Abstract
OBJECTIVE
The complement cascade has been implicated in cerebral ischemia/reperfusion injury. To develop clinically useful therapies that successfully manipulate the complement cascade, the individual roles of its components must be clearly defined. Previous studies have shown that C5 inhibition improves outcome after experimental stroke. In this study, we investigated the role of C5a in stroke injury by inhibiting its activity at the receptor level.
METHODS
C5a receptor antagonist or vehicle was administered to mice before temporary middle cerebral artery occlusion. Stroke outcomes were assessed 24 hours later in all mice using both neurological deficit scores and cerebral infarct volumes.
RESULTS
Animals treated with C5a receptor antagonist experienced significantly decreased infarct volume and demonstrated an improving trend in neurological function.
CONCLUSION
These findings demonstrate that modulation of C5a receptor activity significantly alters the degree of neurological damage after experimental reperfused stroke.
Keywords: C5, C5a, C5a receptor, Complement cascade, Murine, Stroke
Ischemic stroke is the leading cause of disability and the third leading cause of death in the United States and other industrialized nations (10). Its burden is experienced through the loss of quality years of life and health care resources (10, 12–14). As a result, efforts have aimed to discover therapeutic strategies that limit the adverse effects of ischemic stroke.
After cerebral ischemia, reperfusion exacerbates tissue damage by inducing an inflammatory response. One important mediator of this inflammatory event is the complement cascade. Complement activation has been implicated in ischemia/reperfusion injury in various organ systems, including the myocardium, gut, and limb, and has also received attention as a possible target for stroke therapy (3, 5, 6, 9).
Investigations have targeted various components of the complement cascade in an effort to determine their individual roles and contributions to the pathogenesis of cerebral ischemic injury (17). We have previously shown that C3 and C3a play a critical role in the inflammatory cascade after murine reperfused stroke and that deficiency of C3 or inhibition of C3a resulted in significantly improved outcome (17). At the same time, we found that genetic C5 deficiency offered no neuroprotection (17). In contrast, other groups have reported significant reductions in infarct volume after treatment with anti-C5 antibody in a rat model of stroke (8). One possible explanation for this difference is that lifelong deficiency of C5 may confer a constitutionally detrimental effect that masks any potential benefit that acute C5 blockade may have in the context of stroke. It is likely, therefore, that focal C5 inhibition alone, during the peri-ischemic time period, offers neuroprotection in the absence of long-term C5 deficiency.
C5 itself, however, is not the mediator of proinflammatory effects but undergoes cleavage into C5a and C5b by either classic or alternative pathway C5 convertase (1). C5b joins C6 to C9 to form the membrane attack complex, which can cause lysis of cells and bacteria (1). C5a, an anaphylatoxin, is a potent instigator of inflammatory cell recruitment and activation and, therefore, represents an attractive target for modulation of inflammation (1). We hypothesized that the neuroprotective effect of C5 inhibition is attributable, in large part, to the prevention of C5a formation and function. To specifically investigate the role of C5a during cerebral ischemia and reperfusion, we blocked C5a function at its receptor by administering a C5a receptor antagonist (C5aRA) to mice just before transient middle cerebral artery occlusion and compared their outcomes with those of vehicle-treated controls.
MATERIALS AND METHODS
Mice
All experiments were conducted in a humane manner under the supervision and approval of the Columbia University Institutional Animal Care and Use Committee. All experimental animals were male C57Bl/6 mice aged 8 to 10 weeks and weighing between 22 and 26 g at the time of surgery. They were housed in certified animal care barrier facilities in microisolator cages with free access to food and water on a 12-hour light/dark cycle.
Murine Model of Focal Cerebral Ischemia
These studies used the intraluminal filament model described previously, with minor modifications (7). Briefly, mice were anesthetized, intubated, and maintained under general inhalational anesthesia on a mechanical ventilator. Middle cerebral artery occlusion was performed by advancing a heat-blunted, silicon-coated, 7–0 nylon suture via the external carotid artery to the origin of the middle cerebral artery. After 60 minutes of ischemia, the occluding suture was withdrawn to establish reperfusion.
