Summary
DNA double-strand breaks (DSBs) are considered the most cytotoxic form of DNA damage. In human cells, the major pathway for the repair of ionizing radiation (IR)-induced DSBs is non-homologous end joining (NHEJ). Here we discuss recent developments in our understanding of the mechanism of NHEJ, the proteins involved and its regulation.
Keywords: Non-homologous end joining, DNA-PKcs, Ku, DNA repair, ionizing radiation
Introduction
Non-homologous end joining (NHEJ) is the major pathway for the repair of ionizing radiation (IR)-induced DNA double strand breaks (DSBs) in human cells and is also essential for V(D)J recombination and the development of mature T and B cells [1]. Cells compromised for NHEJ therefore have major defects in DSB repair, are radiation sensitive and, at the organismal level, are immune deficient. Given that the majority of cancer patients are treated with IR in the form of radiation therapy and/or with chemotherapeutic drugs that produce DSBs, a thorough understanding of the molecular mechanisms by which cells detect and repair DSBs is of critical importance to improving the effectiveness of existing cancer therapies as well as the development of new approaches to target human malignancies. We recently reviewed the mechanism of NHEJ in mammalian cells [2]. Here, we provide an update to that review, highlighting many of the significant developments in the field since that time.
A model for NHEJ
For convenience NHEJ can be divided into three stages, 1) end-detection and tethering, 2) processing and 3) ligation (Fig. 1). In step one, the Ku70/80 heterodimer detects and binds to the extreme termini of the DSB, acting as the signal for the recruitment of subsequent NHEJ factors, including the DNA-dependent protein kinase catalytic subunit (DNA-PKcs), a serine threonine protein kinase of the phosphatidyl inositol 3-kinase (PIKK) family. Together, DNA-PKcs and Ku tether the DNA ends in a synaptic complex [3]. The interaction with DSB-bound Ku enhances DNA-PKcs’ protein kinase activity [4], which is required for NHEJ [5,6]. The second step in NHEJ involves enzymatic processing of DNA ends. IR frequently produces DNA termini containing non-ligatable 3′-phosphate groups, 3′-phosphoglycolates or 5′-hydroxyl groups that must be converted to 3′-hydroxyls and 5′-phosphates prior to ligation. Removal of these non-ligatable end groups may involve polynucleotide kinase/phosphatase (PNKP), a DNA 3′-phosphatase/5′-kinase [7], and possibly tyrosyl-DNA phosphodiesterase 1 (TDP1), which can convert 3′-phosphoglycolates to 3′-phosphates that are subsequently removed by PNKP [8,9]. Similarly, aprataxin (APTX) may have a role in removal of abortive ligation products at DSB ends [10]. Nucleases such as Exonuclease 1 (Exo1) [11], Mre11 [12], Artemis [13] and the Werner syndrome helicase/exonuclease, WRN [14], may also be involved in processing damaged DNA termini, to reveal regions of DNA sequence micro-homology that help position ends for ligation, while nucleotide gaps or deletions are filled in by DNA polymerases μ and λ [15] (Fig. 1). Processing of DSB ends is predicted to lead to loss or modification of nucleotides from either side of the break, making NHEJ inherently error prone. In the final step of NHEJ, the DNA ends are re-ligated by DNA ligase IV (LIG4) which exists in complex with X-ray cross complementing gene 4 (XRCC4) and XRCC4-like factor (XLF, also known as Cernunnos) [1,2] (Fig. 1).
Figure 1.
A model for NHEJ, showing the three basic steps (end detection, processing and ligation) along with the proteins implicated in each stage. Interactions are shown by black lines with solid arrowheads. Putative interactions are in dashed lines. Blue lines indicate phosphorylation events. Blue, red and black Ps enclosed in circles indicate DNA-PK, ATM and CK2-mediated phosphorylation events, respectively. The AP lyase activity of Ku is indicated by the red arrow. For simplicity, the interaction between Artemis and DNA ligase IV [49,50] is not shown. See text for details.
It is important to note that apart from initial binding of Ku and ultimate ligation by LIG4, there is considerable uncertainty regarding the timing and order of the intervening steps during NHEJ. Indeed, rather that proceed in a stepwise fashion, NHEJ may take place within a dynamic, multi-component protein-DNA complex. Moreover, after DSB detection by Ku, subsequent recruitment of specific end processing factors may depend upon the nature of the lesion to be repaired and/or cell cycle stage. For example, DNA-PKcs may only be required for a subset of more slowly repaired, complex lesions [16], while ATM (Ataxia Telangiectasia mutated), DNA-PKcs and Artemis are required for repair of DSBs in heterochromatic regions [17,18].
Ku
The Ku70/80 heterodimer is regarded as the cornerstone of NHEJ and has been described as a “tool-belt” protein onto which other proteins are recruited [19]. It detects DSBs through its high affinity for double stranded (ds) DNA ends and is required for recruitment of DNA-PKcs to DNA in vitro [20] and to sites of DNA damage in vivo [21]. Small angle X ray scattering (SAXS) revealed that the C-terminal region (CTR) of Ku80 forms a long “arm” that extends from the DNA binding core, suitable for interacting with other proteins [22]. Since the extreme C-terminus of Ku80 interacts with DNA-PKcs [23,24], it is tempting to speculate that it may serve to recruit DNA-PKcs to DSBs, however deletion of the Ku80 CTR did not abolish recruitment of DNA-PKcs to DSBs, DNA-PKcs kinase activity in vitro or DNA-PKcs autophosphorylation on Ser2056 in vivo [13]. Therefore, precisely how DNA-PKcs is recruited to Ku at DSBs and how this leads to activation of DNA-PKcs kinase activity remains unclear.
Ku also interacts with WRN [25], and with XRCC4, which enhances the binding of XRCC4/LIG4 to DNA in vitro, and is required for recruitment of XRCC4 to DSBs in vivo [26,27]. Ku also interacts with XLF, and is also required for XLF recruitment to DSBs in vivo [28,29]. Moreover, Ku is required for recruitment of APLF (APTX and PNK-like factor) to DSBs, which facilitates stability of the repair complex and promotes NHEJ [30] (Fig. 1).
In addition to these critical roles in detection of damage and recruitment of other NHEJ proteins, Ku has 5′-deoxyribose-5-phosphate (5′-dRP)/AP lyase activity, suggesting that it also plays an enzymatic role in processing DNA ends during NHEJ [31]. Ku excises abasic sites near DSBs in vitro, and this activity was highest when the abasic site was within a short 5′ overhang at the DSB end, suggesting that Ku promotes NHEJ fidelity [32]. This novel activity of Ku may be important in repairing IR-induced base damage in the vicinity of the strand breaks, contributing to genomic stability.
