Abstract
Monostratified epithelial cells translocate HIV-1 from the apical to the basolateral surface via vesicular transcytosis. Since acutely transmitted HIV-1 is almost exclusively CCR5-tropic and human intestinal epithelial cells preferentially transcytose CCR5-tropic virus, we established epithelial monolayers using polarized HT-29 cells transduced to express CCR5, and an explant system using normal human rectal mucosa, to characterize biological parameters of epithelial cell transcytosis of HIV-1 and assess antiviral antibody blockade of transcytosis. The amount of cell-free HIV-1 transcytosed through the epithelial monolayer increased linearly in relation to the amount of virus applied to the apical surface, indicating transcytosis efficiency was constant (r2 = 0.9846, P<0.0001). The efficiency of HIV-1 transcytosis ranged between 0.05% and 1.21%, depending on the virus strain, producer cell type and gp120 V1-V3 loop signature. Inoculation of HIV-1 neutralizing antibodies to the immunodominant region (7B2) or the conserved membrane proximal external region (2F5) of gp41 or to cardiolipin (IS4) onto the apical surface of epithelial monolayers prior to inoculation of virus significantly reduced HIV-1 transcytosis. 2F5 was the most potent of these IgG1 mAbs. Dimeric IgA (dIgA) and monomeric IgA (mIgA), but not polymeric IgM, 2F5 antibodies also blocked HIV-1 transcytosis across the epithelium and, importantly, across explanted normal human rectal mucosa, with mIgA substantially more potent than dIgA in effecting transcytosis blockade. These findings underscore the potential role of transcytosis blockade in the prevention of HIV-1 transmission across columnar epithelium such as that of the rectum.
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) enters the host through the mucosa in all transmissions, except those acquired parenterally (1-3). During this process, a key entry event is the translocation of virus across the epithelium. In intestinal, colonic, rectal and endocervical mucosa, the epithelium is composed of a single layer of polarized, columnar epithelial cells with tight junctions that separate the cells into apical and basolateral domains (4). In contrast, ectocervical and vaginal epithelium is composed of pluristratified epithelial cells that lack a polarized plasma membrane and tight junctions, allowing a rich network of intraepithelial dendritic cells (DCs) and Langerhans cells to integrate into the epithelium (3-5). Depending on the site of inoculation, these distinct structural features of mucosal epithelium permit the translocation of HIV-1 by different pathways, including columnar epithelial cell transcytosis (4, 6, 7) and DC/Langerhans cell transport (5, 8, 9).
Transcytosis is a rapid, non-degradative process in which “cargo” is transported in vesicles from the apical to basolateral surface of polarized cells (6, 10, 11). HIV-1 transcytosis across gut and genital epithlelium has been reported to involve viral components, including gp41 (7, 12-14), gp120 (10) and gp160 (10, 11), and host epithelial cell receptor and attachment molecules, including the glycosphingolipid galactosylceramide (GalCer) (12), the co-receptor CCR5 (15), and the heparin sulfate proteoglycan (HSPG) attachment receptors syndecan (10) and agrin (16). Elucidating the biology of HIV-1 transcytosis is important since disruption of the transcytotic process may provide a strategy for inhibiting HIV-1 entry through columnar epithelium and protection against infection. In this regard, the transcytosis of cell-associated HIV-1 can be inhibited in vitro by dimeric IgA (dIgA) and pentameric IgM (pIgM) isolated from HIV-1-infected subjects (7), secretory IgA (S-IgA) specific for gp41 (13, 17), dIgA and pIgM 2F5 and 2G12 antibodies (18), mucosal and serum IgA from HIV-1-exposed seronegative individuals (14, 19), and IgG and S-IgA anti-gp160 (11, 20).
The transcytosis of cell-free HIV-1 across the epithelium has received less investigative attention, although cell-free virus in infected breast milk (21-23) and semen (24-26) likely enters the mucosa of the inoculated recipient through epithelial cell transcytosis. Antibodies to host cell epitopes such as CCR5 and GalCer block cell-free HIV-1 transcytosis across model epithelium and primary epithelial cells (10, 11, 27), but neutralizing antibodies to HIV-1 components reportedly do not block the transcytosis of cell-free virus across HEC-1 cells, which are derived from genital endometrial adenocarcinoma (28). Here we characterized the transcytosis of cell-free HIV-1 through HT-29 cells derived from colon adenocarcinoma epithelium, antibody blockade of HIV-1 transcytosis through the cells, and 2F5 antibody isotype blockade of HIV-1 transcytosis into explanted rectal mucosa.
MATERIALS AND METHODS
HIV-1 molecular clones and viruses
Replication competent clones of R5 viruses, including YU2 (29), NA20.B59, NA353.B27, NA420.B33 and NA420.LN85 (30-32), were prepared by transfection of plasmid DNAs into 293T cells using Fugene 6 (Roche, Indianapolis, IN) per the manufacturer's protocol. After 60 h, the supernatants were harvested, clarified by low speed centrifugation, filtered through a 0.45 μm filter, aliquoted and stored at −80°C. Viruses were titrated using TZM-bl cells (27), and p24 levels were measured by ELISA (PerkinElmer, Boston, MA). The X4 viruses SG3 and NL4-3 were prepared similarly using pSG3 (33) and pNL4-3 plasmids (34), respectively. The YU2 and SG3 plasmids were obtained from Dr. George Shaw, University of Alabama at Birmingham; NA20.B59, NA353.B27, NA420.B33 and NA420.LN85 molecular clones from Dr. Paul Clapham, University of Massachusetts; and pNL4-3 from the AIDS Research and Reference Reagent Program, NIH. The replication competent NL4-3.Balecto was constructed by replacing the ectodomain of the Env gene of the pNL4-3 with that of the R5 virus Bal and produced as above, resulting in a virus with an X4 backbone and an R5 ectodomain (Edmonds, Ochsenbauer and Kappes, manuscript submitted). NL4-3.Balecto was used to assess antibody inhibition of HIV-1 transcytosis as part of a larger (Center for HIV/AIDS Vaccine Immunology [CHAVI], NIH) multi-laboratory investigation of the inhibition of HIV-1 entry in different systems. To examine the effects of viral producer cells on HIV-1 transcytosis, NL4-3.Balecto was propagated in MT4R5 cells and PBMCs, and Bal was grown in PBMCs. SF162 and SHIV.SF162P3 were provided by CHAVI.
