficient animals demonstrated a significant increase in brain water content and greater neurologic impairment at 3 days following ICH (Nakamura et al., 2004). These paradoxic findings were explained by suggesting that activation of C5 may have a beneficial effect in this setting as demonstrated by Mukherjee and Pasinetti in a kainic acid-induced model of hippocampal damage, or perhaps that factors proximal to C5 are critical in mediating damage following ICH (Mukherjee and Pasinetti, 2000; Nakamura et al., 2004; Pasinetti et al., 1996). Alternatively, C5-deficient mice might manifest unanticipated phenotypic compensation for the developmental lack of C5.
Given these initial findings, and our group’s determination of a predominant role for C3 in the pathogenesis of cerebral ischemia-reperfusion injury (Ducruet et al., 2008; Mocco et al., 2006a, b) subsequent work in ICH focused on C3 activation, and in particular the C3 cleavage product C3a, as a critical effector of complement-mediated cerebral injury In a murine model of autologous blood injection, Yang et al. (2006a, b) first demonstrated that C3-deficient mice developed less edema, had reduced heme oxygenase-1 (HO-1) levels, and exhibited neurologic deficits compared to C3-sufficient mice. Thereafter, using a similar murine model developed in our laboratory (Rynkowski et al., 2008, 2009), we demonstrated that brain edema and neurologic deficits were reduced at 72 post-ICH when mice were administered C3a receptor antagonist (C3aRA) at the delayed time point of 6 h following hemorrhage onset (Rynkowski et al., 2008, 2009). Furthermore, mice pretreated with C3aRA had significantly reduced perihematomal granulocyte infiltration, suggesting a critical role for C3aR on inflammatory cell infiltration in experimental ICH. Further support for a central role for C3a derives from additional experimental work examining the time course of injury in experimental models of ICH. Zhang et al. (2006). recently showed that in rats, both brain edema and the inflammatory cytokines TNF-α, IL-6, and NF-κB were maximal at 48 h following ICH. C3 expression peaked at 48–72 h, followed by delayed expression of ICAM-1 and VEGF (which correlates with blood–brain barrier breakdown and vasogenic edema) at 72 h after the hemorrhage. Viewing these studies in combination, C3 appears to play a central role in the pathogenesis of injury following experimental ICH.
Recently, there has been renewed interest in a potential role for the C5a anaphylatoxin in the pathophysiology of ICH induced cerebral injury. In a recent study carried out by our group, Garrett et al. (2009) demonstrated significant improvements in neurological function as assessed by both the corner turn test and a 28-point neurological scale at 24 and 72 h following ICH in mice treated with a small molecule antagonist to the C5a receptor. These animals also demonstrated improved spatial memory retention in the Morris Water Maze relative to vehicle-treated animals. Additionally, mice treated with C5a receptor antagonist demonstrated a decrease in granulocyte infiltration into the hemorrhagic hemisphere. Synergism was seen when combined with C3a receptor antagonist, suggesting that a combined approach of complement inhibition may offer superior neuroprotec-tion. This finding again highlights the complexity of probing individual arms of these potentially redundant cascades, and supports a combined approach using both pharmacologic and genetic deletion strategies to tease out the functional role of specific elements of the complement cascade.
Potential beneficial effects of complement activation following ICH
As with many components of immunologic and inflammatory processes, recent work suggests that complement activation products are multifunctional, serving important physiologic roles in repair and recovery following injury (Alexander et al., 2008). An in-depth understanding of the functional role for complement activation over time is therefore critical to developing an effective translational strategy for ICH.
