Abstract
Neuroprotective therapy targeting the complement cascade may reduce injury associated with intracerebral hemorrhage (ICH). We investigated the role of C3a-receptor antagonist (C3aRA) after ICH in mice. Autologous whole blood was infused into the right striatum of mice that were treated with C3aRA or vehicle, using both a pre- and postinjury dosing regimen. Hematoma volume, brain water content, and inflammatory cell profile were assessed at 72 h post-ICH. Neurologic dysfunction was assessed by evaluating both spatial memory and sensorimotor capacity. Animals pretreated with C3aRA showed significantly improved neurologic function, brain water content, and granulocyte infiltration relative to vehicle-treated animals when assessed at 72 h. There was no significant difference in hemorrhagic/nonhemorrhagic ratio of microglial activation among all groups. Hematoma volumes were also not significantly different between C3aRA-treated and vehicle-treated animals. Administration of C3aRA beginning 6 h postinjury afforded significant amelioration of neurologic dysfunction as well as a reduction in brain water content. Treatment with C3aRA improved neurologic outcome while reducing inflammatory cell infiltration and brain edema formation after experimental ICH in mice. Results of this study suggest that the C3a receptor may be a promising target for therapeutic intervention in hemorrhagic stroke.
Keywords: intracerebral hemorrhage, mouse, complement, C3a receptor antagonist, C3a, C3a receptor
Introduction
Intracerebral hemorrhage (ICH) is a devastating disease process, which accounts for approximately 15% of all strokes in the United States and has a 30 to 50% mortality rate that has not improved over the past two decades (Aronowski and Hall, 2005). Currently available therapies are limited to supportive medical therapy and surgery for only a selected group of patients (Broderick et al, 2007; Morgenstern et al, 1998). Experimental models of ICH have been developed to investigate the mechanisms of neuronal injury and edema formation in an effort to develop therapeutic options for this disease (Aronowski and Hall, 2005). Many techniques have been attempted to model this disease in various species, including collagenase injection and mechanical balloon compression (Andaluz et al, 2002; MacLellan et al, 2008). The advent of models using stereotactic intracerebral injection of autologous blood in the rat has enabled researchers to mimic the natural events that occur with spontaneous ICH in humans.
Experimental studies have identified a number of factors involved in the pathogenesis of ICH, such as the upregulation of leukocyte infiltration, microglial activation, initiation of the complement cascade, apoptosis, and alteration of thrombin, heme oxygenase, and matrix metalloproteinase-9 concentrations (Gong et al, 2001; Hua et al, 2000; Tejima et al, 2007; Wagner et al, 2003; Xi et al, 2004). In recent years, special attention has focused on the complement cascade’s involvement in ICH pathophysiology. Rat studies of ICH showed that complement inhibition or its systemic depletion reduces perihematomal brain edema (Hua et al, 2000; Xi et al, 2001). The development of mice deficient in elements of the complement cascade enabled researchers to investigate the role of these components in the setting of ICH, showing that C3-deficient mice had less, whereas C5-deficient mice had more ICH-induced brain injury (Nakamura et al, 2004; Yang et al, 2006). This experimental evidence suggests that factors upstream from C5, such as C3a and C3b, produced by cleavage of C3, have a detrimental effect in ICH (Yang et al, 2006). Furthermore, there is growing appreciation that signaling of the C3a anaphylatoxin, through its receptor C3aR, may be associated with a variety of inflammatory conditions (Barnum et al, 2002; Ducruet et al, 2008; Gasque et al, 1998; Mocco et al, 2006; Van Beek et al, 2000). Studies have reported an upregulation of C3aR in a model of permanent focal cerebral ischemia in the mouse and rat (Barnum et al, 2002; Van Beek et al, 2000), and administration of C3a-receptor antagonist (C3aRA) has been shown to improve outcomes after transient cerebral ischemia in the mouse (Ducruet et al, 2008).
The C3aR is a G-protein-coupled receptor that is expressed in the central nervous system by neurons, reactive astrocytes, microglia, infiltrating macro-phages, neutrophils, and smooth muscle cells surrounding blood vessels (Martin et al, 1997; Gasque et al, 1998; Nataf et al, 1999). It has also been shown to be important in neutrophil migration postischemia (Ducruet et al, 2008; Gutzmer et al, 2004). Because current evidence suggests that microglial activation and neutrophil infiltration contribute to brain injury after hemorrhagic stroke (Wang and Tsirka, 2005;Xi et al, 2006), it is likely that C3a is important in this mechanism of ICH-induced brain injury. Furthermore, emerging data on newly developed pharmaceuticals targeting the complement cascade in cerebral ischemia confirmed the presence of complement-related inflammatory tissue injury, indicating that complement inhibition may be a promising treatment option for acute stroke (Mocco et al, 2006). Our previous animal studies have showed that administration of a C3aRA resulted in commensurate neurologic improvement and stroke volume reduction in mice subjected to transient focal cerebral ischemia (Ducruet et al, 2008; Mocco et al, 2006). This suggests a central role for C3 in complement-mediated cerebral injury, likely through the formation of C3a, the potent proinflammatory anaphylatoxin.
In the present study, we sought to investigate the role of C3a receptor blockade on ICH-induced brain injury by analyzing brain edema formation, leukocyte infiltration, microglial activation as well as neurologic outcome after experimental ICH.
Materials and methods
Animal Preparation and Intracerebral Infusion
All procedures were approved by the Columbia University Institutional Animal Care and Use Committee. Adult male C57BL/6J mice weighing 23 to 30 g were used in this study. Mice were randomized to receive intraperitoneal injection of either C3aRA (1 mg/kg) (SB290157; Calbiochem, Darmstadt, Germany) diluted in phosphate-buffered saline (PBS) and dimethylsulphoxide (DMSO) (1.16%, v/v), or an equal volume of vehicle (PBS and DMSO, 1.16% DMSO, v/v) either 45 mins before ICH induction or 6 and 12 h after induction of ICH, followed in both cohorts by twice daily doses for 72 h. The most effective dose of C3aRA (1 mg/kg) was determined in a dose–response trial comparing 0.1, 0.5, 1, and 5 mg/kg performed by our lab in a model of focal ischemic stroke (data not shown). Sham animals were administered PBS and DMSO (1.16%, v/v) in the same manner.
Mice were anesthetized with a single intraperitoneal dose of ketamine (90 mg/kg) and xylazine (5 mg/kg). Next, the mice were placed in a stereotactic frame (ASI Instruments Inc., Warren, MI, USA) and subjected to ICH using autologous blood infusion as previously described (Rynkowski et al, 2008). Briefly, a 1-mm burr hole was drilled 2.3mm lateral to the midline and 0.2mm anterior to the bregma. Thirty microliters of autologous whole blood was drawn from the tail artery into a capillary tube. A 30-gauge needle was then advanced stereotactically through the burr hole into the right striatum (coordinates: 0.2mm anterior, 2.3mm lateral to the bregma, 3.5 mm ventral). Using a microinfusion pump (KDS220; KD Scientific Inc., Holliston, MA, USA) (Belayev et al, 2003; Rynkowski et al, 2008), 5 µL was delivered at a rate of 1.5 µL/min. After a 7-mins period, an additional 25 µL was delivered at a constant rate of 1.5 µL/min. After a period of 10 mins, the needle was slowly removed, the burr hole was occluded with bone wax and the skin incision was closed with Nexaband (Abbott Laboratories, North Chicago, IL, USA). Sham animals received only needle insertion. Rectal temperature was maintained at 37°C using a feedback-controlled heating lamp, and the animals recovered in a temperature-controlled incubator for 45 mins after procedure.