Transcranial measurements of cerebral blood flow (CBF) were made with laser-Doppler flowmetry (Periflux System 5000; Perimed AB, Järfälla, Sweden) as previously described (7), using 0.5-mm flexible fiberoptic Doppler probes (Perimed AB) attached with tissue adhesive to the intact skull over previously published landmarks (2 mm posterior to the bregma, 6 mm to each side of midline). Relative CBF measurements were obtained after anesthesia, immediately after occlusion, before reperfusion, and immediately after reperfusion to confirm proper suture placement and adequate reperfusion. Strict criteria were used to prospectively exclude animals that did not experience significant CBF drop-off (>70%), did not maintain consistent levels of CBF decrease, or did not demonstrate adequate reperfusion (>50%). Rectal temperature was monitored and held constant during surgery using a heating lamp. After surgery, normothermia was maintained in an animal incubator for 150 minutes after induction of ischemia.
C5aRA Treatment Experiment
The C5aRA is a cyclic hexapeptide AcF[OpdChaWR] that was synthetically designed from the carbolic acid terminus of C5a (11). Peptide synthesis and cyclization were performed as described previously (11). The antagonist has been shown to specifically block C5a-mediated effects in various rodent disease models (19). The reagent was analyzed for lipopolysaccharide content using a limulus assay (Pyrochrome; Associates of Cape Cod, Inc., East Falmouth, MA) and was found to be below 1.5 ng/mg of protein. A single 1-mg/kg intraperitoneal injection of C5aRA (n = 18) or an equal volume of vehicle (sterile phosphate-buffered saline with 10% ethanol) (n = 17) was administered in a blinded fashion to male C57Bl/6 mice 45 minutes before the onset of ischemia.
Infarct Volume and Neurological Function Assessment
Stroke outcomes were assessed in all mice at 24 hours after ischemia by ascertaining both cerebral infarct volume and neurological deficit. Neurological deficit was quantified using a four-tiered grading system, as described previously (7). All assessments were conducted by an observer (SAS) blinded to the identity of individual animals.
After the neurological examination, mice were sacrificed. Brains were perfused with phosphate-buffered saline via intracardiac injection to remove residual blood products. The brains were then removed intact and sliced into 1-mm sections. Sections were immersed in 2% triphenyltetrazolium chloride in 0.9% saline and incubated for 25 minutes at 37°C. Infarcted brain was identified as an area of unstained tissue (Fig. 1). Infarct volumes were calculated by two blinded independent observers (SAS, MR) from planimetric analysis of digitized images of serial cerebral sections and expressed as the percentage of the ipsilateral hemisphere occupied by infarcted tissue.
FIGURE 1.

Brain sections immersed in 2% triphenyltetrazolium chloride in 0.9% saline. Infarcted brain was identified as an area of unstained tissue in the vehicle-treated (A) and C5aRA-treated (B) specimens.
Statistical Analysis
Between-group differences (infarct volume and neurological deficit scores) were analyzed using two-tailed unpaired Student’s t tests. All values are expressed as means ± standard error, with a P value of less than 0.05 considered statistically significant.
RESULTS
Infarct Volume
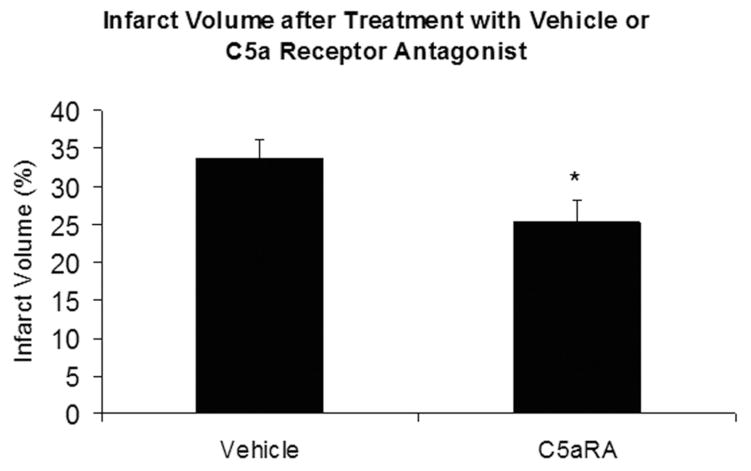
C5aRA-treated mice experienced a significant reduction in infarct volumes. The treated mice demonstrated a 24% decrease in infarct volume (vehicle, 34 ± 2.6%, n = 18; C5aRA, 25.3 ± 2.8%, n = 17; P = 0.02) when compared with vehicle-treated mice (Fig. 2).
