Abstract
Purpose
Ovarian cancer remains a major threat to women's health, partly due to difficulty in early diagnosis and development of metastases. A critical need exists to identify novel targets that curb the progression and metastasis of ovarian cancer. In this study we examined whether the nuclear receptor coregulator PELP1 (proline-, glutamic acid-, leucine-rich protein-1) contributes to progression and metastatic potential of ovarian cancer cells and determined whether blocking of the PELP1 signaling axis had a therapeutic effect.
Experimental Design
Ovarian cancer cells stably expressing PELP1-shRNA (short hairpin RNA) were established. Fluorescent microscopy, Boyden chamber, invasion assays, wound healing, and zymography assays were performed to examine the role of PELP1 in metastasis. Expression analysis of the model cells was conducted using tumor metastasis microarray to identify PELP1 Target genes. Therapeutic potential of PELP1-siRNA in vivo was determined using a nanoliposomal formulation of PELP1-siRNA-DOPC (1,2-dioleoyl-sn-glycero-3-phosphatidylcholine) administered systemically in a xenograft model.
Results
PELP1 knockdown caused cytoskeletal defects and significantly affected the migratory potential of ovarian cancer cells. Microarray analysis revealed that PELP1 affected the expression of selective genes involved in metastasis including Myc, MTA1, MMP2, and MMP9. Zymography analysis confirmed that PELP1 knockdown caused a decrease in the activation of matrix metalloproteases (MMP) 2 and MMP9. Compared with control siRNA-DOPC-treated mice, animals injected with PELP1-siRNA-DOPC had 54% fewer metastatic tumor nodules, exhibited a 51% reduction in tumor growth and an 84% reduction in ascites volume.
Conclusion
The results suggest that PELP1 signaling axis is a potential druggable target and liposomal PELP1-siRNA-DOPC could be used as a novel drug to prevent or treat ovarian metastasis.
Introduction
Ovarian carcinoma continues to be the leading cause of death due to gynecologic malignancy in the United States because it is usually diagnosed in the advanced stage of the disease (1, 2). The standard treatment for epithelial ovarian cancer remains surgical debulking and chemotherapy with a platinum and taxane agent. Although many patients with disseminated tumors respond initially to standard combinations of surgical and cytotoxic therapy, nearly 90% of them develop recurrence (3). A widely recognized behavior of ovarian cancer is its ability to migrate and seed the peritoneal cavity with nests of tumor cells and the formation of ascites (4). The transition from early- to advanced-stage ovarian cancer is a critical determinant of survival; yet, little is known about the molecules that contribute to progression and metastasis of ovarian tumors.
The biological functions of hormones are mediated by nuclear receptors (NR) and several recent studies demonstrated the presence of NRs in about 70% of ovarian epithelial tumors. In the past decade, it has become increasingly clear that the sole existence of NRs is not sufficient for optimal NR function; several coregulatory proteins are also required (5, 6). Many coregulatory proteins are present at rate-limiting levels, are shared by many NRs, and have the potential to control the expression of various subsets of genes to regulate cell processes coordinately such as proliferation and metastasis (7). With the enormous potential of coregulators as master regulators, their deregulation is likely to provide the cancer cells an advantage in proliferation, survival, and metastasis (8, 9). As a consequence, there is a critical need to identify and understand the physiologic role of master coregulators that promote initiation and progression of ovarian cancer and metastasis.
Proline-, glutamic acid-, leucine-rich protein-1 (PELP1; ref. 10) is an NR coregulator that interacts with many NRs including estrogen receptor (11). PELP1 is predominantly localized in the nuclear compartment, interacts with histones and histone-modifying enzymes, and thus plays a role in chromatin remodeling for ligand-bound NRs (11). PELP1 promotes cell proliferation by sensitizing cells to G1→S progression via its interactions with the pRb pathway (12). PELP1 is also unique because it plays important roles in both the genomic (13) and nongenomic actions of the NRs (11). PELP1 is a recently identified proto-oncogene (14). Its expression is deregulated in hormonal-dependent cancers including those of breast, endometrium, and ovaries (15–17).
In this study, we investigated whether PELP1 signaling plays a role in ovarian epithelial cancer cell migration and metastasis by silencing PELP1 expression in ovarian cancer cells and in orthotopic models of ovarian cancer. Our findings revealed that PELP1 modulates metastasis of ovarian cancer cells by regulating expression of a number of genes involved in metastasis. Silencing endogenous PELP1 expression via nanoliposomal siRNA targeted to PELP1 (PELP1-siRNA-DOPC) in vivo significantly reduced tumor growth, number of tumor nodules in the peritoneal cavity and ascites volume. Our results suggest that PELP1 plays a critical role in ovarian cancer progression to metastasis and thus represents a novel therapeutic target for curbing ovarian metastasis.
Materials and Methods
Cell cultures and reagents
OVCAR3 and ES2 cells were purchased from the American Type Culture Collection and maintained in RPMI 1640 supplemented with 20% FBS (Hyclone Laboratories Ltd). The derivation of the ovarian cancer cell line SKOV3ip1 has been described (18). These cells were cultured in RPMI 1640 medium supplemented with 15% FBS and 0.1% gentamicin sulfate (Gemini Bioproducts). Nontumorigenic SV40 Tag-immortalized ovarian surface epithelial-derived cells (IOSE-80) were earlier described (19) and cultured in medium 199:MCDB 105 (1:1; Sigma-Aldrich Corp) containing 15% FBS. Antibodies against vinculin were purchased from Sigma Chemical Co. The PELP1 antibody was purchased from Bethyl Laboratories and antibodies for matrix metalloproteases (MMP) 2, H3K9Ac, and H3K9me2 were purchased from Millipore.
Generation of PELP1-shRNA model cells
Ovarian cancer cells stably expressing PELP1-shRNA (short hairpin RNA) were generated using the FuGENE-6 transfection reagent (Roche) and were selected with G418 selection (500 μg/mL). Pooled clones were used for all the studies. For IOSE cells, electroporation (nucleofection) was used to transfect PELP1 plasmids as previously described (20). The PELP1-specific Sure Silencing shRNA plasmids, catalogue no. KH19454N and control shRNA vector were purchased from SABiosciences. The targeted sequences were PELP1-shRNA1: GGACCAAGGTGTATGCGATAT; PELP1-shRNA2: ATGCTGCTGTCCTC AGAAGAT. The PELP1-shRNA design was based on the GenBank accession number NM_014389. Transient knockdown of PELP1 was achieved using On-Target-PlusSMARTpool siRNA (L-004463-00-0050; Thermo-scientific) and by using oligofectamine transfection (Invitrogen).
Microarray studies
The Human Tumor Metastasis Microarray (Oligo GEArray) was from SABiosciences and contains 113 genes known to be involved in metastasis. Total RNA isolated from OVCAR3 and OVCAR3-PELP1-shRNA cells was used for screening. Probe preparation and hybridization was performed per manufacturer's instructions. Target genes whose expression was differentially regulated (at least 3-fold difference) by PELP1 underexpression were selected and validated using real-time PCR in OVCAR3 and SKOV3ip1 cells. Validated real-time PCR primers were used for validation of PELP1-regulated genes and all primers were purchased from RealTimePrimers.com.
Cell migration, invasion, and MMP reporter gene assays
The cell migration was determined using 8 micromolar pore calorimetric cell migration assay kit (Chemicon) using the manufacturer's protocol. For the cell migration assay, 105 cells per 300 μL of serum-free medium were seeded onto the upper chamber and 0.75 mL of complete growth medium containing 10% FBS was added to each well in the lower chamber (21). Invasion assays were performed using BD Biocoat growth factor–reduced Matrigel invasion chamber (BD Biosciences) as per manufacturer's protocol. The PGL3-MMP9-Luc promoter plasmid that contains 1,305 bp of proximal promoter region of human MMP9 was received from Dr. Xu (22). The PGL3-MMP2-Luc promoter plasmid that contains 1,659 bp of proximal promoter region of human MMP2 was received from Dr. Benveniste (23). Reporter gene assays were performed by transient transfection using FuGENE6 method (Roche) as described (24). Each transfection was carried out in 6-well plates in triplicate and normalized with β-gal activity and the total protein concentration.
Gelatin zymography
The culture supernatant of model cells expressing control or PELP1-shRNA was used to determine the enzymatic activity of MMP2 and MMP9 by using SDS-PAGE gelatin zymography as described (25) using Novex Zymogram Gels (Invitrogen). Recombinant MMP2 and MMP9 (R&D Systems) were used as positive controls.
ChIP assays
Chromatin immunoprecipitation (ChIP) assay was done using antibodies specific for PELP1, H3K9Ac, H3K9me2, or isotype rabbit IgG control in SKOV3ip1 cells as described (24). DNA recovered from ChIP or input controls was subjected to real-time quantitative PCR (qPCR) using primers spanning various regions of MMP9 promoter as described (22). Sequences of MMP promoter primers used: A-F: cttcagagccaggcagttct; A-R: agcctctcgtttcatcctca; B-F: taattgggcctggagatttg; B-R: agcctctcgtttcatcctca; C-F: taagacatttgcccgaggtc; C-R: cctctttttccctccctgac; D-F: ggaggtggtgtaagcccttt; and D-R: agggcagaggtgtctgactg. The promoter occupancy was calculated on the basis of the ratio of ChIP/input control.
Immunofluorescence studies
Confocal microscopy was performed as previously described (21). For these studies, cells were grown on glass cover slips and fixed in 3.7% paraformaldehyde for 15 minutes at room temperature. The fixed cells were then permeabilized with 0.2% TritonX-100 in PBS for 15 minutes followed by blocking with 5% normal goat serum (Sigma) in PBS for 1 hour. The DNA dye 4′,6-diamidino-2-phenylindole (Molecular Probes) was used to costain the DNA (blue). The filamentous actin (F-actin) status was analyzed by phalloidin staining (fluorescein isothiocyanate conjugated from Molecular Probes) for 1 hour at room temperature.
Preparation of liposomal siRNA
For in vivo delivery, PELP1-siRNA was incorporated into 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) nanoliposomes as described previously (26, 27). Briefly, siRNA and DOPC were mixed in excess tertiary butanol at a ratio of 1:10 (w/w), respectively. Subsequently, Tween 20 was added to the mixture at the ratio of 1:19 (Tween 20: siRNA-DOPC). The mixture was vortexed and frozen in an acetone/dry ice bath and lyophilized. For in vivo administration, the mixture was hydrated with 0.9% saline to a concentration of 15 μg/mL, and 200 to 250 μL of the mixture was used for each injection. siRNA for preparation of liposomes were purchased from Sigma. The targeted sequences used were: PELP1: 5′-ccacagagccugacuccua-3′ control: 5′-uucuccgaacgugucacgu-3′.
Nude mice studies
For in vivo injections, the SKOV3ip1 cells (28) were trypsinized, washed twice with PBS, and reconstituted in serum-free HBSS (Invitrogen) at a concentration of 5 × 106 cells/mL, which were then injected intraperitoneally in 5- to 6-week-old female athymic nude mice. To examine the effects of PELP1-siRNA therapy on tumor growth, treatment was initiated 1 week after intraperitoneal injection of tumor cells. Mice were randomly assigned into 2 groups (n = 8 mice per group): (i) control siRNA-DOPC (150 μg/kg i.p. twice weekly) and (ii) PELP1-siRNA-DOPC (150 μg/kg i.p. twice weekly). The mice were monitored daily for adverse affects and the treatment was continued for 27 days. On day 27, mice were euthanized and the total weight, tumor weight, number of metastastic tumor nodules, and volume of ascitic fluid were recorded. Tissue samples were snap frozen for lysate preparation or fixed in formalin for paraffin embedding.
Immunohistochemistry
Immunohistochemical (IHC) analysis was performed as described (20). Briefly, tumor sections were incubated overnight with the primary antibodies [PELP1 (1:500), MMP9 (1:50)]. The sections were then washed 3 times with 0.05% Tween in PBS for 10 minutes, incubated with secondary antibody for 1 hour, washed 3 times with 0.05% Tween in PBS for 10 minutes, developed with 3,3V-diaminobenzidine-H2O2, and counterstained with Mayer's hematoxylin. Proliferating cell nuclear antigen (PCNA; 1:100) from Vector Labs was used in conjunction with proper controls, visualized by DAB (3,3′-diaminobenzidine) substrate, and counterstained with hematoxylin (Vector Lab, Inc.). Proliferative index was calculated as percentage of PCNA-positive cells in 10 randomly selected microscopic fields at 100× per slide. TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) analysis was done using the In situ Cell Death Detection Kit (Roche) as per the manufacturer's protocol and 10 randomly selected microscopic fields in each group were used to calculate the relative ratio of TUNEL-positive cells. MMP9 expression tumors were quantified as the number of positive cells ×100/total number of cells counted under 100× magnification in 10 randomly selected areas in each tumor sample.
