Chemotherapy providers have generally developed and implemented good practices around the safe delivery of intravenous chemotherapy. Nonetheless, practices applying for QOPI certification usually modify or expand one or more processes to meet standards for safe parenteral administration.
Abstract
In 2009, ASCO and the Oncology Nursing Society (ONS) published standards for the safe use of parenteral chemotherapy in the outpatient setting, including issues of practitioner orders, preparation, and administration of medication. In 2011, these were updated to include inpatient facilities. In December 2011, a multistakeholder workgroup met to address the issues associated with orally administered antineoplastics, under the leadership of ASCO and ONS. The workgroup participants developed recommended standards, which were presented for public comment. Public comments informed final edits, and the final standards were reviewed and approved by the ASCO and ONS Boards of Directors. Significant newly identified recommendations include those associated with drug prescription and the necessity of ascertaining that prescriptions are filled. In addition, the importance of patient and family education regarding administration schedules, exception procedures, disposal of unused oral medication, and aspects of continuity of care across settings were identified. This article presents the newly developed standards.
Introduction
Antineoplastic chemotherapy provides great benefit to patients with both malignant and nonmalignant diseases. In general, these medications often have a narrow therapeutic window: the difference between the optimum therapeutic dose and doses that are too low to be effective and so high as to produce overwhelming toxicities is often small. To promote safe administration of this class of medication, ASCO and Oncology Nursing Society (ONS) developed a set of standards addressing the prescription, preparation, and administration of these medications in 2009, using an integrated process including multistakeholder participation and public comments.1 An update expanding the standards to include the inpatient setting was published in 2012.2
These standards only minimally addressed the use of oral chemotherapy. As opposed to more common medications used to treat chronic medical conditions, oral chemotherapy is often administered on a complex schedule of varied days of dosing, and varied dosing even on one day. When medication is administered outside of a controlled setting (whether by patients, their families, or other caregivers), issues of compliance are magnified as compared with the circumstance of treatment facilities, where drug delivery and drug administration are known and observed. In the worst case scenario, patients are given paper or electronic prescriptions and little instruction or help getting the prescriptions filled. At best, there is rarely documentation of medication being ingested, either by patient logs or metabolic testing.3–7
Further, the use of oral chemotherapy is increasing. During an average year, approximately 1.5% of insurance beneficiaries receive treatment with antineoplastic chemotherapy. Based on a Massachusetts claims analysis, in 2010, 16.1% of these patients received oral chemotherapy.8 It is estimated that this will soon reach 25%, as one quarter of the approximately 400 new drugs in development will be orally administered agents.9 Therefore, it is necessary to expand the ASCO/ONS chemotherapy administration safety standards to include oral agents.
Methods
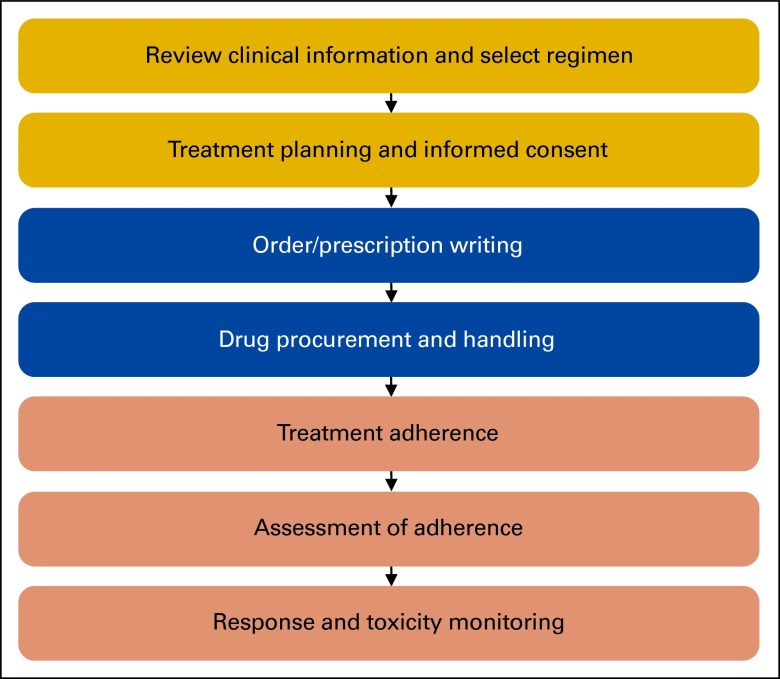
The process for developing the oral chemotherapy administration standards replicated the methodology previously used by ASCO and ONS and has been previously described.1 Briefly, the development process was led by a Steering Group from ASCO and ONS with previous experience in this project (the present authors). Steering group members formalized the scope of the project (Figure 1), and ASCO staff performed a structured literature search to identify relevant literature. The Steering Group reviewed the literature and met via a series of conference calls to generate the draft standards within each domain, to guide review and revision at a multistakeholder consensus workshop.
Figure 1.
Oral chemotherapy administration flow.
Steering Group members invited professional society representatives and individual experts to the workshop meeting held at ASCO headquarters in Alexandria, VA; a list of workshop participants is posted at http://www.asco.org/safety and http://www.ons.org/CNECentral/Chemo/Standards. The relevant literature and the initial draft standards were distributed to workshop participants in advance of the meeting, along with introductory materials regarding the projects goals and scope. Common definitions of terminology were also developed (Table 1). The workshop was convened in December 2011, with 38 participants, including oncologists, nurses, pharmacists, social workers, practice administrators, and patient advocates. Standards were discussed, reviewed, and revised by the workshop participants in small breakout sessions. Resulting standards were reviewed by the full group for redundancy and gaps. Finally, workshop participants voted on the draft standards immediately after the meeting. Voting results and subsequent review by the Steering Group yielded the set of standards released for public comment.
Table 1.
ASCO/ONS Chemotherapy Administration Safety Standards: Definitions
| Term | Definition |
|---|---|
| Adherence | The degree or extent of conformity to the provider's recommendations about day-to-day treatment with respect to timing, dosing, and frequency (synonymous with compliance). |
| Chemotherapy | All antineoplastic agents used to treat cancer, given through oral and parenteral routes or other routes as specified in the standard. Types include targeted agents, alkylating agents, antimetabolites, plant alkaloids and terpenoids, topoisomerase inhibitors, antitumor antibiotics, monoclonal antibodies, and biologics and related agents. Hormonal therapies are not included in the definition of chemotherapy for the standards. |
| Chemotherapy regimen | One or more chemotherapeutic agents used alone or in combination in a well-defined protocol, generally administered cyclically. |
| Chemotherapy setting (site) | All chemotherapy treatment settings (inpatient and outpatient). |
| Clinical encounter | Clinical encounters include each inpatient day, practitioner visits, and chemotherapy administration visits, but not laboratory or administrative visits. |
| Compliance | The degree or extent of conformity to the provider's recommendations about day-to-day treatment with respect to timing, dosing, and frequency (synonymous with adherence). |
| Persistence | The ability of a person to continue to take medication over the prescribed and chronic course of an illness, including getting and taking refills on initial prescriptions, often reflecting education. |
| Practitioner | Licensed independent practitioner, including physicians, advanced practice nurses (nurse practitioner or clinical nurse specialist), and/or physician assistants, as determined by state law. |
Public comment was accomplished using the Zarca Web-based survey tool (Zarca Interactive, Herndon, VA), over a 4-week period in March and April 2012. ASCO, ONS, and organizations that contributed to the workshop encouraged submissions of comments from their members and external stakeholders. All standards were provided, with changes highlighted. Participants were asked, “Should this standard be included in the final set?” with a “yes” or “no” response, and space was provided for comments.
Public comments were reviewed by Steering Group members, and used to select which standards to retain and to inform edits for clarity. Final standards were approved by the ASCO and ONS Boards of Directors in October 2012.
Results
There were 207 responses to the request for public comments; 205 came through the online survey and two through direct communication to ASCO and/or ONS. The frequency of comments ranged from 14 to 50 per measure. The proportion of supporting “yes” votes ranged from 81% to 98%. All comments were reviewed and considered by the steering group, with specific focus paid to standards that scored less than 90% “yes” votes. A summary of the 17 changes resulting from this initiative is found in Table 2. Eight changes were revisions to existing standards, and nine were additions.
Table 2.
Summary of Changes and Additions to the 2011 ASCO/ONS Chemotherapy Administration Safety Standards
| 2011 Standard | Changes | Public Comment (% “yes” responses rounded down) | 2013 Standards |
|---|---|---|---|
| 1D | Added “and safe handling of hazardous chemotherapy agents” | 96%—No changes made. | 1D |
| 1F | Added “in the health care setting” | 93%—No changes made. | 1F |
| New | Proposed fertility discussion/pregnancy screening | 91%—Split into two standards; pregnancy screening added to 2C and fertility education added to 18D. | 2C and 18D |
| New | Proposed assessment of barriers before initiation of oral chemotherapy | 86%—Proposed standard revised on the basis of comments. | 2I |
| New | Proposed standard addressing drug storage | 96%—Minimal edits made on the basis of comments. | 8 |
| 8 | Proposed documentation of changes made to oral chemotherapy regimens | 96%—No changes made. | 9 |
| New | Proposed items to include in a prescription for oral chemotherapy | 92%—Proposed standards revised on the basis of comments. | 12 |
| New | Proposed communication for discontinuation of oral chemotherapy | 91%—Minimal edits made based on comments. | 14 |
| 13 | Additions of labeling requirements specific to oral chemotherapy | 95%—No changes made. | 16 |
| New | Proposed standard to document patient engagement | 80%—Comments raised concerns about the ability to measure engagement. Dropped as a standard; concept incorporated into existing standard. | 18 |
| 15 | Proposed additions to patient education materials. | 98%—Revised to reflect specific needs of oral chemotherapy. | 20 |
| 18 | Proposed verification of IV pump rate if applicable. | 88%—Edited language to improve clarity and the ability to assess. | 21B |
| New | Proposed assessment of adherence to oral chemotherapy | 87%—Considerations raised in public comment were discussed at the workshop. Changed from policy to process in place. | 25 |
| New | Proposed medication reconciliation and drug-drug interaction analysis at each clinical encounter. | 90%—Minor edits from workshop discussion and comments. Revised existing standard. | 27 |
| 25 | Proposed addition of “monitoring visits” to existing standards. | 91%—Proposed change not made, as the standard incorporates all types of visits. | 25 |
| 27 | Proposed changes to enhance communication | 87%—Edited current standard for clarity in response to public comment. | 31 |
| New | Proposed standard to monitor adherence and toxicity in oral chemotherapy | 93%—No changes made. | 35 |
Abbreviation: IV, intravenous.