FIGURE 2.
Mice treated with C5aRA demonstrated a significant decrease (asterisk) in infarct volume after experimental stroke compared with mice treated with vehicle (P = 0.02).
Neurological Function
C5aRA-treated mice demonstrated an improving trend in neurological deficit scores (vehicle, 2.5 ± 0.12, n = 18; C5aRA, 2.1 ± 0.17, n = 17; P = 0.09) compared with controls (Fig. 3).
FIGURE 3.

Mice treated with C5aRA demonstrated a trend toward improvement in neurological function after experimental stroke when compared with mice treated with vehicle (P = 0.09).
DISCUSSION
Inflammation has been shown to play a crucial role in the propagation of injury after experimental stroke. The complement cascade contributes to this process through up-regulation of adhesion molecules, neutrophil recruitment, platelet activation, and generation of reactive oxygen species. Whereas nonspecific inhibition of the complement cascade has demonstrated neuroprotection in the context of stroke, targeted inhibition of specific complement components in experimental stroke models has helped identify which components are primarily responsible for exacerbation of postischemic injury (3). We have recently demonstrated the importance of C3 in the development of injury after acute stroke by showing that C3 knockout mice experience reduction in infarct volumes compared with their wild-type controls. Reconstitution of C3 knockout mice with exogenous C3, in turn, reversed this protection. Moreover, treatment with C3a receptor antagonist resulted in similar attenuation of postischemic injury (17).
C5a, like C3a, is a potent anaphylatoxin that functions as a chemoattractant for neutrophils. C5a stimulation of neutrophils leads to the production of reactive oxygen species and proteinase release, both of which contribute to tissue damage. C5a also stimulates the secretion of proinflammatory cytokines from monocytes and macrophages, further amplifying the inflammatory response (3). Given the efficacy of C3/C3a receptor inhibition in limiting tissue damage after stroke, we expected that C5/C5a receptor inhibition would demonstrate similar benefit. However, previous work from our group revealed that C5 deficiency actually affords mice no neuroprotection after stroke when compared with wild-type controls (17). An obvious explanation for these findings is that C5 is not a critical mediator of cerebral ischemia/reperfusion injury. Alternatively, the lack of neuroprotection in C5-deficient mice might be better explained by phenotypic disadvantages that predispose these mice to enhanced cerebral injury and neurodegeneration. For instance, C5-deficient mice have previously been shown to be more susceptible to intraventricular kainate toxicity through the overproduction of proinflammatory cytokines and alterations of Ca2+ influx (21). In addition, C5-deficient mice subjected to intracerebral hemorrhage exhibited increased cerebral edema (20).
In contrast, animal models using targeted, nongenetic anti-C5 strategies have demonstrated attenuation of neurotoxicity. Administration of anti-C5 monoclonal antibody in a rat model of reperfused stroke resulted in a decrease in cerebral infarct size and edema and an improvement in neurological function (8). Similarly, C5 has been implicated in other organ systems of reperfusion injury. In the kidney, preischemic administration of C5aRA substantially inhibited ischemia/reperfusion-induced renal damage (2). In addition, both anti-C5 antibody and C5aRA have been shown to attenuate reperfusion injury in the ischemic rat intestine (15, 22). These findings suggest that, in contrast to genetic deficiency, modulation of C5 or C5a activity during the peri-ischemic period more specifically targets acute inflammation, thereby limiting reperfusion injury.