Statistical analysis
Statistical differences among groups were analyzed with either t test or ANOVA as appropriate using SPSS software.
Results
PELP1-shRNA clones exhibit defects in cytoskeletal reorganization
Recently, we established OVCAR3 model cells that stably express PELP1-shRNA (20). These cells had less growth potential and distinct flat morphology compared with the morphology of OVCAR3 parental or OVCAR3 vector cells. To further explore this phenotypic observation, we generated 2 additional ovarian cancer cells (SKOV3ip1 and ES2) that stably express PELP1-shRNA. These cells had a 70% to 80% decrease in the expression of PELP1 (Fig. 1A). Interestingly, SKOV3ip1 and ES2 cells expressing PELP1-shRNA also exhibited flat morphology (data not shown). We therefore examined whether the lack of PELP1 expression contributes to cytoskeletal defects in ovarian cancer cells. OVCAR3 (Fig. 1B) and SKOV3ip1 (Fig. 1C) control cells as well as PELP1-shRNA expressing cells were serum starved and treated with or without epidermal growth factor (EGF) for 10 minutes, and F-actin status was analyzed by phalloidin staining. Serum-starved control cells exhibited low F-actin structures with peripheral cortical actin, and EGF stimulation promoted increased formation of ruffles and fillopodia (Fig. 1B and C). Interestingly in PELP1 knockdown cells, EGF treatment did not promote formation of fillopodia or ruffles. However, PELP1-shRNA cells had increased accumulation of stress fibers, which is also an indication of less motile cells (Fig. 1B and C). Similar defects in actin reorganization were also seen in ES2 cells expressing PELP1-shRNA were stimulated with either EGF or estrogen (Fig. 1D). Collectively, these results suggest that PELP1 signaling plays a role in cytoskeletal reorganization and could be required for optimal actin reorganization in ovarian epithelial cells.
Figure 1.
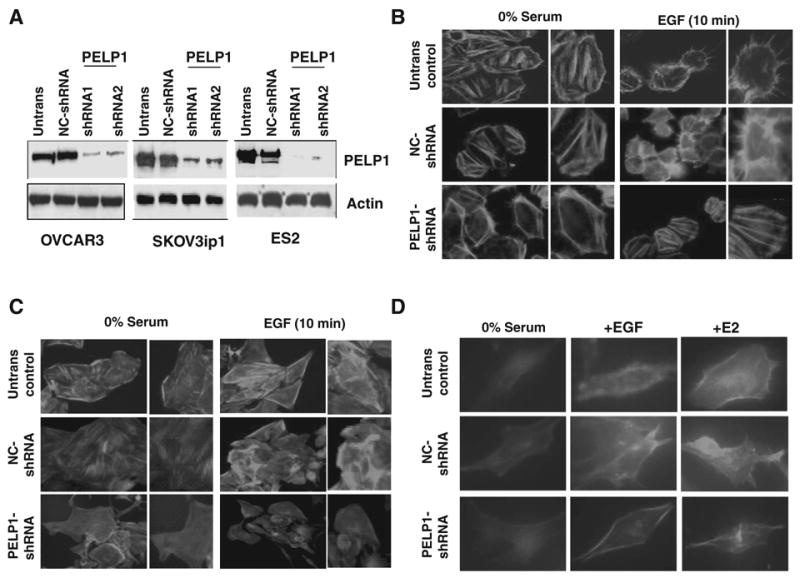
PELP1 knockdown promotes defects in actin reorganization of ovarian epithelial cells. A, OVCAR3, SKOV3ip1, ES2 cells untransfected (Untrans), and or stably expressing negative control (NC)-shRNA vector or PELP1-shRNA were lysed and the expression of PELP1 was analyzed by Western blotting. OVCAR3 (B), SKOV3ip1 (C) cells untransfected, and or stably expressing vector or PELP1-shRNA were treated with EGF (100 ng/μL) for 10 minutes. The status of F-actin was analyzed by confocal microscopy. D, ES2 cells untransfected and or stably expressing vector or PELP1-shRNA were treated with EGF (100 ng/μL) or E2 (10−9 mol/L) for 10 minutes. The status of F-actin was analyzed by confocal microscopy.
PELP1 downregulation affects expression of selective genes involved in metastasis
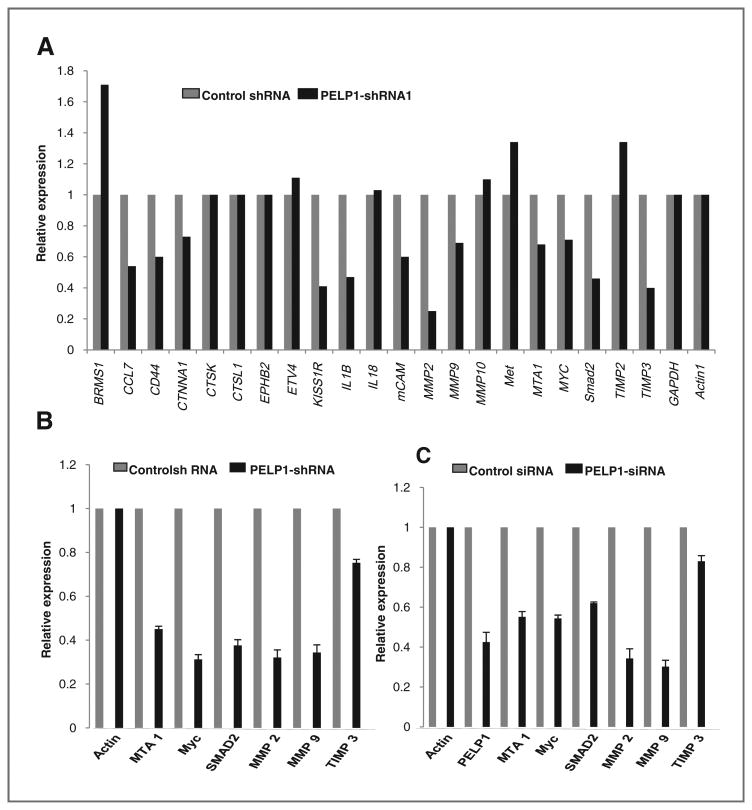
PELP1 plays a critical role in NR genomic functions via chromatin remodeling and by modulating histone code at the NR target promoters (29). Since PELP1 knockdown cells had defects in cell shape and cytoskeletal reorganization, we examined whether the lack of expression of PELP1 affects the expression of genes involved in cell migration/metastasis by using a focused microarray approach. We used the Human Tumor Metastasis Microarray, which contains 113 genes known to be involved in metastasis including genes involved in cell adhesion, ECM (extracellular matrix) components, cell cycle, cell growth and proliferation, apoptosis, transcription factors and regulators, and other genes related to tumor metastasis. Total RNA isolated from OVCAR3 and OVCAR3-PELP1-shRNA cells was used for the array analysis and the results from this screen suggested that PELP1 downregulation substantially reduced the expression of a number of genes, including MMP2, MMP9, MTA1, Myc, and SMAD2, compared with their expression in control cells (Fig. 2A). We validated the changes seen in the array study by measuring gene expression of the top 5 genes that had significant reduction by performing real-time qPCR on the OVCAR3-PELP1-shRNA cells or control shRNA cells (Fig. 2B). The changes in the expression of these genes were also independently confirmed in SKOV3ip1 cells expressing PELP1-siRNA that target a different site in PELP1 as compared with PELP1-shRNA targeting site (Fig. 2C). These results suggest that PELP1 has potential to modulate expression of genes involved in the cell migration/metastasis.