Table 3 details the results of the public comment period. Most common respondents were nurses, accounting for 166, with 14 physicians and 17 pharmacists.
Table 3.
Summary of Public Comment Respondents
| Responding Group | Responses |
Detail | |
|---|---|---|---|
| No. | % | ||
| Nurse | 125 | 125 RN, 12 NP, 28 CNS, 1 LVN | |
| Physician | 14 | ||
| Pharmacist | 17 | ||
| Patient or patient advocate | 1 | ||
| Academic medical center affiliation | 21 | ||
| Private practice affiliation | 89 | 12% independent, 27% hospital associated, 30% ambulatory clinic | |
| Total | 207 | ||
Abbreviations: CNS, clinical nurse specialist; LVN, licensed vocational nurse; NP, nurse practitioner; RN, registered nurse.
Standards receiving the lowest “yes” score or greatest numbers of comments were identified as complicated or cumbersome. If these standards were reasonable in concept, they were identified as impracticable in the current practice environment. Comments indicate that systems are not in place to oversee follow-up, medication adherence, or communication with outside agencies. Standards were revised or removed on the basis of this feedback, as summarized in Table 2. The new standards were integrated into the parent set to create the unified 2013 ASCO/ONS Standards for Safe Chemotherapy Administration (Table 4).
Table 4.
American Society of Clinical Oncology/Oncology Nursing Society Chemotherapy Administration Safety Standards
| Chemotherapy Administration Safety Standards |
|---|
| Explanatory notes or examples are provided in italics, when applicable. |
| Staffing-related standards |
| 1. The practice/institution has policies, procedures, and/or guidelines for verification of training and continuing education for clinical staff. |
| A. Orders for parenteral and oral chemotherapy are written and signed by licensed independent practitioners who are determined to be qualified by the practice/institution according to the practice's/institution's policies, procedures, and/or guidelines. |
| B. Chemotherapy drugs (oral or parenteral) are prepared by a pharmacist, pharmacy technician, or nurse determined to be qualified according to the practice's policies, procedures, and/or guidelines. |
| C. Only qualified physicians, physician assistants, advanced practice nurses, or registered nurses administer chemotherapy. |
| D. The practice/institution has a comprehensive educational program for new staff administering chemotherapy, including a competency assessment, or the practice/institution uses an established educational program regarding chemotherapy administration that ends in competency assessment. Education and competency assessment regarding chemotherapy administration includes all routes of administration used in the practice/institution site (eg, parenteral, oral, intrathecal, intraperitoneal, intravesicular), and safe handling of hazardous chemotherapy agents. |
| An example of an established educational program is the ONS Chemotherapy and Biotherapy Course. |
| E. The practice/institution has a standard mechanism for monitoring chemotherapy administration competency at specified intervals. |
| Annual competency reassessment is recommended. |
| F. There must be at least one clinical staff member who maintains current certification in basic life support on site during chemotherapy administration in the health care setting. |
| Certification should be from a nationally accredited course. Clinical staff includes staff involved in patient care; RNs, MDs, NPs, etc. |
| Chemotherapy planning: Chart documentation standards |
| 2. Before the first administration of a new chemotherapy regimen, chart documentation available to the practice/institution includes: |
| A. Pathologic confirmation or verification of initial diagnosis. If original pathology report is unobtainable, note of explanation is in chart or a reference to primary source pathology. |
| This standard does not imply the need to rebiopsy if not clinically necessary. |
| B. Initial cancer stage or current cancer status. Cancer stage is defined at diagnosis. Cancer status includes a current description of the patient's disease since diagnosis/staging, if relevant (eg, recurrence, metastases). |
| C. Complete medical history and physical examination that includes, at minimum, height, weight, pregnancy screening (when applicable), and assessment of organ-specific function as appropriate for the planned regimen. |
| Example of assessment of organ-specific function as appropriate for the planned regimen: patient plan for cisplatin requires pretreatment assessment of kidney function. |
| D. Presence or absence of allergies and history of other hypersensitivity reactions. |
| E. Documentation of patient's comprehension regarding chemotherapy regimens (and associated medications), including information regarding disease. |
| F. Assessment regarding psychosocial concerns and need for support, with action taken when indicated. |
| Documentation of psychosocial concerns may include copy of distress, depression, or anxiety screening form in the chart; patient self-report of distress, depression, or anxiety; or chart documentation regarding patient coping, adjustment, depression, distress, anxiety, emotional status, family support and care giving, coping style, cultural background, and socioeconomic status. |
| G. The chemotherapy treatment plan, including, at minimum, chemotherapy drugs, doses, anticipated duration, and goals of therapy. |
| H. For oral chemotherapy, the frequency of office visits and monitoring that is appropriate for the individual and the antineoplastic agent and is defined in the treatment plan. |