DNA-PKcs
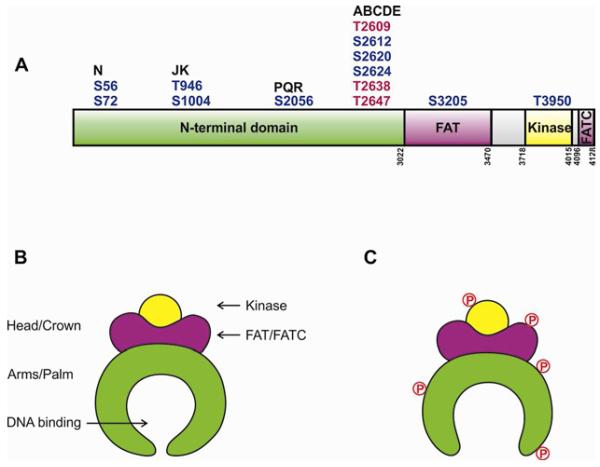
DNA-PKcs is composed of a large N-terminal domain composed largely of helical elements and HEAT (Huntingtin, Elongation Factor 3, PP2A and TOR1) repeats, a conserved region termed the FAT (FRAP, ATM, TRRAP) domain, followed by the kinase domain and a short C-terminal region, termed the FATC domain (Fig. 2A). Structural features of DNA-PKcs inferred from cryo-electron microscopy (cryo-EM) [33,34] and SAXS [22] suggested that the protein is composed of a head or crown domain and a central palm domain with a region of low electron density in the centre, proposed to bind dsDNA [35]. These features were confirmed by the elucidation of the X-ray structure of DNA-PKcs [36]. Although still relatively low-resolution (6.6 Å), this structure significantly extends our understanding of DNA-PKcs structure, revealing that the FAT-kinase-FATC domains are located at the head/crown region while the N-terminal α-helical domain forms the arms of a pincer-shaped structure surrounding an open, central channel that is suggested to bind dsDNA (Fig. 2B). A smaller channel in the head domain may accommodate single stranded (ss) DNA [22,33], possibly reflecting unwinding of dsDNA termini within the DNA-PKcs molecule [3]. Future studies aimed at determining precisely how DNA-PKcs interacts with DNA will be key to understanding its mechanism of action.
Figure 2.
A.Schematic of DNA-PKcs showing major in vivo phosphorylation sites discussed in text. Residues in red are equivalent to residues mutated in the 3A mice [66]. Domain boundaries are shown by vertical numbers. See also [37,62,67].
B. Model for structure of DNA-PKcs, adapted from [36].
C. Model for putative effects of phosphorylation on DNA-PKcs structure, adapted from [37].
Significantly, regions at the apex of the arms of DNA-PKcs were predicted to be highly flexible and the region of DNA-PKcs at the base of the arms contained a gap, suggesting that the arms may open and close around the central DNA binding channel [36]. These findings led us to suggest that phosphorylation of DNA-PKcs (discussed below) might mediate opening and closing of the clamp shaped arms of DNA-PKcs to regulate its interaction with Ku and DNA [37] (Fig. 2C). Since the overall features of the PIKK family of proteins are conserved [38], it seems likely that the overall pincer shaped structure revealed for DNA-PKcs may be conserved in other family members.
XRCC4 and XLF
Although it has no direct catalytic activity, XRCC4 plays an essential role in NHEJ due to its ability to interact with and stabilize LIG4. XRCC4 is constitutively phosphorylated by CK2 on Thr233, which interacts with the fork-head associated (FHA) domains of PNKP, APTX and APLF, suggesting that XRCC4 recruits these factors to sites of DNA damage (Fig. 1). Like XRCC4, XLF has no catalytic activity but is able to facilitate XRCC4-LIG4-mediated end joining, particularly on non-cohesive ends [39-41] and promotes re-adenylation of LIG4, recharging it for the next catalytic cycle [42]. How this occurs is unclear but is proposed to involve XRCC4 and XLF-induced conformational changes in LIG4 [43]. XRCC4 and XLF dimers interact via their head domains to form long, helical filaments that may serve to bridge or align DNA ends for ligation [44-47], which could protect DNA ends from nucleases, regulating resection and possibly pathway choice [48].
DNA ligase IV (LIG4)
Perhaps the most critical step in NHEJ is ligation of the DNA ends to repair the break. LIG4 is composed of N-terminal DNA binding and catalytic domains followed by a C-terminal tandem BRCT domain. The N-terminal DNA binding domain of LIG4 interacts with Artemis, to regulate NHEJ in V(D)J recombination [49,50], while the C-terminal BRCT domains interact with the coiled-coil stalk of XRCC4 [51,52], which is required for LIG4 stability. LIG4 destabilizes XRCC4-XLF filaments, suggesting that it regulates their formation [43,53]. The recently reported high-resolution structures of the N-terminal nucleotidyltransferase [43], DNA binding [50] and BRCT domains [52] of LIG4 in combination with results from SAXS [43,44,53] and cryo-electron microscopy [54] shed light on the overall structures of XRCC4-LIG4 and the XLF-XRCC4-LIG4 complex, however, a more complete understanding of the mechanism of DNA ligation will require higher resolution structures of the complex in association with DSBs.
Removal of Ku from DNA
Since the first demonstration that the Ku70/80 heterodimer forms a basket shaped structure that encircles dsDNA [55], the question of how Ku is removed from the DNA ends prior to ligation has remained enigmatic. Studies in Xenopus laevis egg extracts suggest that polyubiquitylation of Ku80 at Lys48 can mediate its degradation by the proteasome and dissociation from DNA [56,57]. In keeping with this, the E3 ubiquitin ligase RNF8 interacts with Ku80 in human cells, and overexpression of RNF8 decreased Ku80 levels in vivo. Moreover, depletion of RNF8 resulted in prolonged retention of Ku80 at laser-induced DSBs in vivo and reduction of NHEJ repair efficiency, suggesting that ubiquitylation of Ku80 by RNF8 is essential for Ku degradation and possibly its release from DSBs [58]. Other studies suggest that PARP (poly (ADP-ribose) polymerase) is required for retention of Ku since in Dictyostelium discoideum, PARP mutation results in reduced NHEJ efficiency possibly through promoting removal of Ku at DSBs [59]. Intriguingly, in the fission yeast Schizosaccharomyces pombe, the Mre11 nuclease is proposed to nick one strand of the DNA, allowing Exo1-mediated DNA resection and removal of Ku [60]. Whether Mre11 and Exo1 carry out a similar function in mammalian cells remains to be determined.
The role of phosphorylation in NHEJ
Although DNA-PKcs is not conserved in lower eukaryotes, the protein kinase activity of DNA-PK is required for NHEJ in mammalian cells [5] and small molecule inhibitors of DNA-PK kinase activity inhibit DSB repair and induce radiosensitivity [6]. DNA-PKcs undergoes autophosphorylation in vitro [61] and is highly phosphorylated in cells in response to DNA damage (reviewed in [37,62]). In vitro, autophosphorylation results in loss of DNA-PK kinase activity and dissociation of DNA-PKcs from the DNA-Ku complex [63]. Moreover, autophosphorylation induces a dramatic conformational change [22] proposed to facilitate its dissociation from dsDNA [37] (discussed above).
The most well-studied DNA-PKcs phosphorylation sites are located between residues 2023-2056 and 2609-2647, and have been termed the PQR/Ser2056 and ABCDE/Thr2609 clusters, respectively [62,64] (Fig. 2A). Cells expressing DNA-PKcs in which the ABCDE sites have been ablated are extremely sensitive to IR, more so than cells lacking DNA-PKcs [65]. Moreover, mutation of phosphorylation sites in the PQR and ABCDE clusters regulates processing of coding joints during V(D)J recombination in a reciprocal manner, consistent with DNA-PK phosphorylation at these sites regulating end access and processing of DNA ends (reviewed in [62,64]). Significantly, transgenic mice expressing DNA-PKcs in which residues equivalent to Thr2609, Thr2638, and Thr2647 in humans have been mutated to alanine, termed 3A mice, have severe defects in hematopoietic stem cell development and die shortly after birth. These mutations also increased spontaneous DNA lesions in hematopoietic stem cells, as well as hypersensitivity to replication stress inducers [66].
Other phosphorylation sites of DNA-PKcs that are important for function include Thr976/Ser1104 and Ser51/Ser72 in the N-terminal domain (termed JK and N clusters, respectively) [67], and Thr3950 in the catalytic domain [68]. In contrast, Ser3205 in the FAT domain is phosphorylated in an ATM-dependent manner in response to IR but is not required for NHEJ [67]. Fully understanding the effects of DNA-PKcs phosphorylation on its function represents a considerable challenge, as DNA-PKcs is phosphorylated at over 40 sites in vitro (Yu and Lees-Miller, unpublished data) and phosphoproteomics studies have identified over 37 in vivo phosphorylation sites and 17 acetylation sites (reviewed in [37]).
DNA-PKcs phosphorylation is also regulated by protein phosphatases [61,69]. Indeed, DNA-PKcs interacts with protein phosphatases PP5 [70] and PP6 [71,72] and possibly PP1 [73] and PP2A [71], suggesting that it may act as a scaffold to recruit protein phosphatases to sites of DNA damage [74]. Moreover, PP4 and PP2A have been implicated in NHEJ regulation [75,76].
Given that DNA-PK protein kinase activity is required for DSB repair, we and others have long sought to identify physiological substrates of DNA-PKcs. Although DNA-PK phosphorylates Ku70 and Ku80 in vitro, these sites are not required for survival after IR [77]. Similarly although the CTRs of XRCC4 and XLF are phosphorylated in vitro and in vivo [78,79], and although the extreme CTR of XLF is required for interaction with Ku and for recruitment to DSBs in vivo [29], the CTRs of XRCC4 and XLF are not required for cellular survival after X-ray irradiation or for V(D)J recombination [80,81]. However, functional redundancy of the CTRs of may have masked effects of mutation of XLF phosphorylation sites on NHEJ [82], and, in light of this, the effects of XRCC4 and XLF phosphorylation on NHEJ and DSB repair need to be re-evaluated.
ATM and DNA-PK phosphorylate PNKP on Ser114 and Ser126 in vitro, and PNKP phosphorylation in response to DNA damage is largely ATM dependent [83,84]. Phosphorylation of these sites is required for efficient DSB repair, retention of PNKP at sites of DNA damage and cell survival after DNA damage [83,84]. The fact that in vivo phosphorylation of Artemis [85,86], PNKP [83,84], XLF [78] and DNA-PKcs [87] can be ATM-dependent may reflect cross talk between DNA damage signaling and repair pathways or, possibly, ATM-dependent repair of heterochromatic DSBs [17,88].
New substrates and roles of DNA-PKcs
Phosphoproteomics studies have identified hundreds of new DNA-damage induced phosphorylation events in cells [89-92], many of which have been attributed to the activity of ATM [89,92]. However, others are likely phosphorylated by DNA-PK and/or ATR. Moreover, DNA-PK phosphorylates several ATM target proteins in ATM-defective cells, revealing functional redundancy between PIKKs [71,93,94].
Other newly identified in vivo substrates for DNA-PKcs include nuclear receptor 4A (NR4A) [95] (Table 1), which was shown to co-localize with γ-H2AX, a marker of DSBs, and cells in which NR4A was depleted have defects in DSB repair. The heat shock protein Hsp90α [96,97] and the scaffold attachment factor A (SAF-A, also known as heterogenous nuclear ribonucleoprotein, hnRNPU) are also phosphorylated by DNA-PK in vivo in response to DNA damage, [98,99], however whether these phosphorylation events directly impact NHEJ is not known. Moreover, it is becoming clear that DNA-PKcs functions in cellular processes other than NHEJ, such as transcription and mitosis (Table 2), and it is possible that it may target proteins in other pathways in vivo.
Table 1. New in vivo substrates of DNA-PKcs.
Table 2. New functions of DNA-PKcs.
| Function | Action by DNA-PKcs | References |
|---|---|---|
| Mitotic regulation and maintenance of genome stability |
May stabilize centrosome and spindle formation and alignment of chromosomes |
[142-144] |
| Transcriptional regulation of estrogen responsive genes |
Localizes with Ku, PARP1, and Topo II β at the promoter region of this gene |
[145] |
| Regulation during replication stress and control of histone mRNA stability |
Phosphorylation of UPF1 and regulation of histone mRNA stability |
[140] |
| Regulation in response to insulin signaling |
Regulation of FAS gene in response to insulin signaling through phosphorylating USF1 |
[141] |
| Cellular adaptation to hypoxia stress response |
DNA-PKcs phosphorylation on S2056 initiated by histone acetylation in response to hypoxia |
[146] |
Regulation of NHEJ
APLF has emerged as a major player in regulation of NHEJ. It interacts with CK2 phosphorylated XRCC4 [100] and with Ku [30,101] and stimulates NHEJ in vivo by promoting retention of the XRCC4/LIG4 complex at DSBs [30,102]. APLF also interacts with PARP3 [102] and histones [103,104], providing a potential mechanism to interface NHEJ with chromatin. The primate specific DNA methylase, metnase also regulates NHEJ in the context of chromatin, by regulating histone methylation surrounding DSBs [105,106]. Moreover, histone deacetylases HDAC1 and HDAC2 are required for cellular survival after IR, promote NHEJ, regulate retention of Ku, XRCC4 and Artemis at sites of DNA damage, and DNA damage induced phosphorylation of DNA-PKcs [107], linking NHEJ with chromatin structure.
Another important modulator of NHEJ is 53BP1. 53BP1 is recruited to IR-induced foci (IRIF) through RNF8-dependent histone ubiquitylation [108-110] and/or MDC1-dependent histone methylation [111,112], and promotes NHEJ through its ability to bridge DSBs over long ranges of chromatin [113,114]. In addition, 53BP1 promotes NHEJ and suppresses DNA end resection, possibly by forming a chromatin barrier around breaks [115], or by inhibiting Exo1-mediated DNA resection activity [116].
Akt1 (also known as PKBα) has also been shown to regulate NHEJ. Akt1 interacts with DNA-PKcs, promoting DNA-PK kinase activity, and enhances cellular radioresistance possibly by facilitating DNA-PKcs recruitment to DSBs and improving NHEJ repair efficiency (Table 1) [117,118]. DNA-PKcs also interacts with epidermal growth factor receptor (EGFR), and EGFR stabilizes phosphorylation of DNA-PKcs on T2609 and may promote Artemis-mediated end processing at DSBs [119-121].