Figure 2.
PELP1 knockdown affects expression of selective genes involved in metastasis. A, RNA isolated from OVCAR3-shRNA negative control and OVCAR3-PELP1-shRNA expressing cells were hybridized to the human tumor metastasis Oligo GEArray. Changes in the gene expression were analyzed using SABioscience software with actin as a control for normalization. Representative genes downregulated upon PELP1 depletion are shown. B, real-time qPCR validation of the changes in gene expression in OVCAR3 cells stably expressing negative control shRNA or PELP1-shRNA. C, SKOV3ip1 cells were transiently transfected with control or PELP1-specific siRNA and the expression of indicated genes was analyzed real-time qPCR.
PELP1 expression is needed for optimal activation of MMPs
Since PELP1 downregulation altered expression of MMPs and because MMPs are known to promote cancer progression by enhancing cell growth, migration, invasion, and metastasis of ovarian cancer cells (30, 31), we examined whether the reduction in the expression seen in PELP1-shRNA clones translates to lower MMP activity. The activity of MMP2 and MMP9 was determined in SKOV3ip1 and ES2 cells expressing control or PELP1-siRNA by using gelatin zymography. MMP2 and MMP9 activities were lower in PELP1 knockdown cells than in control cells (Fig. 3A). To examine the mechanism by which PELP1 regulate MMP2 and MMP9 expression, we have performed promoter-Luc assays using previously published MMP2 and MMP9 reporter genes (22, 23). In both SKOV3ip1 and ES2 model cells, knockdown of PELP1 significantly reduced the MMP2 and MMP9 reporter gene activation (Fig. 3B). We examined further to check whether PELP1-mediated regulation of MMPs is due to PELP1 recruitment to the promoter regions of MMPs using a ChIP-based assay. Initially, we have used 4 different primers that recognize various regulatory regions reported to activate MMP9. ChIP results showed that PELP1 is uniquely recruited to the proximal promoter region of MMP9 (Fig. 3C) and no recruitment in distal regions was observed. Emerging data implicate that PELP1 associates with acetylases (p300, CBP) and demethylases (KDM1) and participates in epigenetic modifications that are required for optimal transcriptional activation (11). We therefore examined whether PELP1 downregulation affects epigenetic modifications at the MMP9 proximal promoter. ChIP analysis revealed that PELP1 knockdown enhances repressive mark H3K9me2 and decreases activation mark H3K9ac (Fig. 3D). These results suggest that PELP1-mediated genomic actions may play a role in PELP1 modulation of the expression and function of MMPs.
Figure 3.
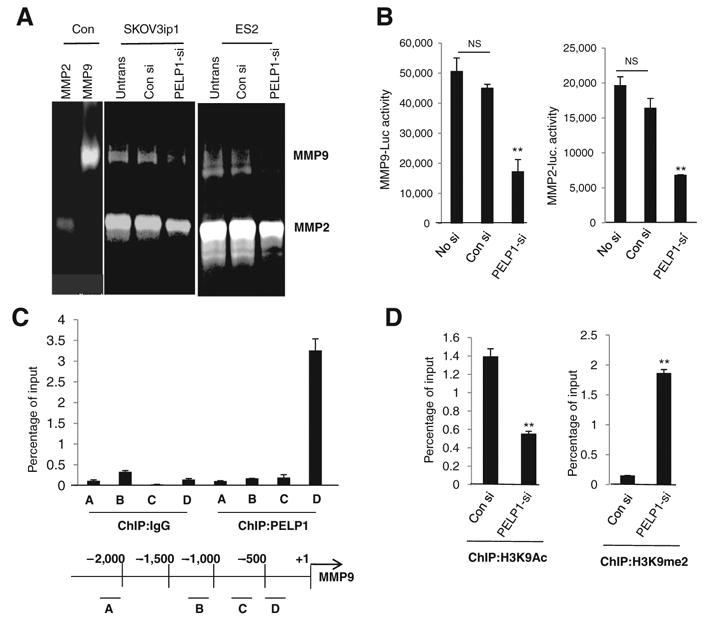
PELP1 regulates the expression and activities of MMPs. A, gelatin zymography analysis of activity of MMP2 and MMP9 in SKOV3ip1 and ES2 cells that were transfected with control siRNA (con si) or PELP1-siRNA (PELP1-si). B, SKOV3ip1 and ES2 cells transfected with MMP9-Luc and MMP2-Luc vectors, treated with control siRNA or PELP1-siRNA, and the reporter gene activity was measured after 72 hours. C, ChIP assay was done using the DNA isolated from SKOV3ip1 cells and by using antibodies specific for PELP1 or isotype rabbit IgG control. DNA recovered from ChIP or input controls was subjected to real-time qPCR using 4 primers (A, B, C, and D) that span the MMP9 promoter region. D, SKOV3ip1 cells were transfected with control or PELP1-siRNA and ChIP assay was done using antibodies specific for H3K9Ac or H3K9me2. DNA recovered from ChIP or input controls was subjected to real-time qPCR using primers that detect proximal MMP9 promoter region. The promoter occupancy was calculated on the basis of the ratio of ChIP/input control. **, P ≤ 0.001, t test. NS, nonsignificant.