| I. Before initiation of an oral chemotherapy regimen, assessment of the patient's ability to obtain the drug and administer it according to the treatment plan is documented, along with a plan to address any identified issues. |
| Assessment includes socioeconomic, psychosocial, financial, administrative and regulatory factors that may influence initiation and/or adherence to prescribed regimen. |
| General chemotherapy practice standards |
| 3. The practice/institution: |
| A. Defines standard chemotherapy regimens by diagnosis with references readily available, and/or |
| B. Identifies source(s) for chemotherapy regimens, including local or centralized institutional review board–approved clinical research protocols or guidelines. |
| 4. For orders that vary from standard chemotherapy regimens, practitioners provide a supporting reference. Reasons for dose modification or exception orders are documented. |
| Exception orders may include notation that standard treatment is contraindicated as a result of pre-existing comorbidity, organ dysfunction, or prior therapy. |
| 5. The practice/institution maintains written statements that determine the appropriate time interval for regimen-specific laboratory tests that are: |
| A. Evidence based when national guidelines exist (eg, American Society of Clinical Oncology or National Comprehensive Cancer Network guidelines), or |
| B. Determined by practitioners at the site. |
| Documentation of regimen-specific laboratory tests may be part of standardized regimen orders. |
| 6. The practice/institution maintains a policy for how informed consent is obtained and documented for chemotherapy. |
| The practice/institution may provide options for consent (eg, use of chart documentation of patient consent or a signed patient consent form) that allow for variation among practitioners in the practice/institution. |
| 7. If the practice/institution administers chemotherapy that is prepared (mixed) off site, the practice/institution maintains a policy for quality control of that chemotherapy. |
| 8. If practice/institution manages its own pharmacy, the practice/institution has a policy regarding the storage of chemotherapy (including separation of look-a-like products, sound-a-like products, and agents available in multiple strengths). Chemotherapy is stored in a designated area according to regulatory guidelines. |
| Chemotherapy order/prescription standards |
| 9. The practice/institution does not allow verbal orders except to hold or stop chemotherapy administration. New orders or changes to orders, including changes to oral chemotherapy regimens (eg, dose adjustments communicated directly to patients), are documented in the medical record. |
| Fax and e-mail orders are considered written orders. |
| 10. The practice/institution maintains and uses standardized, regimen-level, preprinted or electronic forms for parental chemotherapy prescription writing. |
| Standardized forms may be incorporated into e-prescribing software or electronic health records. |
| 11. Order forms inclusively list all chemotherapy agents in the regimen and their individual dosing parameters. All medications within the order set are listed using full generic names and follow Joint Commission standards regarding abbreviations. |
| Brand names should be included in orders only where there are multiple products or when including the brand name otherwise assists in identifying a unique drug formulation. |
| Complete orders must include: |
| A. Patient's full name and a second patient identifier (eg, medical record number, DOB) |
| B. Date |
| C. Diagnosis |
| D. Regimen name and cycle number |
| E. Protocol name and number (if applicable) |
| F. Appropriate criteria to treat (eg, based on relevant laboratory results and toxicities) |
| G. Allergies |
| H. Reference to the methodology of the dose calculation or standard practice equations (eg, calculation of creatinine clearance) |
| I. Height, weight, and any other variables used to calculate the dose |
| J. Dosage |
| Doses do not include trailing zeros; use a leading zero for doses <1 mg. |
| K. Route and rate (if applicable) of administration |
| L. Length of infusion (if applicable) |
| M. Supportive care treatments appropriate for the regimen (including premedications, hydration, growth factors, and hypersensitivity medications) |
| N. Sequence of drug administration (if applicable) |
| Practices/institutions are not expected to be in full compliance with this standard if they currently have electronic ordering systems that prevent compliance. Appropriate changes should be implemented as soon as possible to ensure that electronic ordering systems integrate all of these elements. If the information cannot be captured in the electronic system, it should be documented within the patient record. |
| 12. Complete prescriptions for oral chemotherapy include: |
| A. Patient's full name and a second patient identifier (eg, medical record number, DOB) |
| B. Drug name |
| C. Date |
| D. Reference to methodology of dose calculation, height, weight and other variables (as applicable) |
| E. Dosage |
| F. Quantity to be dispensed |
| G. Doses may be rounded to the nearest tablet size or specify alternating doses each day to obtain the correct overall dosage |
| Doses do not include trailing zeros; use a leading zero for doses <1 mg |
| H. Route and frequency of administration |
| I. Duration of therapy number of days of treatment (if the medication is not to be taken continuously) |
| J. Number of refills (including none) |