DSB pathway choice
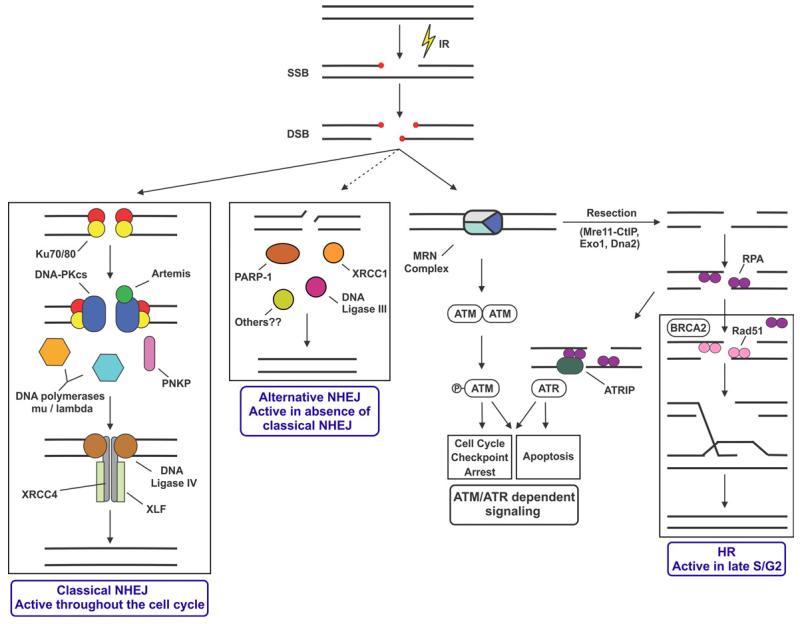
One of the critical questions in DSB repair is what determines whether a DSB is repaired via the classical NHEJ pathway (described here), an alternative NHEJ pathway (Alt-NHEJ, which is characterized by larger deletions and translocations) [122], or the more accurate homologous recombination (HR) pathway [123] (Fig. 3). One major factor is clearly cell cycle stage, as NHEJ occurs in all stages of the cell cycle whereas HR is active only after DNA replication when an undamaged sister chromatid is present as a template. Another major factor in pathway choice is resection of the DNA ends. Current models suggest that blunt or modestly processed DNA ends are targeted by Ku70/80 for NHEJ whereas CtIP-mediated resection by Mre11, Exo 1 and/or other nucleases in late S and early G2, primes cells for loading of Rad51 and consequently initiation of HR [124,125]. In the fission yeast Schizosaccharomyces pombe, Mre11 promotes Exo-1 mediated DNA resection, which promotes HR [60].
Figure 3.
A model for the DNA damage response in mammalian cells indicating competition of DSB ends by classical NHEJ, Alt-NHEJ and HR. Adapted from [139] with permission.
In mammalian cells, DNA-PKcs phosphorylation may also regulate pathway choice. The protein kinase activity of DNA-PKcs suppresses HR and phosphorylation of DNA-PKcs on the JK cluster or Thr3950 inhibits NHEJ and promotes HR [67]. Moreover, cells from 3A mice lacking three of the ABCDE phosphorylation sites (discussed above), showed lower HR activity than those from DNA-PKcs-null mice, and cells expressing phosphoablation mutations in the ABCDE cluster have slower rates of Rad51 foci formation [105,126]. Together, these studies are consistent with phosphorylation of DNA-PKcs regulating access of the DNA ends to resection factors and initiation of HR.
Therapeutic potential of NHEJ
Given the importance of NHEJ in the repair of IR-induced DSBs, NHEJ has been considered a potential therapeutic target for cancer treatment [127]. Indeed, inhibitors of DNA-PKcs kinase activity have been shown to have efficacy in human cells, two are in phase I clinical trials (Table 3) and others are in development [128-131]. A small molecule inhibitor of PNKP that sensitizes cells to camptothecin has also been developed [132,133]. However, the therapeutic implications of targeting NHEJ will depend in part on NHEJ proficiency in tumour versus normal cells. Recent studies have also suggested that NHEJ can modulate the cellular response to other therapeutic strategies. For example, PARP inhibitors have shown considerable potential in the treatment of HR deficient tumors [134] and this effect is dependent on functional NHEJ [135,136]. Moreover, inhibition of NHEJ suppresses sensitivity to interstrand crosslinking agents in Fanconi Anemia [137,138].
Table 3. NHEJ inhibitors.
PI3K, phosphatidyl inositol 3 kinase.
| Inhibitor | Mechanism/Comments | References |
|---|---|---|
| A12B4C3 | PNKP inhibitor, sensitizes cells to camptothecin | [132,133] |
| BTW3 | A small peptide DNA-PK inhibitor, proposed to compete for DNA-PKcs autophosphorylation site. |
[147] |
| KU0060648 | DNA-PK and PI3K inhibitor. | [148] |
| NU7441/KU57788 | DNA-PK inhibitor, competitive with ATP | [6,148] |
| ScFv 18-2 | An antibody-derived DNA-PK inhibitor that can bind to an epitope unique to DNA-PKcs. |
[149] |
| ZSTK474 | DNA-PK and PI3K inhibitor, competitive with ATP; in phase 1 clinical trials (NCT01280487) |
[150-152] |
| CC-115 | Dual inhibitor of DNA-PKcs and mTOR, in phase 1 clinical trials. |
NCT01353625 |
| CC-122 | DNA-PK inhibitor, in phase 1 clinical trials. | NCT01421524 |
Conclusions
In this review we summarized the many recent significant findings related to NHEJ in human cells. Despite these advances, many critical questions remain. One is how the different NHEJ proteins are coordinated to access and dissociate from DSBs in vivo and the role of phosphorylation and other post-translational modifications in this process. Other critical questions include what determines access of Ku and MRN to DSB ends to initiate NHEJ, ATM-mediated signaling and HR, how pathway choice is regulated, and how repair occurs in the context of chromatin. Further understanding of the mechanism of NHEJ is critical to understanding the effects of IR on human cells, which has the potential to improve existing cancer therapies, as well as developing new cancer biomarkers and novel therapeutics.