PELP1 is involved in ovarian epithelial cell migration and invasion
To examine further whether overexpression of PELP1 in IOSE cells increases their migratory potential, we studied IOSE cells overexpressing PELP1 in a Boyden chamber assay. Overexpression of PELP1 substantially enhanced the migratory potential of IOSE cells (Fig. 4A). On the other hand, compared with control cells, PELP1 knockdown in SKOV3ip1 cells resulted in a significantly reduced migration in Boyden chamber assays (Fig. 4B, left). Similarly, ES2-PELP1 knockdown cells also revealed significantly less migration (Fig. 4C, right). PELP1 knockdown also significantly reduced the invasion potential of both SKOVip1 and ES2 cells (Fig. 4C). Collectively, these results suggest that PELP1 has potential to modulate the expression of the genes that are involved in migratory and invasion potential of ovarian epithelial cells.
Figure 4.
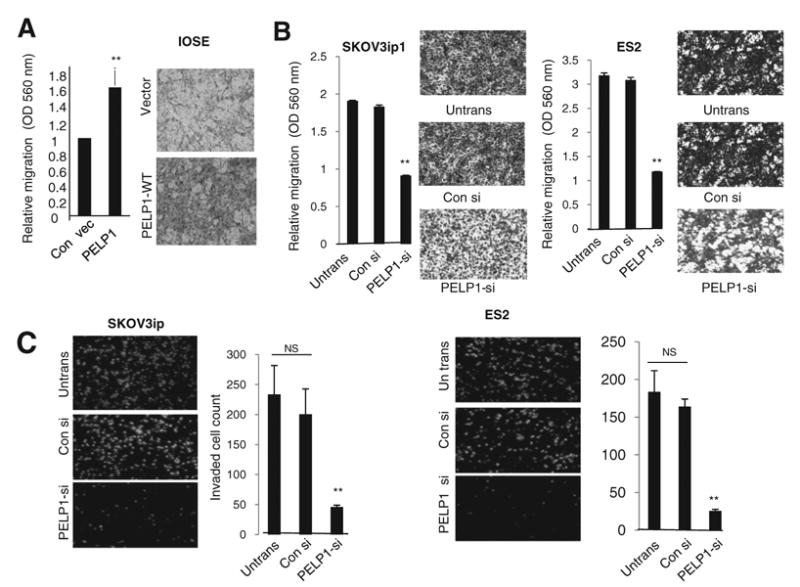
PELP1 knockdown affects the migration and invasion of ovarian cancer cells. A, migratory potential of IOSE cells overexpressing control vector (vec) or PELP1 expression vector was analyzed by using a Boyden chamber assay. B, cell migration potential of SKOV3ip1 and ES2 cells transfected with control of PELP1-siRNA was analyzed by using a Boyden chamber assay. Photomicrographs of migrated cells in various treatments are shown. C, cell invasion potential of SKOV3ip1 and ES2 cells transfected with control of PELP1-siRNA was analyzed using Matrigel invasion chamber assays. Photomicrographs of invaded cells in various treatments are shown. Columns, mean from 3 independent experiments; bars, SEM. **, P < 0.001, t test. WT, wild-type.
PELP1-siRNA reduce ovarian cancer cell growth in vitro and tumor progression in vivo
We next examined whether PELP1 downregulation affects proliferation of ovarian cancer cells in vitro by using a CellTiter-Glo assay. PELP1-siRNA-transfected SKOV3ip1 and ES2 showed substantially reduced cellular proliferation compared with control siRNA-transfected cells (Fig. 5A). We then examined whether systemically administered PELP1-siRNA in a nanoliposomal formulation (PELP1-siRNA-DOPC) reduced tumor growth by using an ovarian cancer xenograft model. Nude mice were injected with SKOV3ip1 ovarian cancer cells (1 × 106 cells i.p). One week later, mice (n = 8/group) were randomly assigned to receive therapy with either 150 μg/kg control siRNA-DOPC or PELP1-siRNA-DOPC every 3 days, based on our previous dose–response experiments for effective downregulation of genes in vivo (26). After 27 days, mice were euthanized. IHC examination of the tumors revealed that PELP1-siRNA-DOPC–treated tumors had low expression of PELP1 (Fig. 5B). No toxicity was observed in behavioral changes, including eating habits and mobility, in animals treated with liposomes and mouse weights were not significantly different between control and PELP1-siRNA treatment groups (Fig. 5C). Compared with control siRNA-DOPC–treated mice, nude mice treated with PELP1-siRNA-DOPC had a significant reduction in metastatic tumor nodules (54%, P < 0.001), a reduction in tumor growth (51%, P < 0.001), and reduction in ascites volume (84%, P < 0.001; Fig. 5C). PCNA staining of the tumor sections revealed a reduction in the proliferation in the PELP1-siRNA-DOPC–treated tumors and 60% of cells in control siRNA–treated tumors were PCNA positive, whereas only 20% of cells were PCNA positive in PELP1-siRNA–treated tumors. (Fig. 5D). TUNEL assay results showed increased apoptosis in PELP1-siRNA–treated cells compared with control siRNA. PELP1-siRNA-DOPC–treated tumors also had lower expression of MMP9 than the control tumors (Fig. 5D). Collectively, these results suggest that PELP1 downregulation decreases tumor cell proliferation and increases apoptosis and that PELP1-siRNA-DOPC can be used therapeutically for reducing ovarian cancer growth in vivo.
Figure 5.