Neurofunction and Behavioral Testing
Sensorimotor function was evaluated by means of the 28-point neurologic function score, corner test, and forelimb placing tests, which were performed preoperatively and at 6, 24, 48, and 72 h after injury (Clark et al, 1997; Hua et al, 2002). Navigational memory was evaluated with the means of a Morris water-maze (MWM) test preoperatively and at 72 h after ICH according to a modified protocol as previously described (Ten et al, 2003). Tests were conducted by an observer masked to the treatment group.
Neurologic Function
Acute neurologic deficits were assessed using a 28-point scoring system including body symmetry, gait, climbing, circling behavior, front limb symmetry, compulsory circling, and whisker response. Each point was graded from 0 to 4. The maximum deficit score was 28 (Clark et al, 1997).
Corner Test
For the corner turn test, the mouse was allowed to walk down a corridor into a 30° corner. To exit the corner, the animal could turn either to the right or left. The mouse’s choice of turn direction was noted. The number of right and left turns out of 10 total attempts was recorded. The laterality index (LI) and normalized LI were calculated as proposed by Bouet et al (2007). The LI was calculated for each mouse, according to the formula: LI = (number of right turns—number of left turns)/(total number of turns). The LI for the day before surgery (LIBS) and each of the postsurgery days was calculated and normalized using the formula: Normalized LI = (LI + 2)/ (LIBS + 2).
Forelimb Placing Test
The second behavioral analysis involved a forelimb placing test. The animals were held by their torsos, which allowed the forelimbs to hang free. The animals were moved gently up and down before the placing test to facilitate muscle relaxation. Each forelimb was tested by brushing the respective vibrissae on the corner of a countertop. Intact animals place the forelimb ipsilateral to the stimulated vibrissae quickly onto the countertop. Each animal was tested 10 times for each forelimb, and the percentage of trials in which the mouse placed the appropriate forelimb on the edge of the table after vibrissae stimulation was determined.
Morris Water-Maze Test
For the MWM test, mice were tested in a pool 80 cm in diameter (Ten et al, 2003). The pool was filled with opaque water (deep-white dye added to the water) at a depth of 20 cm. The water temperature was maintained at 26°C. The rescue-landing platform (3 cm in diameter) was submerged by 0.5 cm under the water surface in one of four quadrants. There were navigational cues placed on the walls of the pool and a small flag on the landing platform. Testing consisted of two parts: pre- and postoperative. The first part comprised five consecutive training days during which three attempts were given to each mouse to find the platform within 120 secs. Each mouse was given 20 mins to rest between swimming trials. The training session occurred at the same time of the day throughout the training and testing periods. A mouse was placed in the water in the quadrant opposite to the landing. The time, in seconds, spent by each animal to find the platform was recorded. If the mouse failed to locate and climb on the platform within the allotted time, it was gently placed and left on the platform for 30 secs. On day 4, the flag was removed from the platform and the mouse was given three attempts to locate the platform using only peripheral navigational cues. On day 5, the landing platform was removed and each mouse was placed only one time in the pool for 60 secs. The time spent in the quadrant where the platform had been situated before was recorded. All the mice that completed the training were operated on the following day. On postoperative day 3, mice were placed in the same swimming pool without the landing platform for 60 secs. The time spent in the quadrant where the landing had been situated before was recorded in a masked fashion. Only one attempt was given to each animal.
Histopathologic study and Determination of Lesion Volume
After killing, brains were perfused with 4% paraformaldehyde in 0.1 mol/L PBS, pH 7.4, rapidly harvested in one piece, and fixed in 4% paraformaldehyde for 12 h at 4°C. Brains were then incubated in 30% sucrose for 2 to 3 days at 4°C. Next, they were frozen in Optimal Cutting Temperature Compound (OCT) Tissue-Tek (no. 4583; Sakura Finetek Inc., Torrance, CA, USA) on dry ice then placed at –80°C. Twenty micrometers coronal sections were cut using a cryostat, and stained using Nissl staining. Striatal lesion volume was determined by digitizing serial coronal sections (50 µm apart) to span the entire hematoma. A masked observer outlined the region of hematoma (defined by the presence of blood) present in the striatum of the animal. Volume of the lesion (mm3) was then determined by summation of the lesion area in each coronal section and integrating over the section depth of 0.5 mm.
Determination of Brain Water Content
Mice were reanesthetized with ketamine (80 to 100 mg/kg intraperitoneal), xylazine (5 to 10 mg/kg intraperitoneal), and killed 3 days after hemorrhage to determine brain water content (sham n = 6, vehicle n = 8, pre-C3aRA n = 8, and post-C3aRA n = 11). Brains were removed immediately en bloc and five 2-mm coronal slices were obtained beginning 2 mm from the frontal pole. The brain slices were divided into two hemispheres along the midline. The cortex of each hemisphere was then carefully dissected from the basal ganglia. The cerebellum was retained as a control. Each of the five sections was then weighed on an electronic analytical balance (Model AG 104; Mettler-Toledo Inc., Columbus, OH, USA) to determine the wet weight. The sections were then placed onto preweighed cover slips and dried overnight in a vacuum oven for 24 h to obtain the dry weight. Brain water content (%) was calculated as: ((wet weight–dry weight)/wet weight) × 100.
Preparation of Brains and Flow Cytometry Analysis
Both cerebral hemispheres were analyzed for inflammatory cells using flow cytometry. Mice were euthanized 72 h after hemorrhagic stroke onset (sham n = 6, vehicle n = 7, and pre-C3aRA n = 8). After transcardiac perfusion with PBS, brains were harvested, divided into ipsilateral and contralateral hemispheres, and minced in RPMI (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) (Invitrogen). The resulting suspension was passed through a microfilter (70 µm), pelleted, resuspended in 30% Percoll (Amersham, Piscataway, NJ, USA) and centrifuged at 27,000g for 30 mins. After centrifugation, the myelin layer was discarded and the remaining suspension was washed with Dulbecco’s PBS containing 1% FBS.
Flow Cytometric Analysis
Granulocytes were isolated and identified using a previously described antibody-based system (Stevens et al, 2002). All antibodies used for flow cytometry were rat antimouse monoclonal antibodies (BD Pharmingen, Franklin Lakes, NJ, USA): flourescein isothiocyanate-conjugated rat antimouse Ly-6G monoclonal antibody, R-phycoerythrin-conjugated rat antimouse CD45 monoclonal antibody, and PerCP-Cy5.5-conjugated rat anti-mouse CD11b monoclonal antibody. Antibodies were diluted in D-PBS containing 1% FBS. Cell extracts were incubated simultaneously with the three antibodies and Fc Block (anti-CD16/CD32) for 30 mins. Flow cytometric analysis was performed using a FACSCalibur (BD Bio-sciences, Franklin Lakes, NJ, USA), and the data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA). An antibody to CD45, a cell-surface marker expressed by all leukocytes as well as microglia, was used to exclude all other cell types. The CD11b marker, expressed by all nonlymphocyte leukocytes, was used to distinguish leukocytes from other cell types. Finally, the Ly-6G marker, expressed primarily by granulocytes and lymphocytes (Nagendra and Schlueter, 2004), was used to identify granulocytes. Using these three markers, the CD45-positive cells were separated into three general subcategories: (1) lymphocytes (CD45-positive, CD11b-negative, Ly-6G-positive), (2) microglia (CD45-positive, CD11b-positive, Ly-6G-negative), and (3) granulocytes (CD45-positive, CD11b-positive, Ly-6G-positive).