Antibodies
Antibodies (Table 1) used to evaluate blockade of HIV-1 entry included 2F5 IgG1 (35), 2F5 dIgA, 2F5 pIgM (18), 7B2, 4E10 (36), IS4 (37), ISQ, 13H11 (38, 39), 5A9 (39), PA-3F (40), Synagis (41) and for control pooled IgG, IgA and IgM (Sigma, St. Louis, MO). For preparation of monomeric IgA, DNA encoding the VH and VL regions of 2F5 antibody was synthesized according to the published coding sequence (42) and cloned into pcDNA3:VHCα and pcDNA3:VLCκ vectors kindly provided by Dr. B. Corthésy (Swiss Institute for Experimental Cancer Research, Lausanne, Switzerland) (43) using the integrated vector system for the expression of antibodies and VHExpress and VKExpress vectors under the control of EF-1α promoter (44). The sequences of the V regions, analyzed after vector construction using IMGT software were identical to the original sequence (42). IgA was obtained from the supernatant after co-transfection of both heavy and light chain vectors into CHO dhfr-cells cultured in RPMI supplemented with 10% fetal calf serum. The antibody was specific for the ELDKWA peptide, as expected, and bound to gp41-MPER at a nM range (0.2 + 0.025). Full description of the IgA is in preparation.
Table I.
Antibodies used to assess inhibition of HIV-1 transcytosis.
| Antibody | Brief Description |
|---|---|
| 2F5 IgG1 | human cluster II mAb to gp41 MPERa, binds ELDKWA |
| 2F5 dIgA | human cluster II mAb to gp41 MPER, binds ELDKWA |
| 2F5 pIgM | human cluster II mAb to gp41 MPER, binds ELDKWA |
| 2F5 mIgA | human cluster II mAb to gp41 MPER, binds ELDKWA |
| 4E10 | human cluster II mAb to gp41 MPER, binds NWFDIT |
| 7B2 | human cluster I mAb to immunodominant region of gp41 |
| IS4 | human anti-cardiolipin mAb, neutralizes HIV-1 in PBMCs |
| ISQ | human anti-cardiolipin mAb, neutralizes HIV-1 in PBMCs |
| 13H11 | mouse cluster II anti-gp41 MPER region mAb, HIV-1 non-neutralizing |
| 5A9 | mouse cluster II anti-gp41 MPER region mAb, HIV-1 non-neutralizing |
| PA-3F11 | mouse anti-anthrax protective antigen mAb |
| Synagis | human anti-RSV mAb |
| Control IgG | polyclonal antibody from pooled normal human serum |
| Control IgA | polyclonal antibody from human colostrum |
| Control IgM | polyclonal antibody from pooled normal human serum |
MPER = membrane proximal external region
Model columnar epithelium
Epithelial monolayers were established in transwell chambers using HT-29 cells (ATCC, Manassas, VA) transduced to constitutively express CCR5. The level of CCR5 expression (15% CCR5+, relative mean fluorescence intensity 485) in the HT-29 cells used here was determined by flow cytometry using anti-CCR5 mAb 2D7 (BD Bioscience, San Jose, CA). To establish the HT-29 monolayers, 3 × 105 HT-29 CCR5+ cells in 100 μL media were transferred to a 6.5 mm diameter transwell in a 24-well plate (Corning, Corning, NY) and allowed to grow into a tight, polarized monolayer on the transwell polycarbonate membrane containing 3.0 μm pores. The monolayer formed apical and basolateral surfaces that mimicked the luminal and basolateral domains of columnar epithelium in vivo. Monolayer integrity was monitored by measuring transepithelial electrical resistance (TEER) with a volt-ohm meter (Millipore Millicell-ERS, Millipore, Concord, MA), and monolayers were used only when the TEER was 390 mΩ/cm2 or greater, consistent with non-permeable, intact tight junctions.
Transcytosis assay and transcytosis blockade in model epithelium
To determine the relationship between input and output virus, HIV-1 (1×105 infectious units) was inoculated onto the apical surface of HT-29 monolayers, 2 h later media in the lower chamber was harvested, and virus that had entered the lower chamber was titrated using TZM-bl cells. Transcytosis efficiency was calculated by dividing the amount of virus (in infectious units) in the lower chamber by the amount of virus inoculated onto the apical surface in the upper chamber.
To analyze antibody blockade of transcytosis, antibodies at 5-fold serial dilutions up to 50 μg/mL were first applied to the apical surface and incubated for 30 min after which virus (11 ng p24) was added to the apical chamber and incubated for 2 h, mimicking the in vivo inoculation of virus onto a mucosal surface containing previously secreted antibody. Virus in the lower chamber was harvested and quantified by p24 ELISA in order to measure inhibition of virus transcytosis and not neutralization. In the indicated experiment, antibodies were added to the lower chamber and incubated for 1 h before adding virus to the upper chamber. Antibody inhibition of HIV-1 transcytosis was expressed as relative transcytosis efficiency, with transcytosis efficiency in the presence of isotype-matched control antibody defined as 100%.
Rectal mucosal explant
The proximal (internal) portion of normal rectum obtained from donors undergoing elective perineal proctectomy for rectal prolapse was used to construct mucosal explants, as we recently described for small intestinal and vaginal explants ((45), Shen, manuscript submitted). Histological analysis of adjacent tissue confirmed that the rectal epithelium was intact and the lamina propria not inflamed. Briefly, the submucosa was removed by mechanical dissection, the resultant mucosa was sectioned into 1 cm2 pieces, and each piece was immediately attached to a disc of filter paper with a central 0.5 cm circular perforation, with the intact apical surface in the superior position to maintain polarity. The filter paper then was sealed at the outer edge to the nylon mesh of a 40 μm cell strainer (BD Falcon, Bedford, MA) with surgical glue. A polystyrene cylinder (1 cm high, 0.6 cm diameter), hereafter referred to as the upper well, was attached with surgical glue to the apical surface of the mucosa, and the mucosal surface in the upper well was bathed in 50 μL RPMI to maintain epithelial cell viability. The explant then was placed in a TC-6 well plate and cultured at 37°C in RPMI containing 10% human AB serum (Atlanta Biological, Norcross, GA).
To measure 2F5 antibody inhibition of HIV-1 transcytosis across rectal epithelium in the mucosal explant, antibodies were added to the upper well and incubated for 30 min prior to the addition of YU2 (55 ng p24) to the well. After a 2 h incubation at 37°C, explants were harvested, washed 3 times with PBS, trypsinized for 10 min, and washed 3 more times with PBS. DNA-free total RNA was isolated using the Qiagen Plus Mini RNeasy kit per the manufacturer's manual and analyzed by quantitative RT-PCR to measure apically applied virus that had entered and remained in the mucosa. Antibody inhibition of HIV-1 entry into the rectal mucosa was expressed as relative transcytosis efficiency as described above.
Real-time RT-PCR
HIV-1 RNA was assessed by real-time PCR, using our previously described protocol (45). Briefly, total RNA was transcribed into cDNA using the iScript cDNA Synthesis Kit (BioRad, Hercules, CA) with oligo (dT) and random primers, and the gag gene was amplified with TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA) (46). The gag-specific primers included 5’-ACATCAAGCAGCCATGCAAAT-3’ and 5’-CTATGTCACTTCCCCTTGGTTCTCT-3’. The gag-specific probe was 5’-6-FAMACCATCAATGAGGAAGCTGCAGAATGGG–TAMRA-3’. Expression of the endogenous housekeeping gene 18S rRNA (Ref. Seq. X03205.1) was determined simultaneously with VIC/TAMRA labelled primer-probe set. Real-time PCR reactions were run for 50 cycles (15 sec 95°C, 60 sec 57.9°C) on a Chromo4 PCR system (BioRad) and analyzed with Opticon Monitor™ software, version 3.1. Relative copy numbers were determined based on a standard curve and normalized to 18S rRNA.