Acknowledgements
We apologize to authors whose work we were not able to cite due to space constraints. The authors acknowledge Alberta-Innovates: Health Solutions and the Engineered Air Chair for Cancer Research for support. Work in the author’s laboratory is funded by the Canadian Institutes of Health Research (MOP13639) and NIH program project grant (P01 CA92584).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mahaney BL, Meek K, Lees-Miller SP. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J. 2009;417:639–650. doi: 10.1042/BJ20080413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].DeFazio LG, et al. Synapsis of DNA ends by DNA-dependent protein kinase. Embo J. 2002;21:3192–3200. doi: 10.1093/emboj/cdf299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gottlieb TM, Jackson SP. The DNA-dependent protein kinase: Requirement for DNA ends and association with ku antigen. Cell. 1993;72:131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- [5].Kurimasa A, et al. Requirement for the kinase activity of human DNA-dependent protein kinase catalytic subunit in DNA strand break rejoining. Mol Cell Biol. 1999;19:3877–3884. doi: 10.1128/mcb.19.5.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhao Y, et al. Preclinical evaluation of a potent novel DNA-dependent protein kinase inhibitor nu7441. Cancer Res. 2006;66:5354–5362. doi: 10.1158/0008-5472.CAN-05-4275. [DOI] [PubMed] [Google Scholar]
- [7].Weinfeld M, et al. Tidying up loose ends: The role of polynucleotide kinase/phosphatase in DNA strand break repair. Trends Biochem Sci. 2011;36:262–271. doi: 10.1016/j.tibs.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Huang SN, Pommier Y, Marchand C. Tyrosyl-DNA phosphodiesterase 1 (tdp1) inhibitors. Expert Opin Ther Pat. 2011;21:1285–1292. doi: 10.1517/13543776.2011.604314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bahmed K, Nitiss KC, Nitiss JL. Yeast tdp1 regulates the fidelity of nonhomologous end joining. Proc Natl Acad Sci U S A. 2010;107:4057–4062. doi: 10.1073/pnas.0909917107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rass U, Ahel I, West SC. Molecular mechanism of DNA deadenylation by the neurological disease protein aprataxin. J Biol Chem. 2008;283:33994–34001. doi: 10.1074/jbc.M807124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bahmed K, et al. End-processing during non-homologous end-joining: A role for exonuclease 1. Nucleic Acids Res. 2011;39:970–978. doi: 10.1093/nar/gkq886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xie A, Kwok A, Scully R. Role of mammalian mre11 in classical and alternative nonhomologous end joining. Nat Struct Mol Biol. 2009;16:814–818. doi: 10.1038/nsmb.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Weterings E, et al. The ku80 carboxy terminus stimulates joining and artemis-mediated processing of DNA ends. Mol Cell Biol. 2009;29:1134–1142. doi: 10.1128/MCB.00971-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kusumoto R, et al. Werner protein cooperates with the xrcc4-DNA ligase iv complex in end-processing. Biochemistry. 2008;47:7548–7556. doi: 10.1021/bi702325t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ramsden DA, Asagoshi K. DNA polymerases in nonhomologous end joining: Are there any benefits to standing out from the crowd? Environ Mol Mutagen. 2012 doi: 10.1002/em.21725. [DOI] [PubMed] [Google Scholar]
- [16].Reynolds P, et al. The dynamics of ku70/80 and DNA-pkcs at dsbs induced by ionizing radiation is dependent on the complexity of damage. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Riballo E, et al. A pathway of double-strand break rejoining dependent upon atm, artemis, and proteins locating to gamma-h2ax foci. Mol Cell. 2004;16:715–724. doi: 10.1016/j.molcel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- [18].Goodarzi AA, Jeggo PA. The heterochromatic barrier to DNA double strand break repair: How to get the entry visa. Int J Mol Sci. 2012;13:11844–11860. doi: 10.3390/ijms130911844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gu J, Lieber MR. Mechanistic flexibility as a conserved theme across 3 billion years of nonhomologous DNA end-joining. Genes Dev. 2008;22:411–415. doi: 10.1101/gad.1646608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yoo S, Dynan WS. Geometry of a complex formed by double strand break repair proteins at a single DNA end: Recruitment of DNA-pkcs induces inward translocation of ku protein. Nucleic Acids Res. 1999;27:4679–4686. doi: 10.1093/nar/27.24.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Uematsu N, et al. Autophosphorylation of DNA-pkcs regulates its dynamics at DNA double-strand breaks. J Cell Biol. 2007;177:219–229. doi: 10.1083/jcb.200608077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hammel M, et al. Ku and DNA-dependent protein kinase dynamic conformations and assembly regulate DNA binding and the initial non-homologous end joining complex. J Biol Chem. 2010;285:1414–1423. doi: 10.1074/jbc.M109.065615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gell D, Jackson SP. Mapping of protein-protein interactions within the DNA-dependent protein kinase complex. Nucleic Acids Res. 1999;27:3494–3502. doi: 10.1093/nar/27.17.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Falck J, Coates J, Jackson SP. Conserved modes of recruitment of atm, atr and DNA-pkcs to sites of DNA damage. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- [25].Karmakar P, et al. Ku heterodimer binds to both ends of the werner protein and functional interaction occurs at the werner n-terminus. Nucleic Acids Res. 2002;30:3583–3591. doi: 10.1093/nar/gkf482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mari PO, et al. Dynamic assembly of end-joining complexes requires interaction between ku70/80 and xrcc4. Proc Natl Acad Sci U S A. 2006;103:18597–18602. doi: 10.1073/pnas.0609061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Costantini S, et al. Interaction of the ku heterodimer with the DNA ligase iv/xrcc4 complex and its regulation by DNA-pk. DNA Repair (Amst) 2007;6:712–722. doi: 10.1016/j.dnarep.2006.12.007. [DOI] [PubMed] [Google Scholar]
- [28].Yano K, et al. Ku recruits xlf to DNA double-strand breaks. EMBO Rep. 2008;9:91–96. doi: 10.1038/sj.embor.7401137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yano KI, et al. Functional significance of the interaction with ku in DNA double-strand break recognition of xlf. FEBS Lett. 2011;585:841–846. doi: 10.1016/j.febslet.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Grundy GJ, et al. Aplf promotes the assembly and activity of non-homologous end joining protein complexes. EMBO J. 2012 doi: 10.1038/emboj.2012.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Roberts SA, et al. Ku is a 5′-drp/ap lyase that excises nucleotide damage near broken ends. Nature. 2010;464:1214–1217. doi: 10.1038/nature08926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Strande N, et al. Specificity of the drp/ap lyase of ku promotes nonhomologous end joining (nhej) fidelity at damaged ends. J Biol Chem. 2012;287:13686–13693. doi: 10.1074/jbc.M111.329730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Williams DR, et al. Cryo-em structure of the DNA-dependent protein kinase catalytic subunit at subnanometer resolution reveals alpha helices and insight into DNA binding. Structure. 2008;16:468–477. doi: 10.1016/j.str.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chiu CY, et al. Cryo-em imaging of the catalytic subunit of the DNA-dependent protein kinase. J Mol Biol. 1998;284:1075–1081. doi: 10.1006/jmbi.1998.2212. [DOI] [PubMed] [Google Scholar]