The number of cells that expressed each leukocyte marker was compared with the percentage of the total CD45-positive cells to obtain a percent concentration of each cell subtype, as previously described (Stevens et al, 2002). Sham-operated animals showed no significant differences in CD45-positive cell populations between hemispheres. There was also no significant difference between the CD45-positive populations between sham hemispheres and hemispheres contralateral to the lesion in the experimental cohorts (data not shown). We, therefore, used the nonhemorrhagic (i.e., contralateral) hemisphere as a control for each animal and normalized the expression of each marker in the hemorrhagic hemisphere by comparing it to the expression level present in the nonhemorrhagic hemisphere.
Statistical Analysis
All data are presented as mean±s.e.m. Comparisons between groups were made using Kruskal–Wallis analysis of variance (ANOVA) on ranks or one-way ANOVA with post hoc Bonferroni test. A value of P<0.05 was considered statistically significant.
Results
Neurologic Function and Behavioral Tests
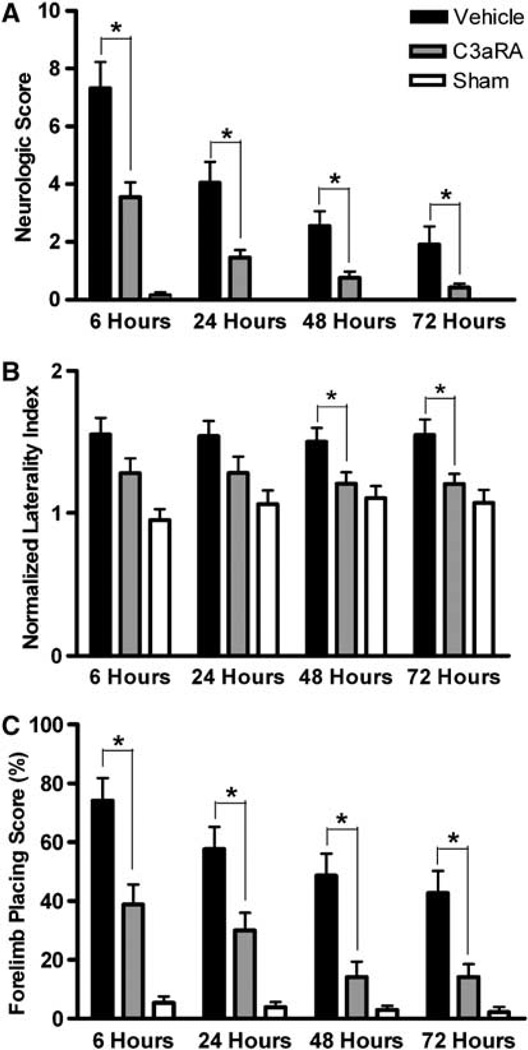
At 6 h after ICH there were marked functional deficits as assessed by the 28-point scoring system, corner turn test, and forelimb placing test (Figure 1). All tests showed a gradual recovery of function in pre-C3aRA-treated mice (n = 24) so that there were no significant deficits compared with sham animals (n = 13) at 72h after ICH. Compared with pre-C3aRA-treated animals, vehicle-treated animals (n = 24) also showed some recovery of function over time, but they remained significantly inferior to both sham and C3aRA-treated mice in 28-point scale, corner test, and forelimb placing test scores at all time points (Figure 1).
Figure 1.
Total neurologic score (A), corner test performance expressed by the normalized laterality index (B), and forelimb placing capacity in the impaired limb (C) in sham (n = 13), vehicle-treated (n = 24), and C3aRA-treated (n = 24) groups at 6, 24, 48, and 72 h after intrastriatal infusion of 30 µL autologous blood. Values are expressed as mean±s.e.m. An asterisk represents significantly different by Kruskal–Wallis ANOVA on ranks (P<0.05).
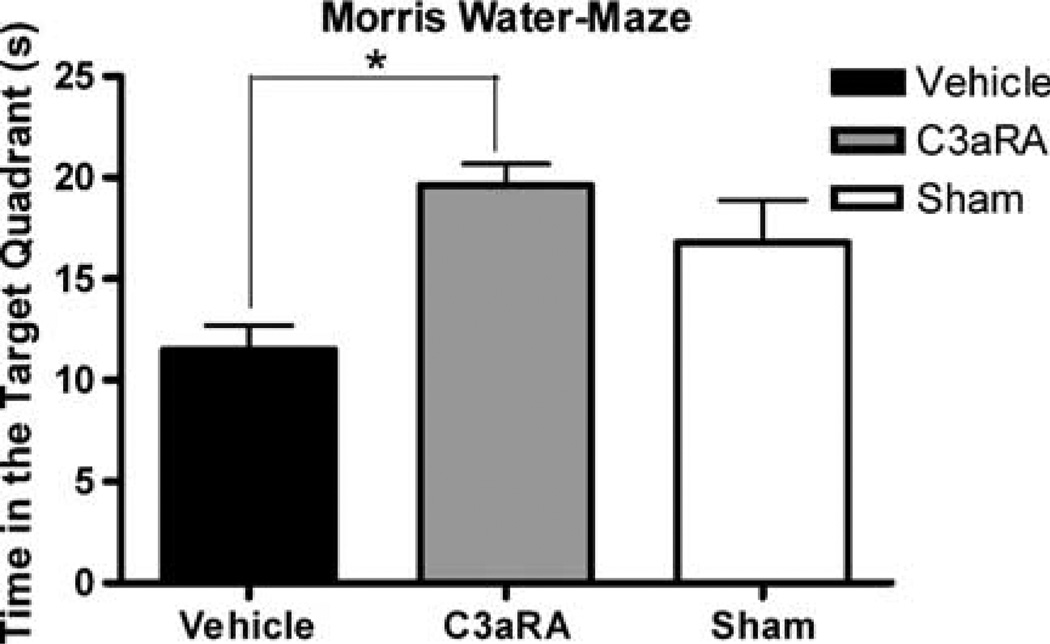
The MWM test showed a significantly better performance in the pre-C3aRA-treated mice (n = 12) compared with vehicle-treated animals (n = 12) at 72h after ICH (19.62±1.08 versus 11.52±1.19 secs, respectively, P<0.05). In addition, when the C3aRA-treated group was compared with sham animals, there were no statistical differences in the time spent in the target quadrant (19.62±1.08 versus 16.80±2.08 secs, respectively, P>0.05; Figure 2).
Figure 2.
Morris water-maze performance in sham (n =8), vehicle-treated (n=12) and C3aRA-treated (n = 12) mice expressed as the time spent in the target quadrant 72 h after ICH (16.80±2.08 versus 11.52±1.19 versus 19.62± 1.08 secs). Values are expressed as mean±s.e.m. An asterisk represents significantly different by repeated-measures analysis of variance (ANOVA) with post hoc Bonferroni test (P<0.05).
Hematoma Volume
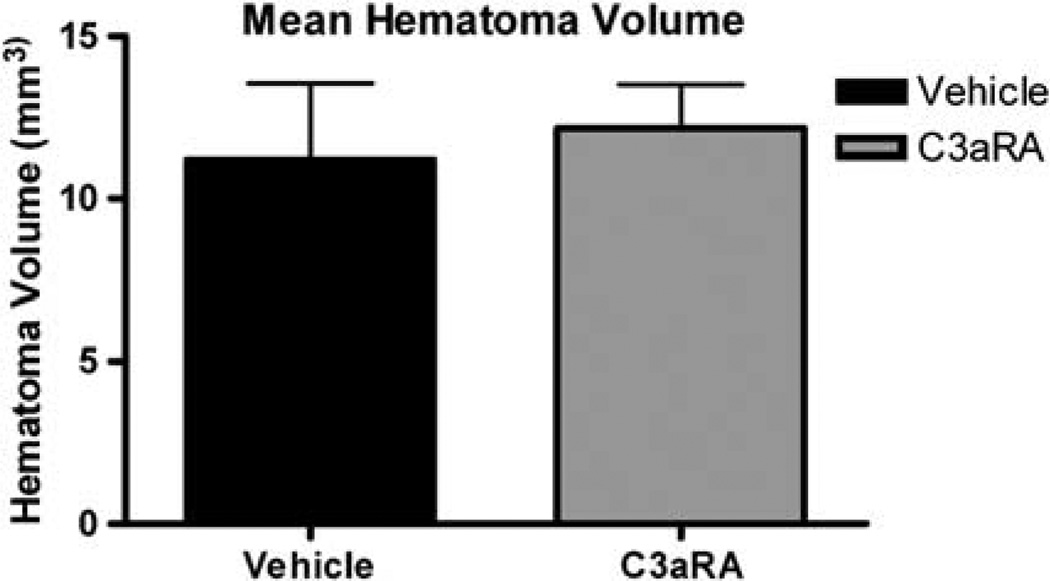
Seventy-two hours after ICH, there was no significant difference in the average hematoma volume between the pre-C3aRA-treated (n = 7) and vehicle-treated (n = 7) animals (12.17±1.33 versus 11.20±2.35 mm3, respectively, P>0.05; Figure 3).
Figure 3.
Mean hematoma volume in vehicle-treated (n = 7) and C3aRA-treated (n = 7) groups obtained 72 h after ICH. There was no significant difference in hematoma volume between vehicle and C3aRA-treated mice (11.20±2.35 versus 12.17±1.33 mm3P=0.38). Values are expressed as mean±s.e.m.
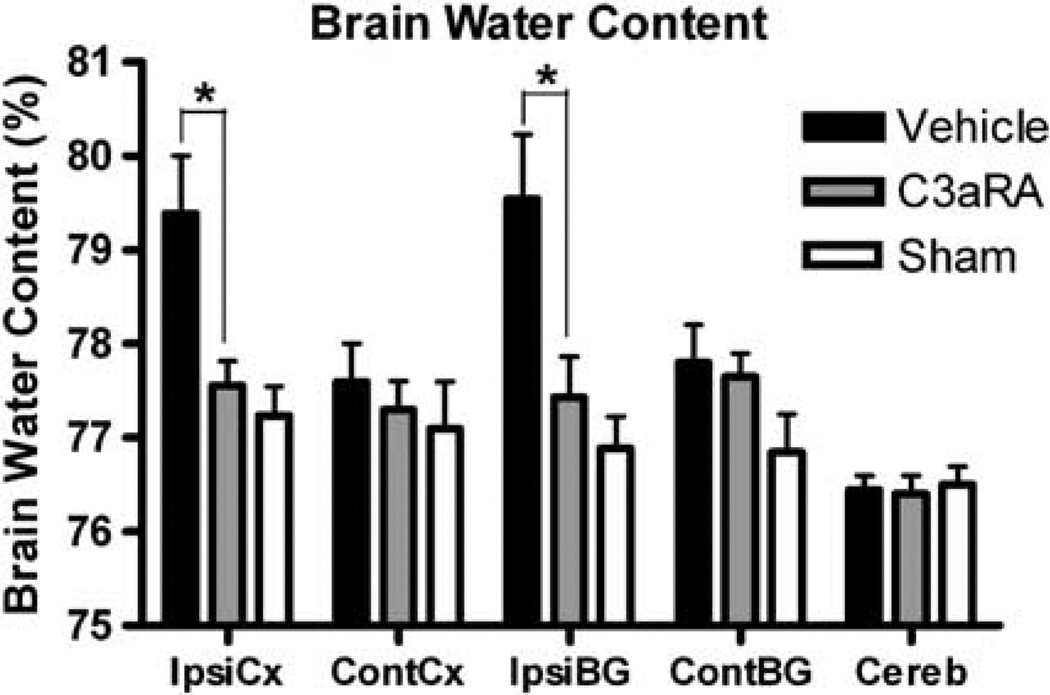
Brain Water Content
Three days after ICH, perihematomal brain edema was less in pre-C3aRA-treated (n = 8) than in vehicle-treated mice (n = 8) (ispilateral cortex: 77.56±0.26 versus 79.39±0.61%, P<0.05; ispilateral basal ganglia: 77.43±0.43 versus 79.54±0.69%, P<0.05; Figure 4). Water content in the ipsilateral cortex and in the ipsilateral basal ganglia were not different between pre-C3aRA-treated and sham mice (ipsilateral cortex: 77.57±0.26 versus 77.24± 0.25%; ipsilateral basal ganglia: 77.43±0.43 versus 76.89±0.34%; P>0.05; Figure 4), whereas vehicle-treated mice had more brain edema than sham mice (n = 6) (ipsilateral cortex: 79.39±0.61 versus 77.24±0.31%, P<0.05; ipsilateral basal ganglia: 79.54±0.69 versus 76.89±0.34%, P<0.05). Water content in the contralateral cortex, basal ganglia, and cerebellum was not different in pre-C3aRA-treated, vehicle-treated, and sham animals (Figure 4). Please note that three mice included in the neurologic testing did not have tissue results. In two of the mice, tissue was lost from the glass slides during processing and thus had to be excluded. During one of the fluorescence-activated cell sorting (FACS) preparations the pellet was lost during resuspension and the mouse was excluded.
Figure 4.
Brain water content at 72h after ICH induction in sham (n = 6), vehicle-treated (n = 8) and C3aRA-treated (n = 8) mice (ipsilateral cortex: 77.24±0.31 versus 79.39±0.61 versus 77.56±0.26%; ipsilateral basal ganglia: 76.89±0.34 versus 79.54±0.69 versus 77.43±0.43%). Values are expressed as mean±s.e.m. An asterisk represents significantly different by repeated-measures analysis of variance (ANOVA) with post hoc Bonferroni test (P< 0.05). Cont-CX, contralateral cortex; Cont-BG, contralateral basal ganglia; Ipsi-CX, ipsilateral cortex; Ipsi-BG, ipsilateral basal ganglia; Cereb, cerebellum.
Granulocyte Infiltration and Microglial Activation
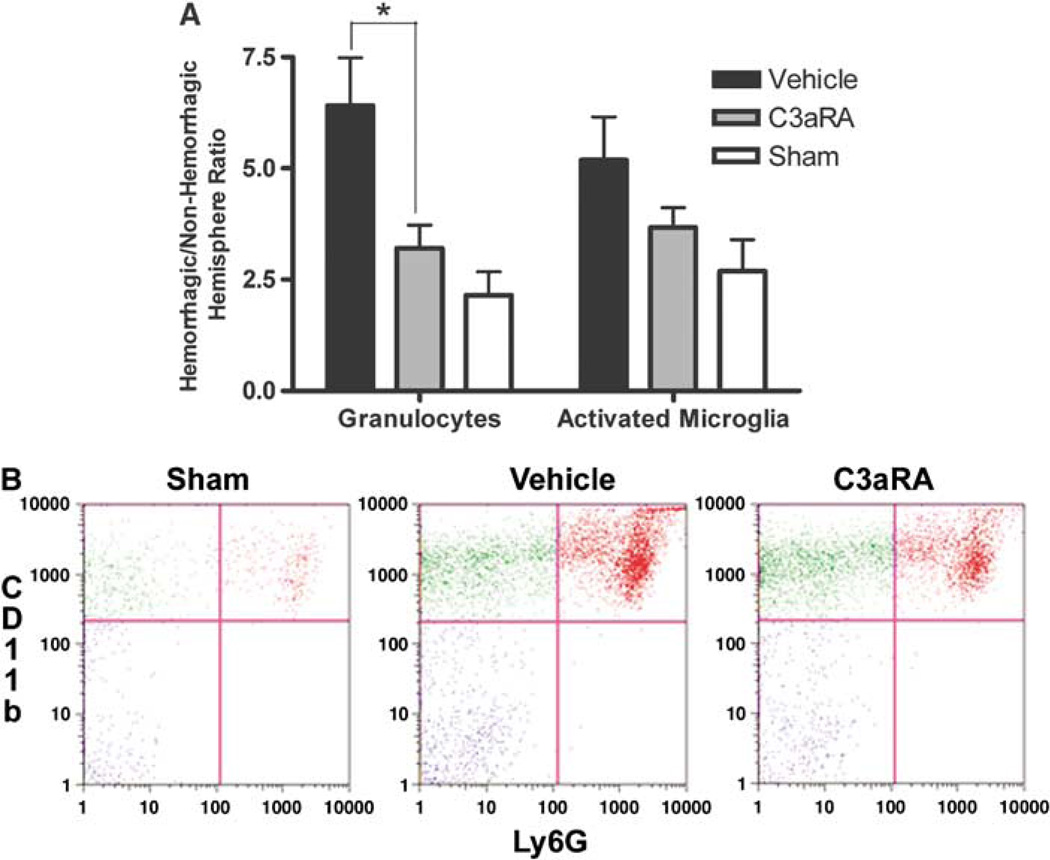
Flow cytometric analysis revealed that the granulo-cyte population, expressed as the hemorrhagic/ nonhemorrhagic ratio of cells at 3 days after ICH was less in pre-C3aRA-treated mice (n = 8) than in vehicle-treated mice (n = 7) (3.21±0.52 versus 6.41±1.08, respectively, P< 0.05). In addition, the sham animals (n = 6) had a significantly smaller ratio compared with the vehicle-treated group (respectively, 2.15±0.53 versus 6.41±1.08, P<0.05). There was no difference in hemorrhagic/nonhemorrhagic ratio of granulocyte infiltration between sham and pre-C3aRA-treated animals (respectively, 2.15±0.53 versus 3.21±0.52, P >0.05; Figure 5). Flow cyto-metric analysis of microglial activation did not show any significant differences among the three groups (pre-C3aRA-treated: 3.67±0.45 versus vehicle-treated: 5.19±0.97 versus sham: 2.69±0.71; P = 0.087; Figure 5).
Figure 5.
(A) Hemorrhagic versus nonhemorrhagic hemisphere ratio of activated microglia and granulocytes in sham-treated (n = 6), vehicle-treated (n =7), and C3aRA-treated (n = 8) mice at 72 h after ICH (granulocytes: 2.15±0.53 versus 6.41±1.08 versus 3.21±0.52; microglia: 2.69±0.71 versus 5.19±0.97 versus 3.67±0.45). Values are expressed as mean±s.e.m. An asterisk represents significantly different by repeated-measures analysis of variance (ANOVA) with post hoc Bonferroni test (P<0.05). (B) FACS plot for all three groups, sorting for CD11b on the Y-axis and Ly6G on the X-axis.
Post-ICH Induction Dosing of C3aRA
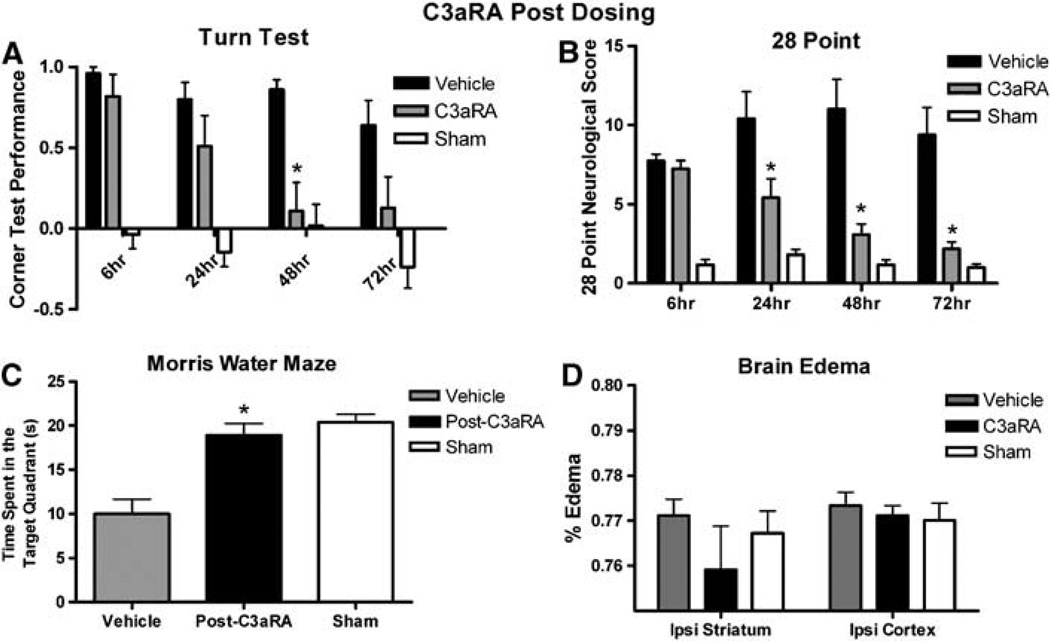
The data above clearly show that antagonism of the C3aR before induction of ICH reduces brain edema and improves neurologic outcome. We then pursued a more clinically relevant model of post-ICH induction dosing of the C3aRA by dosing first at 6 h, followed by a dose at 12 h and then every 12 h for 72 h. Compared with vehicle-treated animals (n = 17), post-C3aRA-treated mice showed significant improvement at 48 h in the turn test, and significant improvement at 24, 48, and 72 h in the 28-point test (Figure 6). Post-C3aRA-treated mice (n = 11) performed significantly better than vehicle-treated animals (n = 10) at 72 h after ICH in the MWM (post-C3aRA: 18.9±1.3 versus 10±1.7secs, P = 0.0002). There was no statistical difference between sham animals (n = 11) and post-C3aRA animals in performance in the MWM (sham: 20.4±0.9 secs, P=NS; Figure 6). Post-C3aRA-treated mice (n = 11) had mildly decreased brain water content than vehicle (n = 12), but this difference did not reach statistical significance (Figure 6).
Figure 6.
(A) Corner test performance expressed by the laterality index in vehicle-treated (n = 17), post-C3aRA-treated (n = 18), and sham-treated (n = 11) mice at 6, 24, 48, and 72h after ICH. First injection administered at 6hafter hemorrhage. (B) Twenty-eight-point neurologic score in vehicle-treated (n = 16), post-C3aRA-treated (n =18), and sham mice (n =11) at 6, 24, 48, and 72h after ICH. (C) Morris water-maze performance in vehicle (n = 10), post-C3aRA-treated (n = 11), and sham mice (n = 11) expressed as the time spent in the target quadrant 72h after ICH. Longer times equate to better performance. (D) Brain water content at 72h after ICH induction in vehicle-treated (n = 12), post-C3aRA-treated (n = 11), and sham (n = 6) mice. Trend towards decreased edema in C3aRA-treated mice. An asterisk signifies a value of P <0.05.
Discussion
Intracranial bleeds, particularly those caused by ruptured aneurysms (subarachnoid hemorrhage) or hemorrhagic stroke (ICH), remain a major treatment challenge. Current evidence shows that components of the complement cascade may be involved in brain inflammation after intracranial hemorrhage (Mack et al, 2007). Inflammatory mediators then lead to brain edema and other forms of secondary brain injury that may be reduced by neuroprotective therapy targeting the complement cascade. Our previous work showed that C3 is a critical effector of complement-mediated ischemic brain injury and that C3aRAs confer a neuroprotective effect in reperfused stroke models (Ducruet et al, 2008; Mocco et al, 2006). On the basis of these findings and on recent data suggesting that elements such as C3a and C3b is detrimental in ICH (Yang et al, 2006), we sought to examine the role of C3a anaphylatoxin in ICH-induced brain injury by blocking its receptor using a commercially available C3aR antagonist.
The findings of this study revealed that ICH-induced brain edema formation and neurologic deficits are reduced in mice treated with C3aRA both before, and after induction of ICH compared with vehicle-treated mice. In addition, granulocyte infiltration is significantly less in pre-C3aRA-treated mice, whereas microglial activation did not differ between groups. These results indicate that C3a anaphylatoxin and its receptor are important factors in complement-mediated brain injury after hemorrhagic stroke, and that the subsequent neutrophil-mediated inflammatory response contributes to this neurotoxic process. Furthermore, we confirmed that administration of C3aRA after induction of ICH also confers a protective effect, highlighting its potential as a clinical therapeutic.
Intracerebral Hemorrhage Model
The development of controllable and reproducible animal models of ICH is essential for the systematic study of the pathophysiology and treatment of hemorrhagic stroke. An ideal animal model would mimic the natural events of hemorrhagic stroke in human beings. In our murine ICH model we used a double-injection method of infusing autologous blood into the striatum. To our knowledge, this is the first study that uses this technique in mice. Previous researchers have used a double-injection method by using donor blood, or they have applied a one-stage technique of autologous blood infusion into murine basal ganglia (Belayev et al, 2003; Nakamura et al, 2004). One concern with these models has been that the induction of inflammatory cascades may differ depending on the origin of the blood product that was injected (Xi et al, 2001). Furthermore, the single-injection method fails to reproducibly create hematomas of equal size due to ventricular rupture or backflow of the injected blood (Yang et al, 1994). Our model appears to closely mimic the natural course of human ICH, and generates reproducible hematoma volumes without additional blood accumulation within the ventricles and subarachnoid space (Rynkowski et al, 2008). It may, therefore, be an advantageous model used in experimental studies of ICH.
Neurologic Deficits
Neurologic deficits are important clinical endpoints for ICH studies. Different tests for assessing acute changes in neurologic function have been applied in rat and mouse models of unilateral brain injury, including cerebral ischemia and ICH (Bederson et al, 1986; Belayev et al, 2003; Bouet et al, 2007; Clark et al, 1997; Hua et al, 2002). The corner test, forelimb placing test and 28-point neurologic scoring system have been extensively used in ICH models and have proven to be useful for evaluating the extent of brain injury (Belayev et al, 2003; Hua et al, 2002). These tests were sensitive enough to detect marked behavioral deficits after blood injections as well as charting the course of recovery over the following 3 days. Our results revealed that animals treated with C3aRA had significantly improved neurologic outcome scores on all 3 days compared with vehicle-treated animals.
To our knowledge, we are the first to present results of the MWM in murine ICH. These results show that ICH within the striatum causes spatial memory deficits that may be attenuated by pharma-cologic blockade of the C3a receptor both before and after ICH induction. Previous animal studies have showed that deficits in MWM performance are linked to damage in the striatum, hippocampus, basal forebrain, cerebellum, and neocortex (D’Hooge and De Deyn, 2001). This test has been extensively performed in different types of striatal injury and has proven to discern impairments of sensorimotor function, simple stimulus–response learning, and cognitive-spatial tasks (Gibson and Murphy, 2004; Whishaw et al, 1987). On the basis of our present results, previous experience and widespread usage of the MWM, we believe that the MWM is a useful neurologic test in animal models of intrastriatal hemorrhage. Nevertheless, further studies of long-term outcome would be warranted to better validate its usefulness in ICH models.
Brain Edema Formation
Brain edema is one of the factors involved in the pathophysiology of ICH formation and subsequent brain injury. In experimental ICH models, brain edema peaks between the third and fourth day after ictus and then slowly declines (Xi et al, 1998; Yang et al, 1994). Available evidence suggests that activation of the complement cascade and the formation of the membrane attack complex contribute to perihe-matomal brain edema formation (Hua et al, 2000). In a rat model of ICH, systemic complement depletion by cobra venom factor reduced brain edema and concentrations of tumor necrosis factor-α, and attenuated the inflammatory response (Xi et al, 2001). Furthermore, Yang et al (2006) have shown that C3 deficient mice had less brain edema after ICH induction than C3 sufficient mice. In the present study, we showed that mice treated with C3aRA before induction of ICH had decreased brain water content compared with vehicle-treated mice, indicating that pharmacologic C3aR blockade protects against brain edema formation. Mice treated with C3aRA after induction of ICH, however, did not show a significant difference in brain water indicating perhaps that the events leading to edema formation occur early in the reperfusion period.
