Abstract
Abnormalities in renal angiotensin type 1 receptor (AT1R), D1 dopamine receptor (D1R) and G protein–coupled receptor kinase 4 (GRK4) are present in polygenic hypertension. Selective renal reduction of AT1R expression by intrarenal cortical infusion of antisense oligodeoxynucleotides (As-Odns) in conscious, uni-nephrectomized, sodium-loaded rats decreases proteinuria, normalizes the glomerular sclerosis index (GSI), increases the sodium excretion (UNaV), and modestly increases blood pressure (BP) in spontaneously hypertensive rats (SHR) but not in normotensive Wistar-Kyoto rats (WKY). In contrast, selective renal reduction of GRK4 expression by infusion of GRK4 As-Odns increases UnaV, attenuates the increase in arterial BP with age, and modestly decreases protein excretion in SHR, but not in WKY. In this study, we report that intrarenal cortical infusion of both GRK4 and AT1R As-Odns decreased BP and increased UNaV in SHR; these effects were also noted in WKY to a lesser extent. Infusion of SHR with this combination of As-Odns resulted in a decrease in proteinuria and improvement of GSI similar to those by AT1R As-Odn only. In contrast to the increased circulating angiotensin II and aldosterone levels induced by AT1R As-Odn alone, the combination of As-Odns decreased both, contributing to greater natriuresis and amelioration of hypertension than by GRK4 or AT1R As-Odn only. Our results indicate an interaction between GRK4-regulated receptors and the renin-angiotensin system in the regulation of renal function and BP.
Keywords: hypertension, kidney, receptors, blood pressure, G protein–coupled receptor kinase 4
Introduction
The renal dopaminergic and renin-angiotensin systems (RAS) have counter regulatory effects on cellular signal transduction, production of reactive oxygen species, renal sodium excretion, and blood pressure (1). The renal dopaminergic system is important in promoting sodium excretion during conditions of sodium excess (2, 3) while the RAS is preeminent in sodium conservation (4–6). Indeed, under basal conditions, angiotensin II is responsible for more than 50% of renal sodium reabsorption (5).
Disruption of genes regulating the RAS, including the angiotensin type 1 receptor (AT1R), in mice decreases blood pressure (7). We have reported, however, that in salt-loaded, uninephrectomized spontaneously hypertensive rats (SHR) the renal cortical inhibition of AT1R expression by intrarenal cortical infusion of AT1R-specific antisense oligodeoxynu-cleotides (As-Odns) increases blood pressure because the treatment increases circulating angiotensin II (Ang II), renin and aldosterone levels by interruption of the renin and Ang II negative feedback loop (8). The treatment also enhances sodium excretion in spontaneously hypertensive rats (SHR), but not in normotensive Wistar-Kyoto (WKY) rats. The major consequence of decreasing renal AT1R expression in SHR is a decrease in proteinuria, probably as a result of amelioration in glomerular histopathology that is independent of blood pressure and circulating renin, Ang II, and aldosterone levels (8).
The dopamine produced by the kidney is natriuretic and antihypertensive (1–3). The D1- (D1R and D5R) and D2-like dopamine receptors (D2R, D3R, and D4R) are responsible for more than 50% of sodium excreted under conditions of moderate sodium excess (1–3). Blockade of D1- and/or D2-like receptors in rodents (1–3, 9, 10) and humans (11) and disruption of any of the dopamine receptor genes in mice increases blood pressure (1–3, 12).
In humans with essential hypertension and rodents with genetic hypertension (SHR and Dahl salt-sensitive rats), the diuretic and natriuretic responses mediated by D1-like receptor agonists are impaired (1–3). The ability of the D1-like receptors to inhibit renal proximal sodium reabsorption is also impaired in humans with essential hypertension (1, 2). Further studies have shown that the impaired inhibitory effect of D1-like receptors on epithelial sodium transport in genetic hypertension is due to an uncoupling of the D1-like receptor from its G-protein/effector complex (1–3, 13, 14). The uncoupling is receptor-specific, organ-selective and nephron-segment-specific, precedes the onset of hypertension and co-segregates with the hypertensive phenotype (1–3). The defective transduction of the renal dopaminergic signal is caused by increased activity of G-protein–coupled receptor kinase type 4 (GRK4), which constitutively desensitizes D1Rs, in part by increasing receptor phosphorylation. In genetically hypertensive rats, selective renal inhibition of the increased GRK4 expression ameliorates the hypertension (13). In humans, the presence of activating variants of the GRK4 gene, by themselves or in conjunction with variants of genes of the RAS, is associated with an increase in blood pressure with age (15) or mental stress (16), and with hypertension in several ethnic groups (Caucasians, Chinese, Africans, and Japanese) (1, 17–20). In mice, overexpression of human GRK4 gene variants causes hypertension either with or without salt sensitivity, depending upon the variant. GRK4 gene variants, by preventing the natriuretic function of the dopaminergic system, may be a cause of salt sensitivity (1, 14, 16, 20).
We have reported that selective inhibition of renal cortical GRK4 expression by the intrarenal cortical infusion of GRK4-specific As-Odns increases sodium excretion, decreases arterial blood pressure, and minimally decreases protein excretion in SHR, and that these effects are not observed in WKY rats (13). The current studies were conducted to determine the renal functional and blood pressure consequences of selective renal cortical inhibition of both GRK4 and AT1R protein expression using specific As-Odns. We hypothesized that the combined selective renal cortical inhibition of GRK4 and AT1R protein expression would normalize blood pressure by ameliorating the blood pressure increase associated with AT1R As-Odn alone. We also predicted that the combined inhibition would decrease blood pressure and increase sodium excretion to a greater extent than by either GRK4 or AT1R As-Odn alone.
Methods
Animals
Four-week-old male WKY and SHR (Japan SLC Inc., Sendai, Japan) were fed 0.26% NaCl chow (Japan CLEA, Tokyo, Japan) and tap water. At 5 weeks of age, under intraperitoneal pentobarbital anesthesia (50 mg/kg), the right kidney was removed, and a catheter (an 8-mm PE 10 tube connected to 30 gauge, BB311-30 medical Teflon microtubing; Scientific Commodities, Inc., Lake Havasu City, USA) was implanted with Bipax epoxy resin glue 3- to 4-mm deep into the lower pole of the remaining left kidney (8, 13). Uninephrectomy was performed to obviate the interstitial infusion of the vehicle and oligodeoxynucleotides (Odns) to both kidneys, which would necessitate extra surgery. An osmotic mini-pump (1 μL/h; Alzet Corporation, Palo Alto, USA) was positioned in the space previously occupied by the right kidney for the continuous interstitial infusion of lactated Ringer’s solution. After the surgery the diet was increased to 4% NaCl in order to suppress the RAS, maintain a high level of AT1AR expression in the kidney (5), increase renal dopamine production, and enhance the natriuretic effect of the D1R (1–3).