Statistics
P values were calculated using Student's T-test.
RESULTS
Parameters of HIV-1 transcytosis through columnar epithelium
Since transmitted and founder viruses are almost exclusively CCR5-tropic (47) and human primary intestinal epithelial cells preferentially translocate CCR5-tropic viruses (27), we established HT-29 CCR5+ epithelial monolayers to investigate the biological properties of R5 virus transcytosis. We first examined whether the amount of HIV-1 that transcytosed through the model epithelium correlated with the amount of virus inoculated onto the apical surface. The inoculation of 1-30 × 105 infectious units of HIV-1 onto the apical surface of the monolayer resulted in the rapid (2 h) translocation of virus into the lower chamber, such that the amount of virus transported through the epithelium increased linearly in relation to the amount of input virus (Fig. 1A), indicating that the efficiency of transcytosis was constant (r2 = 0.9846, p<0.0001 by linear regression). To determine whether HIV-1 transcytosis is dependent on the virus strain, equal amounts of X4 viruses SG3 and NL4-3 and R5 viruses NL4-3.Balecto and YU2 were added in parallel to the apical surfaces of individual epithelial cell monolayers, and transcytosis efficiency was determined. The efficiency of HIV-1 transcytosis was low; mean transcytosis efficiency (±SD) for SG3 was 0.33 ±0.14% and for NL4-3 0.38 ±0.07%, NL4-3.Balecto 0.05 ±0.01% and YU2 0.36 ±0.18% (Fig. 1B). To examine the influence of the variable region of gp120 on HIV-1 transcytosis, we assessed the transcytosis efficiency of four macrophage-tropic viruses (NA20.B59, NA353.B27, NA420.B33 and NA420.LN85) that have the same NL4-3 genomic backbone but different R5 envelope genes (31, 32). The transcytosis efficiency of these viruses ranged between 0.32% and 1.21% (NA20.B59: 0.32 ±0.09%; NA353.B27: 0.54 ±0.11%; NA420.B33: 1.21 ±0.32%; and NA420.LN85: 0.50 ±0.12%) (Fig. 1C), indicating variation in transcytosis efficiency among viruses with different V1-V3 loop signatures.
Fig. 1.
Transcytosis of cell-free HIV-1 through model epithelium. (A) Increasing amounts of NL4-3.Balecto were inoculated onto tight HT-29 monolayers (Input HIV-1), and, 2 h later, media in the basolateral chamber was harvested and analyzed for HIV-1 by titration on TZM-bl cells (Output virus). Transcytosis efficiency across model epithelium was determined for (B) different HIV-1 strains, (C) NL4-3 and molecular clones with the same NL4-3 backbone but different envelope genes, and (D) HIV-1 produced in different cell types. Data are the mean of 3-5 experiments; error bars represent SD. Differences between the *bars and other bars was (B) P<0.02, (C) P<0.02, and (D) P<0.004.
Enveloped viruses released from infected cells may incorporate host cell proteins internally or embed them into the virion envelope (48). HIV-1, in particular, is reported to acquire distinct envelope glycosylation patterns of the producer cell (49), potently modulating the infectivity of progeny virions (50). Therefore, we evaluated whether the production of HIV-1 by different host cells affects the transcytosis efficiency of the virus through epithelial cells. As shown in Fig. 1D, the transcytosis efficiencies of NL4-3.Balecto produced by 293T cells was 0.06 ±0.01%, MTR5 cells 0.03 ±0.02% and PBMCs 0.14 ±0.02%, indicating enhanced trancytosis efficiency for virus produced in primary mononuclear cells.
Effects of HIV-1-specific IgG antibodies on HIV-1 transcytosis
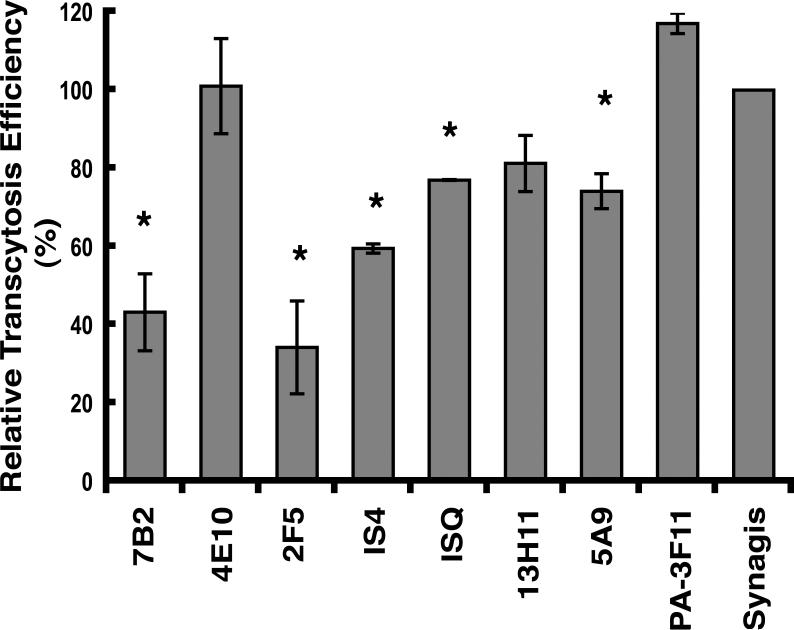
We next investigated the ability of IgG mAbs to HIV-1 to inhibit transcytosis of the virus across HT-29 monolayers. NL4-3.Balecto was used in these experiments to standardize the input virus as part of the multi-laboratory CHAVI investigation of HIV-1 entry blockade. At a concentration of 50 μg/mL, 7B2, an antibody to the immunodominant region of gp41 (39), inhibited the transcytosis of NL4-3.Balecto by 57%, in contrast to 13H11 and 5A9, non-neutralizing antibodies to gp41 cluster II membrane proximal external region (MPER) (39), which exhibited very limited (<20%) inhibition (Fig. 2). The MPER is a region of gp41 crucial for HIV-1 infection and controls cell-associated HIV-1 transcytosis. Concerning cell-free HIV-1 transcytosis, 2F5 IgG1, which recognizes the conserved epitope ELDKWA (35, 51, 52), was the most potent inhibitor of transcytosis among the mAbs tested. In contrast, 4E10, the broadest HIV-1 neutralizing antibody, which binds the conserved epitope NWFDIT in the gp41 MPER (53, 54), displayed no inhibitory effect, suggesting that the more surface-exposed ELDKWA (55) and not NWFDIT is involved in transcytosis. Interestingly, the IS4 and ISQ antibodies to cardiolipin, which neutralize HIV-1 in PBMCs, displayed modest to very low, albeit reproducible, inhibition of NL4-3.Balecto transcytosis, suggesting the cardiolipin epitope is minimally involved in cell free HIV-1 transcytosis. Control antibodies to anthrax protective antigen (PA-3F11) and a respiratory synytia virus antigen (Synagis) had no inhibitory effect. Together, these data suggest that separate epitopes are involved in HIV-1 neutralization and transcytosis, agreeing with the findings of Chomont, et al. (28) and Matoba, et al. (56).
