Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Rouviere-Yaniv J. Amino and carboxy terminal sequences of the DNA-binding protein HU from the Cyanobacterium Synechocystis PCC 6701 (ATCC 27170). Biochem Biophys Res Commun. 1979 Nov 28;91(2):461–467. doi: 10.1016/0006-291x(79)91544-4. [DOI] [PubMed] [Google Scholar]
- Baker T. A., Sekimizu K., Funnell B. E., Kornberg A. Extensive unwinding of the plasmid template during staged enzymatic initiation of DNA replication from the origin of the Escherichia coli chromosome. Cell. 1986 Apr 11;45(1):53–64. doi: 10.1016/0092-8674(86)90537-4. [DOI] [PubMed] [Google Scholar]
- Bear S. E., Court D. L., Friedman D. I. An accessory role for Escherichia coli integration host factor: characterization of a lambda mutant dependent upon integration host factor for DNA packaging. J Virol. 1984 Dec;52(3):966–972. doi: 10.1128/jvi.52.3.966-972.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold V., Geider K. Interaction of DNA with DNA-binding proteins. The characterization of protein HD from Escherichia coli and its nucleic acid complexes. Eur J Biochem. 1976 Dec 11;71(2):443–449. doi: 10.1111/j.1432-1033.1976.tb11132.x. [DOI] [PubMed] [Google Scholar]
- Better M., Lu C., Williams R. C., Echols H. Site-specific DNA condensation and pairing mediated by the int protein of bacteriophage lambda. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5837–5841. doi: 10.1073/pnas.79.19.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Cozzarelli N. R. Use of site-specific recombination as a probe of DNA structure and metabolism in vivo. J Mol Biol. 1987 Mar 20;194(2):205–218. doi: 10.1016/0022-2836(87)90369-x. [DOI] [PubMed] [Google Scholar]
- Bossi L., Smith D. M. Conformational change in the DNA associated with an unusual promoter mutation in a tRNA operon of Salmonella. Cell. 1984 Dec;39(3 Pt 2):643–652. doi: 10.1016/0092-8674(84)90471-9. [DOI] [PubMed] [Google Scholar]
- Broyles S. S., Pettijohn D. E. Interaction of the Escherichia coli HU protein with DNA. Evidence for formation of nucleosome-like structures with altered DNA helical pitch. J Mol Biol. 1986 Jan 5;187(1):47–60. doi: 10.1016/0022-2836(86)90405-5. [DOI] [PubMed] [Google Scholar]
- Busby S., Kolb A., Buc H. Isolation of plasmid-protein complexes from Escherichia coli. Eur J Biochem. 1979 Aug 15;99(1):105–111. doi: 10.1111/j.1432-1033.1979.tb13237.x. [DOI] [PubMed] [Google Scholar]
- Bushman W., Thompson J. F., Vargas L., Landy A. Control of directionality in lambda site specific recombination. Science. 1985 Nov 22;230(4728):906–911. doi: 10.1126/science.2932798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron F., Jacq C., Rouvière-Yaniv J. Characterization of a histone-like protein extracted from yeast mitochondria. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4265–4269. doi: 10.1073/pnas.76.9.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA gyrase and the supercoiling of DNA. Science. 1980 Feb 29;207(4434):953–960. doi: 10.1126/science.6243420. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Nash H. A. E. coli integration host factor binds to specific sites in DNA. Cell. 1984 Dec;39(3 Pt 2):707–716. doi: 10.1016/0092-8674(84)90478-1. [DOI] [PubMed] [Google Scholar]
- Craigie R., Arndt-Jovin D. J., Mizuuchi K. A defined system for the DNA strand-transfer reaction at the initiation of bacteriophage Mu transposition: protein and DNA substrate requirements. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7570–7574. doi: 10.1073/pnas.82.22.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepin M., Cukier-Kahn R., Gros F. Effect of a low-molecular-weight DNA binding protein, H1 factor, on the in vitro transcription of the lactose operon in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jan;72(1):333–337. doi: 10.1073/pnas.72.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukier-Kahn R., Jacquet M., Gros F. Two heat-resistant, low molecular weight proteins from Escherichia coli that stimulate DNA-directed RNA synthesis. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3643–3647. doi: 10.1073/pnas.69.12.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Green G. R., Searcy D. G. A histone-like protein (HTa) from Thermoplasma acidophilum. I. Purification and properties. J Biol Chem. 1981 Jan 25;256(2):900–904. [PubMed] [Google Scholar]
- DeLange R. J., Williams L. C., Searcy D. G. A histone-like protein (HTa) from Thermoplasma acidophilum. II. Complete amino acid sequence. J Biol Chem. 1981 Jan 25;256(2):905–911. [PubMed] [Google Scholar]
- Dean F., Krasnow M. A., Otter R., Matzuk M. M., Spengler S. J., Cozzarelli N. R. Escherichia coli type-1 topoisomerases: identification, mechanism, and role in recombination. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):769–777. doi: 10.1101/sqb.1983.047.01.088. [DOI] [PubMed] [Google Scholar]
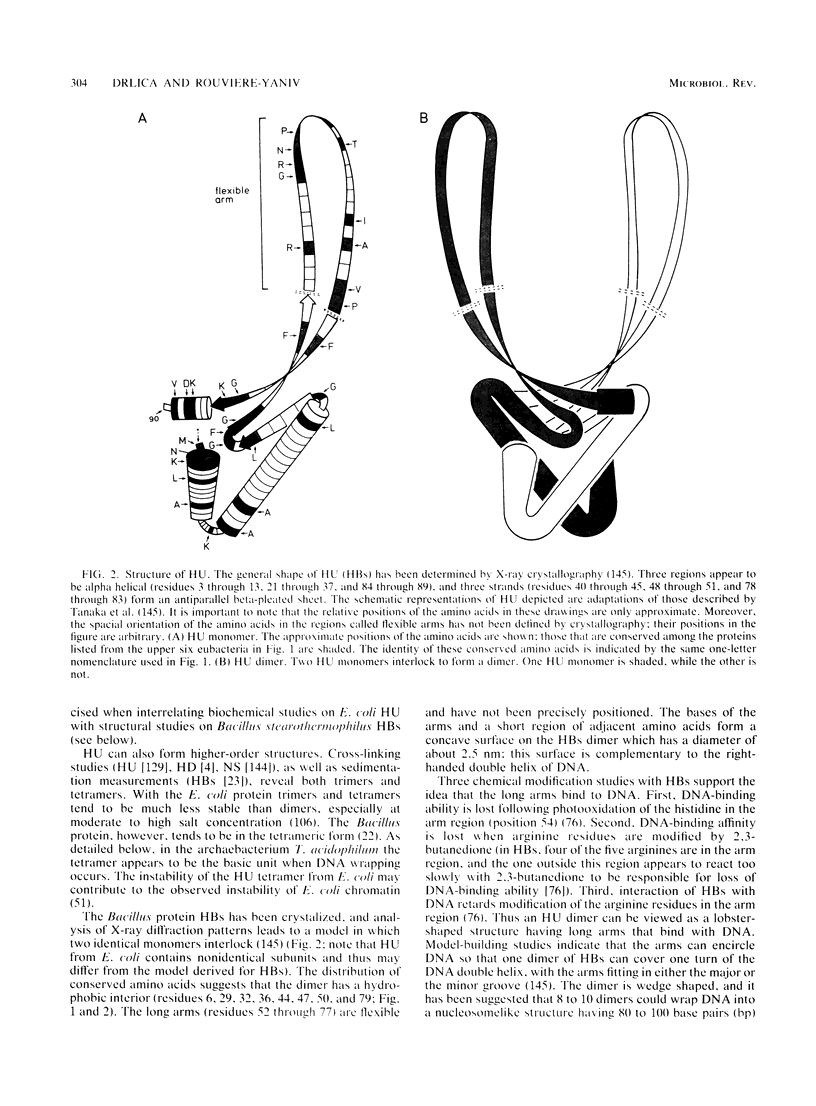
- Dijk J., White S. W., Wilson K. S., Appelt K. On the DNA binding protein II from Bacillus stearothermophilus. I. Purification, studies in solution, and crystallization. J Biol Chem. 1983 Mar 25;258(6):4003–4006. [PubMed] [Google Scholar]
- Dixon N. E., Kornberg A. Protein HU in the enzymatic replication of the chromosomal origin of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(2):424–428. doi: 10.1073/pnas.81.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Snyder M. Superhelical Escherichia coli DNA: relaxation by coumermycin. J Mol Biol. 1978 Apr 5;120(2):145–154. doi: 10.1016/0022-2836(78)90061-x. [DOI] [PubMed] [Google Scholar]
- Echols H., Dodson M., Better M., Roberts J. D., McMacken R. The role of specialized nucleoprotein structures in site-specific recombination and initiation of DNA replication. Cold Spring Harb Symp Quant Biol. 1984;49:727–733. doi: 10.1101/sqb.1984.049.01.082. [DOI] [PubMed] [Google Scholar]
- Echols H. Multiple DNA-protein interactions governing high-precision DNA transactions. Science. 1986 Sep 5;233(4768):1050–1056. doi: 10.1126/science.2943018. [DOI] [PubMed] [Google Scholar]
- Ehbrecht H. J., Pingoud A., Urbanke C., Maass G., Gualerzi C. Linear diffusion of restriction endonucleases on DNA. J Biol Chem. 1985 May 25;260(10):6160–6166. [PubMed] [Google Scholar]
- Feiss M., Frackman S., Sippy J. Essential interaction between lambdoid phage 21 terminase and the Escherichia coli integrative host factor. J Mol Biol. 1985 May 25;183(2):239–246. doi: 10.1016/0022-2836(85)90216-5. [DOI] [PubMed] [Google Scholar]
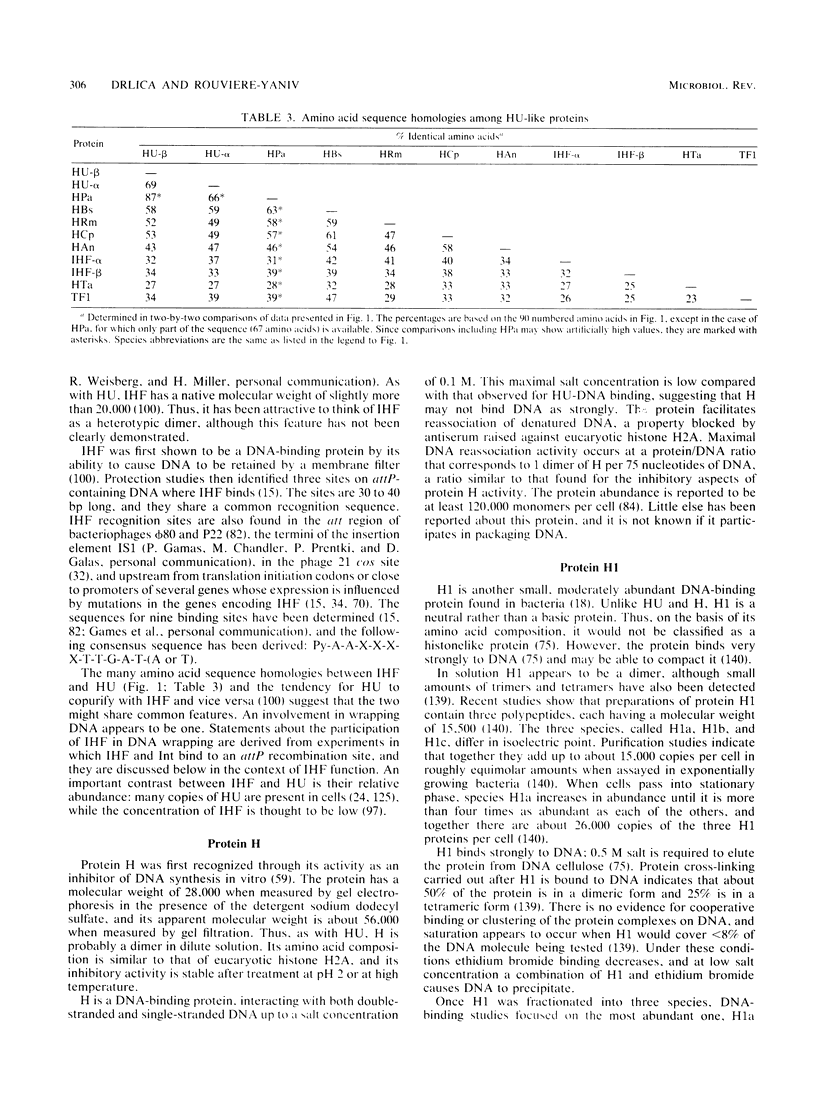
- Flamm E. L., Weisberg R. A. Primary structure of the hip gene of Escherichia coli and of its product, the beta subunit of integration host factor. J Mol Biol. 1985 May 25;183(2):117–128. doi: 10.1016/0022-2836(85)90206-2. [DOI] [PubMed] [Google Scholar]
- Friden P., Voelkel K., Sternglanz R., Freundlich M. Reduced expression of the isoleucine and valine enzymes in integration host factor mutants of Escherichia coli. J Mol Biol. 1984 Feb 5;172(4):573–579. doi: 10.1016/s0022-2836(84)80024-8. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. J., Carver D., Gellert M. Synergistic effect of himA and gyrB mutations: evidence that him functions control expression of ilv and xyl genes. J Bacteriol. 1984 Feb;157(2):484–489. doi: 10.1128/jb.157.2.484-489.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Funnell B. E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984 Oct;38(3):889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Funnell B. E., Baker T. A., Kornberg A. Complete enzymatic replication of plasmids containing the origin of the Escherichia coli chromosome. J Biol Chem. 1986 Apr 25;261(12):5616–5624. [PubMed] [Google Scholar]
- Gardner J. F., Nash H. A. Role of Escherichia coli IHF protein in lambda site-specific recombination. A mutational analysis of binding sites. J Mol Biol. 1986 Sep 20;191(2):181–189. doi: 10.1016/0022-2836(86)90255-x. [DOI] [PubMed] [Google Scholar]
- Geider K., Hoffmann-Berling H. Proteins controlling the helical structure of DNA. Annu Rev Biochem. 1981;50:233–260. doi: 10.1146/annurev.bi.50.070181.001313. [DOI] [PubMed] [Google Scholar]
- Geider K. Interaction of DNA with DNA-binding proteins: protein exchange and complex stability. Eur J Biochem. 1978 Jul 3;87(3):617–622. doi: 10.1111/j.1432-1033.1978.tb12414.x. [DOI] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gellert M., Menzel R., Mizuuchi K., O'Dea M. H., Friedman D. I. Regulation of DNA supercoiling in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):763–767. doi: 10.1101/sqb.1983.047.01.087. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giphart-Gassler M., Goosen T., van Meeteren A., Wijffelman C., van de Putte P. Properties of the recombinant plasmid pGP1 containing part of the early region of bacteriophage mu. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1179–1185. doi: 10.1101/sqb.1979.043.01.133. [DOI] [PubMed] [Google Scholar]
- Goosen N., van Heuvel M., Moolenaar G. F., van de Putte P. Regulation of Mu transposition. II. The escherichia coli HimD protein positively controls two repressor promoters and the early promoter of bacteriophage Mu. Gene. 1984 Dec;32(3):419–426. doi: 10.1016/0378-1119(84)90017-9. [DOI] [PubMed] [Google Scholar]
- Goosen N., van de Putte P. Regulation of Mu transposition. I. Localization of the presumed recognition sites for HimD and Ner functions controlling bacteriophage Mu transcription. Gene. 1984 Oct;30(1-3):41–46. doi: 10.1016/0378-1119(84)90103-3. [DOI] [PubMed] [Google Scholar]
- Green G. R., Searcy D. G., DeLange R. J. Histone-like protein in the Archaebacterium Sulfolobus acidocaldarius. Biochim Biophys Acta. 1983 Nov 17;741(2):251–257. doi: 10.1016/0167-4781(83)90066-0. [DOI] [PubMed] [Google Scholar]
- Greene J. R., Brennan S. M., Andrew D. J., Thompson C. C., Richards S. H., Heinrikson R. L., Geiduschek E. P. Sequence of the bacteriophage SP01 gene coding for transcription factor 1, a viral homologue of the bacterial type II DNA-binding proteins. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7031–7035. doi: 10.1073/pnas.81.22.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D., Nash H. A. Genetic rearrangement of DNA induces knots with a unique topology: implications for the mechanism of synapsis and crossing-over. Proc Natl Acad Sci U S A. 1985 May;82(10):3124–3128. doi: 10.1073/pnas.82.10.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. Visualization of prokaryotic DNA in a regularly condensed chromatin-like fiber. Proc Natl Acad Sci U S A. 1976 Feb;73(2):563–567. doi: 10.1073/pnas.73.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamkalo B. A., Rattner J. B. The structure of mesokaryote chromosome. Chromosoma. 1977 Mar 7;60(1):39–47. doi: 10.1007/BF00330409. [DOI] [PubMed] [Google Scholar]
- Haselkorn R., Rouvière-Yaniv J. Cyanobacterial DNA-binding protein related to Escherichia coli HU. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1917–1920. doi: 10.1073/pnas.73.6.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins A. R., Wootton J. C. A single DNA-binding protein from Pseudomonas aeruginosa homologous to proteins NS1 and NS2 (HU proteins) of Escherichia coli and other bacteria. FEBS Lett. 1981 Aug 3;130(2):275–278. doi: 10.1016/0014-5793(81)81138-6. [DOI] [PubMed] [Google Scholar]
- Holck A., Kleppe K. Affinity of protein HU for different nucleic acids. FEBS Lett. 1985 Jun 3;185(1):121–124. doi: 10.1016/0014-5793(85)80753-5. [DOI] [PubMed] [Google Scholar]
- Hoyt M. A., Knight D. M., Das A., Miller H. I., Echols H. Control of phage lambda development by stability and synthesis of cII protein: role of the viral cIII and host hflA, himA and himD genes. Cell. 1982 Dec;31(3 Pt 2):565–573. doi: 10.1016/0092-8674(82)90312-9. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Lutz H., Kornberg A. Novel histone H2A-like protein of escherichia coli. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5097–5101. doi: 10.1073/pnas.77.9.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imber R., Bächinger H., Bickle T. A. Purification and characterisation of a small DNA-binding protein, HB, from Bacillus globigii. Eur J Biochem. 1982 Mar 1;122(3):627–632. doi: 10.1111/j.1432-1033.1982.tb06485.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Bruist M. F., Simon M. I. Host protein requirements for in vitro site-specific DNA inversion. Cell. 1986 Aug 15;46(4):531–539. doi: 10.1016/0092-8674(86)90878-0. [DOI] [PubMed] [Google Scholar]
- Kaguni J. M., Kornberg A. Replication initiated at the origin (oriC) of the E. coli chromosome reconstituted with purified enzymes. Cell. 1984 Aug;38(1):183–190. doi: 10.1016/0092-8674(84)90539-7. [DOI] [PubMed] [Google Scholar]
- Kano Y., Wada M., Nagase T., Imamoto F. Genetic characterization of the gene hupB encoding the HU-1 protein of Escherichia coli. Gene. 1986;45(1):37–44. doi: 10.1016/0378-1119(86)90129-0. [DOI] [PubMed] [Google Scholar]
- Kano Y., Yoshino S., Wada M., Yokoyama K., Nobuhara M., Imamoto F. Molecular cloning and nucleotide sequence of the HU-1 gene of Escherichia coli. Mol Gen Genet. 1985;201(2):360–362. doi: 10.1007/BF00425687. [DOI] [PubMed] [Google Scholar]
- Karnkowska D., Paterczyk B. Isolation and characterization of chromatin from Caulobacter crescentus. Acta Microbiol Pol. 1985;34(2):95–102. [PubMed] [Google Scholar]
- Kikuchi A., Flamm E., