Abstract
Presynaptic active zones are essential structures for synaptic vesicle release, but the developmental regulation of their number and maintenance during aging at mammalian neuromuscular junctions (NMJs) remains unknown. Here, we analyzed the distribution of active zones in developing, mature, and aged mouse NMJs by immunohistochemical detection of the active zone-specific protein Bassoon. Bassoon is a cytosolic scaffolding protein essential for the active zone assembly in ribbon synapses and some brain synapses. Bassoon staining showed a punctate pattern in nerve terminals and axons at the nascent NMJ on embryonic days 16.5–18.5. Three-dimensional reconstruction of NMJs revealed that the majority of Bassoon puncta within an NMJ were attached to the presynaptic membrane from postnatal day 0 to adulthood, and colocalized with another active zone protein Piccolo. During postnatal development, the number of Bassoon puncta increased as the size of the synapses increased. Importantly, the density of Bassoon puncta remained relatively constant from postnatal day 0 to 54 at 2.3 puncta/μm2, while the synapse size increased 3.3-fold. However, Bassoon puncta density and signal intensity were significantly attenuated at the NMJs of 27-month-old aged mice. These results suggest that synapses maintain the density of synaptic vesicle release sites while the synapse size changes, but this density becomes impaired during aging.
Keywords: Bassoon, neuromuscular junction, synapse formation, aging, unitary assembly
INTRODUCTION
The active zone plays an essential role in synaptic transmission because it is the synaptic vesicle release site of the presynaptic terminal (Couteaux and Pecot-Dechavassine, 1970; Heuser et al., 1979). However, it is largely unknown how the density of the active zone is regulated during the development and maturation of mammalian synapses and whether the structural integrity of active zones is maintained until the end of an animal’s life. Several components of the active zone in vertebrate synapses have been identified, including Bassoon, CAST/Erc2, Munc13, Piccolo, and Rim1 (Cases-Langhoff et al., 1996; Wang et al., 1997; Betz et al., 1998; tom Dieck et al., 1998; Ohtsuka et al., 2002). Deletion of some of these molecules has been shown to cause impairment of the active zones (Dick et al., 2003; Mukherjee et al., 2010; Han et al., 2011; Kaeser et al., 2011). In the current study, we investigated how these synaptic vesicle release sites are organized and controlled when the synapse is changing its morphology and size during development and aging.
In electron micrographs of NMJs, active zones exhibit triangular electron-dense projections that extend from the presynaptic membrane and align with postsynaptic junctional folds (Couteaux and Pecot-Dechavassine, 1970; Hirokawa and Heuser, 1982; Harlow et al., 2001; Nagwaney et al., 2009). The developmental analysis of active zone formation has been reported for the frog NMJ using electron microscopy (Ko, 1985). However, a systematic analysis of active zone development at mammalian NMJs has not been conducted. A light microscopy method is suitable for capturing all of the active zones within a single NMJ, but this approach became feasible only recently following the identification of Bassoon as a marker for the NMJ active zone (Nishimune et al., 2004; Chen et al., 2011). Bassoon is an established active zone marker protein for the synapses of the central nervous system (tom Dieck et al., 1998; Richter et al., 1999; Dick et al., 2001; Khimich et al., 2005; Dondzillo et al., 2010).
NMJs need to sustain their integrity throughout the animal’s life, but some NMJs become denervated in aged animals (Fahim and Robbins, 1982; Banker et al., 1983; Valdez et al., 2010). This phenotype shows that a certain type of motor neuron degeneration starts at NMJs or axon terminals. However, the molecular mechanisms behind this degeneration are not well known. Studies of aging-related changes of synaptic proteins at NMJs have been restricted to a small number of proteins (Balice-Gordon, 1997; Delbono, 2003), and active zone-specific proteins have not been thoroughly studied in aged synapses in either the central or the peripheral nervous system (Xiong and Chen, 2010).
In this study, we used Bassoon immunohistochemistry to analyze the development of active zones of mouse NMJs from embryonic to adult stages. Bassoon immunohistochemistry enabled the study of entire active zones in a given NMJ, which allowed us to evaluate the active zone density and distribution pattern over a large number of synapses. Furthermore, the integrity of active zones at the NMJs was analyzed in aged mice.
MATERIALS AND METHODS
Animals
C57BL/6 wild-type mice (Jackson Laboratory) of both sexes were used in this study. Three or four animals were analyzed at each developmental stage. Aged C57BL/6 mice were obtained from the National Institute of Aging rodent colony and maintained at the University of Kansas Medical Center animal facility until they reached 27 months of age. Four aged mice (two male and two female) were used. Four young adult C57BL/6 mice (one-month-old) were used as controls for aged mouse analysis. All animal studies have been approved by the authors’ institutional review board.
Reagents
All chemicals and reagents were obtained from Sigma (St. Louis, MO), unless otherwise specified.
Antibody characterization
Following antibodies were used in this study, as listed in Table 1.
Table 1.
List of antibodies used.
| Antigen | Immunogen | Manufacture, species, catalog number | Dilution used |
|---|---|---|---|
| Bassoon | Recombinant rat Bassoon (Amino acids 756 – 1001) expressed as a GST fusion protein in E.coli. | Enzo life sciences/Stressgen, Farmingdale, NY Catalog No. ADI-VAM-PS003-D, Clone: SAP7F407, Mouse monoclonal IgG2a | 1/600 |
| Neurofilament | Homogenized hypothalami from Fisher 344 rats, Phosphorylated Neurofilament-M and neurofilament-H | Covance/Sternberger Monoclonals, Princeton, NJ Catalog No. SMI-312R, Clone: SMI312, Mouse monoclonal IgG1 | 1/1000 |
| Piccolo | Recombinant rat Piccolo (Amino acids 4439 – 4776) | Synaptic systems, Gottingen, Germany Catalog No. 142 002 Rabbit polyclonal | 1/2000 |
| SV2 | Synaptic vesicles purified from the Ommata (electric organ) | Developmental Studies Hybridoma Bank Catalog No. SV2, Mouse monoclonal IgG1 | 1/10 |
Bassoon
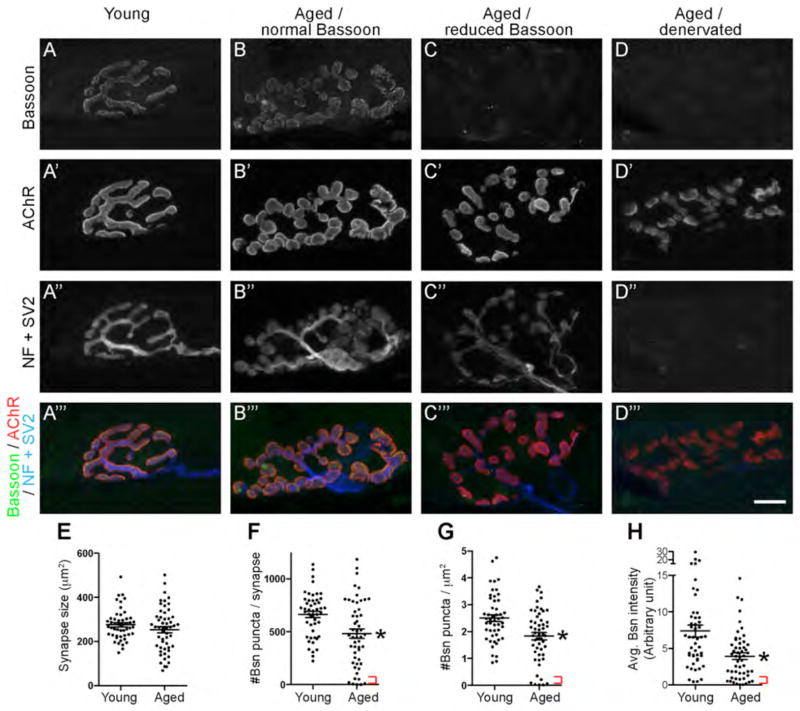
The commercially available Bassoon antibody detects a punctate pattern on cultured rat hippocampal neurons at synapses labeled by an anti-synaptophysin antibody (tom Dieck et al., 1998). By immunohistochemistry, this antibody stains cone photoreceptor terminals at postnatal day 4 (P4) (Regus-Leidig et al., 2009), and the staining is absent in retinae of knockout mice for Bassoon (Dick et al., 2003). We and others have reported that this antibody stains mouse NMJs (Nishimune et al., 2004; Chen et al., 2011; Hagiwara et al., 2011), and we have replicated the same staining pattern in mouse NMJs from embryos to aged animals. The Bassoon signal was absent in the denervated NMJs as shown in Fig. 8 of this study.
Figure 8.
The active zone protein Bassoon was reduced in the NMJs of aged mice. A – D‴: The sternomastoid muscles of aged mice (27-month-old, B – D‴) and young adult mice (1-month-old, A – A‴) were stained using an anti-Bassoon antibody (active zone, A, B, C, D; green in A‴, B‴, C‴, D‴), Alexa Fluor 594-labeled α-bungarotoxin (AChR, A′, B′, C′, D′; red in A‴, B‴, C‴, D‴), and anti-neurofilament plus anti-SV2 antibodies (nerve morphology, A″, B″, C″, D″; blue in A‴, B‴, C‴, D‴). Innervation was confirmed by the nerve morphology visualized using anti-neurofilament plus anti-SV2 antibodies. Panels D – D‴ show a denervated aged NMJ. Bassoon puncta were significantly attenuated in some aged NMJs (C – C‴), while other aged NMJs retained near normal levels of Bassoon staining (B – B‴). Innervated NMJs of aged mice had a similar synapse size (E: young adult mice, 276.2 ± 9.8 μm2; aged mice, 253.3 ± 13.9 μm2; P = 0.19) as the young adult mice. However, the NMJs of aged mice had a decreased number of Bassoon puncta per synapse (F: young adult mice, 664.3 ± 29.9; aged mice, 482.3 ± 43.3; P = 0.0009), a reduced density of Bassoon puncta (G: young adult mice, 2.5 ± 0.1 puncta/μm2; aged mice, 1.8 ± 0.1 puncta/μm2; P = 0.0005), and attenuated Bassoon average signal intensity (H, in arbitrary intensity units: young adult mice, 7.4 ± 0.8; aged mice, 3.9 ± 0.5; P = 0.0003). The red brackets in F – H indicate a subgroup of the NMJs of aged mice with a minuscule level of Bassoon signal at the synapses. The quantifications (E – H) are from a total of 52 NMJs from four aged mice and 49 NMJs from four young adult mice. Asterisks indicate significant differences by unpaired t-test. Scale bar: 10 μm.
Piccolo
The commercially available Piccolo antibody detects ~520 and ~65kDa proteins in Western blots of mouse, rat, and chicken synaptic junctional proteins (Wahlin et al., 2008). Preadsorption of the antibody with the recombinant Piccolo protein abolishes both 520 and 65 kDa bands (Manufacturer’s observation). By immunocytochemistry, this antibody detects a punctate pattern on cultured rat hippocampal neurons that co-localize with the active zone specific protein Bassoon (Dresbach et al., 2006). By immunohistochemistry, we and others have reported that this antibody stains rat NMJs and embryonic mouse NMJs (Juranek et al., 2006; Chen et al., 2011). We replicated the similar staining pattern in adult mouse NMJs in Fig. 2 of this study.
Figure 2.

Bassoon and Piccolo puncta are anchored to the presynaptic membrane in adult NMJs. A – A‴: The active zone-specific protein Piccolo (A′, red in A‴) showed a punctate staining pattern and overlapped with Bassoon staining (A, green in A‴) at a P36 NMJ. Many overlapping puncta were localized on top of the bright lines of Alexa Fluor 647-labeled α-bungarotoxin (A″, blue in A‴). B - B″: The orthogonal views of the NMJ shown in (A‴) with the same layout as the orthogonal views in Fig. 1B. Many overlapping puncta were attached to the α-bungarotoxin signal on the nerve side. Scale bars: 1 μm.
Neurofilament
The commercially available neurofilament monoclonal antibody detects phosphorylated Neurofilament-M and neurofilament-H at molecular weight 200 and 160 kDa in Western blots of mouse brain extracts (Lachyankar et al., 2000). By immunohistochemistry, this antibody detects phosphorylated neurofilaments on axons in fetal human brain (Ulfig et al., 1998; Haynes et al., 2005), and labels motor axons innervating the mouse NMJ (Misgeld et al., 2002). We have reported the same labeling pattern of the motor axons at the mouse NMJs using this antibody (Nishimune et al., 2004; Chen et al., 2011), and replicated the same staining pattern in Fig. 8 of this study. This antibody was used as a marker in this study to label motor axon morphology.
SV2
The commercially available SV2 monoclonal antibody is known to recognize a ~95kDa proteoglycan present at synapses by Western blot, and nerve terminals in the central and peripheral nervous systems of mouse, rat, and chicken by immunohistochemistry (Buckley and Kelly, 1985; Fischer et al., 2008). This antibody also labels the presynaptic terminal at the mouse NMJ (Misgeld et al., 2002). We have reported the same labeling pattern of the presynaptic terminal at the mouse NMJs using this antibody (Nishimune et al., 2004; Chen et al., 2011), and replicated the same staining pattern in Fig. 8 of this study. This antibody was used as a marker in this study to label motor nerve terminal morphology.
Alexa Fluor 488- and 568-conjugated secondary antibodies, and Alexa Fluor 594- or 647-conjugated α-bungarotoxin were obtained from Molecular Probes / Invitrogen (Eugene, OR) and used at a 1:1000 dilution.
Immunohistochemical analysis
The methods employed for the immunohistochemical analyses have been described previously (Nishimune et al., 2004; Nishimune et al., 2008; Chen et al., 2011). In brief, mice were fixed by transcardiac perfusion with 4% paraformaldehyde in PBS (pH 7.1, Dulbecco’s phosphate buffered saline). Sternocleidomastoid and tibialis muscles were removed and post-fixed in the same fixative at room temperature, cryoprotected in 20% sucrose/PBS, frozen in Optimal Cutting Temperature compound (Sakura), and cut using a cryostat. Embryonic tissues were fixed without perfusion. Longitudinal sections were cut at a thickness of 20 μm and blocked in PBS containing 2% BSA, 2% normal goat serum, and 0.1% Triton. Sections were sequentially incubated with primary antibodies overnight at 4°C, washed with PBS, and incubated in a mixture of Alexa Fluor (488 or 568)-conjugated secondary antibodies and Alexa Fluor (594 or 647)-conjugated α-bungarotoxin for 2 hrs. at room temperature. After washing, the sections were mounted with Vectashield (Vector). No staining was observed when primary antibodies or Alexa Fluor-conjugated α-bungarotoxin were omitted.
Confocal microscopy
Sequentially scanned confocal Z-stacks were obtained using a Nikon C1Si confocal microscope (Nikon, Tokyo, Japan) with a 100x objective lens (Apo TIRF, NA=1.49) and immersion medium (refractive index nd = 1.515). The microscope was equipped with following lasers: an argon laser (488 nm), a He-Ne laser (561 nm), and a diode laser (638 nm). The pinhole size was set to 60 μm, following the manufacturer’s recommendation for the lens used, which is smaller than one Airy disk unit and improves the effectiveness of the pinhole (Conchello and Lichtman, 2005). The XY-pixel size (0.050 μm) and Z-steps (0.3 μm/step) were set to follow the Nyquist sampling criterion for each Airy disk unit (XY, 0.21 μm; Z, 0.71 μm) (Conchello and Lichtman, 2005) (Nikon microscopyU, http://www.microscopyu.com/articles/livecellimaging/imagingsystems.html). A smaller zoom (XY-pixel size, 0.062 μm) was used to image aged and one-month-old NMJs because the endplates in aged NMJs were fragmented and occupied wider areas than in young NMJs. However, this did not compromise our ability to resolve puncta. Laser power levels, photomultiplier gain levels, scanning speed, and the confocal pinhole size were kept constant within Figs 3, 4, 6, and 8 for quantification of immunohistochemistry signals. The maximal projection of Z-stacks was obtained using EZ-C1 FreeViewer software (Nikon).
Figure 3.

Bassoon is distributed as puncta in the nascent NMJs. A – B″″: The active zone-specific protein Bassoon (A, A′, green in A‴, A″″, B‴, B″″) and AChRs (A″, red in A‴, A″″, B‴, B″″) were detected at E16.5 (A – A″″) and E18.5 (B – B″″), as described in Fig. 1. Arrowheads in panels A and B indicate Bassoon staining outside of the NMJs in a string-like pattern, suggesting axonal staining. The panels A′ and B′ show highly magnified regions indicated by the arrowheads in A and B. The panels A″″ and B″″ show a highly magnified 5 x 10 μm region indicated by the white dotted box in A‴ and B‴. C – C″: The orthogonal views of a P0 NMJ with the same layout as the orthogonal views in Fig 1B. The presynaptic terminal was placed toward the top of the Z-axis, and the muscle fiber was placed toward the bottom. The anti-Bassoon antibody (green) and Alexa Fluor 594-labeled α-bungarotoxin (red) revealed that most Bassoon puncta are attached to the bungarotoxin signal on the nerve side (white arrowheads), indicating that most Bassoon proteins are anchored to the presynaptic membrane. One Bassoon punctum was detected separate from the bungarotoxin-labeled endplate in YZ- and XZ-orthogonal views (white arrow in panels B′ and B″), suggesting that a small portion of the Bassoon puncta (0.6%, see main text) were floating in the presynaptic terminal at P0. The nascent NMJ lay relatively flat on the muscle fiber compared to the adult NMJs (Fig. 4B), which is shown by the flat α-bungarotoxin signal in the XZ-view (C″) and the A-shape in the YZ-view (C′). D: A representative transmission electron micrograph of a NMJ at E18.5. Active zones (green arrowheads) and a nascent postsynaptic junctional fold (white arrow) were observed. This in vivo data showed one electron-dense material that was not anchored to the presynaptic membrane and was floating in the nerve terminal (green arrow), which resembled the floating Bassoon puncta detected in (C). A magenta-green version of this figure is supplied as Supplementary Figure 2. Scale bars: (A, A″, A‴, B, B″, B‴) 10 μm, (A′, A″″, B′, B″″) 2 μm, (C – C″) 5 μm, (D) 500 nm.
Figure 4.

Most Bassoon puncta were anchored to the presynaptic membrane at NMJs. A – A″: The orthogonal views of P0 NMJs are shown with the same layout as in Fig. 1B. The presynaptic terminal was placed toward the top of Z-axis, and the muscle fiber was placed toward the bottom. The anti-Bassoon antibody (green) and Alexa Fluor 594-labeled α-bungarotoxin (red) revealed that >99% of Bassoon puncta are attached to the bungarotoxin signal on the nerve side (white arrowheads), indicating that the majority of Bassoon proteins are anchored to the presynaptic membrane. The nascent NMJ lay flat on the muscle fiber, which is shown by the flat α-bungarotoxin signal in the YZ- and XZ-views (A′ and A″). B – B″: In the P48 NMJ, all Bassoon puncta are attached to the bungarotoxin signal on the nerve side (white arrowheads). The primary gutter is shown as the U-shaped bungarotoxin staining in the YZ- and XZ-views (B′ and B″). A magenta-green version of this figure is supplied as Supplementary Figure 3. Scale bars: 5 μm.
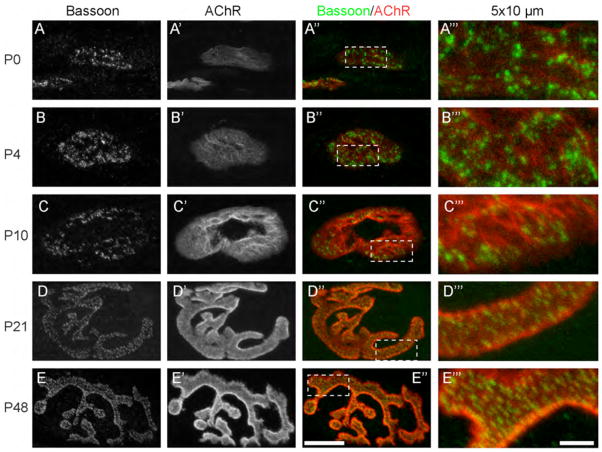
Figure 6.
The postnatal development of the Bassoon puncta distribution at NMJs. The active zone-specific protein Bassoon (A, B, C, D, E; green in the right two columns) and AChRs (A′, B′, C′, D′, E′; red in the right two columns) were detected from P0 (A – A‴), P4 (B – B‴), P10 (C – C‴), P21 (D – D‴), and P48 (E – E‴), as described in Fig. 1. The three left panels show the entire NMJs. The rightmost panels (A‴ – E‴) show a highly magnified 5 x 10 μm region of the NMJs (white dotted boxes in A″ – E″). A magenta-green version of this figure is supplied as Supplementary Figure 4. Scale bars: (left three columns) 10 μm, (the most right column) 2 μm.
Image analysis
Three-dimensional (3D) reconstruction of confocal Z-stacks was performed using NIS-Elements software (Nikon). All other image analyses were conducted using Metamorph (Universal Imaging Corporation). For the quantification of the developing synapse size, the areas of α-bungarotoxin-labeled acetylcholine receptor (AChR) clusters (endplates) were measured from maximal projected confocal images without level adjustment. For the quantification of the Bassoon immunohistochemistry signal at the NMJs, we restricted the measurements to presynaptic terminals overlying α-bungarotoxin-labeled AChR clusters. We measured the number of Bassoon puncta in maximal projected confocal images without level adjustment (1024 x 1024 file size) after thresholding the images for light objects (as explained in the next paragraph). We only took measurements from NMJs in which we could image the entire morphology of the synapse.