One week later the rats were re-anesthetized and the implanted osmotic mini-pump was replaced with another that infused GRK4 and AT1R As-Odns and scrambled Odns (Scr-Odns) (50 nmol/d) or vehicle (lactated Ringer’s solution) at 0.2 μL/h. The infusion was continued for 4 weeks.
Unanesthetized systolic blood pressures were measured twice a week by the tail-cuff method (Blood Pressure Analyzer model BP-8A; Softron, Tokyo, Japan) (8, 13). Urine was collected for 24 h, twice a week. Sodium, potassium and urinary protein concentrations were determined as described previously (8, 13). At the end of 4 weeks the rats were anesthetized, blood was obtained and the heart and remaining kidney were removed. All experimental procedures were approved by the Fukushima Medical University School of Medicine Animal Committee.
Design and Synthesis of Odns
The nucleotide sequences of purified rhodamine-conjugated propyne/phosphorothioate-modified rat GRK4 Odns (Greiner, Tokyo, Japan) were as follows: GRK4 As-Odn, 209-5′ CATGAAGTTCTCCAGTTCCAT-3′-89; GRK4 Scr-Odn, 5′-ATTTTCCATACGCCGCATTAG-3′. GRK4 As-Odns but not GRK4 Scr-Odns have been shown to cause a 50% decrease in GRK4 protein in the kidney, as measured by immunoblotting (13). The phosphorothioate-modified AT1R Odns (Bowman Gray School of Medicine of Wake Forest University, Winston-Salem, USA) were as follows: AT1R As-Odn, 286-5′-AGAGTTAAGGGCCAT-3′-272; AT1R Scr-Odn, 5′-CCCTTTGAAGGTTCC-3′. AT1R As-Odns but not AT1R Scr-Odns cause a 50% decrease in AT1R protein, as measured by immunoblotting (8).
Quantitative Real-Time RT-PCR
Total RNA was prepared from renal cortex tissue using TRI-zol (Invitrogen, Carlsbad, USA) and column-purified after DNase treatment with an RNase-free DNase set (Qiagen, Valencia, USA). Subsequently, one microgram of total RNA was reverse transcribed into cDNA using the oligo(dT) method (iScript cDNA synthesis kit; Bio-Rad, Hercules, USA) in a 20 μL reaction volume. cDNA in 1 μL of reaction mix was used for real-time quantitative PCR using an iQ5 Real-Time PCR Detection System and iQ SYBR Green Supermix (Bio-Rad). Quantification of mRNA was based on the Ct value, normalized to β-actin, and expressed as relative mRNA. Primers for rat AT1R, GRK4 and β-actin were used to replicate cDNAs reverse-transcribed from the experimental, and positive and negative control RNA samples. The primers used were as follows: for AT1R, TGCCTGAATCCTCT-GTTTTATGG (forward) and AGCCTGCGTGTGACTTGG (reverse); for GRK4, ACTTCAGCAGACTGGAAGCA (forward) and GGTGTCCAGGTTGACTCCTT (reverse); and for β-actin, GAGGGAAATCGTGCGTGAC (forward) and CCAGGGAGGAAGAGGATGC (reverse).
Immunoprecipitation and Immunoblotting
Renal cortical proteins were immunoblotted or immunoprecipitated as previously reported (8, 12–14, 21–23). Polyclonal rabbit anti-human AT1R (sc173; Santa Cruz Biotechnology Inc., Santa Cruz, USA), polyclonal anti-rat GRK4, anti-human GRK4 γ/δ (Santa Cruz), anti-rat D1R, anti-phospho-serine (Zymed Laboratories Inc., San Francisco, USA), and monoclonal β-actin antibodies (Santa Cruz) were used. The specificities of the anti-human AT1R (8, 21, 22), anti-rat D1R (13, 14, 22, 23), polyclonal anti-rat GRK4 (13, 14), anti-human GRK4 γ/δ (13, 14) and anti-phosphoserine antibodies (14, 23) have been established. The bands were visualized by ECL reagents (Amersham Biosciences, Piscataway, USA) and quantified by densitometry (Quantiscan, Ferguson, USA).
Histochemistry
Three μm kidney sections were stained with periodic-acid-Schiff reagent and coded to remove evaluator bias (8). The degree of glomerulosclerosis was semi-quantified using a glomerular sclerosis index (GSI) from 0 to 4+. Sclerosis was defined as collapse and/or obliteration of the glomerular capillary tuft in association with increased hyaline matrix. In each single section of the kidney, all glomeruli (i.e., superficial and juxtaglomerular) were assessed for glomerulosclerosis. The severity of sclerosis for each glomerulus was graded from 0 to 4+. No lesion was graded as 0, lesions constituting 10% or less of the glomerulus were graded as 1, lesions of up to 25% of the glomerulus were graded as 2, lesions of up to 50% of the glomerulus were graded as 3, and lesions of up to 100% of the glomerulus were graded as 4.
Hormone Determinations
EDTA-treated blood was centrifuged at 3,000 × g for 15 min at 4°C and the plasma was stored at − 80° C until analyzed. Samples for measurement of Ang II were flash frozen immediately after collection and stored at − 80 °C until analyzed. Plasma renin activity (PRA), Ang II, and aldosterone concentrations were measured by radioimmunoassay.
Statistical Analysis
The data are expressed as the mean±SEM. Comparisons within and among groups were made by repeated measures or factorial ANOVA, respectively, followed by Duncan’s test. Two-group comparisons were performed using the Student’s t-test. A value p < 0.05 was considered statistically significant.
Results
Baseline Data
At the beginning of the study, body weight, water and food intake, urine output, and sodium, potassium, and protein excretions, as well as the weights of the removed kidneys expressed as a percentage of body weight, were similar among the groups, but systolic blood pressures were higher in SHR than in WKY (Table 1).
Table 1.