| Practices/institutions are not expected to be in full compliance with this standard if they currently have electronic ordering systems or electronic prescribing systems that prevent compliance. Appropriate changes should be implemented as soon as possible to ensure that electronic ordering or prescribing systems integrate all of these elements. If the information cannot be captured in the electronic system, it should be documented within the patient record. |
| 13. Orders for parenteral/oral chemotherapy should be written with a time limitation to ensure appropriate evaluation at predetermined intervals. |
| 14. The practice/institution maintains procedures for communicating discontinuation of oral chemotherapy, including patient education regarding time to stop treatment, and patient education regarding disposal of remaining medication. |
| In certain circumstances, it may be appropriate to alert the dispensing pharmacy when the oral chemotherapy is discontinued. |
| Drug preparation |
| 15. A second person (a practitioner or other personnel approved by the practice/institution to prepare or administer chemotherapy) independently verifies each order for chemotherapy before preparation, including confirming: |
| A. Two patient identifiers |
| B. Drug names |
| C. Drug dose |
| D. Drug volume |
| E. Route of administration |
| F. Rate of administration |
| G. The calculation for dosing (including the variables used in this calculation) |
| H. Treatment cycle and day of cycle |
| 16. Chemotherapy drugs are labeled immediately upon preparation, including, at minimum: |
| A. Patient's full name and a second patient identifier (eg, medical record number, DOB) |
| B. Full generic drug name |
| C. Drug administration route |
| D. Total dose to be given |
| E. Total volume required to administer this dosage |
| F. Date of administration |
| G. Date and time of preparation |
| H. Date and time of expiration when not for immediate use |
| Immediate use must be defined by institutional policy, state, and federal regulations (eg, use within 2 h) |
| I. Special handling instructions as appropriate |
| J. Administration instructions (oral agents) |
| K. Number of refills (oral agents) |
| L. Prescriber name (oral agents) |
| Practices/institutions are not expected to be in full compliance with this standard if they currently have electronic systems that are unable to meet these labeling requirements. Appropriate changes should be implemented as soon as possible to ensure that electronic labels integrate all of these elements. |
| 17. Practices/institutions that administer intrathecal medication maintain policies specifying that intrathecal medication will: |
| A. Not be prepared during preparation of any other agents |
| B. Be stored, once prepared, in an isolated container or location with a uniquely identifiable intrathecal medication label |
| C. Be delivered to the patient only with other medication intended for administration into the CNS |
| Patient consent and education |
| 18. Before initiation of a chemotherapy regimen, each patient is given written documentation, including, at minimum: |
| A. Information regarding his or her diagnosis |
| B. Goals of therapy |
| C. Planned duration of chemotherapy, drugs, and schedule |
| D. Information on possible short- and long-term adverse effects, including infertility risks |
| E. Regimen- or drug-specific risks or symptoms that require notification and emergency contact information, including: |
| • How to contact the practice or organization |
| • Symptoms that should trigger a call |
| • Who should be called in specific circumstances (oncologist or other provider) |
| F. Plan for monitoring and follow-up, including appointments with practitioners or laboratory testing |
| Patient education materials should be appropriate for the patient's reading level/literacy and patient-caregiver understanding. Documentation should include patient feedback reflecting understanding and engagement. |
| 19. Informed consent for chemotherapy must be documented prior to initiation of a chemotherapy regimen. |
| The consent process should follow appropriate professional and legal guidelines. For more information and sample forms, see http://www.asco.org/consent. |
| 20. All patients who are prescribed oral chemotherapy are provided written or electronic patient education materials about the oral chemotherapy before or at the time of prescription. |
| A. Patient education includes: |
| • The storage, handling, preparation, administration, and disposal of oral chemotherapy |
| • Concurrent cancer treatment and supportive care medications/measures (when applicable) |
| • Possible drug/drug and drug/food interactions |
| • The plan for missed doses |
| B. The education plan includes family, caregivers, or others based on the patient's ability to assume responsibility for managing therapy. |
| Patient education materials should be appropriate for the patient's reading level/literacy and patient-caregiver understanding. Documentation should include patient feedback reflecting understanding and engagement. |
| Chemotherapy administration |
| 21. Before chemotherapy administration: |
| A. A practitioner who is administering the chemotherapy confirms with the patient his/her planned treatment prior to each cycle |
| B. At least two practitioners or personnel approved by the practice/institution to prepare or administer chemotherapy, verify the accuracy of: |
| • Drug name |
| • Drug dose |
| • Drug volume |
| • Rate of administration |
| • Expiration dates/times, if applicable; expiration date/time is not required if for immediate use |
| (Immediate use must be defined by intuitional policy, state, federal regulations, eg, use within 2 h) |
| • Appearance and physical integrity of the drugs |
| • Rate set on infusion pump, when utilized |