We previously used one unified threshold value for the quantification of Bassoon immunohistochemistry signals for all of the analyses in our publication (Chen et al., 2011). However, it was difficult to define a unified threshold value for the developmental analysis of Bassoon puncta among a wide range of ages due to the dynamic changes in the signal intensity of Bassoon immunohistochemistry (Fig. 6). In a similar situation, different researchers have used different image analysis methods for the quantification of Bassoon fluorescence intensity, area, or punctum number. One study used manual thresholding (Dondzillo et al., 2010), and another study used background subtraction from the entire image combined with the estimation of Bassoon puncta using a two-dimensional Gaussian distribution (Frank et al., 2010). To threshold the images as objectively as possible in the current study, we used a function in the Metamorph software called “auto threshold for light objects”. Briefly, this algorithm utilizes the distribution of a histogram of pixel gray values and defines a background as the gray values with the largest peak. The algorithm defines the objects as peaks of the histogram on the bright side of the background. Then it locates a trough in the histogram between the objects and the background and defines a threshold value (Narayan et al., 2007). The anti-Bassoon antibody staining produced a high signal-to-noise ratio with a low background. Thus, this automated method produced threshold values that accurately covered the Bassoon-stained puncta. All Bassoon images were thresholded by this method prior to the quantification of the area.
The number of Bassoon puncta per synapse was determined by dividing the area of each Bassoon punctum by the averaged Bassoon punctum size and rounding to the nearest whole number. The average Bassoon punctum size for the developmental NMJ analysis (0.082 ± 0.0023 μm2, mean ± SEM) was quantified by manually identifying isolated puncta of Bassoon staining at P21 (153 puncta) and P48 (161 puncta) in maximal projected and auto-thresholded confocal images. The average Bassoon punctum size for aged NMJ analysis (0.092 ± 0.0023 μm2) was quantified by manually identifying isolated puncta of Bassoon staining at young adult (131 puncta) and aged NMJs (115 puncta) in maximal projected and auto-thresholded confocal images. These average sizes of Bassoon puncta (thresholded) were smaller than the point spread function (PSF), suggesting that most Bassoon puncta in the maximal projected confocal images are similar to or smaller than the PSF size (0.141 μm2 = 3.14 x ((1.22x519nm)/(1.49(NA)x2))2 ). Thus, we focused our analysis on the number of the Bassoon puncta, but not the size of these puncta because fluorescent objects under the size of a PSF will not accurately reflect its original size in fluorescent microscopy (Conchello and Lichtman, 2005; Heilemann et al., 2009).
We did not observe a significant fluorescence intensity decline along the Z-axis of the stained sections (20 μm). However, to avoid potential complications caused by antibody penetration or light scattering due to the thickness of the tissue section, we imaged NMJs in the superficial two-thirds of the section and averaged the data from 31–44 NMJs over 9–12 sections from 3 or 4 animals for each developmental age.
For the quantification of aged NMJs, we quantified only the fully innervated NMJs that were stained by anti-neurofilament plus anti-SV2 antibodies. For the quantification of the Bassoon signal intensity of aged and the young adult NMJs, we used a unified threshold value that was defined by averaging the auto-threshold values generated by Metamorph software for NMJs of aged and the young adult mice. The average Bassoon signal intensity was determined by dividing the total Bassoon signal intensity at a single NMJ by the synapse size. We averaged data obtained from 49 NMJs from four mice at 1-month of age and 52 NMJs from four mice at 27-month of age.
Ultrastructural analysis
The methods employed for the electron microscopy analysis have been described previously (Fernandez-Chacon et al., 2004; Nishimune et al., 2004). In brief, sternocleidomastoid muscles were fixed in 5% glutaraldehyde and 4% paraformaldehyde in PBS, washed, refixed in 1% OsO4, dehydrated, and embedded in resin. Ultrathin sections were stained with uranyl acetate and lead citrate and systematically scanned using a transmission electron microscope. All NMJ profiles encountered in the micrographs were counted. Two to four animals were analyzed per age, with 35–119 NMJs profiles per age. An active zone was defined as the protrusion of electron-dense material from the presynaptic membrane where synaptic vesicles aggregated. Postsynaptic junctional folds were defined as the synaptic muscle membranes that morphologically invaginated into the muscle fibers and contained the basal lamina.
Statistics
All statistics were performed using GraphPad Prism software version 5.0c. Significance was assessed by an unpaired t-test to compare changes between two groups, and by a one-way ANOVA plus Bonferroni’s multiple comparison test for changes among multiple groups. The P values are described in the text and figure legends. All data shown are the mean ± SEM.
Figure preparation
For the representative images shown in the figures, except Fig. 1, levels were adjusted and cropped using Adobe Photoshop CS3 (Adobe Systems, San Jose, CA). For Fig. 1, the maximal projected confocal images and the orthogonal views are shown without level adjustment. The images and the graphs were assembled using Adobe Illustrator CS3 and exported in Tiff file format at 300dpi.
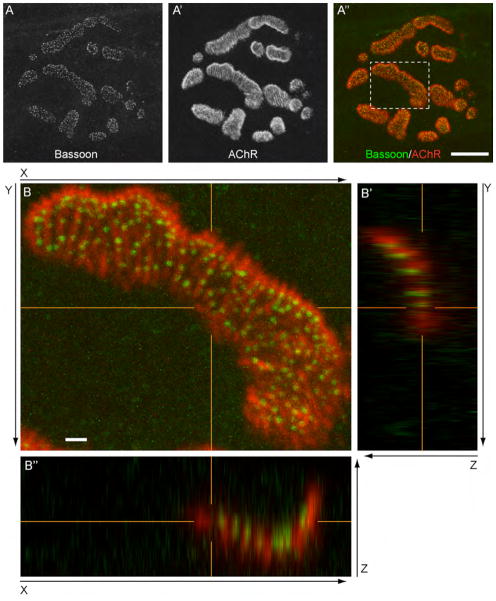
Figure 1.
Active zone protein Bassoon localizes at NMJ active zones. A - A″: The active zone-specific protein Bassoon forms puncta that are aligned with postsynaptic junctional folds in adult NMJs. Bassoon and acetylcholine receptors (AChRs) were visualized by fluorescent immunohistochemistry using an anti-Bassoon antibody (green) and Alexa Fluor 594-labeled α-bungarotoxin (red) in the sternomastoid muscle of P36 mice. Many Bassoon puncta were localized on top of the postsynaptic folds, which were visualized as bright lines of α-bungarotoxin staining. B - B″: The orthogonal views of the dotted area in the NMJ shown in (A″). The presynaptic terminal was placed toward the top of Z-axis, and the muscle fiber was placed toward the bottom. The panel B shows the XY-view of a maximal projected image. Panels B′ and B″ show YZ- and XZ-views of orthogonal single optical planes at the positions indicated by the orange lines in the panel B, XY-view. The orange lines in each image indicate the matching X, Y, and Z coordinates, respectively. The anti-Bassoon antibody (green) and Alexa Fluor 594-labeled α-bungarotoxin (red) revealed that all Bassoon puncta are attached to the α-bungarotoxin signal on the nerve side in the XZ- and YZ-orthogonal views, suggesting their anchoring to the presynaptic membrane. In the mature NMJ, the nerve terminal sinks into the muscle fiber and forms the primary gutter (Desaki and Uehara, 1987), which is shown as the U-shaped bungarotoxin staining in the XZ- and YZ-views. The maximal projected confocal images and the orthogonal views are shown without level adjustment. A magenta-green version of this figure is supplied as Supplementary Figure 1. Scale bars: (A – A″) 10 μm, (B – B″) 1 μm.
RESULTS
The Bassoon antibody labels the active zones of NMJs
We visualized the active zones of NMJs by immunohistochemistry using an anti-Bassoon antibody. Bassoon is a large cytosolic scaffolding protein that is specifically concentrated at the presynaptic active zones of synapses in the central and peripheral nervous system (tom Dieck et al., 1998; Nishimune et al., 2004). Immunohistochemical detection of the Bassoon protein in adult NMJs revealed the following features. First, Bassoon signals exhibited a small punctate pattern that was only distributed within the NMJs in adult mice (Figs. 1, 6), and Bassoon was not detected at denervated endplates (Fig. 8D – D‴). These results suggested a presynaptic origin for the anti-Bassoon antibody signal. Second, the punctate staining pattern of Bassoon was different from the diffuse immunohistochemistry signal pattern of the synaptic vesicle-associated protein SV2 (Fig. 8A″), in accordance with the localization of the Bassoon protein at active zones. Third, the Bassoon puncta were distributed relatively evenly within a synapse. These data were in good agreement with the scattered distribution pattern of active zones determined from reconstructions of serial electron micrographs of NMJs (Rowley et al., 2007). Fourth, the Bassoon puncta aligned well with the postsynaptic junctional folds at adult NMJs (Fig. 1). The junctional folds were visualized as bright lines by the α-bungarotoxin staining, which are caused by the accumulation of fluorescent signals in the depth axis of the junctional folds (Marques et al., 2000). The alignment of Bassoon puncta and junctional folds strongly supports the hypothesis that these Bassoon puncta are active zones because the active zones detected by transmission electron microscopy align well with the postsynaptic junctional folds at adult NMJs (Couteaux and Pecot-Dechavassine, 1970; Hirokawa and Heuser, 1982; Harlow et al., 2001; Nagwaney et al., 2009). We tested the reliability of the Bassoon immunohistochemistry-based active zone visualization by the following two methods: 3D analysis of Bassoon puncta localization within the nerve terminals and colocalization analysis with another active zone protein Piccolo.
First, we validated the Bassoon puncta location within the nerve terminal by analyzing the confocal Z-stacks in 3D reconstructions. We investigated where Bassoon puncta localize within the presynaptic terminal by analyzing the relative position of the Bassoon puncta and the α-bungarotoxin-labeled postsynaptic AChR clusters. In mature NMJs at P36, all Bassoon puncta were attached to the α-bungarotoxin signal on the nerve side in both XZ- and YZ-orthogonal views (Fig. 1B′, B″), which is consistent with the location of the active zone detected by electron microscopy (Fig. 5B, B′).
Figure 5.

A-B′: Representative transmission electron micrographs showing active zones and postsynaptic junctional folds at the NMJs of sternocleidomastoid muscles at E18.5 (A) and P21 (B). Higher magnification images of A and B are shown in A′ and B′. Arrowheads indicate electron-dense material at the active zones that are aligned (black) or not aligned (white) with the junctional folds. The white arrows in panels A and A′ indicate the nascent junctional folds at E18.5. C: The quantification of the alignment between active zones and postsynaptic junctional folds by electron microscopy revealed an increase in the alignment during early postnatal development. The quantifications are from a total of 35 to 119 NMJ profiles per age. Analysis using a one-way ANOVA revealed that the increment between P4 and 2-week (wk)-old mice was significant (P < 0.05), but the increments between other immediately adjacent ages were not significant. Scale bars: (A – B′) 500 nm.