Characteristics of 4.5 Week Old WKY and SHRs Prior to Unilateral Nephrectomy and Insertion of an Intracortical Catheter into the Remaining Kidney
| Variables | WKY rats
|
SHRs
|
||||
|---|---|---|---|---|---|---|
| Vehicle (n=6) | Scrambled- Odn (n=6) | Antisense-Odn (n=6) | Vehicle (n=5) | Scrambled-Odn (n=6) | Antisense-Odn (n=5) | |
| BW(g) | 75±3 | 78±4 | 73±1 | 77±3 | 77±4 | 75±5 |
| Kidney weight (% BW) | 0.83±0.06 | 0.83±0.08 | 0.88±0.05 | 0.72±0.04 | 0.70 ±0.04 | 0.72±0.06 |
| Systolic BP | 92±2 | 96±1 | 95±1 | 115±2* | 111±2* | 112±2* |
| Water intake (mL/d) | 13.5±0.7 | 13.8±10.6 | 12.5±1.0 | 11.6±0.9 | 12.2±0.8 | 11.8±1.2 |
| Food intake (g/d) | 8.8±1.2 | 10.0±0.9 | 9.7±0.4 | 9.4±1.3 | 9.2±0.8 | 9.4±1.4 |
| Urine output (mL/d) | 2.36±0.43 | 1.77±0.43 | 1.63±0.46 | 3.05±0.76 | 2.94±0.51 | 2.44±0.38 |
| Na+ excretion (mmol/d) | 0.35±0.05 | 0.37±0.08 | 0.35±0.04 | 0.48±0.04 | 0.47±0.06 | 0.45±0.04 |
| K+ excretion (mmol/d) | 0.95±0.17 | 0.76±0.15 | 0.67±0.19 | 0.53±0.04 | 0.58±0.09 | 0.72±0.17 |
| Proteinuria (mg/d) | 0.52±0.10 | 0.46±0.06 | 0.41±0.12 | 0.46±0.10 | 0.40±0.08 | 0.34±0.04 |
p < 0.05 vs. WKY.
BW, body weight; BP, blood pressure; WKY, Wistar Kyoto; SHRs, spontaneously hypertensive rats; Odn, oligo-deoxynucleotide.
Effect of Treatment with GRK4 and AT1R Odns
Infusion of vehicle, combined GRK4 and AT1R Scr-Odns (GRK4-AT1R Scr-Odns), or combined GRK4 and AT1R As-Odns (GRK4-AT1R As-Odns) had no effect on body or heart weight, weight of the remaining kidney, or food and water intake (Table 2). Body weight and heart weight (as a percentage of body weight) were lower in WKY than in SHR. Serum creatinine was normal in all groups, although the values were lower in SHR than in WKY (Table 2).
Table 2.
Characteristics of WKY and SHRs at the End of the Study
| Variables | WKY rats
|
SHRs
|
||||
|---|---|---|---|---|---|---|
| Vehicle (n=6) | Scrambled-Odn (n=6) | Antisense-Odn (n=6) | Vehicle (n=5) | Scrambled-Odn (n=6) | Antisense-Odn (n=5) | |
| BW(g) | 250±6 | 255±6 | 247±4 | 229±3* | 225±3* | 230±1* |
| Heart weight (%BW) | 0.41±0.01 | 0.41±0.01 | 0.43±0.01 | 0.55±0.01* | 0.55±0.02* | 0.55±0.02* |
| Remaining kidney weight (%BW) | 0.71±0.01 | 0.70±0.01 | 0.69±0.02 | 0.68±0.02 | 0.68±0.02 | 0.65±0.01 |
| Water intake (mL/d) | 48.8±2.5 | 52.2±4.1 | 49.5±4.4 | 54.8±0.9 | 50.5±1.2 | 51.4±2.6 |
| Food intake (g/d) | 17.8±0.9 | 18.7±1.4 | 19.2±0.6 | 18.2±1.0 | 17.2±0.6 | 17.7±1.0 |
| Serum creatinine (mg/dL) | 0.27±0.01 | 0.27±0.01 | 0.26±0.005 | 0.23±0.01* | 0.23±0.01* | 0.22±0.01* |
| Urine output (mL/d) | 31.5±2.5 | 31.0±2.8 | 33.5±1.3 | 23.5±1.3* | 24.2±1.9* | 29.8±1.8† |
| K+ excretion (mmol/d) | 3.38±0.31 | 3.45±0.30 | 3.86±0.55 | 2.77±0.25 | 2.92±0.15 | 3.08±0.14 |
| PRA (ng/mL/h) | 2.68 ±0.43 | 3.13±0.48 | 0.95±0.28† | 4.46±0.48* | 5.02±0.68* | 1.60±0.37† |
| PAC (mg/dL) | 50.7±2.8 | 60.4±4.5 | 49.5±3.6 | 74.1±6.5* | 74.9±5.7* | 47.5±3.1† |
| Ang II (pg/mL) | 9.0±1.3 | 9.3±1.7 | 3.0±0.07† | 6.2±0.7 | 6.8±0.8 | 4.20±.6† |
p < 0.05 vs. WKY,
p < 0.05 vs. others within their respective groups, ANOVA, Duncan’s test.
BW, body weight; PRA, plasma renin activity; PAC, plasma aldosterone concentration; Ang II, angiotensin II; WKY, Wistar Kyoto; SHRs, spontaneously hypertensive rats; Odn, oligodeoxynucleotide.
GRK4 Expression
GRK4 mRNA was similar in vehicle- and GRK4-AT1R Scr-Odn–treated WKY and SHR but was significantly decreased by GRK4-AT1R As-Odn treatment (Fig. 1). GRK4 protein was greater in SHR than in WKY. GRK4 mRNA was not increased in these rats, suggesting that the increase in GRK4 protein was a consequence of post-transcriptional modification. Vehicle treatment or GRK4-AT1R Scr-Odns had no effect on GRK4 protein in either WKY or SHR (Fig. 2A). GRK4-AT1R As-Odns decreased renal GRK4 protein levels in WKY and SHR, but the absolute values remained higher in SHR than in WKY.
Fig. 1.