Fig. 2.
Inhibition of HIV-1 transcytosis across model epithelium by a panel of IgG antibodies. Antibodies at a concentration of 50 μg/mL were applied to HT-29 epithelial monolayers for 30 min prior to the inoculation and incubation (2 h) of HIV-1 NL4-3.Balecto. Virus transcytosed into the basolateral chamber was quantified by p24 ELISA with transcytosis efficiency in the presence of control antibody defined as 100%. Results are the mean of three experiments; error bars represent SD. Difference between the *bars and control antibody was P<0.01.
IgG and dIgA, but not pIgM, 2F5 antibodies substantially reduce HIV-1 transcytosis
Since antibody isotypes to the same epitope may vary in their fine specificity (57) and avidity (58) to certain antigens, we next determined whether IgG1, dIgA and pIgM isotypes of 2F5 also inhibited cell-free HIV-1 transcytosis. 2F5 antibodies at 5-fold serial dilutions were applied to the epithelial surface, followed 30 min later by virus, and 2 h later supernatant in the lower chamber was harvested and virus quantified by p24 ELISA. IgG1 and dIgA, but not pIgM, 2F5 antibodies inhibited HIV-1 transcytosis through the epithelium in a dose-dependent manner (Fig. 3); 2F5 IgG reduced transcytosis by 66% and dIgA by 61% at a concentration of 50 μg/mL. Even at a low concentration of 0.4 μg/mL, IgG1 and dIgA 2F5 antibodies inhibited transcytosis by 45% and 34%, respectively (Fig. 3). 2F5 antibodies added to the basolateral chamber did not inhibit HIV-1 transcytosis (data not shown).
Fig. 3.
2F5 isotype antibody inhibition of HIV-1 transcytosis through model epithelium. (A) IgG1, dIgA and pIgM 2F5 antibodies at the indicated concentrations were applied to the apical surface of HT-29 epithelial monolayers for 30 min prior to the inoculation and incubation (2 h) of HIV-1 NL4-3.Balecto. Virus transcytosed into the basolateral chamber was quantified by p24 ELISA with transcytosis efficiency in the presence of control antibody defined as 100%. Results are the mean of three experiments; error bars represent SD.
To determine whether 2F5 antibody inhibition of HIV-1 transcytosis depends on the HIV-1 strain, we next measured the efficiency with which a panel of viruses transcytose across HT-29 monolayers in the presence of 2F5 antibodies at 50 μg/mL. Both IgG1 and dIgA 2F5 antibodies potently inhibited SF162 and NL4-3.Balecto, R5 viruses; IgG1 2F5 antibodies reduced transcytosis 22%-64% (Fig. 4A) and dIgA 2F5 antibodies 16%-53% (Fig. 4B). Again, pIgM 2F5 had no inhibitory effect on epithelial cell transcytosis of these viruses (data not shown).
Fig. 4.
2F5 antibody inhibition of transcytosis of multiple isolates of HIV-1 across model epithelium. IgG1 (A) and dIgA (B) 2F5 antibody inhibition of HIV-1 transcytosis was assessed by comparing p24 input and output levels with transcytosis efficiency in the presence of control antibody defined as 100%. Results are the mean of three experiments; error bars represent SD.
Monomeric IgA 2F5 antibodies potently inhibit HIV-1 transcytosis
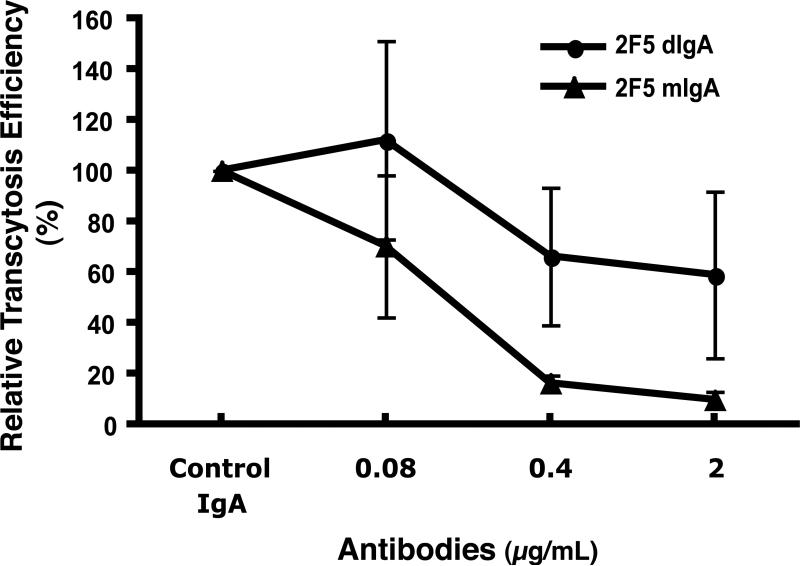
Since both secretory and systemic IgA have been shown to inhibit cell-associated HIV-1 transcytosis across model epithelium (7, 13, 19, 20, 59), we compared the ability of dIgA and mIgA 2F5 antibodies to inhibit cell-free HIV-1 transcytosis. Over a wide range of antibody concentrations, the mIgA 2F5 antibodies inhibited NL4-3.Balecto transcytosis significantly more than dIgA 2F5 antibodies: at 2 μg/mL, mIgA blocked trancytosis by 90% but dIgA by only 41%, and at 0.08 μg/mL, mIgA antibodies inhibited transcytosis by 30% but dIgA antibodies had no inhibitory effect (Fig. 5). Thus, compared to dIgA 2F5 anti-HIV-1 antibodies, mIgA 2F5 antibodies more potently reduced HIV-1 transcytosis across model epithelium.
Fig. 5.
Inhibition of HIV-1 transcytosis across model epithelium by mIgA and dIgA 2F5 antibodies. Antibody inhibition of NL4-3.Balecto transcytosis was assessed as described in the Materials and Methods. Results are the mean of three experiments; error bars represent SD.