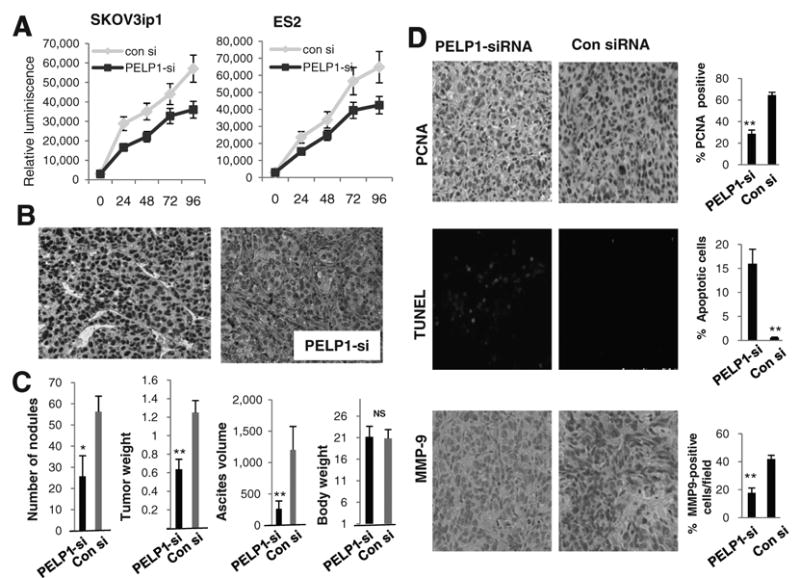
PELP1-siRNA reduce ovarian cancer cell growth in vitro and tumor progression in vivo. A, cell proliferation capacity of SKOV3ip1 and ES2 cells were analyzed after treating the cells with control or PELP1-siRNA using CellTiter-Glo assay. B, IHC analysis of PELP1 expression in tumors treated with control or PELP1-siRNA liposomes. C, number of tumor nodules, mean tumor weight (mg), and ascites volume (ml) in control siRNA-DOPC- or PELP1-siRNADOPC-treated groups (n = 8/group). Column, mean tumor weights (g); bars, SEM. *, P ≤ 0.05; **, P ≤ 0.001, t test. D, PCNA expression as a marker of proliferation, TUNEL staining as a marker of apoptosis, and MMP9 expression was analyzed by immunohistochemistry in tumors (n = 8/group) treated with control or PELP1-siRNA-DOPC; quantitation was done as described in the “Materials and Methods” section; bars, SEM. **, P < 0.001.
Discussion
Among the gynecologic malignancies reported for Western countries, ovarian cancer has the highest mortality rate and is the most common (32). Despite modest improvements in response rates, overall survival rates remain disappointing for patients with advanced ovarian cancer (33). A widely recognized behavior of ovarian cancer is its ability to migrate and seed the peritoneal cavity with nests of tumor cells and the formation of ascites (4). We found that (i) PELP1 plays a role in ovarian tumor cell cytoskeletal reorganization, (ii) PELP1 downregulation affects expression of genes involved in metastasis, (iii) PELP1 is recruited to promoters of MMPs and is required for optimal activation of MMPs, (iv) PELP1 overexpression in normal IOSE cells promote cell migration, (v) PELP1 knockdown substantially affected ovarian cancer ability to migrate in Boyden chamber and invasion assays, and (vi) downregulation of PELP1 in vivo by PELP1-siRNA-DOPC significantly reduced ovarian tumor progression in xenograft models. Collectively, these results suggest that PELP1 signaling confers growth and metastatic advantages to ovarian epithelial cells.
Even though individual proto-oncogenes may play a role in oncogenesis, master regulators that control activities of multiple oncogenes and NRs can provide a growth advantage to cancer cells. Emerging findings suggest that NR coregulator proteins have the potential to be differentially expressed in malignant tumors, and that their functions may be altered, leading to tumor progression (7). A few recent studies examined the status of NR coregulators in ovarian tumors and found deregulation of a few coregulators including AIB1, SRA, and ARA70 (34–36). Furthermore, AIB1 knockout mice studies demonstrated that normal expression of the coregulator AIB1 is required for initiation of tumorigenesis by carcinogens and oncogenes (37, 38), suggesting that coregulators can play a vital role in tumor initiation and/or progression. PELP1 is a unique NR coregulator that interacts with a number of NRs (11). Its expression is deregulated in ovarian cancers (20). Using a metastasis array, we have identified a close linkage between PELP1 expression in ovarian cells with the activation of many genes that are implicated in metastasis. Our results suggest that PELP1 is a central NR coregulator that plays a critical role in ovarian cancer progression and metastasis.
A number of oncogenes that allow cells to grow independently from the host signals have been identified in ovarian cancer. The proto-oncogene c-Src is of interest in ovarian tumors as overexpression of Src has been demonstrated in 93% of advanced-stage ovarian tumors (39) and inhibition of c-Src in preclinical models can inhibit ovarian tumor growth (40). Disruptions of the p16-CDK4/cyclin D1-pRb pathway (RB pathway) also play an important role in the development of ovarian cancers (41). PELP1 couples NR signaling to cell-cycle progression via the pRb pathway (12) and PELP1 is a recently identified substrate of cyclin-dependent kinases (CDK; ref. 24). Emerging evidence suggests that PELP1 is a proto-oncogene (14) and collaborates with activation of other oncogenes including Src (11). Growth factor and hormonal signals promote PELP1 interactions with cytosolic kinases leading to activation of MAPK (mitogen-activated protein kinase) and AKT pathways. Our results that PELP1 knockdown substantially affects the growth of ovarian cancer cells in vitro and in vivo also supports a role of PELP1 in providing growth advantage to ovarian cancer cells. PELP1-mediated crosstalk with cytosolic kinases and cell-cycle proteins may provide growth advantage in PELP1 deregulated ovarian cancer cells.
Accumulating evidence suggest that PELP1 functions as a large scaffolding protein and modulates gene transcription via protein–protein interactions with histone-modifying acetylases/deacetylases (42) and methyltransferases/demethylases. A recent study identified PELP1 as a component of the MLL1 methyltransferase complex (43). We found that PELP1 functions as a modifier of dimethyl modification of histones (29). Our results from the current study demonstrated that PELP1 signaling affects the ovarian cancer cell cytoskeleton and their migration. Gene array and expression analyses found that PELP1 has the potential to activate the transcription of genes involved in metastasis. ChIP studies revealed that PELP1 is recruited to the proximal promoter region of MMM9 and PELP1 knockdown enhances repressive mark H3K9me2 and decreases activation mark H3K9ac at the proximal promoter. These results suggest that PELP1-mediated genomic functions play a role in expression of genes involved in metastasis.
Currently, no known drugs inhibit PELP1 functions. Therefore, in this study, we developed PELP1-siRNA nanoliposomes to examine the in vivo therapeutic potential of inhibiting PELP1. We examined the ability of systemically administered PELP1-siRNA to silence PELP1 gene expression in vivo using a xenograft model. The advantage of DOPC nanoliposomes is that they have no electrical charge. This neutrality provides an advantage over positively or negatively charged liposomes when it comes to binding with and penetrating cells (28). Another advantage of DOPC nanoliposomes is their small size (65–125 nm) that enables their transport through blood vessels. Several recently published studies validated in vivo delivery of siRNA using DOPC-based liposomes and proved that this method is therapeutically efficacious (26, 44, 45). We found that PELP1-siRNA-DOPC can effectively reduce PELP1 expression and significantly reduced the tumor growth in ovarian xenografts. Peritoneal surfaces are the most common site of ovarian metastases. The reduction in the number of tumor nodules found in the PELP1-siRNA-DOPC–treated nude mice indicate that PELP1-siRNA-DOPC has potential to block ovarian metastasis. These results suggest that PELP1-siRNA-DOPC can be used as a novel drug for therapeutic targeting of PELP1 in ovarian cancer.