In the present study, we sought to define the role of C5a in reperfused stroke. A role for C5a in postischemic cerebral injury has been suggested by previous demonstration of up-regulation of C5aR expression after permanent focal ischemia (4). Both constitutive neural expression and up-regulation on infiltrating leukocytes accounted for the increase in receptor density (4). In addition, human stroke patients have been shown to exhibit a marked increase in C5a plasma levels after cerebral ischemia (18). Therefore, we hypothesized that focal C5a inhibition with C5aRA would limit reperfusion injury and improve neurological outcome after murine stroke. As expected, C5aRA-treated mice experienced smaller stroke volumes and improved neurological scores when compared with vehicle-treated controls.
In comparison with previous investigations of C3a receptor blockade, however, C5a receptor blockade resulted in a smaller reduction in infarct volume; and whereas C3a receptor inhibition led to statistically significant improvements in neurological score, C5a receptor blockade did not (17). Further efforts to determine the distinct contributions of C3a and C5a will require dosing experiments and trials of various permutations of C5a and C3a blockade to see whether more comprehensive suppression of anaphylatoxin activity offers further benefit.
CONCLUSION
Although C3a has been shown to mediate ischemia/reperfusion injury in the brain by recruiting and activating neutrophils, the exact mechanisms by which the C5a receptor mediates its effects are still unknown. We monitored temperature and CBF, but physiological changes in blood pressure, heart rate, or arterial blood gas levels could have affected stroke volume without our knowledge. Similarly, our control solution of phosphate-buffered saline and ethanol leaves the possibility that the C5aRA solution may work by means of a mechanism other than inhibition of C5a receptor. Future studies are necessary to elucidate the mechanism by which the C5a receptor is up-regulated in the brain after ischemia/reperfusion injury and the specific targets through which it mediates its effects. In addition, efforts to validate postischemic efficacy should be pursued. Defining the therapeutic window for C5a receptor inhibition in stroke will be of critical importance in assessing its potential as a clinically relevant target of stroke therapy. Moreover, in light of the recently characterized clinical efficacy and safety of anti-C5 therapies in subgroups of myocardial ischemia patients (16), investigation of the role of C5a in cerebral ischemia has the potential to lead to profound and expedient advances in the treatment of stroke.
Contributor Information
Grace H. Kim, Department of Neurological Surgery, Columbia University, New York, New York
J Mocco, Department of Neurological Surgery, Columbia University, New York, New York
David K. Hahn, Department of Neurological Surgery, Columbia University, New York, New York
Christopher P. Kellner, Department of Neurological Surgery, Columbia University, New York, New York
Ricardo J. Komotar, Department of Neurological Surgery, Columbia University, New York, New York
Andrew F. Ducruet, Department of Neurological Surgery, Columbia University, New York, New York
William J. Mack, Department of Neurological Surgery, Columbia University, New York, New York
E. Sander Connolly, Jr., Department of Neurological Surgery, Columbia University, New York, New York
References
- 1.Arumugam TV, Magnus T, Woodruff TM, Proctor LM, Shiels IA, Taylor SM. Complement mediators in ischemia-reperfusion injury. Clin Chim Acta. 2006;374:33–45. doi: 10.1016/j.cca.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Arumugam TV, Shiels IA, Strachan AJ, Abbenante G, Fairlie DP, Taylor SM. A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int. 2003;63:134–142. doi: 10.1046/j.1523-1755.2003.00737.x. [DOI] [PubMed] [Google Scholar]
- 3.Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM. The role of the complement system in ischemia-reperfusion injury. Shock. 2004;21:401–409. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Barnum SR, Ames RS, Maycox PR, Hadingham SJ, Meakin J, Harrison D, Parsons AA. Expression of the complement C3a and C5a receptors after permanent focal ischemia: An alternative interpretation. Glia. 2002;38:169–173. doi: 10.1002/glia.10069. [DOI] [PubMed] [Google Scholar]