2F5 antibodies inhibit HIV-1 entry into rectal mucosa
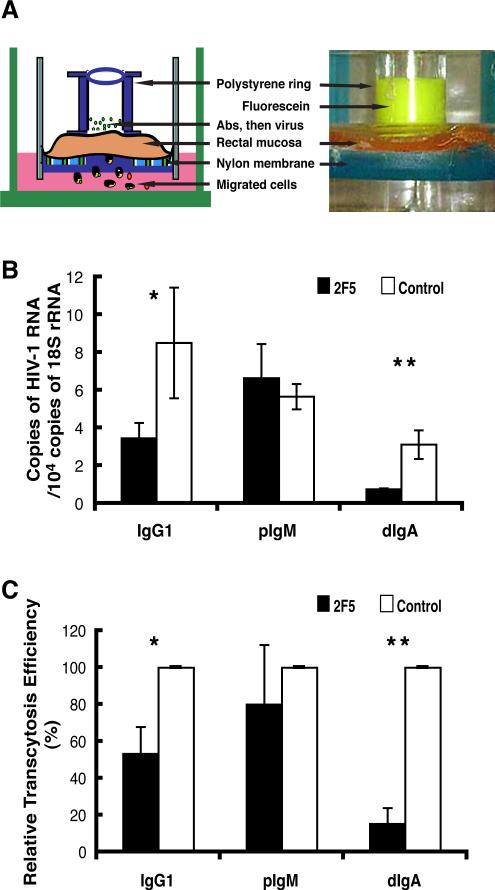
To more closely mimic mucosal HIV-1 transmission in vivo, we established a leakproof rectal explant system (Fig. 6A) similar to our previously described intestinal and vaginal explant system in which virus transcytoses across columnar epithelium to enter the mucosa ((45), Shen, manuscript submitted). Using this system, we determined whether 2F5 antibodies blocked HIV-1 transcytosis across rectal epithelium, thus entry into rectal mucosa. Total RNA was isolated from the explanted tissue at the conclusion of the entry assay, subjected to quantitative RT-PCR, and the relative copy number of HIV-1 RNA was determined according to the standard curve method by normalizing to 18S rRNA. Compared to control antibodies, IgG1 and dIgA 2F5 isotype antibodies significantly decreased the HIV-1 RNA copy number detected in explanted rectal mucosa (Fig. 6B). The decreased copy number in rectal mucosa from three separate tissues corresponded to IgG1- and dIgA-mediated reductions in the efficiency of HIV-1 transcytosis into rectal mucosa by 47% and 85%, respectively (Fig. 6C). Interestingly, pIgM, which did not inhibit transcytosis in model epithelium (Fig. 2), caused a 20% reduction in transcytosis in rectal mucosa (Fig. 6C). Having shown that mIgA 2F5 antibodies more potently inhibited HIV-1 transcytosis across model epithelium (Fig. 4), we next compared the abilities of mIgA and dIgA 2F5 to block HIV-1 transcytosis across rectal mucosal epithelium (Fig. 7). Compared to control IgA and dIgA, mIgA 2F5 antibodies at a final concentration of 2.5 μg/mL significantly decreased the number of HIV-1 RNA copies detected in rectal mucosa (Fig. 7A). mIgA 2F5 inhibited HIV-1 transcytosis and entry into rectal mucosa by 77%, whereas dIgA 2F5 inhibited transcytosis and entry by only 18% (Fig. 7B). Thus, 2F5 isotype antibodies, especially mIgA, inhibited HIV-1 transcytosis across rectal epithelium and thus entry into the subepithelial lamina propria.
Fig. 6.
Antibody inhibition of HIV-1 entry into rectal mucosa. (A) Schematic representation (left panel) and photograph 2 h after inoculation of fluorescene dye into upper chamber (right panel) of the explant system. (B) 2F5 antibody inhibition of HIV-1 entry into rectal mucosa. Antibodies were incubated for 30 min on the apical surface of rectal explants, after which virus was added and incubated for 2 h. Total RNA was isolated from the explanted tissue and analyzed by quantitative real-time RT-PCR for relative copy number of HIV-1 RNA normalized to the housekeeping gene 18S rRNA. Results are representative of three separate experiments with three different donor tissues; error bars represent SD (*P=0.07, **P=0.003). (C) HIV-1 transcytosis into rectal mucosa in the presence of 2F5 antibodies compared to transcytosis in the presence of control antibodies (defined as 100%). Values are the mean results from three separate donor tissues; error bars represent SD (*P=0.0014, **P=0.0003).
Fig. 7.
(A) IgA antibody inhibition of HIV-1 entry into rectal mucosa. IgA antibodies were incubated for 30 min on the apical surface of rectal explants, after which virus was added and incubated for 2 h. Total RNA isolated from the tissue was analyzed by quantitative real-time RT-PCR for relative copy number of HIV-1 RNA normalized to the housekeeping gene 18S rRNA. Results are representative of duplicate assays; error bars represent SD (P<0.01 compared to control IgA and dIgA 2F5). (B) Relative efficiency of HIV-1 transcytosis into rectal mucosa in the presence of IgA antibodies. The transcytosis efficiency in the presence of control IgA was defined as 100%. Values are the mean from duplicate assays; error bars represent SD (P<0.03 compared to control IgA and 2F5 dIgA).
DISCUSSION
Excluding parenteral transmissions, virtually all HIV-1 infections are acquired via the mucosal surfaces of the genital and gastrointestinal tracts (1-3), underscoring the importance of targeting mucosal transmission pathways, including transcytosis, in the prevention of HIV-1 infection. To model the transcytosis of HIV-1 across columnar epithelial cells (4, 6, 7), we constructed a monostratified epithelium using HT-29 cells transduced to express CCR5 because CCR5-tropic viruses are the dominant viruses transmitted in vivo (47) and are preferentially transcytosed across intestinal epithelial cells in vitro (27). We identified the following features of transcytosis in this model system. First, the amount of HIV-1 transcytosed across the monolayer increased linearly in relation to the amount of virus inoculated onto the apical surface. Second, the efficiency with which cell-free HIV-1 transcytosed across the epithelial monolayer ranged between 0.05% and 1.21%, depending on the HIV-1 strain. Third, the efficiency of HIV-1 transcytosis varied 4-fold among macrophage-tropic viruses with different envelope genes. Fourth, the cell source of HIV-1 affected transcytosis efficiency, as virus produced by PBMCs transcytosed more efficiently than virus produced by cell lines.
The HIV-1 inoculated onto the epithelial monolayers and explanted rectal mucosa in the experiments reported here was cell-free virus. Cell-free HIV-1, along with cell-associated virus, is present in breast milk (9, 49) and semen (1, 24, 26) and thus available for transcytosis across recipient small intestinal and rectal columnar epithelium during vertical and homosexual transmission, respectively. Presumably, transcytosis through HT-29 model epithelium is initiated by viral envelope binding to the epithelial surface. In this connection, the V3 region is reported to be important in HIV-1 transcytosis across pluristratified vaginal and ectocervical epithelium (10). Here, we showed that transcytosis efficiency varied among four viruses (NA20.B59, NA353.B27, NA420.B33 and NA420.LN85) with different V1-V3 regions. The charge of the V3 regions for the four viruses was the same, but sequence alignment of the envelopes of these viruses (30) revealed substitution of leucine for the more conserved phenylalanine at residue 315 in the V3 region of NA420.B33, the most efficiently transcytosed of the four viruses, raising the possibility that transcytosis efficiency was dependent, in part, on the V3 amino acid sequence. However, our findings do not exclude a role for other envelope determinants in HIV-1 transcytosis through columnar epithelium.