In the case of acute tissue injury, such as occurs following ICH, it is likely that necrotic cell debris from uncleared apoptotic cells exacerbates the pro-inflammatory response, although this pathway remains incompletely defined (Fadok, 1999). Complement, particularly early components such as C1q, C3b, and C4b, functions prominently in the process of opsonization and subsequent apoptotic cell clearance (Fishelson et al., 2001; Mevorach, 1999, 2003). Previous work in other disease models suggests that complement inhibition might exacerbate pathology as a result of a decrease in clearance of cellular debris. This has been observed in transgenic models of neurodegeneration in both systemic lupus erythematosus and Alzheimer’s disease (Alexander et al., 2005; Wyss-Coray et al., 2002). Furthermore, it appears that the process of phagocytosis of apoptotic cells has a profound role in directing the shift in cytokine milieu from proinflammatory to anti-inflammatory in the setting of injury (De Simone et al., 2003; Fadok and Henson, 1998, Savill, 1997). Given this established critical function for complement in the clearance of apoptotic bodies and cellular debris, inhibition of complement in the subacute to chronic phase of ICH might impair the physiologic process of recovery.
Previous work has demonstrated a marked recovery of neurologic function in the days to weeks following experimental ICH (Hua et al., 2002). It is unclear whether this progressive recovery results from the resumption of function by remaining neurons or from the development of new neurons. In support of the latter, neurogenesis has been demonstrated in specimens obtained from patients with primary brain hemorrhage (Shen et al., 2008), and recent work in a rat model of ICH (Yang et al., 2008) has demonstrated evidence of immature neuronal cells. Nevertheless, it remains unclear what, if any, functional role is served by the production and development of these endogenous precursor cells following ICH.
Interestingly, a recent study has proposed a role for complement, specifically for the anaphylatoxin C3a, in neurogenesis following ischemic stroke (Rahpeymai et al., 2006). In that study, mice genetically deficient for the C3a receptor, as well as mice treated with an antagonist to the C3a receptor, exhibited impaired basal neurogenesis in the hippocampus. Furthermore, C3 knockout mice exhibited impaired ischemia-induced neurogenesis in the subventri-cular zone following permanent occlusion of the middle cerebral artery. These findings suggest a role for C3a in both basal and ischemia-induced neurogenesis. The role of complement components in neurogenesis following ICH, while a logical extension of this work, remains unexplored.
Therapeutic strategies for complement inhibition in ICH
A multitude of pharmacologic strategies exist for targeting the complement cascade as a therapeutic intervention in a particular disease process. Given the redundancy of the proximal pathways of activation, the possibility for cascade-independent cleavage of C3, and available evidence derived from experimental models, strategies targeting C3 are currently the most attractive choice for complement inhibition following ICH. This strategy would serve to inhibit the formation of both anaphylatoxins, as well as the downstream process of MAC-mediated erythrocyte lysis, and concomitant heme toxicity. However, given the known role for complement in the clearance of apoptotic cell bodies, as well as its putative role in ICH-induced neurogenesis, therapeutic complement inhibition must be carried out in a temporally targeted fashion to maximize the beneficial effects of complement inhibition while minimizing the potential negative aspects of this treatment.
Conclusion
While there exists little data on the activation of complement in human patients following ICH, the importance of complement activation, and in particular C3/C3a, has been shown repeatedly in the pathogenesis of other neurosurgical diseases (Kasuya and Shimizu, 1989; Mack et al., 2007). Additionally, there is compelling animal data for an integral role for complement activation in mediating cerebral injury after ICH. Currently there are no effective pharmacological therapies for the treatment of ICH in humans. Selective and temporally targeted complement inhibition, particularly of C3, therefore represents an extremely attractive strategy for reducing inflammation, cerebral edema, and brain injury following human ICH, with the goal of improving functional outcomes.