Second, we examined the distribution pattern of another active zone-specific protein Piccolo, which is a cytosolic scaffolding protein that shares some domain structure similarity with Bassoon but differs in having two C2 domains and a PDZ domain and in residing on a different chromosome compared to Bassoon (Fenster et al., 2000). We have shown the co-localization of Bassoon and Piccolo in the mouse NMJs at embryonic day 18.5 (E18.5) (Chen et al., 2011). Here, we compared the distribution pattern of Piccolo and Bassoon in adult NMJs. The anti-Piccolo antibody showed punctate staining pattern similar to, but slightly larger than, the Bassoon puncta in the maximal projected confocal images at the NMJs of P36 mice (Fig. 2A – A‴). We validated the Bassoon and Piccolo puncta location within the nerve terminal by analyzing the confocal Z-stacks in 3D reconstructions (Fig. 2B – B″). Many Bassoon and Piccolo puncta overlapped and were attached to the α-bungarotoxin signal on the nerve side in both XZ- and YZ-orthogonal views. These data suggested that Bassoon and Piccolo are localized at the active zones of NMJs.
The above data suggest that the anti-Bassoon antibody labels the active zones at adult NMJs. Immunohistochemistry of active zone proteins enables the study of the entire active zones in a given NMJ and the analysis of active zones over a large number of synapses. Because of the crisp signal and low background noise obtained by the anti-Bassoon antibody compared to the anti-Piccolo antibody, we chose Bassoon immunohistochemistry to study the active zones in developing, mature, and aged NMJs.
The unitary assembly of active zones at nascent NMJs
We first analyzed the distribution pattern of Bassoon in the developing NMJs to visualize the initial stage of active zone formation. The motor axons and aneural AChR clusters on muscle fibers initially contact each other after E13–E14 in mouse diaphragms, and all AChR clusters are innervated by nerve terminals by E16.5–E18.5 (Lin et al., 2001; Misgeld et al., 2005). Thus, we analyzed the distribution of the Bassoon protein in nascent NMJs from E16.5 (Fig. 3A – A″″). The Bassoon protein was already distributed in a punctate pattern within the nascent synapse at E16.5. As in adults, these puncta were relatively evenly distributed within the presynaptic terminal. The punctate Bassoon signal was also observed in a linear structure outside of the NMJ, which suggested axonal transport of Bassoon proteins in aggregates. These non-synaptic Bassoon puncta were observed between E16.5 and P0, but rarely at P4 and later stages (Figs. 3A – C″ ,6). At E16.5, the α-bungarotoxin signal was very weak in the NMJ compared to later stages, suggesting a lower density of AChRs. At E18.5, bright Bassoon puncta were also observed in NMJs, but the inter-puncta distance seemed greater than that in E16.5 embryos (Fig. 3B – B″″). At P0, the Bassoon puncta showed variations in size and signal intensity (Figs. 3C – 3C″ ,4A – 4A″, 6A – 6A‴).
To analyze the distribution pattern of Bassoon puncta in nascent NMJs, we reconstructed the confocal Z-stacks in 3D. Among the nascent NMJs, we chose to analyze NMJs at P0 for the following reasons. First, the axonal Bassoon staining was still present at P0, suggesting that the NMJs were still developing the Bassoon distribution pattern. Second, P0 NMJs had larger nerve terminals compared to embryonic NMJs, which aided the 3D analysis of Bassoon puncta distribution. We analyzed the relative position between the Bassoon puncta and the α-bungarotoxin-labeled postsynaptic AChR clusters. At P0, most Bassoon puncta were attached to the α-bungarotoxin signal. However, a small number of Bassoon puncta were located in the vicinity of the α-bungarotoxin signal, but not attached to this signal, in both YZ- and XZ-views (Fig. 3C′, C″), indicating that few Bassoon protein aggregates were floating in the presynaptic terminal.
Consistent with the 3D reconstruction analysis of the confocal images of nascent NMJs, an electron microscopy analysis of NMJs at E18.5 revealed electron-dense materials surrounded by many synaptic vesicles, which resided at the nerve terminal but were not attached to the presynaptic membrane (Fig. 3D). These structures were similar to the precursor spheres described in the retina (Regus-Leidig et al., 2009). A unitary assembly of the presynaptic active zone has been proposed for synapses of the retina and cultured hippocampal neurons based on the transport aggregates of active zone proteins and synaptic vesicles (Ahmari et al., 2000; Shapira et al., 2003; Regus-Leidig et al., 2009). The unitary assembly of the active zones at NMJs was suggested by the detection of following structures within the presynaptic terminal at the initial stage of NMJ formation: the punctate distribution pattern of Bassoon and floating Bassoon puncta by immunohistochemistry, and the precursor sphere-like structures by electron microscopy.
Next, we investigated the rate of floating Bassoon puncta within the presynaptic terminal by analyzing the relative position of the Bassoon puncta and the α-bungarotoxin-labeled postsynaptic AChR clusters in 3D. On average, 1.1 floating Bassoon puncta were observed per synapse at P0, which was only 0.6% of the total number of puncta per synapse (with an average of 183 puncta per synapse, Fig. 7B). These results indicated that most Bassoon puncta were attached to the presynaptic membrane (Fig. 4A – A″). At P4, the average number of floating Bassoon puncta was 0.7 puncta per synapse, suggesting that almost all Bassoon puncta were anchored to presynaptic membranes. At P36 and P48, all Bassoon puncta were attached to the α-bungarotoxin signal on the nerve side in both XZ- and YZ-orthogonal views (Figs. 1B – B″, 2B – B″, 4B – B″). These 3D analyses revealed that >99% of the Bassoon puncta are anchored to the presynaptic membrane, and the floating puncta are a very minor portion of the entire Bassoon puncta in the postnatal NMJs.
Figure 7.

The constant density of the Bassoon puncta at postnatal NMJs. A: The synapse size showed constant growth from the embryonic to adult stages, which was determined by the α-bungarotoxin stained area (P0: 89.7 ± 5.5; P4: 125.9 ± 7.8; P10: 179.2 ± 7.3; P21: 217.9 ± 9.5; P48 –54: 295.2 ± 17.6 μm2). B: The number of Bassoon puncta increased as the synapse size increased. The average number of Bassoon puncta in a synapse constantly increased from P0 to P54 (P0: 183.0 ± 12.7; P4: 304.3 ± 25.4; P10: 365.7 ± 24.8; P21: 553.2 ± 24.5; P48–54: 780.2 ± 45.4). C: The density of the Bassoon puncta stayed at a relatively constant level (2.3 puncta/μm2) during the postnatal development of the NMJs (P0: 2.0 ± 0.09; P4: 2.3 ± 0.1; P10: 1.9 ± 0.1; P21: 2.5 ± 0.1; P54: 2.6 ± 0.1 puncta/μm2). These quantifications were from 31–39 NMJs from 3 mice at each stage. Asterisks indicate significance by one-way ANOVA analysis (P < 0.05). (A) The synapse size at P10 and P48–54 was significantly larger than the immediately adjacent younger age groups. (B) The Bassoon puncta number per synapse was significantly larger than the immediately adjacent younger age groups at P4, P21, and P54. (C) Significant differences were detected between P10 and P21.
The alignment of active zones and postsynaptic junctional folds
After confirming that the majority of the Bassoon puncta are anchored to the presynaptic membrane, we compared the Bassoon puncta location during development to the active zone location detected by transmission electron microscopy, which is the gold standard for detecting active zones at NMJs. In electron micrographs of adult NMJs, active zones exhibit triangular electron-dense projections extending from presynaptic membranes that align with postsynaptic junctional folds (Couteaux and Pecot-Dechavassine, 1970; Hirokawa and Heuser, 1982; Harlow et al., 2001; Nagwaney et al., 2009). We first analyzed the alignment rate of active zones and junctional folds during development by transmission electron microscopy. At E18.5, typical active zones were observed at presynaptic terminals, with electron-dense materials anchored to the presynaptic membrane and synaptic vesicles accumulated in their vicinity (Fig. 5A, A′). Nascent postsynaptic junctional folds were present at E18.5, but only 3% of the active zones apposed junctional folds (Fig. 5C). The percent alignment increased from 26% in one-week-old mice to 63% in two-week-old mice. By three weeks of age, 73% of active zones apposed junctional folds (Fig. 5B, B′, and C). The percent alignment remained at this level in mature NMJs (Fig. 5C).
We next analyzed the alignment of the Bassoon puncta and junctional folds detected by immunohistochemistry. At P0, nascent primary gutters and junctional folds were stained as brighter spots or lines of α-bungarotoxin (Fig. 6A′). However, these structures within an ovoid endplate did not have a clearly organized pattern as seen in mature NMJs. The majority of the Bassoon puncta were distributed relatively evenly within the endplates, and few puncta aligned with the nascent junctional folds at P0 (Fig. 6A – A‴). These junctional folds develop from the perinatal stage, mainly at postnatal stages (Matthews-Bellinger and Salpeter, 1983; Missias et al., 1997; Marques et al., 2000). At P4, while the NMJs were multiply innervated, the number of junctional folds increased and more Bassoon puncta were distributed on top of these nascent folds; however, many Bassoon puncta still resided at areas without apparent junctional folds. At P10, which is near the end of the synapse elimination period (Brown et al., 1976; Balice-Gordon and Lichtman, 1993; Walsh and Lichtman, 2003), the postsynaptic junctional folds became organized and could be visualized as bright lines of α-bungarotoxin staining running parallel to the short-axis of the AChR clusters in several endplate domains. In these areas, the Bassoon puncta were often found on the bright α-bungarotoxin lines, indicating the alignment of Bassoon puncta and postsynaptic junctional folds. At P21–P54, endplates became pretzel-shaped and entirely covered with junctional folds. Most Bassoon puncta aligned with the junctional folds at these stages (Fig. 6D – E‴). The trend of alignment between the Bassoon puncta and the junctional folds correlated with the alignment between the active zones and the junctional folds detected by electron microscopy during development, which strengthens the idea that the Bassoon puncta represent active zones. The quantification from electron micrographs and observation by light microcopy demonstrated that the formation of active zones precedes the formation of junctional folds, and the active zones and the folds align mainly in the second postnatal week.
The constant density of Bassoon puncta during NMJ development
We subsequently investigated how the number of active zones changes while NMJ size increases and morphology changes during the postnatal development. We used Bassoon immunohistochemistry to analyze the number and density of active zones in postnatal NMJs (Figs. 6, 7). To estimate the number of Bassoon puncta in the NMJs, we first determined the unit size of an isolated Bassoon punctum in P21 NMJs (153 puncta) and P48 NMJs (161 puncta) where all Bassoon puncta were attached to the presynaptic membrane. The average size of a Bassoon punctum was 0.082 ± 0.0023 μm2, which was similar to the active zone size (0.146 μm2) calculated from the reconstructions of transmission electron micrographs of rat diaphragm NMJs (Rowley et al., 2007).