Cell-free HIV-1 transcytosis also has been investigated using endothelial cells as a model for heterosexual transmission (11, 28). Using HEC-1 endothelial monolayers with a transepithelial resistance of >300 Ohms/cm2, Chomont, et al., showed that 13 HIV-1-specific monoclonal antibodies, including broadly neutralizing 2F5 and gp120-specific 2G12 and IgG1b12, did not inhibit HIV-1 transcytosis (28). Two explanations address the difference between these findings and our results. First, in our system, IgG1 2F5 was preincubated on the epithelial cell surface prior to inoculation of virus to more closely recapitulate in vivo conditions, whereas Chaumont et al. added virus (50 pg/mL p24) and the IgG 2F5 antibodies together. Second, our HT-29 colon epithelial monolayers had a resistance of >390 Ohms/cm2, and 2F5 IgG1 antibodies blocked the transcytosis of seven HIV-1 isolates by 16-66%. However, at a resistance of 300 Ohms/cm2, 2F5 IgG1 antibodies at the same concentration did not block HIV-1 transcytosis in the HT-29 monolayers (data not shown), suggesting the source of the cell line, as well as transepithelial resistance and experimental design, are important considerations in assessing antibody inhibition of HIV-1 transcytosis through model epithelium.
Using HT-29 epithelial monolayers, we showed that IgG1 and dIgA, but not pIgM, 2F5 antibodies applied to the apical surface substantially reduced HIV-1 transcytosis through monostratified epithelium. The addition of antibodies to the basolateral chamber had no inhibitory effect, despite the previously demonstrated ability of dIgA and pIgM applied to the basolateral surface to neutralize cell-associated HIV-1 intracellularly (7, 18). Importantly, we used p24 to quantify input and output virus in the HT-29 assays, indicating that reduction in measurable virus in the basolateral chamber was due to the inhibition of transcytosis and not virus neutralization.
Finally, we developed an explant system using normal human rectal mucosa to test antibody inhibition of HIV-1 transcytosis across rectal mucosa ex vivo. IgG1 and dIgA isotypes of 2F5 antibody to ELDKWA in the gp41 MPER significantly inhibited HIV-1 transcytosis across the rectal mucosa. Compared to the IgG isotype, dIgA 2F5 displayed more potent inhibition of HIV-1 entry into rectal mucosa, reflecting the role of IgA as the predominant Ig isotype in gut secretions, in contrast to IgG, the dominant Ig isotype in female genital secretions (60, 61). These findings provide support for vaccine strategies directed at eliciting mucosal antibodies to the MPER of HIV-1 gp41 (17, 56).
IgA and IgG anti-HIV-1 antibodies have been detected in nearly all external secretions (60-62). IgA is actively transported across the epithelium via the polymeric immunoglobulin receptor (pIgR) (63) to protect against the entry of certain pathogens. Systemic and intravaginal administration of neutralizing IgG antibodies effectively prevented mucosal SHIV infection in macaques (64-66), consistent with the significant contribution of circulating IgG to genital IgG and IgG being the predominant isotype in genital secretions (60, 61). Here we report that IgG1 and dIgA 2F5 antibodies at 50 μg/mL, and mIgA 2F5 antibodies at 2.5 μg/mL, substantially reduced the transcytosis of HIV-1 through human rectal mucosa. (A single concentration of antibody was used due to the limited availability of normal rectal mucosa.) Importantly, mIgA 2F5 antibodies inhibited HIV-1 transcytosis across both model epithelium and human rectal mucosa substantially more efficiently than dIgA antibodies. The more effective blockade of HIV-1 transcytosis efficiency by mIgA was not due to an increase in avidity but more likely was due to the better access of the smaller IgA molecule to the MPER in the virus spike. The ability of MPER-specific antibodies to inhibit HIV-1 transcytosis suggests that this region of the viral envelope plays a crucial role in HIV-1 transcytosis of both cell-free (this study) and cell-associated HIV-1 (13-15). In this regard, the MEPR is the key structural component that binds to epithelial cell galactosylceramide, the first step in the transcytosis process (12). Thus, our findings suggest that antibody inhibition of HIV-1 transcytosis is achievable in intact rectal mucosa and should be considered a potential strategy for the prevention of HIV-1 transmission by the rectal route.
Footnotes
This work was supported by NIH grants (DK-47322, AI-83027DK-54495, AI-83539, RR-20136), the Center for HIV-1/AIDS Vaccine Immunology (AI-67854), UAB Mucosal HIV and Immunobiology Center (DK-64400) and grants from the French National Agency for AIDS Research, SIDACTION Ensemble Contra le SIDA, and the Research Service of the Veterans Administration.
REFERENCES
- 1.Royce RA, Sena A, Cates W, Jr., Cohen MS. Sexual transmission of HIV. N. Engl. J. Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 2.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, Kiwanuka N, Kigozi G, Kiddugavu M, Lutalo T, Nalugoda F, Wabwire-Mangen F, Meehan MP, Quinn TC. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 3.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 4.Bomsel M, Alfsen A. Entry of viruses through the epithelial barrier: pathogenic trickery. Nat. Rev. Mol. Cell Biol. 2003;4:57–68. doi: 10.1038/nrm1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nature reviews. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 7.Bomsel M, Heyman M, Hocini H, Lagaye S, Belec L, Dupont C, Desgranges C. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity. 1998;9:277–287. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]
- 8.Pope M. Mucosal dendritic cells and immunodeficiency viruses. J. Infect. Dis. 1999;179:S427–S430. doi: 10.1086/314798. [DOI] [PubMed] [Google Scholar]
- 9.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bobardt MD, Chatterji U, Selvarajah S, Van der Schueren B, David G, Kahn B, Gallay PA. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J. Virol. 2007;81:395–405. doi: 10.1128/JVI.01303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hocini H, Becquart P, Bouhlal H, Chomont N, Ancuta P, Kazatchkine MD, Bélec L. Active and selective transcytosis of cell-free human immunodeficiency virus through a tight polarized monolayer of human endometrial cells. J. Virol. 2001;75:5370–5374. doi: 10.1128/JVI.75.11.5370-5374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alfsen A, Bomsel M. HIV-1 gp41 envelope residues 650-685 exposed on native virus act as a lectin to bind epithelial cell galactosyl ceramide. J. Biol. Chem. 2002;277:25649–25659. doi: 10.1074/jbc.M200554200. [DOI] [PubMed] [Google Scholar]