- 5.Cannon RO., 3rd Mechanisms, management and future directions for reperfusion injury after acute myocardial infarction. Nat Clin Pract Cardiovasc Med. 2005;2:88–94. doi: 10.1038/ncpcardio0096. [DOI] [PubMed] [Google Scholar]
- 6.Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion: A review. Acta Cir Bras. 2005;20:336–343. doi: 10.1590/s0102-86502005000400013. [DOI] [PubMed] [Google Scholar]
- 7.Connolly ES, Jr, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD, Stern DM, Solomon RA, Gutierrez-Ramos JC, Pinsky DJ. Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion: Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest. 1996;97:209–216. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa C, Zhao L, Shen Y, Su X, Hao L, Colgan SP, Stahl GL, Zhou T, Wang Y. Role of complement component C5 in cerebral ischemia/reperfusion injury. Brain Res. 2006;1100:142–151. doi: 10.1016/j.brainres.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 9.D’Ambrosio AL, Pinsky DJ, Connolly ES. The role of the complement cascade in ischemia/reperfusion injury: Implications for neuroprotection. Mol Med. 2001;7:367–382. [PMC free article] [PubMed] [Google Scholar]
- 10.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 11.Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J Med Chem. 1999;42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- 12.Fisher M. Recommendations for advancing development of acute stroke therapies: Stroke Therapy Academic Industry Roundtable 3. Stroke. 2003;34:1539–1546. doi: 10.1161/01.STR.0000072983.64326.53. [DOI] [PubMed] [Google Scholar]
- 13.Fisher M, Ratan R. New perspectives on developing acute stroke therapy. Ann Neurol. 2003;53:10–20. doi: 10.1002/ana.10407. [DOI] [PubMed] [Google Scholar]
- 14.Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: Lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 15.Heller T, Hennecke M, Baumann U, Gessner JE, zu Vilsendorf AM, Baensch M, Boulay F, Kola A, Klos A, Bautsch W, Köhl J. Selection of a C5a receptor antagonist from phage libraries attenuating the inflammatory response in immune complex disease and ischemia/reperfusion injury. J Immunol. 1999;163:985–994. [PubMed] [Google Scholar]
- 16.Mahaffey KW, Van de Werf F, Shernan SK, Granger CB, Verrier ED, Filloon TG, Todaro TG, Adams PX, Levy JH, Hasselblad V, Armstrong PW. Effect of pexelizumab on mortality in patients with acute myocardial infarction or undergoing coronary artery bypass surgery: A systematic overview. Am Heart J. 2006;152:291–296. doi: 10.1016/j.ahj.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Mocco J, Mack WJ, Ducruet AF, Sosunov SA, Sughrue ME, Hassid BG, Nair MN, Laufer I, Komotar RJ, Claire M, Holland H, Pinsky DJ, Connolly ES., Jr Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circ Res. 2006;99:209–217. doi: 10.1161/01.RES.0000232544.90675.42. [DOI] [PubMed] [Google Scholar]
- 18.Mocco J, Wilson DA, Komotar RJ, Sughrue ME, Coates K, Sacco RL, Elkind MS, Connolly ES., Jr Alterations in plasma complement levels after human ischemic stroke. Neurosurgery. 2006;59:28–33. doi: 10.1227/01.NEU.0000219221.14280.65. [DOI] [PubMed] [Google Scholar]
- 19.Morikis D, Roy M, Sahu A, Troganis A, Jennings PA, Tsokos GC, Lambris JD. The structural basis of compstatin activity examined by structure-function-based design of peptide analogs and NMR. J Biol Chem. 2002;277:14942–14953. doi: 10.1074/jbc.M200021200. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Xi G, Hua Y, Schallert T, Hoff JT, Keep RF. Intracerebral hemorrhage in mice: Model characterization and application for genetically modified mice. J Cereb Blood Flow Metab. 2004;24:487–494. doi: 10.1097/00004647-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Pasinetti GM, Tocco G, Sakhi S, Musleh WD, DeSimoni MG, Mascarucci P, Schreiber S, Baudry M, Finch CE. Hereditary deficiencies in complement C5 are associated with intensified neurodegenerative responses that implicate new roles for the C-system in neuronal and astrocytic functions. Neurobiol Dis. 1996;3:197–204. doi: 10.1006/nbdi.1996.0020. [DOI] [PubMed] [Google Scholar]
- 22.Wada K, Montalto MC, Stahl GL. Inhibition of complement C5 reduces local and remote organ injury after intestinal ischemia/reperfusion in the rat. Gastroenterology. 2001;120:126–133. doi: 10.1053/gast.2001.20873. [DOI] [PubMed] [Google Scholar]