In summary, our results demonstrate that the NR coregulator PELP1 plays a critical role in ovarian cancer cell migration and modulates expression of several genes involved in metastasis. Even through earlier studies found that PELP1 plays a role in the proliferation of hormone-driven tumors, this study demonstrated that it also has the potential to promote metastasis of ovarian epithelial cells. Furthermore, our study results provide the first in vivo evidence that PELP1-siRNA-DOPC can be used as a line of therapy in ovarian cancer metastasis. Since multiple signaling pathways are involved in optimal generation of biological outcomes, targeting master regulators, such as PELP1, is clinically relevant and will have better therapeutic effect.
Translational Relevance.
Ovarian carcinoma is the leading cause of death due to gynecologic malignancy. In this study, we investigated whether PELP1 (proline-, glutamic acid-, leucine-rich protein-1) signaling plays a role in ovarian epithelial cancer cell migration/metastasis and determined the therapeutic potential of PELP1-siRNA in vivo using a nanoliposomal formulation of PELP1-siRNA-DOPC (1,2-dioleoyl-sn-glycero-3-phosphatidylcholine). Our results demonstrate that PELP1 plays a critical role in ovarian cancer cell migration and modulates expression of several genes involved in metastasis. We found that PELP1-siRNA-DOPC can effectively reduce PELP1 expression and significantly reduce the tumor growth in ovarian xenografts. The results suggest that PELP1 signaling axis is a potential druggable target and liposomal PELP1-siRNA-DOPC could be used as a novel drug to prevent or treat ovarian metastasis.
Acknowledgments
Grant Support: This study was supported by NIH-CA0095681, W81XWH-06-1-0398, CTRC P30CA54174, U54CA151668, CA083639, and OCRF grants.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Landen CN, Jr, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26:995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 2.Ozols RF, Bookman MA, Connolly DC, Daly MB, Godwin AK, Schilder RJ, et al. Focus on epithelial ovarian cancer. Cancer Cell. 2004;5:19–24. doi: 10.1016/s1535-6108(04)00002-9. [DOI] [PubMed] [Google Scholar]
- 3.Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol. 2006;33:S3–11. doi: 10.1053/j.seminoncol.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5:355–66. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- 5.McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science. 2002;296:1642–4. doi: 10.1126/science.1071884. [DOI] [PubMed] [Google Scholar]
- 6.McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–44. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 7.O'Malley BW. Molecular biology. Little molecules with big goals. Science. 2006;313:1749–50. doi: 10.1126/science.1132509. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–28. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 9.O'Malley BW. Coregulators: from whence came these “master genes”. Mol Endocrinol. 2007;21:1009–13. doi: 10.1210/me.2007-0012. [DOI] [PubMed] [Google Scholar]
- 10.Vadlamudi RK, Wang RA, Mazumdar A, Kim Y, Shin J, Sahin A, et al. Molecular cloning and characterization of PELP1, a novel human coregulator of estrogen receptor alpha. J Biol Chem. 2001;276:38272–9. doi: 10.1074/jbc.M103783200. [DOI] [PubMed] [Google Scholar]
- 11.Vadlamudi RK, Kumar R. Functional and biological properties of the nuclear receptor coregulator PELP1/MNAR. Nucl Recept Signal. 2007;5:e004. doi: 10.1621/nrs.05004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balasenthil S, Vadlamudi RK. Functional interactions between the estrogen receptor coactivator PELP1/MNAR and retinoblastoma protein. J Biol Chem. 2003;278:22119–27. doi: 10.1074/jbc.M212822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nair SS, Mishra SK, Yang Z, Balasenthil S, Kumar R, Vadlamudi RK. Potential role of a novel transcriptional coactivator PELP1 in histone H1 displacement in cancer cells. Cancer Res. 2004;64:6416–23. doi: 10.1158/0008-5472.CAN-04-1786. [DOI] [PubMed] [Google Scholar]
- 14.Rajhans R, Nair S, Holden AH, Kumar R, Tekmal RR, Vadlamudi RK. Oncogenic potential of the nuclear receptor coregulator proline-, glutamic acid-, leucine-rich protein 1/modulator of the nongenomic actions of the estrogen receptor. Cancer Res. 2007;67:5505–12. doi: 10.1158/0008-5472.CAN-06-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vadlamudi RK, Balasenthil S, Broaddus RR, Gustafsson JA, Kumar R. Deregulation of estrogen receptor coactivator proline-, glutamic acid-, and leucine-rich protein-1/modulator of nongenomic activity of estrogen receptor in human endometrial tumors. J Clin Endocrinol Metab. 2004;89:6130–8. doi: 10.1210/jc.2004-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habashy HO, Powe DG, Rakha EA, Ball G, Macmillan RD, Green AR, et al. The prognostic significance of PELP1 expression in invasive breast cancer with emphasis on the ER-positive luminal-like subtype. Breast Cancer Res Treat. 2010;120:603–12. doi: 10.1007/s10549-009-0419-9. [DOI] [PubMed] [Google Scholar]
- 17.Nair S, Vadlamudi RK. Emerging significance of ER-coregulator PELP1/MNAR in cancer. Histol Histopathol. 2007;22:91–6. doi: 10.14670/hh-22.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90:447–54. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 19.Choi KC, Auersperg N, Leung PC. Expression and antiproliferative effect of a second form of gonadotropin-releasing hormone in normal and neoplastic ovarian surface epithelial cells. J Clin Endocrinol Metab. 2001;86:5075–8. doi: 10.1210/jcem.86.10.8100. [DOI] [PubMed] [Google Scholar]
- 20.Dimple C, Nair SS, Rajhans R, Pitcheswara PR, Liu J, Balasenthil S, et al. Role of PELP1/MNAR signaling in ovarian tumorigenesis. Cancer Res. 2008;68:4902–9. doi: 10.1158/0008-5472.CAN-07-5698. [DOI] [PubMed] [Google Scholar]