- 13.Alfsen A, Iniguez P, Bouguyon E, Bomsel M. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J. Immunol. 2001;166:6257–6265. doi: 10.4049/jimmunol.166.10.6257. [DOI] [PubMed] [Google Scholar]
- 14.Tudor D, Derrien M, Diomede L, Drillet AS, Houimel M, Moog C, Reynes JM, Lopalco L, Bomsel M. HIV-1 gp41-specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4+ cell infection: an IgA gene and functional analysis. Mucosal Immunol. 2009;5:412–426. doi: 10.1038/mi.2009.89. [DOI] [PubMed] [Google Scholar]
- 15.Bomsel M, Pastori C, Tudor D, Alberti C, Garcia S, Ferrari D, Lazzarin A, Lopalco L. Natural mucosal antibodies reactive with first extracellular loop of CCR5 inhibit HIV-1 transport across human epithelial cells. AIDS. 2007;21:13–22. doi: 10.1097/QAD.0b013e328011049b. [DOI] [PubMed] [Google Scholar]
- 16.Alfsen A, Yu H, Magerus-Chatinet A, Schmitt A, Bomsel M. HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Mol. Biol. Cell. 2005;16:4267–4279. doi: 10.1091/mbc.E05-03-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matoba N, Magerus A, Geyer BC, Zhang Y, Muralidharan M, Alfsen A, Arntzen CJ, Bomsel M, Mor TS. A mucosally targeted subunit vaccine candidate eliciting HIV-1 transcytosis-blocking Abs. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13584–13589. doi: 10.1073/pnas.0405297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolbank S, Kunert R, Stiegler G, Katinger H. Characterization of human class-switched polymeric (immunoglobulin M [IgM] and IgA) anti-human immunodeficiency virus type 1 antibodies 2F5 and 2G12. J. Virol. 2003;77:4095–4103. doi: 10.1128/JVI.77.7.4095-4103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devito C, Broliden K, Kaul R, Svensson L, Johansen K, Kiama P, Kimani J, Lopalco L, Piconi S, Bwayo JJ, Plummer F, Clerici M, Hinkula J. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J. Immunol. 2000;165:5170–5176. doi: 10.4049/jimmunol.165.9.5170. [DOI] [PubMed] [Google Scholar]
- 20.Hocini H, Bélec L, Iscaki S, Garin B, Pillot J, Becquart P, Bomsel M. High-level ability of secetory IgA to block HIV type 1 transcytosis: contrasting secertory IgA and IgG responses to glycoprotein 160. AIDS Res. Hum. Retro. 1997;13:1179–1185. doi: 10.1089/aid.1997.13.1179. [DOI] [PubMed] [Google Scholar]
- 21.Lewis P, Nduati R, Kreiss JK, John GC, Richardson BA, Mbori-Ngacha D, Ndinya-Achola J, Overbaugh J. Cell-free human immunodeficiency virus type 1 in breast milk. J Infect Dis. 1998;177:34–39. doi: 10.1086/513816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koulinska IN, Villamor E, Chaplin B, Msamanga G, Fawzi W, Renjifo B, Essex M. Transmission of cell-free and cell-associated HIV-1 through breast-feeding. J Acquir Immune Defic Syndr. 2006;41:93–99. doi: 10.1097/01.qai.0000179424.19413.24. [DOI] [PubMed] [Google Scholar]
- 23.Lehman DA, Chung MH, John-Stewart GC, Richardson BA, Kiarie J, Kinuthia J, Overbaugh J. HIV-1 persists in breast milk cells despite antiretroviral treatment to prevent mother-to-child transmission. AIDS research and human retroviruses. 2008;22:1475–1485. doi: 10.1097/QAD.0b013e328302cc11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borzy MS, Connell RS, Kiessling AA. Detection of human immunodeficiency virus in cell-free seminal fluid. J. Acquir. Immune. Defic. Syndr. 1988;1:419–424. [PubMed] [Google Scholar]
- 25.Rasheed S, Li Z, Xu D. Human immunodeficiency virus load. Quantitative assessment in semen from seropositive individuals and in spiked seminal plasma. J. Reprod. Med. 1995;40:747–757. [PubMed] [Google Scholar]
- 26.Kiessling AA. Isolation of human immunodeficiency virus type 1 from semen and vaginal fluids. Methods Mol Biol. 2005;304:71–86. doi: 10.1385/1-59259-907-9:071. [DOI] [PubMed] [Google Scholar]
- 27.Meng G, Wei X, Wu X, Sellers MT, Decker JM, Moldoveanu Z, Orenstein JM, Graham MF, Kappes JC, Mestecky J, Shaw GM, Smith PD. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat. Med. 2002;8:150–156. doi: 10.1038/nm0202-150. [DOI] [PubMed] [Google Scholar]
- 28.Chomont N, Hocini H, Gody JC, Bouhlal H, Becquart P, Krief-Bouillet C, Kazatchkine M, Belec L. Neutralizing monoclonal antibodies to human immunodeficiency virus type 1 do not inhibit viral transcytosis through mucosal epithelial cells. Virology. 2008;370:246–254. doi: 10.1016/j.virol.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Kappes JC, Conway JA, Price RW, Shaw GM, Hahn BH. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J. Virol. 1991;65:3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duenas-Decamp MJ, Peters PJ, Burton D, Clapham PR. Determinants flanking the CD4 binding loop modulate macrophage tropism of human immunodeficiency virus type 1 R5 envelopes. J Virol. 2009;83:2575–2583. doi: 10.1128/JVI.02133-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters PJ, Sullivan WM, Duenas-Decamp MJ, Bhattacharya J, Ankghuambom C, Brown R, Luzuriaga K, Bell J, Simmonds P, Ball J, Clapham PR. Non-macrophage-tropic human immunodeficiency virus type 1 R5 envelopes predominate in blood, lymph nodes, and semen: implications for transmission and pathogenesis. J. Virol. 2006;80:6324–6332. doi: 10.1128/JVI.02328-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters PJ, Bhattacharya J, Hibbitts S, Dittmar MT, Simmons G, Bell J, Simmonds P, Clapham PR. Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages. J. Virol. 2004;78:6915–6926. doi: 10.1128/JVI.78.13.6915-6926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh SK, Fultz PN, Keddie E, Saag MS, Sharp PM, Hahn BH, Shaw GM. A molecular clone of HIV-1 tropic and cytopathic for human and chimpanzee lymphocytes. Virology. 1993;194:858–864. doi: 10.1006/viro.1993.1331. [DOI] [PubMed] [Google Scholar]
- 34.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A, et al. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retroviruses. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 37.Zhu M, Olee T, Le DT, Roubey RA, Hahn BH, Woods VL, Jr., Chen PP. Characterization of IgG monoclonal anti-cardiolipin/anti-beta2GP1 antibodies from two patients with antiphospholipid syndrome reveals three species of antibodies. Br. J. Haematol. 1999;105:102–109. [PubMed] [Google Scholar]