References
- Alexander JJ, Jacob A, Bao L, Macdonald RL, Quigg RJ. Complement-dependent apoptosis and inflammatory gene changes in murine lupus cerebritis. J Immunol. 2005;175:8312–8319. doi: 10.4049/jimmunol.175.12.8312. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Anderson AJ, Barnum SR, Stevens B, Tenner AJ. The complement cascade: Yin-Yang in neuroinflammation–neuro-protection and -degeneration. J Neurochem. 2008;107:1169–1187. doi: 10.1111/j.1471-4159.2008.05668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara U, Rittirsch D, Flierl M, Bruckner U, Klos A, Gebhard F, Lambris JD, Huber-Lang M. Interaction between the coagulation and complement system. Adv Exp Med Biol. 2008;632:71–79. doi: 10.1007/978-0-387-78952-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27:268–279. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- Bellander BM, Singhrao SK, Ohlsson M, Mattsson P, Svensson M. Complement activation in the human brainafter traumatic head injury. J Neurotrauma. 2001;18:1295–1311. doi: 10.1089/08977150152725605. [DOI] [PubMed] [Google Scholar]
- Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, Mayberg M, Morgenstern L, Ogilvy CS, Vespa P, Zuccarello M. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001–2023. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- Chavez-Cartaya RE, DeSola GP, Wright L, Jamieson NV, White DJ. Regulation of the complement cascade by soluble complement receptor type 1. Protective effect in experimental liver ischemia and reperfusion. Transplantation. 1995;59:1047–1052. doi: 10.1097/00007890-199504150-00023. [DOI] [PubMed] [Google Scholar]
- Clark A, Weymann A, Hartman E, Turmelle Y, Carroll M, Thurman JM, Holers VM, Hourcade DE, Rudnick DA. Evidence for non-traditional activation of complement factor C3 during murine liver regeneration. Mol Immunol. 2008;45:3125–3132. doi: 10.1016/j.molimm.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone R, Ajmone-Cat MA, Tirassa P, Minghetti L. Apoptotic PC12 cells exposing phosphatidylserine promote the production of anti-inflammatory and neuroprotective molecules by microglial cells. J Neuropathol Exp Neurol. 2003;62:208–216. doi: 10.1093/jnen/62.2.208. [DOI] [PubMed] [Google Scholar]
- Ducruet AF, Hassid BG, Mack WJ, Sosunov SA, Otten ML, Fusco DJ, Hickman ZL, Kim GH, Komotar RJ, Mocco J, Connolly ES. C3a receptor modulation of granulocyte infiltration after murine focal cerebral ischemia is reperfusion dependent. J Cereb Blood Flow Metab. 2008;28:1048–1058. doi: 10.1038/sj.jcbfm.9600608. [DOI] [PubMed] [Google Scholar]
- Fadok VA. Clearance: the last and often forgotten stage of apoptosis. J Mammary Gland Biol Neoplasia. 1999;4:203–211. doi: 10.1023/a:1011384009787. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Henson PM. Apoptosis: getting rid of the bodies. Curr Biol. 1998;8:R693–695. doi: 10.1016/s0960-9822(98)70438-5. [DOI] [PubMed] [Google Scholar]
- Fishelson Z, Attali G, Mevorach D. Complement and apoptosis. Mol Immunol. 2001;38:207–219. doi: 10.1016/s0161-5890(01)00055-4. [DOI] [PubMed] [Google Scholar]
- Foreman KE, Glovsky MM, Warner RL, Horvath SJ, Ward PA. Comparative effect of C3a and C5a on adhesion molecule expression on neutrophils and endothelial cells. Inflammation. 1996;20:1–9. doi: 10.1007/BF01487740. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Tanaka R, Takeuchi S, Koike T, Minakawa T, Sasaki O. Hematoma enlargement in spontaneous intracerebral hemorrhage. J Neurosurg. 1994;80:51–57. doi: 10.3171/jns.1994.80.1.0051. [DOI] [PubMed] [Google Scholar]
- Ganz RE, Faustmann PM. Central autonomic dysorganization in the early stages of experimental meningitis in rabbits induced by complement-C5A-fragment: a pathophysiological validation of the largest lyapunov exponent of heart rate dynamics. Int J Neurosci. 1994;76:177–184. doi: 10.3109/00207459408986002. [DOI] [PubMed] [Google Scholar]
- Garrett MC, Otten ML, Starke RM, Komotar RJ, Magotti P, Lambris JD, Rynkowski MA, Connolly ES. Synergistic neuroprotective effects of C3a and C5a receptor blockade following intracerebral hemorrhage. Brain Res. 2009 doi: 10.1016/j.brainres.2009.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasque P, Singhrao SK, Neal JW, Wang P, Sayah S, Fontaine M, Morgan BP. The receptor for complement anaphylatoxin C3a is expressed by myeloid cells and nonmyeloid cells in inflamed human central nervous system: analysis in multiple sclerosis and bacterial meningitis. J Immunol. 1998;160:3543–3554. [PubMed] [Google Scholar]
- Gong Y, Xi GH, Keep RF, Hoff JT, Hua Y. Complement inhibition attenuates brain edema and neurological deficits induced by thrombin. Acta Neurochir, Suppl. 2005;95:389–392. doi: 10.1007/3-211-32318-x_79. [DOI] [PubMed] [Google Scholar]
- Hill JH, Ward PA. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med. 1971;133:885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Lindsay TF, Ortiz F, Yeh CG, Hechtman HB, Moore FD., Jr Soluble complement receptor type 1 ameliorates the local and remote organ injury after intestinal ischemia-reperfusion in the rat. J Immunol. 1992;149:1723–1728. [PubMed] [Google Scholar]
- Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol Rev. 2008;223:300–316. doi: 10.1111/j.1600-065X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- Hua Y, Xi G, Keep R, Xiang J, Hoff J. Complement C9 accumulation, membrane attack complex (MAC) formation and clusterin upregulation following intracer-ebral hemorrhage. J Cereb Blood Flow Metab. 1999;19 (Suppl 1):S670. [Google Scholar]
- Hua Y, Xi G, Keep RF, Hoff JT. Complement activation in the brain after experimental intracerebral hemorrhage. J Neurosurg. 2000;92:1016–1022. doi: 10.3171/jns.2000.92.6.1016. [DOI] [PubMed] [Google Scholar]
- Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke. 2007;38:759–762. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- Ikai M, Itoh M, Joh T, Yokoyama Y, Okada N, Okada H. Complement plays an essential role in shock following intestinal ischaemia in rats. Clin Exp Immunol. 1996;106:156–159. doi: 10.1046/j.1365-2249.1996.d01-817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya H, Shimizu T. Activated complement components C3a and C4a in cerebrospinal fluid and plasma following subarachnoid hemorrhage. J Neurosurg. 1989;71:741–746. doi: 10.3171/jns.1989.71.5.0741. [DOI] [PubMed] [Google Scholar]
- Leinhase I, Rozanski M, Harhausen D, Thurman JM, Schmidt OI, Hossini AM, Taha ME, Rittirsch D, Ward PA, Holers VM, Ertel W, Stahel PF. Inhibition of the alternative complement activation pathway in traumatic brain injury by a monoclonal anti-factor B antibody: a randomized placebo-controlled study in mice. J Neuroinflammation. 2007;4:13. doi: 10.1186/1742-2094-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack WJ, Ducruet AF, Hickman ZL, Garrett MC, Albert EJ, Kellner CP, Mocco J, Connolly ES., Jr Early plasma complement C3a levels correlate with functional outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007;61:255–260. doi: 10.1227/01.NEU.0000255518.96837.8E. discussion 260–251. [DOI] [PubMed] [Google Scholar]
- Mevorach D. The immune response to apoptotic cells. Ann NY Acad Sci. 1999;887:191–198. doi: 10.1111/j.1749-6632.1999.tb07933.x. [DOI] [PubMed] [Google Scholar]
- Mevorach D. Systemic lupus erythematosus and apoptosis: a question of balance. Clin Rev Allergy Immunol. 2003;25:49–60. doi: 10.1385/CRIAI:25:1:49. [DOI] [PubMed] [Google Scholar]
- Mocco J, Mack WJ, Ducruet AF, Sosunov SA, Sughrue ME, Hassid BG, Nair MN, Laufer I, Komotar RJ, Claire M, Holland H, Pinsky DJ, Connolly ES., Jr Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circ Res. 2006a;99:209–217. doi: 10.1161/01.RES.0000232544.90675.42. [DOI] [PubMed] [Google Scholar]
- Mocco J, Wilson DA, Komotar RJ, Sughrue ME, Coates K, Sacco RL, Elkind MS, Connolly ES., Jr Alterations in plasma complement levels after human ischemic stroke. Neurosurgery. 2006b;59:28–33. doi: 10.1227/01.NEU.0000219221.14280.65. discussion 28–33. [DOI] [PubMed] [Google Scholar]
- Mollnes TE, Song WC, Lambris JD. Complement in inflammatory tissue damage and disease. Trends Immunol. 2002;23:61–64. doi: 10.1016/s1471-4906(01)02129-9. [DOI] [PubMed] [Google Scholar]
- Morgenstern LB, Frankowski RF, Shedden P, Pasteur W, Grotta JC. Surgical treatment for intracerebral hemorrhage (STICH): A single-center, randomized clinical trial. Neurology. 1998;51:1359–1363. doi: 10.1212/wnl.51.5.1359. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Pasinetti GM. The role of complement anaphylatoxin C5a in neurodegeneration: Implications in Alzheimer’s disease. J Neuroimmunol. 2000;105:124–130. doi: 10.1016/s0165-5728(99)00261-1. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Xi G, Hua Y, Schallert T, Hoff JT, Keep RF. Intracerebral hemorrhage in mice: model characterization and application for genetically modified mice. J Cereb Blood Flow Metab. 2004;24:487–494. doi: 10.1097/00004647-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Okusawa S, Yancey KB, van der Meer JW, Endres S, Lonnemann G, Hefter K, Frank MM, Burke JF, Dinarello CA, Gelfand JA. C5a stimulates secretion of tumor necrosis factor from human mononuclear cells in vitro. Comparison with secretion of interleukin 1 beta and interleukin 1 alpha. J Exp Med. 1988;168:443–448. doi: 10.1084/jem.168.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinetti GM, Tocco G, Sakhi S, Musleh WD, DeSimoni MG, Mascarucci P, Schreiber S, Baudry M, Finch CE. Hereditary deficiencies in complement C5 are associated with intensified neurodegenerative responses that implicate new roles for the C-system in neuronal and astrocytic functions. Neurobiol Dis. 1996;3:197–204. doi: 10.1006/nbdi.1996.0020. [DOI] [PubMed] [Google Scholar]
- Pemberton M, Anderson G, Vetvicka V, Justus DE, Ross GD. Microvascular effects of complement blockade with soluble recombinant CR1 on ischemia/reperfusion injury of skeletal muscle. J Immunol. 1993;150:5104–5113. [PubMed] [Google Scholar]
- Rahpeymai Y, Hietala MA, Wilhelmsson U, Fotheringham A, Davies I, Nilsson AK, Zwirner J, Wetsel RA, Gerard C, Pekny M, Pekna M. Complement: a novel factor in basal and ischemia-induced neurogenesis. EMBO J. 2006;25:1364–1374. doi: 10.1038/sj.emboj.7601004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynkowski MA, Kim GH, Komotar RJ, Otten ML, Ducruet AF, Zacharia BE, Kellner CP, Hahn DK, Merkow MB, Garrett MC, Starke RM, Cho BM, Sosunov SA, Connolly ES. A mouse model of intracerebral hemorrhage using autologous blood infusion. Nat Protoc. 2008;3:122–128. doi: 10.1038/nprot.2007.513. [DOI] [PubMed] [Google Scholar]
- Rynkowski MA, Kim GH, Garrett MC, Zacharia BE, Otten ML, Sosunov SA, Komotar RJ, Hassid BG, Ducruet AF, Lambris JD, Connolly ES. C3a receptor antagonist attenuates brain injury after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2009;29:98–107. doi: 10.1038/jcbfm.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–380. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- Shen J, Xie L, Mao X, Zhou Y, Zhan R, Greenberg DA, Jin K. Neurogenesis after primary intracerebral hemorrhage in adult human brain. J Cereb Blood Flow Metab. 2008;28:1460–1468. doi: 10.1038/jcbfm.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabayashi T, Vannier E, Clark BD, Margolis NH, Dinarello CA, Burke JF, Gelfand JA. A new biologic role for C3a and C3a desArg: regulation of TNF-alpha and IL-1 beta synthesis. J Immunol. 1996;156:3455–3460. [PubMed] [Google Scholar]