Inflammatory Response
Inflammation represents one of the major components of the brain’s response to ICH in animals, which involves complement cascade activation (Aronowski and Hall, 2005; Hua et al, 2000; Xi et al, 2001; Yang et al, 2006). The anaphylatoxins C3a and C5a represent some of the most potent pro-inflammatory stimuli discovered to date (D’Ambrosio et al, 2001). The receptor for C3a is expressed in the central nervous system by astrocytes, neurons, microglia, oligodendrocytes, and endothelial cells (Gasque et al, 1998; Nataf et al, 1999), and its upregulation after brain injury has been reported by other investigators (Barnum et al, 2002; Van Beek et al, 2000). In addition, C3a is capable of attracting leukocytes and increasing vascular permeability (Foreman et al, 1996). Furthermore, current evidence suggests that microglial activation and neutrophil infiltration with the release of vasoactive and cytotoxic mediators, along with complement activation, contribute to brain injury after hemorrhagic stroke (Aronowski and Hall, 2005; Wang and Dore, 2007; Wang and Tsirka, 2005; Xi et al, 2006; Yang et al, 2006).
In the present study, we have shown that mice pretreated with C3aRA show significantly reduced ratios of granulocytes in hemorrhagic hemisphere versus nonhemorrhagic hemisphere compared with vehicle-treated mice. These data suggest that C3aRA has a strong effect on inflammatory cell migration after experimental ICH. C3aRA administration inhibited the influx of granulocytes into the hemorrhagic hemisphere and led to neurofunctional improvement. This suggests that the presence of inflammatory cells such as granulocytes may have a negative functional consequence. The present study also shows increased microglial activation in ispilateral hemisphere among all the experimental groups. The slightly smaller effect of C3aRA administration on microglial activation compared with its effect on granulocyte infiltration may be due to the chosen time point for data assessment in this study. Recent studies have showed in a mouse ICH model that microglial activation is prominent at day 1, peaks at day 7, and returns to normal by 21 days (Wang and Tsirka, 2005). Therefore, long-term studies involving C3aR administration are warranted to further investigate this phenomenon in ICH.
This study is not without its limitations. Recent evidence has led to speculation regarding receptor-density-dependent effects of the C3aRA (SB 290157) in vitro (Therien, 2005). However, SB 290157 has been used as an antagonist by several other groups, and has consistently showed the ability to act as an in vivo antagonist in a number of organ systems and disease models through the attenuation of downstream inflammatory products (Proctor et al, 2006; Zhang et al, 2007; Bao et al, 2005). Furthermore, its efficacy in ameliorating outcome and inflammation in our model of stroke supports a net antagonist effect for SB 290157 in ischemic brain tissue (Ducruet et al, 2008). It is also unclear how C3aRA, and other anticomplement strategies, will affect long-term outcome. This is particularly complex in light of recent evidence showing a potential role for complement in modulation of endogenous neurogenesis (Rahpeymai et al, 2006). Thus it is possible that although acute inhibition of C3aR is beneficial, long-term inhibition may mitigate this effect. Further work examining the long-term functional outcomes in the ICH model is essential for understanding the myriad effects of C3a in the injured brain and critical in the translation of anticomplement strategies.
In summary, the results of this study suggest that C3 is involved in complement-mediated cerebral injury after ICH induction, specifically through the formation of potent proinflammatory anaphylatoxin, C3a, and subsequent binding of C3a to the C3a receptor. These data also indicate that the acute inflammatory response to ICH is characterized by granulocyte infiltration and brain edema formation. This complement-mediated neuroinflammatory response is attenuated through C3aRA blockade, leading to marked improvement in neurologic function and reduction in brain edema formation. Efficacy with post-ICH administration of C3aR blockade shows the potential of this mechanism of complement blockade to be used as a therapeutic in ICH. Further studies using genetically modified mice and long-term outcomes are needed to fully understand the pathophysiology of complement-mediated inflammatory damage after ICH, and to realize the promise of complement inhibition in this setting.
Acknowledgements
Dr Rynkowski was supported by The Kosciuszko Foundation Postdoctoral Research Fellowship.
References
- Andaluz N, Zuccarello M, Wagner KR. Experimental animal models of intracerebral hemorrhage. Neurosurg Clin N Am. 2002;13:385–393. doi: 10.1016/s1042-3680(02)00006-2. [DOI] [PubMed] [Google Scholar]
- Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27:268–279. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- Bao L, Osawe I, Haas M, Quigg RJ. Signaling through up-regulated C3a receptor is key to the development of experimental lupus nephritis. J Immunol. 2005;110:228–236. doi: 10.4049/jimmunol.175.3.1947. [DOI] [PubMed] [Google Scholar]
- Barnum SR, Ames RS, Maycox PR, Hadingham SJ, Meakin J, Harrison D, Parsons AA. Expression of the complement C3a and C5a receptors after permanent focal ischemia: an alternative interpretation. Glia. 2002;38:169–173. doi: 10.1002/glia.10069. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke4. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Belayev L, Saul I, Curbelo K, Busto R, Belayev A, Zhang Y, Riyamongkol P, Zhao W, Ginsberg MD. Experimental intracerebral hemorrhage in the mouse: histological, behavioral, and hemodynamic characterization of a double-injection model. Stroke. 2003;34:2221–2227. doi: 10.1161/01.STR.0000088061.06656.1E. [DOI] [PubMed] [Google Scholar]
- Bouet V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol. 2007;203:555–567. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, Mayberg M, Morgenstern L, Ogilvy CS, Vespa P, Zuccarello M. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001–2023. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- Clark WM, Lessov NS, Dixon MP, Eckenstein F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol Res. 1997;19:641–648. doi: 10.1080/01616412.1997.11740874. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio AL, Pinsky DJ, Connolly ES. The role of the complement cascade in ischemia/reperfusion injury: implications for neuroprotection. Mol Med. 2001;7:367–382. [PMC free article] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Ducruet AF, Hassid BG, Mack WJ, Sosunov SA, Otten ML, Fusco DJ, Hickman ZL, Kim GH, Komotar RJ, Mocco J, Connolly ES. C3a receptor modulation of granulocyte infiltration after murine focal cerebral ischemia is reperfusion dependent. J Cereb Blood Flow Metab. 2008;28:1048–1058. doi: 10.1038/sj.jcbfm.9600608. [DOI] [PubMed] [Google Scholar]
- Foreman KE, Glovsky MM, Warner RL, Horvath SJ, Ward PA. Comparative effect of C3a and C5a on adhesion molecule expression on neutrophils and endothelial cells. Inflammation. 1996;20:1–9. doi: 10.1007/BF01487740. [DOI] [PubMed] [Google Scholar]