- [35].Boskovic J, et al. Visualization of DNA-induced conformational changes in the DNA repair kinase DNA-pkcs. Embo J. 2003;22:5875–5882. doi: 10.1093/emboj/cdg555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sibanda BL, Chirgadze DY, Blundell TL. Crystal structure of DNA-pkcs reveals a large open-ring cradle comprised of heat repeats. Nature. 2010;463:118–121. doi: 10.1038/nature08648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dobbs TA, Tainer JA, Lees-Miller SP. A structural model for regulation of nhej by DNA-pkcs autophosphorylation. DNA Repair (Amst) 2010;9:1307–1314. doi: 10.1016/j.dnarep.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lempiainen H, Halazonetis TD. Emerging common themes in regulation of pikks and pi3ks. EMBO J. 2009;28:3067–3073. doi: 10.1038/emboj.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gu J, et al. Xrcc4:DNA ligase iv can ligate incompatible DNA ends and can ligate across gaps. Embo J. 2007;26:1010–1023. doi: 10.1038/sj.emboj.7601559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lu H, et al. Length-dependent binding of human xlf to DNA and stimulation of xrcc4.DNA ligase iv activity. J Biol Chem. 2007;282:11155–11162. doi: 10.1074/jbc.M609904200. [DOI] [PubMed] [Google Scholar]
- [41].Tsai CJ, Kim SA, Chu G. Cernunnos/xlf promotes the ligation of mismatched and noncohesive DNA ends. Proc Natl Acad Sci U S A. 2007;104:7851–7856. doi: 10.1073/pnas.0702620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Riballo E, et al. Xlf-cernunnos promotes DNA ligase iv-xrcc4 re-adenylation following ligation. Nucleic Acids Res. 2009;37:482–492. doi: 10.1093/nar/gkn957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ochi T, et al. Structural insights into the role of domain flexibility in human DNA ligase iv. Structure. 2012;20:1212–1222. doi: 10.1016/j.str.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hammel M, et al. Xrcc4 interactions with xrcc4-like factor (xlf) create an extended grooved scaffold for DNA ligation and double-strand break repair. J Biol Chem. 2011;286:32638–32650. doi: 10.1074/jbc.M111.272641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ropars V, et al. Structural characterization of filaments formed by human xrcc4-cernunnos/xlf complex involved in nonhomologous DNA end-joining. Proc Natl Acad Sci U S A. 2011;108:12663–12668. doi: 10.1073/pnas.1100758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wu Q, et al. Non-homologous end-joining partners in a helical dance: Structural studies of xlf-xrcc4 interactions. Biochem Soc Trans. 2011;39:1387–1392. doi: 10.1042/BST0391387. suppl 1382 p following 1392. [DOI] [PubMed] [Google Scholar]
- [47].Andres SN, et al. A human xrcc4-xlf complex bridges DNA. Nucleic Acids Res. 2012;40:1868–1878. doi: 10.1093/nar/gks022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mahaney BL, et al. Xrcc4 and xlf form long helical protein filaments suitable for DNA end protection and alignment to facilitate DNA double strand break repair. Biochemistry and Cell Biology. doi: 10.1139/bcb-2012-0058. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Malu S, et al. Artemis c-terminal region facilitates v(d)j recombination through its interactions with DNA ligase iv and DNA-pkcs. J Exp Med. 2012;209:955–963. doi: 10.1084/jem.20111437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].De Ioannes P, et al. Structural basis of DNA ligase iv-artemis interaction in nonhomologous end-joining. Cell Rep. 2012 doi: 10.1016/j.celrep.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sibanda BL, et al. Crystal structure of an xrcc4-DNA ligase iv complex. Nat Struct Biol. 2001;8:1015–1019. doi: 10.1038/nsb725. [DOI] [PubMed] [Google Scholar]
- [52].Wu PY, et al. Structural and functional interaction between the human DNA repair proteins DNA ligase iv and xrcc4. Mol Cell Biol. 2009;29:3163–3172. doi: 10.1128/MCB.01895-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hammel M, et al. Xlf regulates filament architecture of the xrcc4.Ligase iv complex. Structure. 2010;18:1431–1442. doi: 10.1016/j.str.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Recuero-Checa MA, et al. Electron microscopy of xrcc4 and the DNA ligase iv-xrcc4 DNA repair complex. DNA Repair (Amst) 2009;8:1380–1389. doi: 10.1016/j.dnarep.2009.09.007. [DOI] [PubMed] [Google Scholar]
- [55].Walker JR, Corpina RA, Goldberg J. Structure of the ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- [56].Postow L, et al. Ku80 removal from DNA through double strand break-induced ubiquitylation. J Cell Biol. 2008;182:467–479. doi: 10.1083/jcb.200802146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Postow L. Destroying the ring: Freeing DNA from ku with ubiquitin. FEBS Lett. 2011;585:2876–2882. doi: 10.1016/j.febslet.2011.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Feng L, Chen J. The e3 ligase rnf8 regulates ku80 removal and nhej repair. Nat Struct Mol Biol. 2012;19:201–206. doi: 10.1038/nsmb.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Couto CA, et al. Parp regulates nonhomologous end joining through retention of ku at double-strand breaks. J Cell Biol. 2011;194:367–375. doi: 10.1083/jcb.201012132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Langerak P, et al. Release of ku and mrn from DNA ends by mre11 nuclease activity and ctp1 is required for homologous recombination repair of double-strand breaks. PLoS Genet. 2011;7:e1002271. doi: 10.1371/journal.pgen.1002271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Douglas P, et al. Identification of in vitro and in vivo phosphorylation sites in the catalytic subunit of the DNA-dependent protein kinase. Biochem J. 2002;368:243–251. doi: 10.1042/BJ20020973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Neal JA, Meek K. Choosing the right path: Does DNA-pk help make the decision? Mutat Res. 2011;711:73–86. doi: 10.1016/j.mrfmmm.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chan DW, Lees-Miller SP. The DNA-dependent protein kinase is inactivated by autophosphorylation of the catalytic subunit. J Biol Chem. 1996;271:8936–8941. doi: 10.1074/jbc.271.15.8936. [DOI] [PubMed] [Google Scholar]
- [64].Meek K, Dang V, Lees-Miller SP. DNA-pk: The means to justify the ends? Adv Immunol. 2008;99:33–58. doi: 10.1016/S0065-2776(08)00602-0. [DOI] [PubMed] [Google Scholar]
- [65].Ding Q, et al. Autophosphorylation of the catalytic subunit of the DNA-dependent protein kinase is required for efficient end processing during DNA double-strand break repair. Mol Cell Biol. 2003;23:5836–5848. doi: 10.1128/MCB.23.16.5836-5848.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhang S, et al. Congenital bone marrow failure in DNA-pkcs mutant mice associated with deficiencies in DNA repair. J Cell Biol. 2011;193:295–305. doi: 10.1083/jcb.201009074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Neal JA, et al. Inhibition of homologous recombination by DNA-dependent protein kinase requires kinase activity, is titratable, and is modulated by autophosphorylation. Mol Cell Biol. 2011;31:1719–1733. doi: 10.1128/MCB.01298-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Douglas P, et al. The DNA-dependent protein kinase catalytic subunit (DNA-pkcs) is phosphorylated in vivo on threonine 3950, a highly conserved amino acid in the protein kinase domain. Mol Cell Biol. 2007;27:1581–1591. doi: 10.1128/MCB.01962-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Douglas P, et al. Protein phosphatases regulate DNA-dependent protein kinase activity. J Biol Chem. 2001;276:18992–18998. doi: 10.1074/jbc.M011703200. [DOI] [PubMed] [Google Scholar]