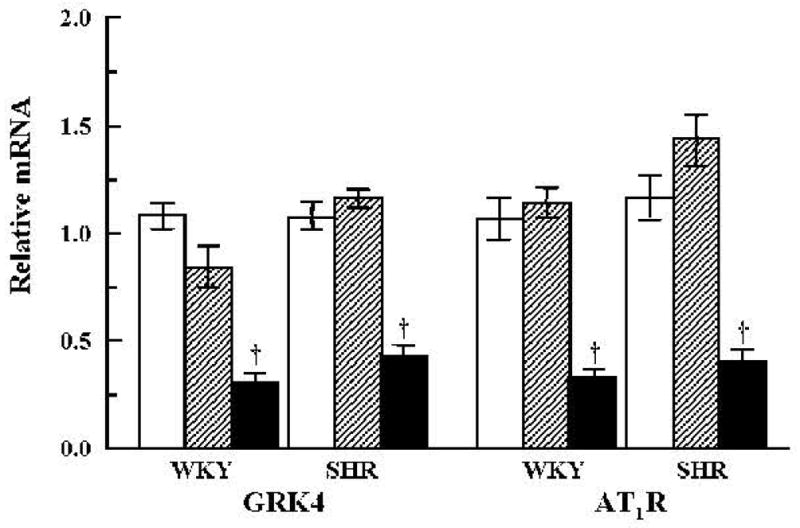
Effect of vehicle or combined GRK4 and AT1R oligodeoxynucleotides on the expression of GRK4 mRNA and AT1R mRNA, as determined by quantitative RT-PCR in the renal cortex. Open bars: vehicle; hatched bars: Scr-Odns; closed bars: As-Odns. †p < 0.05 vs. all others in the same group by factorial ANOVA and Duncan’s test; n = 5–6 per group.
Fig. 2.
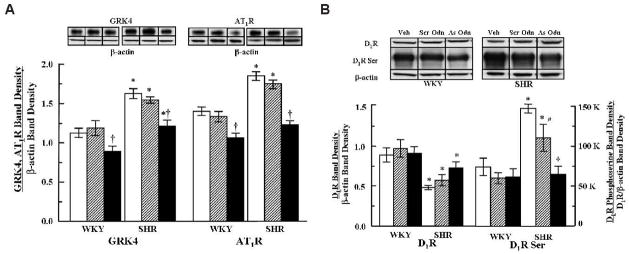
Effect of vehicle or combined GRK4 andAT1R oligodeoxynucleotides on the protein expression of GRK4, AT1R, D1R, and serine-phosphorylated D1R expression in renal cortical membranes. A: Top: immunoblots of GRK4 (54 kDa), AT1R, and β-actin are shown. Bottom: quantification of GRK4 and AT1R protein expression in cortical membranes. GRK4 and AT1R band densities were corrected by the respective band densities of β-actin. B: Top: immunoblots of D1R protein and serine phosphorylated D1R expression are shown. Bottom: quantification of D1R protein and serine phosphorylated D1R expression in cortical membranes. D1R band densities were corrected by the respective band densities of β-actin. Serine-phosphorylated D1R is expressed as a ratio of the respective D1R band density corrected by actin. Open bars: vehicle; hatched bars: Scr-Odns; closed bars: As-Odns. *p < 0.05 vs. WKY, †p < 0.05 vs. all others in the same group, #p < 0.05 vs. SHR Veh (vehicle) by factorial ANOVA and Duncan’s test; n = 5–6 per group.
AT1R Expression
The expression of AT1R mRNA was not affected by either vehicle or GRK4-AT1R Scr-Odn treatments but decreased in both groups of rats after treatment with GRK4-AT1R As-Odns (Fig. 1). The level of AT1R protein was greater in SHR than in WKY (Fig. 2A). Vehicle or GRK4-AT1R Scr-Odns had no effect on AT1R protein in either WKY or SHR. GRK4-AT1R As-Odns decreased AT1R in both groups; AT1R protein in SHR decreased to the levels seen in vehicle- and Scr-Odn–treated WKY and became approximately the same as that observed in As-Odn–treated WKY.
D1R Expression and Serine-Phosphorylation
D1R protein was lower in SHR than in WKY (Fig. 2B). In a previous study, we did not find decreased D1R protein in renal cortical homogenates in SHR relative to WKY (13). Probably depending upon membrane preparation, renal cortical or proximal tubule D1R expression may (Hussain and Lokhand-wala (3) and the current study) or may not be (13) decreased in SHR relative to WKY. GRK4-AT1R Scr-Odns or GRK4-AT1R As-Odns had no effect on renal D1R protein in either group, although there was a slight but nonsignificant tendency for D1R protein to increase in SHR treated with GRK4-AT1R As-Odns (Fig. 2B).
Serine-phosphorylated D1R was greater in vehicle- and GRK4-AT1R Scr-Odn–treated SHR than in WKY, as in a previous report (23). GRK4-AT1R Scr-Odns had no effect on serine-phosphorylated D1R in WKY but modestly decreased it in SHR, although the levels remained higher than those observed in WKY. GRK4-AT1R As-Odns also had no effect on the level of serine-phosphorylated D1R in WKY but decreased it in SHR so that the levels were no longer different between the two rat strains (Fig. 2B).
PRA, Ang II, and Aldosterone Levels
PRA and plasma aldosterone were higher in vehicle-treated SHR than in vehicle-treated WKY and their levels were not modified by treatment with GRK4-AT1R Scr-Odns. Although samples for plasma Ang II were obtained in the absence of specific protease inhibitors and results should be interpreted with caution, plasma Ang II levels were similar in both rat strains treated with vehicle or with GRK4-AT1R Scr-Odns and were also decreased in both strains by GRK4-AT1R As-Odns treatment. Plasma aldosterone concentrations were also decreased in SHR but not in WKY (Table 2).
Blood Pressure
Blood pressure increased with age to a greater extent in SHR than in WKY (Table 1 and Fig. 3). Infusion of GRK4-AT1R Scr-Odns had no effect on blood pressure in either group. In SHR, GRK4-AT1R As-Odns treatment decreased blood pressure from 1.5 weeks of treatment (data not shown) until the end of the study (Fig. 3). In WKY the blood pressure–lowering effect of GRK4-AT1R As-Odns was noted at 2.5 weeks (data not shown) after starting the treatment and was evident until the end of the study (Fig. 3).
Fig. 3.
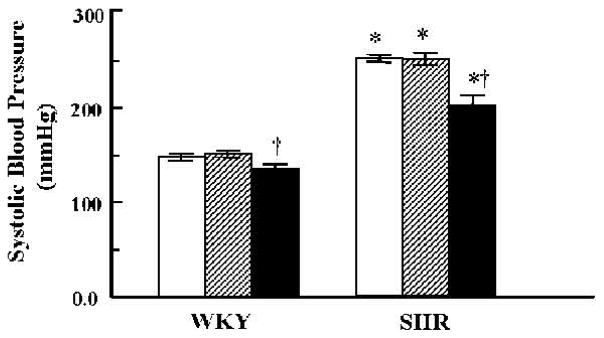
Effect of vehicle or combined GRK4 and AT1R oligodeoxynucleotides on systolic blood pressure. Blood pressures were measured by the tail-cuff method. *p < 0.05 vs. WKY, †p < 0.05 vs. all others in the same group by factorial ANOVA and Duncan’s test; n = 5–6 per group. Open bars: vehicle; hatched bars: Scr-Odns; closed bars: As-Odns.
Urine Flow, Sodium and Potassium Excretion, and Sodium Balance
Urine output was lower in vehicle- or GRK4-AT1R Scr-Odn–treated SHR than in WKY (Table 2). In SHR, GRK4-AT1R As-Odns increased urine output relative to vehicle and GRK4-AT1R Scr-Odn treatment and approached the values observed in SHR. Urine volume was not affected by the treatments in WKY (Table 2).
Sodium (Table 1 and Fig. 4A) and potassium excretions increased with age in all groups (Tables 1 and 2). Potassium excretion was similar in WKY and SHR and unaffected by Odn treatment (Table 2).
Fig. 4.
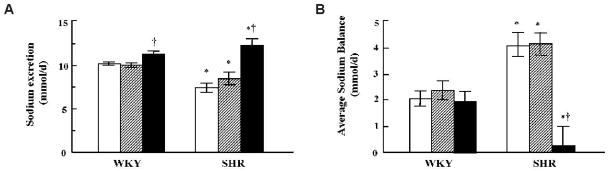
Effect of vehicle or combined GRK4 and AT1R oligodeoxynucleotides on urinary sodium excretion and sodium balance. A: Sodium concentrations were measured in urine collected over 24 h and expressed as mmol/d. B: Average daily sodium balance during the last 2 weeks of the study. Values are expressed in mmol/d. Open bars: vehicle; hatched bars: Scr-Odns; closed bars: As-Odns. *p < 0.05 vs. WKY, †p < 0.05 vs. all others in the same group by Factorial ANOVA and Duncan’s test; n = 5–6 per group.
At the end of the study sodium excretion was lower in vehicle- and GRK4-AT1R Scr-Odn–treated SHR than in WKY (Fig. 4A). Sodium excretion increased in GRK4-AT1R As-Odn–treated SHR after 1.5 weeks of treatment (data not shown) and remained increased until the end of the study; sodium excretion was also increased by GRK4-AT1R As-Odn treatment in WKY, but to a lesser degree (Fig. 4A).
Average daily sodium balance during the last 2 weeks of the study was greater in vehicle- and GRK4-AT1R Scr-Odn–treated SHR than in vehicle or Odn-treated WKY. In contrast, GRK4-AT1R As-Odns decreased sodium balance in SHR to values lower than those observed in WKY (Fig. 4B).
Urine Protein Excretion
Protein excretion became greater in SHR than in WKY at 5.5 weeks of age (data not shown). GRK4-AT1R As-Odn treatment substantially decreased protein excretion in SHR. The treatment also modestly decreased urinary protein excretion in WKY to levels that were slightly but not significantly lower than those observed in SHR (Fig. 5).
Fig. 5.
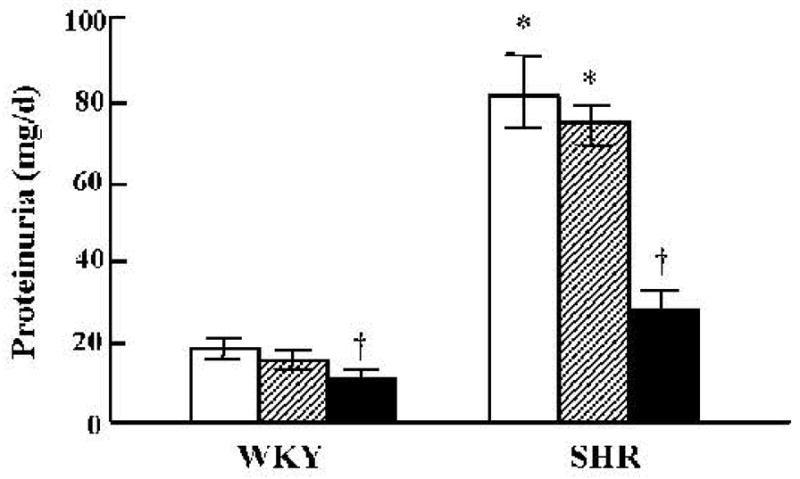
Effect of vehicle or combined GRK4 and AT1R oligodeoxynucleotides on urinary protein. Protein concentrations were measured in urine collected over 24 h and expressed as mg/d. Open bars: vehicle; hatched bars: Scr-Odns; closed bars: As-Odns. *p < 0.05 vs. WKY, †p < 0.05 vs. all others in the same group by factorial ANOVA and Duncan’s test; n = 5–6 per group.
Histochemistry
WKY glomeruli appeared normal and were not affected by Odns (Fig. 6A–C). In contrast, glomerulosclerosis was evident in vehicle (Fig. 6D)- and GRK4-AT1R Scr-Odn–treated SHR (Fig. 6E). However, in GRK4-AT1R As-Odn–treated SHR, glomeruli appeared almost normal (Fig. 6F) (8, 13) and the glomerular sclerosis index was similar to that in WKY, with or without Odn treatment (Fig. 7); As-Odns minimally increased the glomerular sclerosis index in WKY.
Fig. 6.
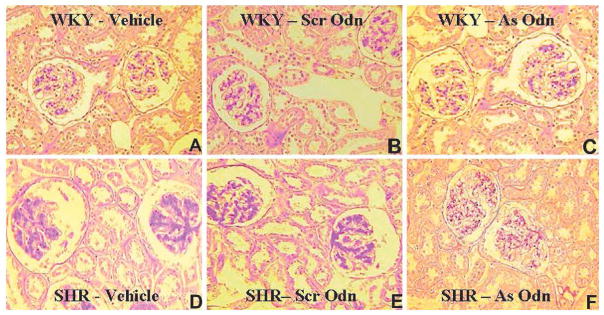
Effect of vehicle or combined GRK4 and AT1R oligonucleotides on renal glomerular morphology. Three μm sections were stained with periodic-acid-Schiff (PAS) reagent. At 10 weeks of age, the glomeruli in WKY were normal in appearance and not affected by Odn treatment (A–C). In contrast, in SHR at 10 weeks of age, glomerulosclerosis was evident in vehicle (D)- and combined GRK4 and AT1R Scr-Odn–treated SHR (E). However, GRK4 and AT1R As-Odn–treated SHR had almost normal-appearing glomeruli (F).
Fig. 7.
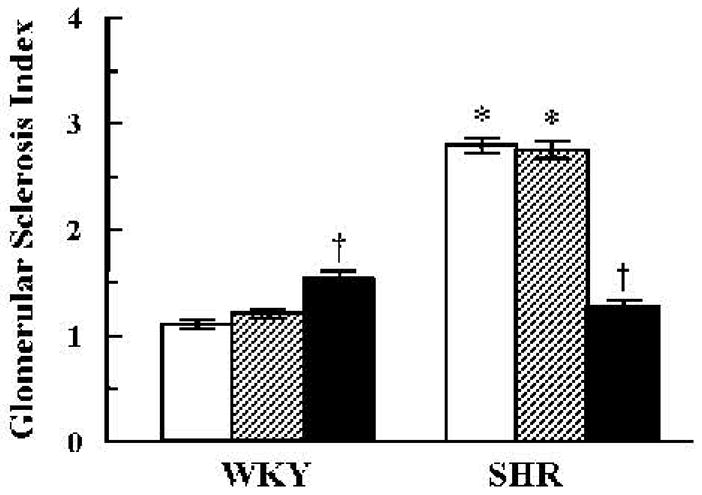
Effect of vehicle or combined GRK4 and AT1R oligodeoxynucleotides on the glomerular sclerosis index. The severity of sclerosis for each glomerulus was graded from 0 to 4+ as described in the Methods. Open bars: vehicle; hatched bars: Scr-Odns; closed bars: As-Odns. *p < 0.05 vs. WKY, †p < 0.05 vs. all others in the same group by factorial ANOVA and Duncan’s test; n = 5–6 per group.
Discussion
This study evaluated the contributions of the renal dopaminergic and angiotensin systems in the regulation of blood pressure and sodium excretion by the selective intrarenal cortical infusion of combined GRK4 and AT1R As-Odns (8, 13). The D1R is important in the regulation of blood pressure, because disruption of the D1R gene in mice increases blood pressure (1, 2). However, the intrarenal cortical infusion of D1R As-Odn only transiently increases sodium excretion and does not affect blood pressure in WKY (24). GRK4 desensitizes a few G protein-coupled receptors, including D1R (25–31). Heterologous expression of GRK4 gene variants in Chinese hamster ovary cells impairs D1R function (14). Overexpression of the human GRK4γ gene variant, GRK4γ A142V, but not the human GRK4γ wild-type gene in mice impairs the natriuretic effect of D1R and produces hypertension in human GRK4γ A142V transgenic mice fed a normal NaCl diet (14). Moreover, overexpression of human GRK4γ A486V, but not the human GRK4γ wild-type gene, in mice causes hypertension in human GRK4γ A486V transgenic mice fed a high NaCl diet (32).
Although the infusion of GRK4 As-Odns increases sodium excretion, and decreases sodium balance and blood pressure in SHR (13), blood pressure is not normalized, indicating the importance of renal factors (e.g., AT1R) not alleviated by inhibiting renal GRK4 function or non-renal mechanisms that participate in hypertension (7, 33–35). The intrarenal cortical infusion of GRK4 As-Odns also does not alter sodium excretion or blood pressure in salt-loaded WKY (13). Renal GRK4 expression is not increased in WKY and therefore, inhibition of renal GRK4 expression would not be expected to increase sodium excretion in this rat strain.
In this study, when the expressions of both GRK4 and AT1R were inhibited by combined GRK4-AT1R As-Odn treatment in SHR, sodium excretion was increased and sodium balance was decreased to a greater extent in SHR than when rats were treated with either As-Odn alone (8, 13). Combined GRK4-AT1R As-Odn treatment increased sodium excretion not only in SHR but also in WKY. GRK4-AT1R As-Odn treatment also decreased blood pressure in WKY, an effect not observed with either GRK4 As-Odn or AT1R As-Odn treatments (8, 13). At first glance, one may conclude that in SHR, blood pressure decreases to a similar degree with GRK4-AT1R As-Odns and with GRK4 As-Odn treatment alone (13). However, because AT1R As-Odn treatment, alone, increases blood pressure in SHR (8) one can conclude that combined GRK4-AT1R As-Odn treatment does decrease blood pressure in SHR to a greater extent than GRK4 As-Odn treatment alone. Therefore, these studies suggest that the interaction between GRK4 and AT1R may be important in the overall regulation of sodium balance and blood pressure.
Selective renal inhibition of AT1R expression in SHR does not decrease blood pressure because the interruption of the renin-angiotensin negative feedback loop results in increased circulating renin and Ang II (8). We did not study the effect of selective renal inhibition of GRK4 alone on the RAS. However, the combined inhibition of GRK4 and AT1R expression unexpectedly decreased circulating PRA and Ang II in both WKY and SHR (and plasma aldosterone concentration in SHR but not in WKY). Renin activity and Ang II levels should have increased in both strains with AT1R As-Odn treatment only (8). The inhibition of GRK4 expression should have allowed D1R activity to become manifest, resulting in an increase in renin secretion; D1R stimulates renin secretion under certain circumstances (33, 36). A greater effect should have occurred in the SHR whose D1R function was impaired, because of the constitutively increased GRK4 activity in these animals (13). One possible explanation for these results is that they occur through an effect of the D3R that inhibits renin secretion (37). We have preliminary data indicating that GRK4 also regulates the D3R (unpublished results). D1R and D3R co-localize in mouse juxtaglomerular cells (33, 37). The dopaminergic stimulation of renin secretion (via the D1R) is more apparent under sodium deficit and the stimulatory effect is decreased during sodium excess (38, 39). If the inhibitory effect of D3R on renin secretion becomes manifest during sodium excess, then the lack of desensitization of D1R and D3R that results from the decrease in the expression of GRK4 should decrease renin secretion and circulating Ang II. The decrease in Ang II levels could explain the decrease in plasma aldosterone concentrations with GRK4-AT1R As-Odn treatment. An alternative explanation for our findings may be related to tubuloglomerular feedback (38); the decrease in sodium transport caused by enhanced D1R and D3R functions and decreased AT1R function increases the amount of sodium presented to the macula densa, resulting in the inhibition of renin release. This assumes, however, that the GRK4 and AT1R As-Odn treatment does not alter distal nephron transport.
The intrarenal cortical administration of GRK4 As-Odn in SHR prevents by more than 50% the increase in blood pressure with age but only modestly reduces the age-related increase in urinary protein excretion (13). In contrast, the intrarenal cortical administration of AT1R As-Odns that results in an increase rather than in a decrease in blood pressure in SHR causes a dramatic reduction in protein excretion (8). These data suggest that the antiproteinuric effect of AT1R As-Odn treatment may be a result of a reduction of AT1R action in the glomerulus, as AT1R As-Odns do reach the glomeruli (data not shown), while the modest decrease in proteinuria afforded by GRK4 As-Odns may be the result of the reduction in blood pressure. In the current studies, GRK4-AT1R As-Odns decreased proteinuria to an extent similar to that obtained by AT1R As-Odn only. Angiotensin-converting enzyme inhibitors, alone or in combination with AT1R blockers, decrease urine protein excretion, even in normotensive rats and human subjects, independent of blood pressure (40, 41). In diabetic subjects whose aldosterone levels are increased during angiotensin-converting enzyme inhibition, the addition of an aldosterone blocker decreases urinary protein even further, without changes in blood pressure (42). Our earlier studies also indicate that the antiproteinuric effect of AT1R suppression is independent of aldosterone levels and predominates over any proteinuria-promoting effect of aldosterone (8). The absence of an added antiproteinuric effect of GRK4 As-Odns with concomitant AT1R As-Odn treatment is not surprising, because GRK4 is not expressed in glomeruli (13). Similarly, in the current studies, GRK4-AT1R As-Odns improved the GSI to the same extent as observed by AT1R As-Odn-treatment alone (8). Because GRK4 As-Odns do not affect GSI in SHR (13), it is likely that the AT1R As-Odns are mainly responsible for the decrease in the GSI resulting from combined GRK4-AT1R As-Odn treatment.
In conclusion, combined treatment with both GRK4 and AT1 As-Odns decreased blood pressure and increased sodium excretion in both normotensive and hypertensive rats, and the effects on hypertensive rats appeared to be greater than those observed with either of the As-Odns alone. These effects may have been at least partly mediated by a decrease in circulating renin, Ang II and aldosterone, which would indicate an interaction among GRK4-regulated receptors and the RAS. These results may have important therapeutic implications in salt sensitivity and/or hypertension.
Acknowledgments
These studies were supported in part by grants from the National Institutes of Health (Nos. HL074940, HL23081, DK39308, and HL068686).
References
- 1.Felder RA, Jose PA. The role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol. 2006;2:637–650. doi: 10.1038/ncpneph0301. [DOI] [PubMed] [Google Scholar]
- 2.Zeng C, Sanada H, Watanabe H, Eisner GM, Felder RA, Jose PA. Functional genomics of the dopaminergic system in hypertension. Physiol Genomics. 2004;19:233–246. doi: 10.1152/physiolgenomics.00127.2004. [DOI] [PubMed] [Google Scholar]
- 3.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med. 2003;228:134–142. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- 4.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system–an endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 5.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1–7) Hypertension. 1997;30:535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- 7.Crowley SD, Gurley SB, Oliverio MI, et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoneda M, Sanada H, Yatabe J, et al. Differential effects of angiotensin II type-1 receptor antisense oligonucleotides on renal function in spontaneously hypertensive rats. Hypertension. 2005;46:58–65. doi: 10.1161/01.HYP.0000171587.44736.ba. [DOI] [PubMed] [Google Scholar]
- 9.Shigetomi S, Ueno S, Kohno H, et al. Role of renal dopamine receptor in the pathogenesis of hypertension after sodium loading. Nippon Naibunpi Gakkai Zasshi. 1986;62:26–33. doi: 10.1507/endocrine1927.62.1_26. [DOI] [PubMed] [Google Scholar]
- 10.Luippold G, Zimmermann C, Mai M, et al. Dopamine D3 receptors and salt-dependent hypertension. J Am Soc Nephrol. 2001;12:2272–2279. doi: 10.1681/ASN.V12112272. [DOI] [PubMed] [Google Scholar]
- 11.Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology (Berl) 2001;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- 12.Bek MJ, Wang X, Asico LD, et al. Angiotensin-II type 1 receptor–mediated hypertension in D4 dopamine receptor–deficient mice. Hypertension. 2006;47:288–295. doi: 10.1161/01.HYP.0000198427.96225.36. [DOI] [PubMed] [Google Scholar]
- 13.Sanada H, Yatabe J, Midorikawa S, et al. Amelioration of genetic hypertension by suppression of renal G protein–coupled receptor kinase type 4 expression. Hypertension. 2006;47:1131–1139. doi: 10.1161/01.HYP.0000222004.74872.17. [DOI] [PubMed] [Google Scholar]
- 14.Felder RA, Sanada H, Xu J, et al. G protein–coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci U S A. 2002;99:3872–3877. doi: 10.1073/pnas.062694599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu H, Lu Y, Wang X, Treiber FA, et al. The G protein–coupled receptor kinase 4 gene affects blood pressure in young normotensive twins. Am J Hypertens. 2006;19:61–66. doi: 10.1016/j.amjhyper.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Zhu H, Lu Y, Wang X, et al. The G protein–coupled receptor kinase 4 gene modulates stress-induced sodium excretion in black normotensive adolescents. Pediatr Res. 2006;60:440–442. doi: 10.1203/01.pdr.0000238250.64591.44. [DOI] [PubMed] [Google Scholar]