- 38.Liao HX, Sutherland LL, Xia SM, Brock ME, Scearce RM, Vanleeuwen S, Alam SM, McAdams M, Weaver EA, Camacho Z, Ma BJ, Li Y, Decker JM, Nabel GJ, Montefiori DC, Hahn BH, Korber BT, Gao F, Haynes BF. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology. 2006;353:268–282. doi: 10.1016/j.virol.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alam SM, Scearce RM, Parks RJ, Plonk K, Plonk SG, Sutherland LL, Gorny MK, Zolla-Pazner S, Vanleeuwen S, Moody MA, Xia SM, Montefiori DC, Tomaras GD, Weinhold KJ, Karim SA, Hicks CB, Liao HX, Robinson J, Shaw GM, Haynes BF. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection. J. Virol. 2008;82:115–125. doi: 10.1128/JVI.00927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staats HF, Alam SM, Scearce RM, Kirwan SM, Zhang JX, Gwinn WM, Haynes BF. In vitro and in vivo characterization of anthrax anti-protective antigen and anti-lethal factor monoclonal antibodies after passive transfer in a mouse lethal toxin challenge model to define correlates of immunity. Infect. Immun. 2007;75:5443–5452. doi: 10.1128/IAI.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Driver LC, Oertel MD. Synagis: an anti-RSV monoclonal antibody. Pediatr. Nurs. 1999;25:527–530. [PubMed] [Google Scholar]
- 42.Kunert R, Ruker F, Katinger H. Molecular characterization of five neutralizing anti-HIV type 1 antibodies: identification of nonconventional D segments in the human monoclonal antibodies 2G12 and 2F5. AIDS Res. Hum. Retroviruses. 1998;14:1115–1128. doi: 10.1089/aid.1998.14.1115. [DOI] [PubMed] [Google Scholar]
- 43.Berdoz J, Blanc CT, Reinhardt M, Kraehenbuhl JP, Corthesy B. In vitro comparison of the antigen-binding and stability properties of the various molecular forms of IgA antibodies assembled and produced in CHO cells. Proc. Natl. Acad. Sci. U.S.A. 1999;96:3029–3034. doi: 10.1073/pnas.96.6.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Persic L, Roberts A, Wilton J, Cattaneo A, Bradbury A, Hoogenboom HR. An integrated vector system for the eukaryotic expression of antibodies or their fragments after selection from phage display libraries. Gene. 1997;187:9–18. doi: 10.1016/s0378-1119(96)00628-2. [DOI] [PubMed] [Google Scholar]
- 45.Shen R, Richter HE, Clements RH, Novak L, Huff K, Sankaran-Walters S, Dandekar S, Clapham PR, Smythies LE, Smith PD. Macrophages in vaginal but not in intestinal mucosa are monocyte-like and permissive to HIV-1. J. Virol. 2009;83:3258–3267. doi: 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moroney SM, Heller LC, Widen RH. Evaluation of two TaqMan PCR assays for the detection of HIV-1 proviral DNA in blood samples. J. Microbiol. Methods. 2006;65:350–353. doi: 10.1016/j.mimet.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. USA. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cantin R, Methot S, Tremblay MJ. Plunder and stowaways: incorporation of cellular proteins by enveloped viruses. J Virol. 2005;79:6577–6587. doi: 10.1128/JVI.79.11.6577-6587.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho YS, Abecasis AB, Theys K, Deforche K, Dwyer DE, Charleston M, Vandamme AM, Saksena NK. HIV-1 gp120 N-linked glycosylation differs between plasma and leukocyte compartments. Virol J. 2008;5:14–24. doi: 10.1186/1743-422X-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato K, Aoki J, Misawa N, Daikoku E, Sano K, Tanaka Y, Koyanagi Y. Modulation of human immunodeficiency virus type 1 infectivity through incorporation of tetraspanin proteins. J Virol. 2008;82:1021–1033. doi: 10.1128/JVI.01044-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pai EF, Klein MH, Chong P, Pedyczak A. W. I. P. Organization; 2000. [Google Scholar]
- 52.Ofek G, Tang M, Sambor A, Katinger H, Mascola JR, Wyatt R, Kwong PD. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. World Intellectual Propert Organization patent WO-00/61618. J. Virol. 2004;78:10724–10737. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cardoso RM, Zwick MB, Stanfield RL, Kunert R, Binley JM, Katinger H, Burton DR, Wilson IA. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22:163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PW. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dennison SM, Stewart SM, Stempel KC, Liao HX, Haynes BF, Alam SM. Stable docking of neutralizing human immunodeficiency virus type 1 gp41 membrane-proximal external region monoclonal antibodies 2F5 and 4E10 is dependent on the membrane immersion depth of their epitope regions. J. Virol. 2009;83:10211–10223. doi: 10.1128/JVI.00571-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matoba N, Griffin TA, Mittman M, Doran JD, Alfsen A, Montefiori DC, Hanson CV, Bomsel M, Mor TS. Transcytosis-blocking abs elicited by an oligomeric immunogen based on the membrane proximal region of HIV-1 gp41 target non-neutralizing epitopes. Curr. HIV Res. 2008;6:218–229. doi: 10.2174/157016208784324994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torres M, May R, Scharff MD, Casadevall A. Variable-region-identical antibodies differing in isotype demonstrate differences in fine specificity and idiotype. J. Immunol. 2005;174:2132–2142. doi: 10.4049/jimmunol.174.4.2132. [DOI] [PubMed] [Google Scholar]
- 58.Pritsch O, Magnac C, Dumas G, Bouvet JP, Alzari P, Dighiero G. Can isotype switch modulate antigen-binding affinity and influence clonal selection? Eur. J. Immunol. 2000;30:3387–3395. doi: 10.1002/1521-4141(2000012)30:12<3387::AID-IMMU3387>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 59.Belec L, Ghys PD, Hocini H, Nkengasong JN, Tranchot-Diallo J, Diallo MO, Ettiegne-Traore V, Maurice C, Becquart P, Matta M, Si-Mohamed A, Chomont N, Coulibaly IM, Wiktor SZ, Kazatchkine MD. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1-seronegative African women. J. Infect. Dis. 2001;184:1412–1422. doi: 10.1086/324375. [DOI] [PubMed] [Google Scholar]
- 60.Mestecky J, Moldoveanu Z, Russell MW. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am. J. Reprod. Immunol. 2005;53:208–214. doi: 10.1111/j.1600-0897.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 61.Mestecky J. Humoral immune responses to the human immunodeficiency virus type-1 (HIV-1) in the genital tract compared to other mucosal sites. J. Reproduct. Immunol. 2006;72:1–17. doi: 10.1016/j.jri.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 62.Moldoveanu Z, Huang WQ, Kulhavy R, Pate MS, Mestecky J. Human male genital tract secretions: both mucosal and systemic immune compartments contribute to the humoral immunity. J. Immunol. 2005;175:4127–4136. doi: 10.4049/jimmunol.175.6.4127. [DOI] [PubMed] [Google Scholar]
- 63.Apodaca G, Bomsel M, Arden J, Breitfeld PP, Tang K, Mostov KE. The polymeric immunoglobulin receptor. A model protein to study transcytosis. J. Clin. Invest. 1991;87:1877–1882. doi: 10.1172/JCI115211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 65.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 66.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]