| C. A practitioner who is administering the chemotherapy documents that the verification in B was done |
| D. At least two individuals, in the presence of patient, verify the patient identification using at least two identifiers (eg, medical record number, DOB) |
| 22. Extravasation management procedures are defined and align with current literature and guidelines; antidote order sets and antidotes are accessible. |
| 23. A licensed independent practitioner is on site and immediately available during all chemotherapy administration in licensed infusion centers and acute care settings. |
| A licensed practitioner must be on site for the initiation of first doses of parenteral chemotherapy and should remain available throughout the administration unless the patient is transitioned to a home care or nonacute facility. Patients/caregivers are educated in procedures for unplanned events and circumstances when subsequent doses are administered in either a home care or nonacute facility. |
| Monitoring and assessment |
| 24. The practice/institution maintains protocols for response to life-threatening emergencies, including escalation of patient support beyond basic life support. |
| It is recommended that emergency protocols be reviewed annually. |
| 25. The practice/institution maintains a written policy and/or procedure to complete an initial assessment of patients' adherence to oral chemotherapy. The policy must include a plan for clinical staff to address any issues identified within a time frame appropriate to the patient and regimen. |
| Examples of assessment for adherence to an oral chemotherapy treatment plan include: |
| • Confirmation that the patient filled the prescription as written |
| • Inquiry regarding concerns about treatment costs |
| • Verification that the patient understands how to take the prescribed oral chemotherapy (eg, frequency, with/without food, whole or crushed, etc) |
| • Verification that the patient understands what to do in case of missed doses |
| • Assessment for potential toxicity |
| 26. On each clinical visit or day of treatment during chemotherapy administration, staff: |
| A. Assess and document clinical status and/or performance status |
| B. Document vital signs and weight |
| C. Verify allergies, previous reactions, and treatment-related toxicities |
| D. Assess and document psychosocial concerns and need for support, taking action when indicated |
| This standard applies to all clinical encounters (including each inpatient day, practitioner visits, and chemotherapy administration visits, but not laboratory or administrative visits). |
| 27. At each clinical encounter, staff review and document the patient's current medications, including over-the-counter medications and complementary and alternative therapies. Any change in the patient's medications prompts a review for drug-drug interactions. |
| This standard applies to all clinical encounters (including each inpatient day, practitioner visits, and chemotherapy administration visits, but not laboratory or administrative visits). |
| 28. The practice/institution maintains referral resources for psychosocial and other supportive care services. |
| 29. The practice/institution has a procedure for documentation and follow-up for patients who miss or cancel scheduled visits and/or chemotherapy treatments. |
| 30. The practice/institution evaluates and documents treatment-related toxicities using standard definitions or criteria selected by that practice/institution. |
| Examples include NCI Common Toxicity Criteria and WHO Toxicity Criteria. |
| 31. The practice/institution has policies and procedures that identify: |
| A. A process to provide 24/7 triage to a practitioner (eg, on-call practitioner, emergency department) for care of toxicities |
| B. Consistent documentation and communication of toxicities, modifications in dose or schedule, or discontinuation of treatment, within the practice/institution |
| 32. The practice/institution has a system in place to promote a safe handoff between all sites of care, including evaluating and communicating appropriateness of, and schedule for, chemotherapy administration in another setting. |
| 33. Toxicity assessment documentation is available for planning subsequent treatment cycles. |
| 34. The practice/institution has a process to track cumulative doses of chemotherapy agents associated with a risk of cumulative toxicity. |
| 35. The practice/institution maintains a plan for ongoing and regimen-specific assessment of each patient's oral chemotherapy adherence and toxicity. The policy includes, at minimum, patient assessment for adherence and toxicity at each clinical encounter at the practice/institution, as well as a plan for clinical staff to address any issues identified. |
| 36. The practice/institution uses standard, disease-specific processes to monitor treatment response (e.g., use of evaluations, laboratory results, or scans/imaging) that are based on published literature/guidelines or are determined by the practice/institution. |
| 37. The practice/institution encourages the reporting of errors and near misses and has a formal process for evaluating the data. Error and near-miss reports are reviewed and evaluated at least semiannually. |
NOTE. The current version of these consensus standards reflects modifications that are intended to extend the standards to address the safe use of oral chemotherapeutic agents. The American Society of Clinical Oncology/Oncology Nursing Society (ASCO/ONS) standards are intended to reflect current thinking on best practices and, as such, are intended to be a “living” document; future modifications are expected.