We then calculated the number and density of Bassoon puncta in postnatal NMJs using the average Bassoon punctum size. During the postnatal development of NMJs, the synapse size increased 3.3 fold between P0 and P54 (Fig. 7A). Concomitantly, the postsynaptic AChR cluster changed its morphology from an ovoid plaque shape at birth to a perforated shape, then to a C-shaped aggregate, and finally into a pretzel-shaped endplate (Fig. 6) (Slater, 1982; Desaki and Uehara, 1987; Lupa and Hall, 1989; Marques et al., 2000; Nishimune et al., 2008). Accordingly, the number of Bassoon puncta per synapse increased from 183 puncta per synapse at P0 to 780 puncta per synapse at P54 (Fig. 7B). This is consistent with the developmental increase of miniature endplate potential (mEPP) frequency from E18.5 to P43 (Knight et al., 2003; Kretschmannova and Zemkova, 2004; Liu et al., 2009). Importantly, while the synapses were changing their size and morphology, the density of Bassoon puncta stayed relatively constant at 2.3 puncta/μm2 between P0 and P54 (Fig. 7C). These results suggested that the presynaptic terminal maintains the density of active zones during the postnatal morphological change and the size increase of NMJs.
NMJs mature from having multiple innervations to a single innervation through a process called synapse elimination, which ends around 2 weeks of age (Brown et al., 1976; Balice-Gordon and Lichtman, 1993; Walsh and Lichtman, 2003). Although we did not precisely analyze the Bassoon puncta in the remaining axon terminal versus the terminals that would be pruned, the overall Bassoon puncta density per synapse decreased transiently at P10, suggesting a remodeling of the presynaptic terminal toward the end of the synapse elimination period (Fig. 7C).
Decreased density and intensity of Bassoon puncta at aged NMJs
The above data demonstrate that the density of active zones is maintained during postnatal development; we therefore sought to determine whether this density is maintained during aging. The integrity of the active zones at NMJs needs to be maintained throughout the animal’s life because these synapses are stable unless they become injured (Sanes and Lichtman, 1999). We analyzed aged mice close to the end of their life span to investigate this question in vivo. The survival rate of C57BL/6 mice significantly reduces beyond two years of age (Turturro et al., 1999). Thus, we analyzed 27-month-old C57BL/6 mice to examine the aged NMJs.
In aged mice, denervation of NMJs has been reported at frequencies ranging from 4% to 35%, depending on the muscle type and the age of the mouse (approximately 24–30 months of age) (Fahim and Robbins, 1982; Banker et al., 1983; Balice-Gordon, 1997; Valdez et al., 2010). In our study, an average of 45% of the NMJs in the sternomastoid muscle of 27-month-old mice completely or partially lacked staining for anti-neurofilament or anti-SV2 antibodies. These NMJs were likely to be completely or partially denervated and did not show Bassoon signals in these denervated areas (Fig. 8D – D‴).
At the fully innervated NMJs of aged mice, the synapse size did not show a significant reduction compared to that of young adults (Fig. 8B – C‴, E). The Bassoon protein also showed a small, discrete punctate pattern and was aligned with the junctional folds, similar to the NMJ of young adult mice. However, the Bassoon signal intensity at the innervated, aged synapses was much more variable than in young NMJs (Fig. 8B – C‴). Some aged NMJs had normal levels of Bassoon signal, but a few aged NMJs had very low levels of Bassoon in the presynaptic terminal (Fig. 8C – C‴, red brackets in F – H). Notably, the number of Bassoon puncta at the innervated, aged NMJs were reduced by 27% on average compared to young adult NMJs (Fig. 8F). Consistently, the density of the Bassoon puncta in the innervated, aged NMJs was also reduced by 28% compared to young adult NMJs (Fig. 8G). The average Bassoon signal intensity in innervated, aged NMJs was reduced by 47% compared to young adult NMJs (Fig. 8H). These results suggested that the presynaptic active zones of NMJs are stably maintained in matured NMJs but many are disassembled in aged mice.
DISCUSSION
Little is known about the developmental regulation of the distribution pattern of active zone-specific proteins at the mammalian NMJ. In this study, we described in vivo data demonstrating the following features of active zones at the NMJ. (1) The active zone protein Bassoon was localized as discrete puncta at NMJs from the initial stage of synapse formation, and a precursor sphere-like structure was detected in motor nerve terminals. These data suggested the unitary assembly of active zones at NMJs. (2) Active zone formation preceded the formation of postsynaptic junctional folds, and both occurred independently. The rate of alignment between the two increased in the second postnatal week. (3) The number of active zones increased as the size of the synapse increased, but the density of the active zones was maintained at a relatively constant level between P0 and P54. (4) Active zone density was impaired in the aged NMJ, suggesting that the active zone is not a stable structure. These results suggested that presynaptic active zones of NMJs are maintained by a mechanism that controls their density and sustains their structural integrity; however, this mechanism may be jeopardized in aged NMJs.
Active zone assembly at NMJs
Active zone proteins form a multi-protein complex and are preassembled as precursor spheres for photoreceptor ribbon synapses of the retina (Regus-Leidig et al., 2009) and as Piccolo/Bassoon transport vesicles or multi-vesicle transport aggregates for synapses of cultured hippocampal neurons (Zhai et al., 2001; Tao-Cheng, 2007). This preassembly suggests the unitary assembly of presynaptic active zones for these synapses (Ahmari et al., 2000; Shapira et al., 2003; Regus-Leidig et al., 2009). In electron micrographs, both precursor spheres and multi-vesicle transport aggregates were surrounded by synaptic vesicles. However, the precursor spheres possess non-membranous densities in their centers, while the multi-vesicle transport aggregates possess dense-core vesicles in their centers. In the current study, we showed punctate Bassoon signals in axons and motor nerve terminals, and floating bassoon puncta within nerve terminals during embryonic development. These results can be explained by the anterograde transport and anchoring of preassembled units of active zone proteins at the presynaptic terminal. Consistently, we detected vesicle aggregates nucleated by electron-dense material in the motor nerve terminal that were not anchored to the presynaptic membrane at E18.5. These aggregates resembled the precursor spheres with non-membranous central densities that are detected at photoreceptor synapses. To our knowledge, this is the first in vivo report describing the existence of a precursor sphere-like structure at the NMJ, suggesting that the unitary assembly of active zones occurs similarly at NMJs.
Bassoon puncta versus active zones
In the current study, we detected the punctate localization of Bassoon proteins at the motor nerve terminal from the initial stage of NMJ formation. Previously, we also detected the punctate pattern of active zone specific proteins Piccolo and ELKS at the embryonic mouse NMJs (Chen et al., 2011). However, others have reported diffuse distribution patterns of the active zone proteins Piccolo, ELKS, and CAST/Erc2/ELKS2 at rodent NMJs (Juranek et al., 2006; Tokoro et al., 2007). The reason for the difference of the detected patterns of these active zone proteins is unknown. However, the following observations support the discrete punctate localization of the active zone proteins at the NMJ. (1) Freeze-fracture electron microscopy and 3D reconstructions of transmission electron micrographs revealed active zones at discrete locations within the presynaptic terminal of the rodent NMJ (Ellisman et al., 1976; Fukuoka et al., 1987; Rowley et al., 2007; Nagwaney et al., 2009). (2) The 3D-reconstructed confocal Z-stacks of rat Calyx of Held synapses revealed active zones at discrete locations using Bassoon and Piccolo immunohistochemistry (Dondzillo et al., 2010). (3) The proteins mentioned above are detected specifically at active zones but not diffusely within presynaptic terminals by immuno-electron microscopy (Landis et al., 1988; Satzler et al., 2002; Siksou et al., 2007; Tao-Cheng, 2007; Regus-Leidig et al., 2009; Dondzillo et al., 2010). (4) We also observed the same punctate distribution pattern of active zone proteins at NMJs using epi-fluorescence microscopy, proving that the punctate pattern is not due to an under representation of the immunohistochemistry signals by insufficient scanning conditions of confocal microscopy (data not shown). In summary, these various analysis methods and analyses of different synapses suggest that the active zone-specific proteins distribute mainly at discrete punctate locations at synapses.
Bassoon immuno-electronmicroscopy demonstrated its localization in the presynaptic active zone of synapses in the central nervous system (tom Dieck et al., 1998), but Bassoon immuno-electronmicroscopy of NMJs has not been reported. In this study, we tried to visualize the active zones of NMJs by Bassoon immunohistochemistry. In adult NMJs, Bassoon puncta aligned well with the α-bungarotoxin-labeled postsynaptic AChR clusters in 3D-reconstructed confocal images. P/Q-type voltage-dependent calcium channels (VDCCs) are the major type of presynaptic VDCCs in mature NMJs (Uchitel et al., 1992; Protti and Uchitel, 1993; Urbano et al., 2003) and thought to localize at active zones. However, we could not co-label Bassoon and P/Q-type VDCCs because of a lack of a reliable commercial anti-calcium channel antibody. Importantly, Bassoon puncta did overlap with another active zone-specific protein Piccolo at the presynaptic terminal, indicating that both proteins are localized at the active zones of NMJs.
Bassoon immunohistochemistry provides a convenient way to label the active zones at NMJs. However, a resolution limit of the conventional confocal microscopy makes it difficult to resolve structures that are similar to or smaller than the PSF. Using freeze-fracture electron microscopy, developmental analysis of frog NMJs has revealed an elongated arrangement of active zone particles parallel to the postsynaptic junctional folds (Ko, 1985). In mammals, Ellisman et al. (1976) described active zones of rat NMJs as two parallel arrays of 10~12 nm particles arranged in two rows, with each active zone containing 20 particles (Ellisman et al., 1976). The unit size of these active zone particles in mouse was 80 nm x 73 nm (Fukuoka et al., 1987). This description of an active zone particle unit is supported by the electron tomography analysis of mouse NMJs (Nagwaney et al., 2009), and by the freeze-fracture electron microscopy analysis of human NMJs (Fukunaga et al., 1982). The size of a mouse active zone particle unit is below the PSF of a conventional confocal microscope, so the number of such unit within a Bassoon punctum is not resolvable. In our study using Bassoon immunohistochemistry, the Bassoon puncta showed variations in size at perinatal stages. This variation may represent differences of active zone size, aggregation of multiple active zones, or differences of Bassoon protein distribution from one immature active zone. However, freeze-fracture electron microscopy or super-resolution microscopy techniques are required to distinguish between these possibilities (Kittel et al., 2006; Rust et al., 2006; Willig et al., 2006).