- [70].Wechsler T, et al. DNA-pkcs function regulated specifically by protein phosphatase 5. Proc Natl Acad Sci U S A. 2004;101:1247–1252. doi: 10.1073/pnas.0307765100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Douglas P, et al. Protein phosphatase 6 interacts with the DNA-dependent protein kinase catalytic subunit and dephosphorylates gamma-h2ax. Mol Cell Biol. 2010;30:1368–1381. doi: 10.1128/MCB.00741-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mi J, et al. Activation of DNA-pk by ionizing radiation is mediated by protein phosphatase 6. PLoS One. 2009;4:e4395. doi: 10.1371/journal.pone.0004395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Moorhead GB, et al. Displacement affinity chromatography of protein phosphatase one (pp1) complexes. BMC Biochem. 2008;9:28. doi: 10.1186/1471-2091-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Douglas P, et al. Choreographing the DNA damage response: Pp6 joins the dance. Cell Cycle. 2010:9. doi: 10.4161/cc.9.7.11321. [DOI] [PubMed] [Google Scholar]
- [75].Wang Q, et al. A nonhomologous end-joining pathway is required for protein phosphatase 2a promotion of DNA double-strand break repair. Neoplasia. 2009;11:1012–1021. doi: 10.1593/neo.09720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lee DH, et al. Phosphoproteomic analysis reveals that pp4 dephosphorylates kap-1 impacting the DNA damage response. EMBO J. 2012;31:2403–2415. doi: 10.1038/emboj.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Douglas P, et al. DNA-pk-dependent phosphorylation of ku70/80 is not required for non-homologous end joining. DNA Repair (Amst) 2005;4:1006–1018. doi: 10.1016/j.dnarep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- [78].Yu Y, et al. DNA-pk and atm phosphorylation sites in xlf/cernunnos are not required for repair of DNA double strand breaks. DNA Repair (Amst) 2008;7:1680–1692. doi: 10.1016/j.dnarep.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yu Y, et al. DNA-pk phosphorylation sites in xrcc4 are not required for survival after radiation or for v(d)j recombination. DNA Repair (Amst) 2003;2:1239–1252. doi: 10.1016/s1568-7864(03)00143-5. [DOI] [PubMed] [Google Scholar]
- [80].Malivert L, et al. Delineation of the xrcc4-interacting region in the globular head domain of cernunnos/xlf. J Biol Chem. 2010;285:26475–26483. doi: 10.1074/jbc.M110.138156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Grawunder U, et al. Requirement for an interaction of xrcc4 with DNA ligase iv for wild-type v(d)j recombination and DNA double-strand break repair in vivo. J Biol Chem. 1998;273:24708–24714. doi: 10.1074/jbc.273.38.24708. [DOI] [PubMed] [Google Scholar]
- [82].Roy S, et al. Xrcc4’s interaction with xlf is required for coding (but not signal) end joining. Nucleic Acids Res. 2012;40:1684–1694. doi: 10.1093/nar/gkr1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zolner AE, et al. Phosphorylation of polynucleotide kinase/ phosphatase by DNA-dependent protein kinase and ataxia-telangiectasia mutated regulates its association with sites of DNA damage. Nucleic Acids Res. 2011;39:9224–9237. doi: 10.1093/nar/gkr647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Segal-Raz H, et al. Atm-mediated phosphorylation of polynucleotide kinase/phosphatase is required for effective DNA double-strand break repair. EMBO Rep. 2011;12:713–719. doi: 10.1038/embor.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chen L, et al. Ataxia-telangiectasia-mutated dependent phosphorylation of artemis in response to DNA damage. Cancer Sci. 2005;96:134–141. doi: 10.1111/j.1349-7006.2005.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Poinsignon C, et al. Phosphorylation of artemis following irradiation-induced DNA damage. Eur J Immunol. 2004;34:3146–3155. doi: 10.1002/eji.200425455. [DOI] [PubMed] [Google Scholar]
- [87].Chen BP, et al. Ataxia telangiectasia mutated (atm) is essential for DNA-pkcs phosphorylations at the thr-2609 cluster upon DNA double strand break. J Biol Chem. 2007;282:6582–6587. doi: 10.1074/jbc.M611605200. [DOI] [PubMed] [Google Scholar]
- [88].Beucher A, et al. Atm and artemis promote homologous recombination of radiation-induced DNA double-strand breaks in g2. Embo J. 2009;28:3413–3427. doi: 10.1038/emboj.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bensimon A, et al. Atm-dependent and -independent dynamics of the nuclear phosphoproteome after DNA damage. Sci Signal. 2010;3:rs3. doi: 10.1126/scisignal.2001034. [DOI] [PubMed] [Google Scholar]
- [90].Bennetzen MV, et al. Site-specific phosphorylation dynamics of the nuclear proteome during the DNA damage response. Mol Cell Proteomics. 2010;9:1314–1323. doi: 10.1074/mcp.M900616-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Stokes MP, et al. Profiling of uv-induced atm/atr signaling pathways. Proc Natl Acad Sci U S A. 2007;104:19855–19860. doi: 10.1073/pnas.0707579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Matsuoka S, et al. Atm and atr substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- [93].Callén E, et al. Essential role for DNA-pkcs in DNA double-strand break repair and apoptosis in atm-deficient lymphocytes. Molecular Cell. 2009;34:285–297. doi: 10.1016/j.molcel.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Stiff T, et al. Atm and DNA-pk function redundantly to phosphorylate h2ax after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- [95].Malewicz M, et al. Essential role for DNA-pk-mediated phosphorylation of nr4a nuclear orphan receptors in DNA double-strand break repair. Genes Dev. 2011;25:2031–2040. doi: 10.1101/gad.16872411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Quanz M, et al. Heat shock protein 90alpha (hsp90alpha) is phosphorylated in response to DNA damage and accumulates in repair foci. J Biol Chem. 2012;287:8803–8815. doi: 10.1074/jbc.M111.320887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Solier S, et al. Heat shock protein 90alpha (hsp90alpha), a substrate and chaperone of DNA-pk necessary for the apoptotic response. Proc Natl Acad Sci U S A. 2012;109:12866–12872. doi: 10.1073/pnas.1203617109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Britton S, et al. Cell nonhomologous end joining capacity controls saf-a phosphorylation by DNA-pk in response to DNA double-strand breaks inducers. Cell Cycle. 2009;8:3717–3722. doi: 10.4161/cc.8.22.10025. [DOI] [PubMed] [Google Scholar]
- [99].Berglund FM, Clarke PR. Hnrnp-u is a specific DNA-dependent protein kinase substrate phosphorylated in response to DNA double-strand breaks. Biochem Biophys Res Commun. 2009;381:59–64. doi: 10.1016/j.bbrc.2009.02.019. [DOI] [PubMed] [Google Scholar]
- [100].Kanno S, et al. A novel human ap endonuclease with conserved zinc-finger-like motifs involved in DNA strand break responses. Embo J. 2007;26:2094–2103. doi: 10.1038/sj.emboj.7601663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Macrae CJ, et al. Aplf (c2orf13) facilitates nonhomologous end-joining and undergoes atm-dependent hyperphosphorylation following ionizing radiation. DNA Repair (Amst) 2008;7:292–302. doi: 10.1016/j.dnarep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- [102].Rulten SL, et al. Parp-3 and aplf function together to accelerate nonhomologous end-joining. Mol Cell. 2011;41:33–45. doi: 10.1016/j.molcel.2010.12.006. [DOI] [PubMed] [Google Scholar]