Although the ASCO/ONS standards were not developed to address this issue, ASCO and ONS endorse the safe handling of chemotherapy agents. Published guidelines define the expectations for organizations and health care workers related to the use of safe handling precautions (American Society of Health System Pharmacists: Am J Health Syst Pharm 63:1172-1193, 2006; National Institute for Occupational Safety and Health: DHHS publication No. 2004-165, 2004; Occupational Safety and Health Administration: OSHA technical manual, 1995; Polovich M et al: Pittsburgh, PA, Oncology Nursing Society, 2009; US Pharmacopeial Convention, Rockville, MD, 2008). Education, training, and competency validation for chemotherapy administration must necessarily include this aspect of practice. Organizations should focus on a culture of safety, because of the relationship between patient and health care worker safety (Friese CR et al: BMJ Qual Saf, 2011; Polovich M, Clark PC: Oncology Nursing Forum, 2012).
The standards are not deemed comprehensive and do not account for individual patient variation. It is the responsibility of each administering agent to determine the best methods for chemotherapy administration for each patient. The standards are not medical advice or legal advice. To the extent that the standards conflict with applicable federal, state, or local legal requirements, practitioners should comply with those requirements. The administering agent is solely responsible for, and assumes all risks of, administering chemotherapy drugs notwithstanding any adherence to the standards herein. ASCO and ONS disclaim any and all liability with respect to the standards and the execution of the standards by any party.
Abbreviations: DOB, date of birth; NCI, National Cancer Institute; NP, nurse practitioner; RN, registered nurse.
Discussion
This most recent version of the ASCO/ONS Chemotherapy Administration Safety Standards provides a consensus-based set of recommendations created through review of available evidence and a formal process for obtaining expert stakeholder input. As compared with the 2011 update, changes primarily address the unique issues presented by the increasing use of oral therapies for cancer treatment. This focus grew out of the recognition by the Steering Committee that the previous standards inadequately addressed safety issues in oral cancer therapy.2
Existing cancer treatment practices are being challenged by the incorporation of oral agents.10 The administration of oral antineoplastics is sufficiently complex that some have suggested that specialty clinics, analogous to anticoagulation management programs, to supervise compliance and monitor for toxicity may be beneficial.11 Issues associated with oral medication administration include the requirement for patient and caregiver education regarding safe handling and reliable administration as well as procurement and disposal of medication. For the most part, hospitals, infusion centers, and physician practices have developed and implemented good practices and processes around the safe delivery of intravenous chemotherapy. Nonetheless, practices applying for ASCO's Quality Oncology Practice Initiative Certification12 (a program that requires compliance with a subset of the ASCO/ONS safety standards) almost always modify or expand one or more processes to meet standards for safe parenteral administration. Whether oral medication are administered by health care professionals or self-administered by patients, there may be much greater latitude in the competency assessment of those giving the medication.13 We know of no specific competency requirements for individuals authorized to administer oral chemotherapy versus other oral medications; this may be worth consideration and implementation.14 The lack of a compensation model for the prescription and supervision of complex oral chemotherapy represents an opportunity for payers and providers to work together to create solutions that fairly reimburse oncology practices for the time needed to guarantee patient safety. When chemotherapy is administered parenterally, patients interact with practitioners at regularly determined intervals before chemotherapy is ordered, prepared, dispensed and administered. In contrast, oral chemotherapy is dispensed to patients in a variety of ways, including specialty, local national, local private, and “mail-order” pharmacies. The start of therapy may be delayed by problems with drug acquisition. The frequency of follow-up is drug and patient specific. In addition, adherence to therapy is correlated with treatment success,15 there are many factors associated with nonadherence, and monitoring patients is difficult.16 Given the degree of oversight necessary for these recommended processes, it is important to consider barriers to their implementation, including (but not limited to) issues of drug handling such as storage and disposal, as well as the provenance of drugs provided to patients through varied procedures, pathways, and pharmacies which may be unknown to the prescribing physician.
One such barrier is the high cost of oral agents and different reimbursement schedule for self-administered oral chemotherapy. For a variety of reasons, copayments and other costs to patients may be greater for oral as opposed to parenteral therapies. In addition, although a variety of state laws address this issue,17 these high costs may serve as impediments to prescribing oral antineoplastic agents.18 Because medication adherence is influenced by patient education, and symptom management and monitoring by the health care team, it is critically important that mechanisms that promote these processes be supported as part of the safe oral administration of chemotherapy. Ideally, payers will provide support of these critical services through benefit design. Communication can be a barrier as practitioners provide care to patients receiving oral therapies across multiple settings. These challenges, however, should not interfere with standards implementation.