Constant active zone density at developing and mature NMJs
The most important finding in our study is the constant active zone density of postnatal NMJs. To our knowledge, the analysis of active zone density throughout the development of mammalian NMJs has not been previously reported. The density of Bassoon puncta was maintained at 2.3 puncta/μm2 during the 3.3 fold increase in NMJ size between P0 and P54. During development, muscle fibers acquire slow or fast fiber type characteristics, and NMJs differ in morphology and size between these two fiber types (Narusawa et al., 1987; Prakash et al., 1996; Chakkalakal et al., 2010). In this study, we did not differentiate the NMJs on the basis of fast or slow fibers because the density of the active zone does not change between these fiber types (Rowley et al., 2007). Thus, the active zone density at NMJs is maintained at a constant level between synapses of different sizes regardless of the muscle fiber types or the developmental growth of the NMJs.
How is the active zone density controlled? We have shown that the extracellular interaction of the synapse organizer laminin β2 and presynaptic P/Q-type VDCCs are required for active zone organization (Nishimune et al., 2004). Furthermore, we have demonstrated direct interactions between VDCC β subunits and Bassoon, suggesting a mechanism that links an extracellular organizer to active zone proteins through VDCC α+β subunit complexes (Chen et al., 2011). Among the synaptic laminin subunits that form multimers with laminin β2, the concentration of laminin α4 in the synaptic cleft is lower near the active zones and higher between the active zones (Patton et al., 2001). Laminin α4 knockout mice exhibits mislocalization of active zones at NMJs without any changes in the total number of active zones, suggesting that laminin α4 controls the location of active zones (Patton et al., 2001). In Drosophila, the number and spacing of NMJ active zones are controlled by Rab3, an endocytosic mechanism, or postsynaptic spectrin (Dickman et al., 2006; Pielage et al., 2006; Graf et al., 2009). However, Rab3abcd quadruple knockout mice did not exhibit an active zone phenotype, suggesting that the intracellular mechanism controlling active zone density is different between Drosophila and mice (Schluter et al., 2004). βIII spectrin knockout mice showed impaired synaptogenesis in the cerebellum with ataxic and seizure phenotypes, but the NMJ phenotype remains unknown (Stankewich et al., 2010). Thus, the roles of Rab3 proteins and spectrins in mammalian active zone organization are currently unclear. In summary, molecules known to affect the active zone density in mammalian NMJs are laminin β2 and α4.
The maintenance of active zone density has also been shown at Drosophila NMJs and rat calyx of Held synapses (Meinertzhagen et al., 1998; Reiff et al., 2002; Sigrist et al., 2002; Graf et al., 2009; Dondzillo et al., 2010). The maintenance of constant active zone density at mouse NMJs during development is potentially advantageous for neurotransmission. In the presynaptic terminal, a regulated distance between active zones will ensure access to synaptic vesicles and Ca2+ buffering systems (Neher, 1998). In the synaptic cleft, the local concentration of the neurotransmitter will be kept under a certain level by the constant density of active zones and aid the effective clearance of transmitters by acetylcholinesterase (Massoulie and Bon, 1982). On the postsynaptic side, muscle cells can maintain the density of acetylcholine receptors at a fixed level during development to secure the safety factor of neurotransmission (Kelly, 1978) if the density of presynaptic active zones is constant. At NMJs, the constant density of active zones will aid these synaptic transmission machineries during the developmental increase of active zone numbers (Fig. 7). These possibilities need confirmation by future analyses.
Impaired active zone maintenance at aged NMJs
To our knowledge, this is the first analysis to show the decrease of an active zone-specific protein in aged NMJs. Ultrastructural analysis of aged NMJs has been reported, but the quantitative analysis of active zones has not been demonstrated (Cardasis and LaFontaine, 1987). We discovered a significant reduction of the active zone protein Bassoon and the number of active zones at motor nerve terminals in aged mice. This decreased number of active zones is consistent with the attenuated mEPP frequency at the NMJs of aged mice and rats compared to young adults (Gutmann et al., 1971; Banker et al., 1983; Alshuaib and Fahim, 1991; Fahim, 1997).
The reduction of the Bassoon protein at aged NMJs is not due to partial or full denervation. We observed a reduction of Bassoon signal intensity at the innervated, aged NMJs that possessed synaptic vesicle-related protein SV2 at a level similar to our observations in young NMJs. Moreover, denervated NMJs showed a complete absence of Bassoon signal. The low levels of Bassoon in the aged NMJs is less likely to be due to regeneration after denervation because terminal sprouting of motor axons is less robust in aged muscle tissue (Wernig and Herrera, 1986), and muscle fiber regeneration is depressed in aged animals (Faulkner et al., 1990). These observations suggest that the level of Bassoon decreases at aged NMJs prior to denervation.
What is the consequence of a decreased level of Bassoon protein or a decreased number of active zones at the synapse? Bassoon knockout mice have demonstrated its roles in the delivery of rapidly releasable vesicles to the active zones of cerebellar mossy fiber granule cell synapses and in the structural integrity of active zones at ribbon synapses (Dick et al., 2003; Frank et al., 2010; Hallermann et al., 2010); however, the role of Bassoon in NMJ neurotransmission remains unknown. The synapse organizer laminin β2 and presynaptic P/Q-type VDCCs play essential roles in active zone organization (Nishimune et al., 2004). Laminin β2 knockout mice and P/Q-type VDCC knockout mice have a decreased number of active zones (Noakes et al., 1995; Nishimune et al., 2004), and attenuated mEPP frequency and quantum content at NMJs (Knight et al., 2003; Urbano et al., 2003). These analyses suggest that a decrease in the active zone number at NMJs have significant effects on neurotransmission. Thus, a loss of Bassoon may have a significant effect on aged NMJs.
Our results from aged NMJs suggest that active zones require a mechanism to maintain their structural integrity. Presynaptic active zones are not stable structures after their initial formation during early developmental stages. Patients with Lambert-Eaton myasthenia syndrome (LEMS) have fewer active zones (Fukunaga et al., 1982). Mouse models of LEMS, generated by injecting IgGs from LEMS patients, exhibit active zone loss in adult mice (Fukunaga et al., 1983). Decreasing the external calcium concentration leads to the disruption of active zones in adult frog NMJs (Meriney et al., 1996; Wachman et al., 2004). Inhibition of the interaction between presynaptic VDCCs and the synapse organizer laminin β2 causes a decrease in the active zone numbers of mice after only two days of inhibitor injection (Nishimune et al., 2004). All of these observations suggest that the active zones of adult NMJs require maintenance mechanisms for their integrity.
Supplementary Material
Acknowledgments
We thank N.E.J. Berman and C.D. Klaassen for the aged mouse tissue, B. Fegley and B. Yoo for technical assistance, and J. Livet, M.J. Werle and K. Kiyosue for comments on the manuscript. The monoclonal SV2 antibody developed by K.M. Buckley was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
Grant support: Grant sponsor: NIH-NCRR; Grant number: P20RR024214; Grant sponsor: Whitehall foundation: Kansas IDDRC: P30 NICHD HD 002528 (all to H.N.).
Footnotes
The authors have no competing interests to declare.
LITERATURE CITED
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3(5):445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- Alshuaib WB, Fahim MA. Depolarization reverses age-related decrease of spontaneous transmitter release. J Appl Physiol. 1991;70(5):2066–2071. doi: 10.1152/jappl.1991.70.5.2066. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon R, Lichtman J. In vivo observations of pre- and postsynaptic changes during the transition from multiple to single innervation at developing neuromuscular junctions. The Journal of Neuroscience. 1993;13(2):834–855. doi: 10.1523/JNEUROSCI.13-02-00834.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balice-Gordon RJ. Age-related changes in neuromuscular innervation. Muscle Nerve Suppl. 1997;5:S83–87. doi: 10.1002/(sici)1097-4598(1997)5+<83::aid-mus20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Banker BQ, Kelly SS, Robbins N. Neuromuscular transmission and correlative morphology in young and old mice. The Journal of physiology. 1983;339:355–377. doi: 10.1113/jphysiol.1983.sp014721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Sudhof TC, Rettig J, Brose N. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21(1):123–136. doi: 10.1016/s0896-6273(00)80520-6. [DOI] [PubMed] [Google Scholar]
- Brown MC, Jansen JK, Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. The Journal of physiology. 1976;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley K, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. The Journal of cell biology. 1985;100(4):1284–1294. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardasis CA, LaFontaine DM. Aging rat neuromuscular junnctions: A morphometric study of cholinesterase-stained whole mounts and ultrastructure. Muscle & Nerve. 1987;10(3):200–213. doi: 10.1002/mus.880100303. [DOI] [PubMed] [Google Scholar]
- Cases-Langhoff C, Voss B, Garner AM, Appeltauer U, Takei K, Kindler S, Veh RW, De Camilli P, Gundelfinger ED, Garner CC. Piccolo, a novel 420 kDa protein associated with the presynaptic cytomatrix. Eur J Cell Biol. 1996;69(3):214–223. [PubMed] [Google Scholar]
- Chakkalakal JV, Nishimune H, Ruas JL, Spiegelman BM, Sanes JR. Retrograde influence of muscle fibers on their innervation revealed by a novel marker for slow motoneurons. Development. 2010;137(20):3489–3499. doi: 10.1242/dev.053348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Billings SE, Nishimune H. Calcium Channels Link the Muscle-Derived Synapse Organizer Laminin beta2 to Bassoon and CAST/Erc2 to Organize Presynaptic Active Zones. The Journal of Neuroscience. 2011;31(2):512–525. doi: 10.1523/JNEUROSCI.3771-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conchello J-A, Lichtman JW. Optical sectioning microscopy. Nat Meth. 2005;2(12):920–931. doi: 10.1038/nmeth815. [DOI] [PubMed] [Google Scholar]
- Couteaux R, Pecot-Dechavassine M. Synaptic vesicles and pouches at the level of “active zones” of the neuromuscular junction. C R Acad Sci Hebd Seances Acad Sci D. 1970;271(25):2346–2349. [PubMed] [Google Scholar]