- Taylor CL, Selman WR, Ratcheson RA. Brain attack. The emergent management of hypertensive hemorrhage. Neurosurg Clin N Am. 1997;8:237–244. [PubMed] [Google Scholar]
- Thrane AS, Skehan JD, Thrane PS. A novel interpretation of immune redundancy and duality in reperfusion injury with important implications for intervention in ischaemic disease. Med Hypotheses. 2007;68:1363–1370. doi: 10.1016/j.mehy.2006.10.044. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: Role in cerebral hemorrhage. J Cereb Blood Flow Metab. 2003;23:629–652. doi: 10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- Wang X, Mori T, Sumii T, Lo EH. Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons: caspase activation and oxidative stress. Stroke. 2002;33:1882–1888. doi: 10.1161/01.str.0000020121.41527.5d. [DOI] [PubMed] [Google Scholar]
- Wu G, Huang FP. Effects of venom defibrase on brain edema after intracerebral hemorrhage in rats. Acta Neurochir. 2005;(Suppl 95):381–387. doi: 10.1007/3-211-32318-x_78. [DOI] [PubMed] [Google Scholar]
- Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Yan F, Lin AH, Lambris JD, Alexander JJ, Quigg RJ, Masliah E. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc Natl Acad Sci U S A. 2002;99:10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89:991–996. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- Xi G, Hua Y, Keep RF, Younger JG, Hoff JT. Systemic complement depletion diminishes perihematomal brain edema in rats. Stroke. 2001;32:162–167. doi: 10.1161/01.str.32.1.162. [DOI] [PubMed] [Google Scholar]
- Xi G, Hua Y, Keep RF, Younger JG, Hoff JT. Brain edema after intracerebral hemorrhage: the effects of systemic complement depletion. Acta Neurochir. 2002a;(Suppl 81):253–256. doi: 10.1007/978-3-7091-6738-0_66. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Pathophysiology of brain edema formation. Neurosurg Clin N Am. 2002b;13:371–383. doi: 10.1016/s1042-3680(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Yang S, Nakamura T, Hua Y, Keep RF, Younger JG, Hoff JT, Xi G. Intracerebral hemorrhage in complement C3-deficient mice. Acta Neurochir. 2006a;(Suppl 96):227–231. doi: 10.1007/3-211-30714-1_49. [DOI] [PubMed] [Google Scholar]
- Yang S, Nakamura T, Hua Y, Keep RF, Younger JG, He Y, Hoff JT, Xi G. The role of complement C3 in intracerebral hemorrhage-induced brain injury. J Cereb Blood Flow Metab. 2006b;26:1490–1495. doi: 10.1038/sj.jcbfm.9600305. [DOI] [PubMed] [Google Scholar]
- Yang S, Song S, Hua Y, Nakamura T, Keep RF, Xi G. Effects of thrombin on neurogenesis after intracerebral hemorrhage. Stroke. 2008;39:2079–2084. doi: 10.1161/STROKEAHA.107.508911. [DOI] [PubMed] [Google Scholar]
- Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, Armbrecht AM, Dhillon B, Deary IJ, Redmond E, Bird AC, Moore AT. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- Zamora MR, Davis RD, Keshavjee SH, Schulman L, Levin J, Ryan U, Patterson GA. Complement inhibition attenuates human lung transplant reperfusion injury: a multicenter trial. Chest. 1999;116:46S. doi: 10.1378/chest.116.suppl_1.46s. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li H, Hu S, Zhang L, Liu C, Zhu C, Liu R, Li C. Brain edema after intracerebral hemorrhage in rats: the role of inflammation. Neurol India. 2006;54:402–407. doi: 10.4103/0028-3886.28115. [DOI] [PubMed] [Google Scholar]