The ASCO/ONS standards require regular updating and maintenance, and some stakeholders may identify gaps in practice issues addressed by the standards. Others may focus on the pragmatic difficulty of their implementation. As before, we urge all clinicians involved in cancer treatment to assess compliance with these standards. We also urge clinicians to provide feedback to ASCO and/or ONS to help inform future revisions, which will be necessary as best practices for patient safety evolve.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Michael N. Neuss, American Society of Clinical Oncology (U); Kristen McNiff, American Society of Clinical Oncology (C); Terry R. Gilmore, American Society of Clinical Oncology (C); Kristine B. LeFebvre, Oncology Nursing Society, Pittsburgh PA (C); Lisa Schulmeister, American Society of Clinical Oncology (C) Consultant or Advisory Role: Peg Esper, Pfizer- Speakers Bureau (C), Novartis Speaker Bureau (C), AVEO Speakers Bureau (C) Stock Ownership: None Honoraria: Michael N. Neuss, Tennessee Oncology Practice Society, South Carolina Oncology Society; Peg Esper, Pfizer Speaker Bureau, Novartis Speaker Bureau, AVEO Speakers Bureau Research Funding: None Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: All authors
Administrative support: Kristen McNiff, Terry R. Gilmore, Kristine B. LeFebvre
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Jacobson JO, Polovich M, McNiff KK, et al. American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards. J Clin Oncol. 2009;27:5469–5475. doi: 10.1200/JCO.2009.25.1264. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson JO, Polovich M, Gilmore TR, et al. Revisions to the 2009 American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards: Expanding the scope to include inpatient settings. J Oncol Pract. 2012;8:2–6. doi: 10.1200/JOP.2011.000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halfdanarson TR, Jatoi A. Oral cancer chemotherapy: The critical interplay between patient education and patient safety. Curr Oncol Rep. 2010;12:247–252. doi: 10.1007/s11912-010-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irshad S, Maisey N. Considerations when choosing oral chemotherapy: Identifying and responding to patient need. Eur J Canc Care. 2010;19:5–511. http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2354.2010.01199.x/abstract. [Google Scholar]
- 5.Weingart SN, Toro J, Spencer J, et al. Medication errors involving oral chemotherapy. Cancer. 2010;116:2455–52464. doi: 10.1002/cncr.25027. [DOI] [PubMed] [Google Scholar]
- 6.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 7.Hartigan K. Patient education: The cornerstone of successful oral chemotherapy treatment. Clin J Oncol Nurs. 2003;7:21–524. doi: 10.1188/03.CJON.S6.21-24. [DOI] [PubMed] [Google Scholar]
- 8.Highland J, Clemens H, et al. Mandated benefit review of SB 1070: An act relative to oral cancer therapy. Compass Health Analytic. 2012. www.mass.gov/chia/docs/r/pubs/12/mb-oral-cancer-therapy.doc.
- 9.Weingart S, Brown E, Bach PB, et al. NCCN Task Force Report: Oral chemotherapy. J Natl Compr Canc Netw. 2008;6(suppl 3):S1–S14. [PubMed] [Google Scholar]
- 10.Liu G, Franssen E, Fitch MI, et al. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15:110–5115. doi: 10.1200/JCO.1997.15.1.110. [DOI] [PubMed] [Google Scholar]
- 11.Wong SF, Nguyen CP, et al. Outcome assessment of an oral chemotherapy management clinic: A preliminary report. J Clin Oncol. 2012;30 (suppl 34; abstr 105), http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=126&abstractID=104550. [Google Scholar]
- 12.McNiff KK, Bonelli KR, Jacobson JO. Quality Oncology Practice Initiative certification program: Overview, measure scoring methodology, and site assessment standards. J Oncol Pract. 2009;5:270–276. doi: 10.1200/JOP.091045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedell C. A changing paradigm for cancer treatment: The advent of new oral chemotherapy agents. Clin J Oncol Nurs. 2003;7:5–9. doi: 10.1188/03.CJON.S6.5-9. [DOI] [PubMed] [Google Scholar]
- 14.Markert A, Thierry V, Kieber M, et al. Chemotherapy safety and severe adverse events in cancer patients: Strategies to efficiently avoid chemotherapy errors in in-and outpatient treatment. Intl J Canc. 2009;124:722–728. doi: 10.1002/ijc.23991. [DOI] [PubMed] [Google Scholar]
- 15.Lilleyman JS, Lennard L. Non-compliance with oral chemotherapy in childhood leukaemia. BMJ. 1996;313:1219. doi: 10.1136/bmj.313.7067.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landier W. Adherence to oral chemotherapy in childhood acute lymphoblastic leukemia: An evolutionary concept analysis. Oncol Nurs Forum. 2011;38:343–352. doi: 10.1188/11.ONF.343-352. [DOI] [PubMed] [Google Scholar]
- 17.Hede K. Increase in oral cancer drugs raises thorny issues for oncology practices. J Natl Cancer Inst. 2009;101:1534–51536. doi: 10.1093/jnci/djp421. [DOI] [PubMed] [Google Scholar]
- 18.Neugut A, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29:2534–2542. doi: 10.1200/JCO.2010.33.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]