- 17.Speirs HJ, Katyk K, Kumar NN, et al. Association of G-protein–coupled receptor kinase 4 haplotypes, but not HSD3B1 or PTP1B polymorphisms, with essential hypertension. J Hypertens. 2004;22:931–936. doi: 10.1097/00004872-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Gu D, Su S, Ge D, et al. Association study with 33 single-nucleotide polymorphisms in 11 candidate genes for hypertension in Chinese. Hypertension. 2006;47:1147–1154. doi: 10.1161/01.HYP.0000219041.66702.45. [DOI] [PubMed] [Google Scholar]
- 19.Williams SM, Ritchie MD, Phillips JA, 3rd, et al. Multilocus analysis of hypertension: a hierarchical approach. Hum Hered. 2004;57:28–38. doi: 10.1159/000077387. [DOI] [PubMed] [Google Scholar]
- 20.Sanada H, Yatabe J, Midorikawa S, et al. Single-nucleotide polymorphisms for diagnosis of salt-sensitive hypertension. Clin Chem. 2006;52:352–360. doi: 10.1373/clinchem.2005.059139. [DOI] [PubMed] [Google Scholar]
- 21.Zeng C, Luo Y, Asico LD, et al. Perturbation of D1 dopamine and AT1 receptor interaction in spontaneously hypertensive rats. Hypertension. 2003;42:787–792. doi: 10.1161/01.HYP.0000085334.34963.4E. [DOI] [PubMed] [Google Scholar]
- 22.Zeng C, Yang Z, Wang Z, et al. Interaction of AT1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension. 2005;45:804–810. doi: 10.1161/01.HYP.0000155212.33212.99. [DOI] [PubMed] [Google Scholar]
- 23.Yu P, Asico LD, Luo Y, et al. D1 dopamine receptor hyperphosphorylation in renal proximal tubules in hypertension. Kidney Int. 2006;70:1072–1079. doi: 10.1038/sj.ki.5001708. [DOI] [PubMed] [Google Scholar]
- 24.Wang ZQ, Felder RA, Carey RM. Selective inhibition of the renal dopamine subtype D1A receptor induces antinatriuresis in conscious rats. Hypertension. 1999;33:504–510. doi: 10.1161/01.hyp.33.1.504. [DOI] [PubMed] [Google Scholar]
- 25.Rankin ML, Marinec PS, Cabrera DM, et al. The D1 dopamine receptor is constitutively phosphorylated by G protein-coupled receptor kinase 4. Mol Pharmacol. 2006;69:759–769. doi: 10.1124/mol.105.019901. [DOI] [PubMed] [Google Scholar]
- 26.Iacovelli L, Salvatore L, Capobianco L, et al. Role of G protein–coupled receptor kinase 4 and β-arrestin 1 in agonist-stimulated metabotropic glutamate receptor 1 internalization and activation of mitogen-activated protein kinases. J Biol Chem. 2003;278:12433–12442. doi: 10.1074/jbc.M203992200. [DOI] [PubMed] [Google Scholar]
- 27.Pi M, Oakley RH, Gesty-Palmer D, et al. Beta-arrestin– and G protein receptor kinase–mediated calcium-sensing receptor desensitization. Mol Endocrinol. 2005;19:1078–1087. doi: 10.1210/me.2004-0450. [DOI] [PubMed] [Google Scholar]
- 28.Perroy J, Adam L, Qanbar R, Chenier S, Bouvier M. Phosphorylation-independent desensitization of GABAB receptor by GRK4. EMBO J. 2003;22:3816–3824. doi: 10.1093/emboj/cdg383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oppermann M, Diverse-Pierluissi M, Drazner MH, et al. Monoclonal antibodies reveal receptor specificity among G-protein–coupled receptor kinases. Proc Natl Acad Sci U S A. 1996;93:7649–7654. doi: 10.1073/pnas.93.15.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Premont RT, Macrae AD, Stoffel RH, et al. Characterization of the G protein–coupled receptor kinase GRK4. Identification of four splice variants. J Biol Chem. 1996;271:6403–6410. doi: 10.1074/jbc.271.11.6403. [DOI] [PubMed] [Google Scholar]
- 31.Virlon B, Firsov D, Cheval L, et al. Rat G protein–coupled receptor kinase GRK4: identification, functional expression, and differential tissue distribution of two splice variants. Endocrinology. 1998;139:2784–2789. doi: 10.1210/endo.139.6.6078. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Asico LD, Escano CS, et al. Human G protein–coupled receptor kinase type 4γ (hGRK4γ) wild-type prevents salt sensitivity while its variant, hGRK4γ496V, promotes salt sensitivity in transgenic mice: role of genetic background. Hypertension. 2006;48:e27. [Google Scholar]
- 33.Yamaguchi I, Yao L, Sanada H, et al. Dopamine D1A receptors and renin release in rat juxtaglomerular cells. Hypertension. 1997;29:962–968. doi: 10.1161/01.hyp.29.4.962. [DOI] [PubMed] [Google Scholar]
- 34.Veerasingham SJ, Sellers KW, Raizada MK. Functional genomics as an emerging strategy for the investigation of central mechanisms in experimental hypertension. Prog Biophys Mol Biol. 2004;84:107–123. doi: 10.1016/j.pbiomolbio.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 35.DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens. 2002;11:197–200. doi: 10.1097/00041552-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Yai B, Fan X, Harris RC, Zhang MZ. Dopamine stimulation of renin expression is only seen when renal COX-2 activity is inhibited. J Am Soc Nephrol. 2007;18:163A. [Google Scholar]
- 37.Sanada H, Yao L, Jose PA, Carey RM, Felder RA. Dopamine D3 receptors in rat juxtaglomerular cells. Clin Exp Hypertens. 1997;19:93–105. doi: 10.3109/10641969709080807. [DOI] [PubMed] [Google Scholar]
- 38.Williams BC, Eglen A, Duncan FM, Edwards CR. The effect of sodium intake on the renin response to dopamine in superfused rat renal cortical cells. J Hypertens. 1985;3 (Suppl 3):S267–S268. [PubMed] [Google Scholar]
- 39.Persson AE, Ollerstam A, Liu R, Brown R. Mechanisms for macula densa cell release of renin. Acta Physiol Scand. 2004;181:471–474. doi: 10.1111/j.1365-201X.2004.01320.x. [DOI] [PubMed] [Google Scholar]
- 40.Mervaala E, Muller DN, Schmidt F, et al. Blood pressure–independent effects in rats with human renin and angiotensinogen genes. Hypertension. 2000;35:587–594. doi: 10.1161/01.hyp.35.2.587. [DOI] [PubMed] [Google Scholar]
- 41.Parving HH, Lehnert H, Bröchner-Mortensen J, et al. Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group: The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 42.Panos J, Michelis MF, De Vita MV, Lavie RH, Wilkes BM. Combined converting enzyme inhibition and angiotensin receptor blockade reduce proteinuria greater than converting enzyme inhibition alone: insights into mechanism. Clin Nephrol. 2003;60:13–21. doi: 10.5414/cnp60013. [DOI] [PubMed] [Google Scholar]