- Delbono O. Neural control of aging skeletal muscle. Aging Cell. 2003;2(1):21–29. doi: 10.1046/j.1474-9728.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- Desaki J, Uehara Y. Formation and maturation of subneural apparatuses at neuromuscular junctions in postnatal rats: a scanning and transmission electron microscopical study. Dev Biol. 1987;119(2):390–401. doi: 10.1016/0012-1606(87)90044-3. [DOI] [PubMed] [Google Scholar]
- Dick O, Hack I, Altrock WD, Garner CC, Gundelfinger ED, Brandstätter JH. Localization of the presynaptic cytomatrix protein Piccolo at ribbon and conventional synapses in the rat retina: Comparison with Bassoon. The Journal of Comparative Neurology. 2001;439(2):224–234. doi: 10.1002/cne.1344. [DOI] [PubMed] [Google Scholar]
- Dick O, tom Dieck S, Altrock WD, Ammermuller J, Weiler R, Garner CC, Gundelfinger ED, Brandstatter JH. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37(5):775–786. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- Dickman DK, Lu Z, Meinertzhagen IA, Schwarz TL. Altered synaptic development and active zone spacing in endocytosis mutants. Curr Biol. 2006;16(6):591–598. doi: 10.1016/j.cub.2006.02.058. [DOI] [PubMed] [Google Scholar]
- Dondzillo A, Sätzler K, Horstmann H, Altrock WD, Gundelfinger ED, Kuner T. Targeted three-dimensional immunohistochemistry reveals localization of presynaptic proteins Bassoon and Piccolo in the rat calyx of Held before and after the onset of hearing. The Journal of Comparative Neurology. 2010;518(7):1008–1029. doi: 10.1002/cne.22260. [DOI] [PubMed] [Google Scholar]
- Dresbach T, Torres V, Wittenmayer N, Altrock WD, Zamorano P, Zuschratter W, Nawrotzki R, Ziv NE, Garner CC, Gundelfinger ED. Assembly of active zone precursor vesicles: obligatory trafficking of presynaptic cytomatrix proteins Bassoon and Piccolo via a trans-Golgi compartment. The Journal of biological chemistry. 2006;281(9):6038–6047. doi: 10.1074/jbc.M508784200. [DOI] [PubMed] [Google Scholar]
- Ellisman MH, Rash JE, Staehelin LA, Porter KR. Studies of excitable membranes. II. A comparison of specializations at neuromuscular junctions and nonjunctional sarcolemmas of mammalian fast and slow twitch muscle fibers. The Journal of cell biology. 1976;68(3):752–774. doi: 10.1083/jcb.68.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim MA. Endurance exercise modulates neuromuscular junction of C57BL/6NNia aging mice. J Appl Physiol. 1997;83(1):59–66. doi: 10.1152/jappl.1997.83.1.59. [DOI] [PubMed] [Google Scholar]
- Fahim MA, Robbins N. Ultrastructural studies of young and old mouse neuromuscular junctions. J Neurocytol. 1982;11(4):641–656. doi: 10.1007/BF01262429. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Brooks SV, Zerba E. Skeletal muscle weakness and fatigue in old age: underlying mechanisms. Annu Rev Gerontol Geriatr. 1990;10:147–166. doi: 10.1007/978-3-662-38445-9_9. [DOI] [PubMed] [Google Scholar]
- Fenster SD, Chung WJ, Zhai R, Cases-Langhoff C, Voss B, Garner AM, Kaempf U, Kindler S, Gundelfinger ED, Garner CC. Piccolo, a presynaptic zinc finger protein structurally related to bassoon. Neuron. 2000;25(1):203–214. doi: 10.1016/s0896-6273(00)80883-1. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Wolfel M, Nishimune H, Tabares L, Schmitz F, Castellano-Munoz M, Rosenmund C, Montesinos ML, Sanes JR, Schneggenburger R, Sudhof TC. The synaptic vesicle protein CSP alpha prevents presynaptic degeneration. Neuron. 2004;42(2):237–251. doi: 10.1016/s0896-6273(04)00190-4. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Foster S, Scott MA, Sherwood P. Transient expression of LIM-domain transcription factors is coincident with delayed maturation of photoreceptors in the chicken retina. The Journal of Comparative Neurology. 2008;506(4):584–603. doi: 10.1002/cne.21578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank T, Rutherford MA, Strenzke N, Neef A, Pangrsic T, Khimich D, Fetjova A, Gundelfinger ED, Liberman MC, Harke B, Bryan KE, Lee A, Egner A, Riedel D, Moser T. Bassoon and the Synaptic Ribbon Organize Ca2+ Channels and Vesicles to Add Release Sites and Promote Refilling. Neuron. 2010;68(4):724–738. doi: 10.1016/j.neuron.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga H, Engel AG, Lang B, Newsom-Davis J, Vincent A. Passive transfer of Lambert-Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Proceedings of the National Academy of Sciences of the United States of America. 1983;80(24):7636–7640. doi: 10.1073/pnas.80.24.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga H, Engel AG, Osame M, Lambert EH. Paucity and disorganization of presynaptic membrane active zones in the lambert-eaton myasthenic syndrome. Muscle & Nerve. 1982;5(9):686–697. [Google Scholar]
- Fukuoka T, Engel AG, Lang B, Newsom-Davis J, Prior C, Wray DW. Lambert-Eaton myasthenic syndrome: I. Early morphological effects of IgG on the presynaptic membrane active zones. Ann Neurol. 1987;22(2):193–199. doi: 10.1002/ana.410220203. [DOI] [PubMed] [Google Scholar]
- Graf ER, Daniels RW, Burgess RW, Schwarz TL, DiAntonio A. Rab3 Dynamically Controls Protein Composition at Active Zones. Neuron. 2009;64(5):663–677. doi: 10.1016/j.neuron.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E, Hanzlikova V, Vysokocil F. Age changes in cross striated muscle of the rat. The Journal of physiology. 1971;216(2):331–343. doi: 10.1113/jphysiol.1971.sp009528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara A, Harada K, Hida Y, Kitajima I, Ohtsuka T. Distribution of serine/threonine kinase SAD-B in mouse peripheral nerve synapse. Neuroreport. 2011;22(7):319–325. doi: 10.1097/WNR.0b013e328346013c. [DOI] [PubMed] [Google Scholar]
- Hallermann S, Fejtova A, Schmidt H, Weyhersmüller A, Silver RA, Gundelfinger ED, Eilers J. Bassoon Speeds Vesicle Reloading at a Central Excitatory Synapse. Neuron. 2010;68(4):710–723. doi: 10.1016/j.neuron.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kaeser PS, Sudhof TC, Schneggenburger R. RIM Determines Ca2+ Channel Density and Vesicle Docking at the Presynaptic Active Zone. Neuron. 2011;69(2):304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow ML, Ress D, Stoschek A, Marshall RM, McMahan UJ. The architecture of active zone material at the frog’s neuromuscular junction. Nature. 2001;409(6819):479–484. doi: 10.1038/35054000. [DOI] [PubMed] [Google Scholar]
- Haynes RL, Borenstein NS, Desilva TM, Folkerth RD, Liu LG, Volpe JJ, Kinney HC. Axonal development in the cerebral white matter of the human fetus and infant. The Journal of Comparative Neurology. 2005;484(2):156–167. doi: 10.1002/cne.20453. [DOI] [PubMed] [Google Scholar]
- Heilemann M, Dedecker P, Hofkens J, Sauer M. Photoswitches: Key molecules for subdiffraction-resolution fluorescence imaging and molecular quantification. Laser & Photonics Review. 2009;3(1–2):180–202. [Google Scholar]
- Heuser JE, Reese TS, Dennis MJ, Jan Y, Jan L, Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. The Journal of cell biology. 1979;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Heuser JE. Internal and external differentiations of the postsynaptic membrane at the neuromuscular junction. Journal of Neurocytology. 1982;11(3):487–510. doi: 10.1007/BF01257990. [DOI] [PubMed] [Google Scholar]
- Juranek J, Mukherjee K, Rickmann M, Martens H, Calka J, Sudhof TC, Jahn R. Differential expression of active zone proteins in neuromuscular junctions suggests functional diversification. European Journal of Neuroscience. 2006;24(11):3043–3052. doi: 10.1111/j.1460-9568.2006.05183.x. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Sudhof TC. RIM Proteins Tether Ca2+ Channels to Presynaptic Active Zones via a Direct PDZ-Domain Interaction. Cell. 2011;144(2):282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SS. The effect of age on neuromuscular transmission. The Journal of physiology. 1978;274:51–62. doi: 10.1113/jphysiol.1978.sp012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khimich D, Nouvian R, Pujol R, Tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434(7035):889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, Hell SW, Buchner E, Heckmann M, Sigrist SJ. Bruchpilot Promotes Active Zone Assembly, Ca2+ Channel Clustering, and Vesicle Release. Science (New York, NY. 2006;312(5776):1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- Knight D, Tolley LK, Kim DK, Lavidis NA, Noakes PG. Functional analysis of neurotransmission at beta2-laminin deficient terminals. The Journal of physiology. 2003;546(Pt 3):789–800. doi: 10.1113/jphysiol.2002.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CP. Formation of the active zone at developing neuromuscular junctions in larval and adult bullfrogs. J Neurocytol. 1985;14(3):487–512. doi: 10.1007/BF01217757. [DOI] [PubMed] [Google Scholar]
- Kretschmannova K, Zemkova H. Characterization of neuromuscular transmission in mice with progressive motoneuronopathy. Physiol Res. 2004;53(5):541–548. [PubMed] [Google Scholar]
- Lachyankar MB, Sultana N, Schonhoff CM, Mitra P, Poluha W, Lambert S, Quesenberry PJ, Litofsky NS, Recht LD, Nabi R, Miller SJ, Ohta S, Neel BG, Ross AH. A Role for Nuclear PTEN in Neuronal Differentiation. The Journal of Neuroscience. 2000;20(4):1404–1413. doi: 10.1523/JNEUROSCI.20-04-01404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis DM, Hall AK, Weinstein LA, Reese TS. The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron. 1988;1(3):201–209. doi: 10.1016/0896-6273(88)90140-7. [DOI] [PubMed] [Google Scholar]
- Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee K-F. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410(6832):1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- Liu Y, Oppenheim RW, Sugiura Y, Lin W. Abnormal development of the neuromuscular junction in Nedd4-deficient mice. Developmental Biology. 2009;330(1):153–166. doi: 10.1016/j.ydbio.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupa MT, Hall ZW. Progressive restriction of synaptic vesicle protein to the nerve terminal during development of the neuromuscular junction. The Journal of Neuroscience. 1989;9(11):3937–3945. doi: 10.1523/JNEUROSCI.09-11-03937.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques MJ, Conchello JA, Lichtman JW. From plaque to pretzel: fold formation and acetylcholine receptor loss at the developing neuromuscular junction. The Journal of Neuroscience. 2000;20(10):3663–3675. doi: 10.1523/JNEUROSCI.20-10-03663.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoulie J, Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annual review of neuroscience. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- Matthews-Bellinger JA, Salpeter MM. Fine structural distribution of acetylcholine receptors at developing mouse neuromuscular junctions. The Journal of Neuroscience. 1983;3(3):644–657. doi: 10.1523/JNEUROSCI.03-03-00644.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinertzhagen IA, Govind CK, Stewart BA, Carter JM, Atwood HL. Regulated spacing of synapses and presynaptic active zones at larval neuromuscular junctions in different genotypes of the flies Drosophila and Sarcophaga. The Journal of Comparative Neurology. 1998;393(4):482–492. doi: 10.1002/(sici)1096-9861(19980420)393:4<482::aid-cne7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Meriney SD, Wolowske B, Ezzati E, Grinnell AD. Low calcium-induced disruption of active zone structure and function at the frog neuromuscular junction. Synapse (New York, NY. 1996;24(1):1–11. doi: 10.1002/(SICI)1098-2396(199609)24:1<1::AID-SYN1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Misgeld T, Burgess RW, Lewis RM, Cunningham JM, Lichtman JW, Sanes JR. Roles of neurotransmitter in synapse formation: development of neuromuscular junctions lacking choline acetyltransferase. Neuron. 2002;36(4):635–648. doi: 10.1016/s0896-6273(02)01020-6. [DOI] [PubMed] [Google Scholar]