- [103].Rulten SL, et al. Aplf (c2orf13) is a novel component of poly(adp-ribose) signaling in mammalian cells. Mol Cell Biol. 2008;28:4620–4628. doi: 10.1128/MCB.02243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Mehrotra PV, et al. DNA repair factor aplf is a histone chaperone. Mol Cell. 2011;41:46–55. doi: 10.1016/j.molcel.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Beck BD, et al. Biochemical characterization of metnase’s endonuclease activity and its role in nhej repair. Biochemistry. 2011;50:4360–4370. doi: 10.1021/bi200333k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Fnu S, et al. Methylation of histone h3 lysine 36 enhances DNA repair by nonhomologous end-joining. Proc Natl Acad Sci U S A. 2011;108:540–545. doi: 10.1073/pnas.1013571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Miller KM, et al. Human hdac1 and hdac2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat Struct Mol Biol. 2010;17:1144–1151. doi: 10.1038/nsmb.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Huen MS, et al. Rnf8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell. 2007;131:901–914. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Mailand N, et al. Rnf8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131:887–900. doi: 10.1016/j.cell.2007.09.040. [DOI] [PubMed] [Google Scholar]
- [110].Kolas NK, et al. Orchestration of the DNA-damage response by the rnf8 ubiquitin ligase. Science. 2007;318:1637–1640. doi: 10.1126/science.1150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Xie A, et al. Distinct roles of chromatin-associated proteins mdc1 and 53bp1 in mammalian double-strand break repair. Mol Cell. 2007;28:1045–1057. doi: 10.1016/j.molcel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Pei H, et al. Mmset regulates histone h4k20 methylation and 53bp1 accumulation at DNA damage sites. Nature. 2011;470:124–128. doi: 10.1038/nature09658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Dimitrova N, et al. 53bp1 promotes non-homologous end joining of telomeres by increasing chromatin mobility. Nature. 2008;456:524–528. doi: 10.1038/nature07433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Difilippantonio S, et al. 53bp1 facilitates long-range DNA end-joining during v(d)j recombination. Nature. 2008;456:529–533. doi: 10.1038/nature07476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell. 2012;47:497–510. doi: 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- [116].Tomimatsu N, et al. Exo1 plays a major role in DNA end resection in humans and influences double-strand break repair and damage signaling decisions. DNA Repair (Amst) 2012;11:441–448. doi: 10.1016/j.dnarep.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Bozulic L, et al. Pkbalpha/akt1 acts downstream of DNA-pk in the DNA double-strand break response and promotes survival. Mol Cell. 2008;30:203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- [118].Toulany M, et al. Akt promotes post-irradiation survival of human tumor cells through initiation, progression, and termination of DNA-pkcs-dependent DNA double-strand break repair. Mol Cancer Res. 2012;10:945–957. doi: 10.1158/1541-7786.MCR-11-0592. [DOI] [PubMed] [Google Scholar]
- [119].Das AK, et al. Somatic mutations in the tyrosine kinase domain of epidermal growth factor receptor (egfr) abrogate egfr-mediated radioprotection in non-small cell lung carcinoma. Cancer Res. 2007;67:5267–5274. doi: 10.1158/0008-5472.CAN-07-0242. [DOI] [PubMed] [Google Scholar]
- [120].Dittmann K, et al. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005;280:31182–31189. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- [121].Javvadi P, et al. Threonine 2609 phosphorylation of the DNA-dependent protein kinase is a critical prerequisite for epidermal growth factor receptor-mediated radiation resistance. Mol Cancer Res. 2012;10:1359–1368. doi: 10.1158/1541-7786.MCR-12-0482-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Simsek D, et al. DNA ligase iii promotes alternative nonhomologous end-joining during chromosomal translocation formation. PLoS Genet. 2011;7:e1002080. doi: 10.1371/journal.pgen.1002080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- [124].Symington LS, Gautier J. Double-strand break end resection and repair pathway choice. Annu Rev Genet. 2011;45:247–271. doi: 10.1146/annurev-genet-110410-132435. [DOI] [PubMed] [Google Scholar]
- [125].Yun MH, Hiom K. Ctip-brca1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 2009;459:460–463. doi: 10.1038/nature07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Shibata A, et al. Factors determining DNA double-strand break repair pathway choice in g2 phase. EMBO J. 2011 doi: 10.1038/emboj.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Helleday T, et al. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- [128].Cano C, et al. DNA-dependent protein kinase (DNA-pk) inhibitors. Synthesis and biological activity of quinolin-4-one and pyridopyrimidin-4-one surrogates for the chromen-4-one chemotype. J Med Chem. 2010;53:8498–8507. doi: 10.1021/jm100608j. [DOI] [PubMed] [Google Scholar]
- [129].Clapham KM, et al. DNA-dependent protein kinase (DNA-pk) inhibitors: Structure-activity relationships for o-alkoxyphenylchromen-4-one probes of the atp-binding domain. Bioorg Med Chem Lett. 2011;21:966–970. doi: 10.1016/j.bmcl.2010.12.047. [DOI] [PubMed] [Google Scholar]
- [130].Cano C, et al. Atropisomeric 8-arylchromen-4-ones exhibit enantioselective inhibition of the DNA-dependent protein kinase (DNA-pk) Org Biomol Chem. 2010;8:1922–1928. doi: 10.1039/b926245h. [DOI] [PubMed] [Google Scholar]
- [131].Clapham KM, et al. Potent enantioselective inhibition of DNA-dependent protein kinase (DNA-pk) by atropisomeric chromenone derivatives. Org Biomol Chem. 2012;10:6747–6757. doi: 10.1039/c2ob26035b. [DOI] [PubMed] [Google Scholar]
- [132].Freschauf GK, et al. Mechanism of action of an imidopiperidine inhibitor of human polynucleotide kinase/phosphatase. J Biol Chem. 2010;285:2351–2360. doi: 10.1074/jbc.M109.055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Freschauf GK, et al. Identification of a small molecule inhibitor of the human DNA repair enzyme polynucleotide kinase/phosphatase. Cancer Res. 2009;69:7739–7746. doi: 10.1158/0008-5472.CAN-09-1805. [DOI] [PubMed] [Google Scholar]
- [134].Fong PC, et al. Inhibition of poly(adp-ribose) polymerase in tumors from brca mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- [135].Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(adp-ribose) polymerase (parp) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci U S A. 2011;108:3406–3411. doi: 10.1073/pnas.1013715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Williamson CT, et al. Enhanced cytotoxicity of parp inhibition in mantle cell lymphoma harbouring mutations in both atm and p53. EMBO Mol Med. 2012 doi: 10.1002/emmm.201200229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Pace P, et al. Ku70 corrupts DNA repair in the absence of the fanconi anemia pathway. Science. 2010;329:219–223. doi: 10.1126/science.1192277. [DOI] [PubMed] [Google Scholar]
- [138].Adamo A, et al. Preventing nonhomologous end joining suppresses DNA repair defects of fanconi anemia. Molecular Cell. 2010;39:25–35. doi: 10.1016/j.molcel.2010.06.026. [DOI] [PubMed] [Google Scholar]
- [139].Weinfeld M, Lees-Miller SP. DNA double strand break repair by non-homologous end joining and its clinical relevance. In: Kelley MR, editor. DNA repair in cancer therapy: Molecular targets and clinical applications: Academic Press; 2012. pp. 161–190. [Google Scholar]