- Gasque P, Singhrao SK, Neal JW, Wang P, Sayah S, Fontaine M, Morgan BP. The receptor for complement anaphylatoxin C3a is expressed by myeloid cells and nonmyeloid cells in inflamed human central nervous system: analysis in multiple sclerosis and bacterial meningitis. J Immunol. 1998;160:3543–3554. [PubMed] [Google Scholar]
- Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab. 2004;24:805–813. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF. Intracerebral hemorrhage-induced neuronal death. Neurosurgery. 2001;48:875–882. doi: 10.1097/00006123-200104000-00037. discussion 882-73. [DOI] [PubMed] [Google Scholar]
- Gutzmer R, Lisewski M, Zwirner J, Mommert S, Diesel C, Wittmann M, Kapp A, Werfel T. Human mono-cyte-derived dendritic cells are chemoattracted to C3a after up-regulation of the C3a receptor with interferons. Immunology. 2004;111:435–443. doi: 10.1111/j.1365-2567.2004.01829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- Hua Y, Xi G, Keep RF, Hoff JT. Complement activation in the brain after experimental intracerebral hemorrhage. J Neurosurg. 2000;92:1016–1022. doi: 10.3171/jns.2000.92.6.1016. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Ducruet AF, Hickman ZL, Garrett MC, Albert EJ, Kellner CP, Mocco J, Connolly ES., Jr Early plasma complement C3a levels correlate with functional outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007;61:255–260. doi: 10.1227/01.NEU.0000255518.96837.8E. discussion 260-51. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Silasi G, Poon CC, Edmundson CL, Buist R, Peeling J, Colbourne F. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab. 2008;28:516–525. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- Martin U, Bock D, Arseniev L, Tornetta MA, Ames RS, Bautsch W, Kohl J, Ganser A, Klos A. The human C3a receptor is expressed on neutrophils and mono-cytes, but not on B or T lymphocytes. J Exp Med. 1997;186:199–207. doi: 10.1084/jem.186.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocco J, Mack WJ, Ducruet AF, Sosunov SA, Sughrue ME, Hassid BG, Nair MN, Laufer I, Komotar RJ, Claire M, Holland H, Pinsky DJ, Connolly ES., Jr Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circ Res. 2006;99:209–217. doi: 10.1161/01.RES.0000232544.90675.42. [DOI] [PubMed] [Google Scholar]
- Morgenstern LB, Frankowski RF, Shedden P, Pasteur W, Grotta JC. Surgical treatment for intracerebral hemorrhage (STICH): a single-center, randomized clinical trial. Neurology. 1998;51:1359–1363. doi: 10.1212/wnl.51.5.1359. [DOI] [PubMed] [Google Scholar]
- Nagendra S, Schlueter AJ. Absence of cross-reactivity between murine Ly-6C and Ly-6G. Cytometry A. 2004;58:195–200. doi: 10.1002/cyto.a.20007. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Xi G, Hua Y, Schallert T, Hoff JT, Keep RF. Intracerebral hemorrhage in mice: model characterization and application for genetically modified mice. J Cereb Blood Flow Metab. 2004;24:487–494. doi: 10.1097/00004647-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Nataf S, Stahel PF, Davoust N, Barnum SR. Complement anaphylatoxin receptors on neurons: new tricks for old receptors? Trends Neurosci. 1999;22:397–402. doi: 10.1016/s0166-2236(98)01390-3. [DOI] [PubMed] [Google Scholar]
- Proctor LM, Strachan AJ, Woodruff TM, Mahadevan IB, Williams HM, Shiels IA, Taylor SM. Complement inhibitors selectively attenuate injury following administration of cobra venom factor to rats. Int Immuno-pharmacol. 2006;6:1224–1232. doi: 10.1016/j.intimp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Rahpeymai Y, Hietala MA, Wilhelmsson U, Fotheringham A, Davies I, Nilsson AK, Zwirner J, Wetsel RA, Gerard C, Pekny M, Pekna M. Complement: a novel factor in basal and ischemia-induced neurogenesis. EMBO J. 2006;25:1364–1374. doi: 10.1038/sj.emboj.7601004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynkowski MA, Kim GH, Komotar RJ, Otten ML, Ducruet AF, Zacharia BE, Kellner CP, Hahn DK, Merkow MB, Garrett MC, Starke RM, Cho BM, Sosunov SA, Connolly ES. A mouse model of intracerebral hemorrhage using autologous blood infusion. Nat Protoc. 2008;3:122–128. doi: 10.1038/nprot.2007.513. [DOI] [PubMed] [Google Scholar]
- Stevens SL, Bao J, Hollis J, Lessov NS, Clark WM, Stenzel-Poore MP. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 2002;932:110–119. doi: 10.1016/s0006-8993(02)02292-8. [DOI] [PubMed] [Google Scholar]
- Tejima E, Zhao BQ, Tsuji K, Rosell A, van Leyen K, Gonzalez RG, Montaner J, Wang X, Lo EH. Astrocytic induction of matrix metalloproteinase-9 and edema in brain hemorrhage. J Cereb Blood Flow Metab. 2007;27:460–468. doi: 10.1038/sj.jcbfm.9600354. [DOI] [PubMed] [Google Scholar]
- Ten VS, Bradley-Moore M, Gingrich JA, Stark RI, Pinsky DJ. Brain injury and neurofunctional deficit in neonatal mice with hypoxic-ischemic encephalopathy. Behav Brain Res. 2003;145:209–219. doi: 10.1016/s0166-4328(03)00146-3. [DOI] [PubMed] [Google Scholar]
- Therien AG. Agonist activity of the small molecule C3aR ligand SB 290157. J Immunol. 2005;174:7479. doi: 10.4049/jimmunol.174.12.7479. [DOI] [PubMed] [Google Scholar]
- Van Beek J, Bernaudin M, Petit E, Gasque P, Nouvelot A, MacKenzie ET, Fontaine M. Expression of receptors for complement anaphylatoxins C3a and C5a following permanent focal cerebral ischemia in the mouse. Exp Neurol. 2000;161:373–382. doi: 10.1006/exnr.1999.7273. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab. 2003;23:629–652. doi: 10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- Wang J, Tsirka SE. Tuftsin fragment 1–3 is beneficial when delivered after the induction of intracerebral hemorrhage. Stroke. 2005;36:613–618. doi: 10.1161/01.STR.0000155729.12931.8f. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Mittleman G, Bunch ST, Dunnett SB. Impairments in the acquisition, retention and selection of spatial navigation strategies after medial caudate-putamen lesions in rats. Behav Brain Res. 1987;24:125–138. doi: 10.1016/0166-4328(87)90250-6. [DOI] [PubMed] [Google Scholar]
- Xi G, Fewel ME, Hua Y, Thompson BG, Jr, Hoff JT, Keep RF. Intracerebral hemorrhage: pathophysiology and therapy. Neurocrit Care. 2004;1:5–18. doi: 10.1385/ncc:1:1:5. [DOI] [PubMed] [Google Scholar]
- Xi G, Hua Y, Keep RF, Younger JG, Hoff JT. Systemic complement depletion diminishes perihematomal brain edema in rats. Stroke. 2001;32:162–167. doi: 10.1161/01.str.32.1.162. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89:991–996. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood–brain barrier permeability in rats. J Neurosurg. 1994;81:93–102. doi: 10.3171/jns.1994.81.1.0093. [DOI] [PubMed] [Google Scholar]
- Yang S, Nakamura T, Hua Y, Keep RF, Younger JG, He Y, Hoff JT, Xi G. The role of complement C3 in intracerebral hemorrhage-induced brain injury. J Cereb Blood Flow Metab. 2006;26:1490–1495. doi: 10.1038/sj.jcbfm.9600305. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kimura Y, Fang C, Shou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC. Regulation of toll like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]