- Misgeld T, Kummer TT, Lichtman JW, Sanes JR. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(31):11088–11093. doi: 10.1073/pnas.0504806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missias AC, Mudd J, Cunningham JM, Steinbach JH, Merlie JP, Sanes JR. Deficient development and maintenance of postsynaptic specializations in mutant mice lacking an ‘adult’ acetylcholine receptor subunit. Development. 1997;124(24):5075–5086. doi: 10.1242/dev.124.24.5075. [DOI] [PubMed] [Google Scholar]
- Mukherjee K, Yang X, Gerber SH, Kwon H-B, Ho A, Castillo PE, Liu X, Sudhof TC. Piccolo and bassoon maintain synaptic vesicle clustering without directly participating in vesicle exocytosis. Proceedings of the National Academy of Sciences. 2010;107(14):6504–6509. doi: 10.1073/pnas.1002307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagwaney S, Harlow ML, Jung JH, Szule JA, Ress D, Xu J, Marshall RM, McMahan UJ. Macromolecular connections of active zone material to docked synaptic vesicles and presynaptic membrane at neuromuscular junctions of mouse. The Journal of Comparative Neurology. 2009;513(5):457–468. doi: 10.1002/cne.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan PJ, Gibbons HM, Mee EW, Faull RL, Dragunow M. High throughput quantification of cells with complex morphology in mixed cultures. Journal of neuroscience methods. 2007;164(2):339–349. doi: 10.1016/j.jneumeth.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Narusawa M, Fitzsimons RB, Izumo S, Nadal-Ginard B, Rubinstein NA, Kelly AM. Slow myosin in developing rat skeletal muscle. The Journal of cell biology. 1987;104(3):447–459. doi: 10.1083/jcb.104.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20(3):389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Nishimune H, Sanes JR, Carlson SS. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature. 2004;432(7017):580–587. doi: 10.1038/nature03112. [DOI] [PubMed] [Google Scholar]
- Nishimune H, Valdez G, Jarad G, Moulson CL, Muller U, Miner JH, Sanes JR. Laminins promote postsynaptic maturation by an autocrine mechanism at the neuromuscular junction. The Journal of cell biology. 2008;182(6):1201–1215. doi: 10.1083/jcb.200805095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature. 1995;374(6519):258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Takao-Rikitsu E, Inoue E, Inoue M, Takeuchi M, Matsubara K, Deguchi-Tawarada M, Satoh K, Morimoto K, Nakanishi H, Takai Y. Cast: a novel protein of the cytomatrix at the active zone of synapses that forms a ternary complex with RIM1 and munc13-1. The Journal of cell biology. 2002;158(3):577–590. doi: 10.1083/jcb.200202083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton BL, Cunningham JM, Thyboll J, Kortesmaa J, Westerblad H, Edstrom L, Tryggvason K, Sanes JR. Properly formed but improperly localized synaptic specializations in the absence of laminin alpha4. Nat Neurosci. 2001;4(6):597–604. doi: 10.1038/88414. [DOI] [PubMed] [Google Scholar]
- Pielage J, Fetter RD, Davis GW. A postsynaptic Spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J Cell Biol. 2006;175(3):491–503. doi: 10.1083/jcb.200607036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Miller SM, Huang M, Sieck GC. Morphology of diaphragm neuromuscular junctions on different fibre types. J Neurocytol. 1996;25(2):88–100. doi: 10.1007/BF02284788. [DOI] [PubMed] [Google Scholar]
- Protti DA, Uchitel OD. Transmitter release and presynaptic Ca2+ currents blocked by the spider toxin omega-Aga-IVA. Neuroreport. 1993;5(3):333–336. doi: 10.1097/00001756-199312000-00039. [DOI] [PubMed] [Google Scholar]
- Regus-Leidig H, tom Dieck S, Specht D, Meyer L, Brandstätter JH. Early steps in the assembly of photoreceptor ribbon synapses in the mouse retina: The involvement of precursor spheres. The Journal of Comparative Neurology. 2009;512(6):814–824. doi: 10.1002/cne.21915. [DOI] [PubMed] [Google Scholar]
- Reiff DF, Thiel PR, Schuster CM. Differential Regulation of Active Zone Density during Long-Term Strengthening of Drosophila Neuromuscular Junctions. Journal of Neuroscience. 2002;22(21):9399–9409. doi: 10.1523/JNEUROSCI.22-21-09399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Langnaese K, Kreutz MR, Olias G, Zhai R, Scheich H, Garner CC, Gundelfinger ED. Presynaptic cytomatrix protein bassoon is localized at both excitatory and inhibitory synapses of rat brain. The Journal of Comparative Neurology. 1999;408(3):437–448. doi: 10.1002/(sici)1096-9861(19990607)408:3<437::aid-cne9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Rowley KL, Mantilla CB, Ermilov LG, Sieck GC. Synaptic Vesicle Distribution and Release at Rat Diaphragm Neuromuscular Junctions. Journal of neurophysiology. 2007;98(1):478–487. doi: 10.1152/jn.00251.2006. [DOI] [PubMed] [Google Scholar]
- Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat Meth. 2006;3(10):793–796. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annual review of neuroscience. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Satzler K, Sohl LF, Bollmann JH, Borst JG, Frotscher M, Sakmann B, Lubke JH. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. The Journal of Neuroscience. 2002;22(24):10567–10579. doi: 10.1523/JNEUROSCI.22-24-10567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter OM, Schmitz F, Jahn R, Rosenmund C, Sudhof TC. A Complete Genetic Analysis of Neuronal Rab3 Function. The Journal of Neuroscience. 2004;24(29):6629–6637. doi: 10.1523/JNEUROSCI.1610-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira M, Zhai RG, Dresbach T, Bresler T, Torres VI, Gundelfinger ED, Ziv NE, Garner CC. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron. 2003;38(2):237–252. doi: 10.1016/s0896-6273(03)00207-1. [DOI] [PubMed] [Google Scholar]
- Sigrist SJ, Thiel PR, Reiff DF, Schuster CM. The Postsynaptic Glutamate Receptor Subunit DGluR-IIA Mediates Long-Term Plasticity in Drosophila. The Journal of Neuroscience. 2002;22(17):7362–7372. doi: 10.1523/JNEUROSCI.22-17-07362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siksou L, Rostaing P, Lechaire J-P, Boudier T, Ohtsuka T, Fejtova A, Kao H-T, Greengard P, Gundelfinger ED, Triller A, Marty S. Three-Dimensional Architecture of Presynaptic Terminal Cytomatrix. The Journal of Neuroscience. 2007;27(26):6868–6877. doi: 10.1523/JNEUROSCI.1773-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater CR. Postnatal maturation of nerve-muscle junctions in hindlimb muscles of the mouse. Dev Biol. 1982;94(1):11–22. doi: 10.1016/0012-1606(82)90063-x. [DOI] [PubMed] [Google Scholar]
- Stankewich MC, Gwynn B, Ardito T, Ji L, Kim J, Robledo RF, Lux SE, Peters LL, Morrow JS. Targeted deletion of betaIII spectrin impairs synaptogenesis and generates ataxic and seizure phenotypes. Proceedings of the National Academy of Sciences. 2010;107(13):6022–6027. doi: 10.1073/pnas.1001522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH. Ultrastructural localization of active zone and synaptic vesicle proteins in a preassembled multi-vesicle transport aggregate. Neuroscience. 2007;150(3):575–584. doi: 10.1016/j.neuroscience.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokoro T, Higa S, Deguchi-Tawarada M, Inoue E, Kitajima I, Ohtsuka T. Localization of the active zone proteins CAST, ELKS, and Piccolo at neuromuscular junctions. Neuroreport. 2007;18(4):313–316. doi: 10.1097/WNR.0b013e3280287abe. [DOI] [PubMed] [Google Scholar]
- tom Dieck S, Sanmarti-Vila L, Langnaese K, Richter K, Kindler S, Soyke A, Wex H, Smalla KH, Kampf U, Franzer JT, Stumm M, Garner CC, Gundelfinger ED. Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals. The Journal of cell biology. 1998;142(2):499–509. doi: 10.1083/jcb.142.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54(11):B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Uchitel OD, Protti DA, Sanchez V, Cherksey BD, Sugimori M, Llinas R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(8):3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N, Nickel J, Bohl J. Monoclonal antibodies SMI 311 and SMI 312 as tools to investigate the maturation of nerve cells and axonal patterns in human fetal brain. Cell Tissue Res. 1998;291(3):433–443. doi: 10.1007/s004410051013. [DOI] [PubMed] [Google Scholar]
- Urbano FJ, Piedras-Renteria ES, Jun K, Shin HS, Uchitel OD, Tsien RW. Altered properties of quantal neurotransmitter release at endplates of mice lacking P/Q-type Ca2+ channels. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(6):3491–3496. doi: 10.1073/pnas.0437991100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Kang H, Clemenson GD, Jr, Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachman ES, Poage RE, Stiles JR, Farkas DL, Meriney SD. Spatial Distribution of Calcium Entry Evoked by Single Action Potentials within the Presynaptic Active Zone. The Journal of Neuroscience. 2004;24(12):2877–2885. doi: 10.1523/JNEUROSCI.1660-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlin KJ, Moreira EF, Huang H, Yu N, Adler R. Molecular dynamics of photoreceptor synapse formation in the developing chick retina. The Journal of Comparative Neurology. 2008;506(5):822–837. doi: 10.1002/cne.21582. [DOI] [PubMed] [Google Scholar]
- Walsh MK, Lichtman JW. In Vivo Time-Lapse Imaging of Synaptic Takeover Associated with Naturally Occurring Synapse Elimination. Neuron. 2003;37(1):67–73. doi: 10.1016/s0896-6273(02)01142-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Okamoto M, Schmitz F, Hofmann K, Sudhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388(6642):593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- Wernig A, Herrera AA. Sprouting and remodelling at the nerve-muscle junction. Prog Neurobiol. 1986;27(3):251–291. doi: 10.1016/0301-0082(86)90023-7. [DOI] [PubMed] [Google Scholar]
- Willig KI, Rizzoli SO, Westphal V, Jahn R, Hell SW. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature. 2006;440(7086):935–939. doi: 10.1038/nature04592. [DOI] [PubMed] [Google Scholar]
- Xiong XD, Chen GH. Research progress on the age-related changes in proteins of the synaptic active zone. Physiol Behav. 2010;101(1):1–12. doi: 10.1016/j.physbeh.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Zhai RG, Vardinon-Friedman H, Cases-Langhoff C, Becker B, Gundelfinger ED, Ziv NE, Garner CC. Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron. 2001;29(1):131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.