- 21.Chakravarty D, Nair SS, Santhamma B, Nair BC, Wang L, Bandyopadhyay A, et al. Extranuclear functions of ER impact invasive migration and metastasis by breast cancer cells. Cancer Res. 2010;70:4092–101. doi: 10.1158/0008-5472.CAN-09-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin L, Liao L, Redmond A, Young L, Yuan Y, Chen H, et al. The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-medicated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Mol Cell Biol. 2008;28:5937–50. doi: 10.1128/MCB.00579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin H, Sun Y, Benveniste EN. The transcription factors Sp1, Sp3, and AP-2 are required for constitutive matrix metalloproteinase-2 gene expression in astroglioma cells. J Biol Chem. 1999;274:29130–7. doi: 10.1074/jbc.274.41.29130. [DOI] [PubMed] [Google Scholar]
- 24.Nair BC, Nair SS, Chakravarty D, Challa R, Manavathi B, Yew PR, et al. Cyclin-dependent kinase-mediated phosphorylation plays a critical role in the oncogenic functions of PELP1. Cancer Res. 2010;70:7166–75. doi: 10.1158/0008-5472.CAN-10-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lengyel E, Gum R, Juarez J, Clayman G, Seiki M, Sato H, et al. Induction of M(r) 92,000 type IV collagenase expression in a squamous cell carcinoma cell line by fibroblasts. Cancer Res. 1995;55:963–7. [PubMed] [Google Scholar]
- 26.Landen CN, Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–8. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 27.Mangala LS, Zuzel V, Schmandt R, Leshane ES, Halder JB, Armaiz-Pena GN, et al. Therapeutic targeting of ATP7B in ovarian carcinoma. Clin Cancer Res. 2009;15:3770–80. doi: 10.1158/1078-0432.CCR-08-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 1998;17:279–84. doi: 10.1023/a:1006140513233. [DOI] [PubMed] [Google Scholar]
- 29.Nair SS, Nair BC, Cortez V, Chakravarty D, Metzger E, Schüle R, et al. PELP1 is a reader of histone H3 methylation that facilitates oestrogen receptor-alpha target gene activation by regulating lysine demethylase 1 specificity. EMBO Rep. 2010;11:438–44. doi: 10.1038/embor.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stack MS, Ellerbroek SM, Fishman DA. The role of proteolytic enzymes in the pathology of epithelial ovarian carcinoma. Int J Oncol. 1998;12:569–76. doi: 10.3892/ijo.12.3.569. [DOI] [PubMed] [Google Scholar]
- 31.Naylor MS, Stamp GW, Davies BD, Balkwill FR. Expression and activity of MMPS and their regulators in ovarian cancer. Int J Cancer. 1994;58:50–6. doi: 10.1002/ijc.2910580110. [DOI] [PubMed] [Google Scholar]
- 32.Perez RP, Godwin AK, Hamilton TC, Ozols RF. Ovarian cancer biology. Semin Oncol. 1991;18:186–204. [PubMed] [Google Scholar]
- 33.Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22:255–88. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- 34.Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–8. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 35.Li AJ, Lerner DL, Gapuzan ME, Karlan BY. AIB1 polymorphisms predict aggressive ovarian cancer phenotype. Cancer Epidemiol Biomarkers Prev. 2005;14:2919–22. doi: 10.1158/1055-9965.EPI-05-0540. [DOI] [PubMed] [Google Scholar]
- 36.Quezada S, Avellaira C, Johnson MC, Gabler F, Fuentes A, Vega M. Evaluation of steroid receptors, coregulators, and molecules associated with uterine receptivity in secretory endometria from untreated women with polycystic ovary syndrome. Fertil Steril. 2006;85:1017–26. doi: 10.1016/j.fertnstert.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 37.Kuang SQ, Liao L, Wang S, Medina D, O'Malley BW, Xu J. Mice lacking the amplified in breast cancer 1/steroid receptor coactivator-3 are resistant to chemical carcinogen-induced mammary tumorigenesis. Cancer Res. 2005;65:7993–8002. doi: 10.1158/0008-5472.CAN-05-1179. [DOI] [PubMed] [Google Scholar]
- 38.Kuang SQ, Liao L, Zhang H, Lee AV, O'Malley BW, Xu J. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res. 2004;64:1875–85. doi: 10.1158/0008-5472.can-03-3745. [DOI] [PubMed] [Google Scholar]
- 39.Wiener JR, Windham TC, Estrella VC, Parikh NU, Thall PF, Deavers MT, et al. Activated SRC protein tyrosine kinase is overexpressed in late-stage human ovarian cancers. Gynecol Oncol. 2003;88:73–9. doi: 10.1006/gyno.2002.6851. [DOI] [PubMed] [Google Scholar]
- 40.Han LY, Landen CN, Trevino JG, Halder J, Lin YG, Kamat AA, et al. Antiangiogenic and antitumor effects of SRC inhibition in ovarian carcinoma. Cancer Res. 2006;66:8633–9. doi: 10.1158/0008-5472.CAN-06-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashiguchi Y, Tsuda H, Yamamoto K, Inoue T, Ishiko O, Ogita S. Combined analysis of p53 and RB pathways in epithelial ovarian cancer. Hum Pathol. 2001;32:988–96. doi: 10.1053/hupa.2001.27115. [DOI] [PubMed] [Google Scholar]
- 42.Choi YB, Ko JK, Shin J. The transcriptional corepressor, PELP1, recruits HDAC2 and masks histones using two separate domains. J Biol Chem. 2004;279:50930–41. doi: 10.1074/jbc.M406831200. [DOI] [PubMed] [Google Scholar]
- 43.Dou Y, Milne TA, Tackett AJ, Smith ER, Fukuda A, Wysocka J, et al. Physical association and coordinate function of the H3 K4 methyltransferase MLL1 and the H4 K16 acetyltransferase MOF. Cell. 2005;121:873–85. doi: 10.1016/j.cell.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 44.Halder J, Kamat AA, Landen CN, Jr, Han LY, Lutgendorf SK, Lin YG, et al. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res. 2006;12:4916–24. doi: 10.1158/1078-0432.CCR-06-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landen CN, Merritt WM, Mangala LS, Sanguino AM, Bucana C, Lu C, et al. Intraperitoneal delivery of liposomal siRNA for therapy of advanced ovarian cancer. Cancer Biol Ther. 2006;5:1708–13. doi: 10.4161/cbt.5.12.3468. [DOI] [PubMed] [Google Scholar]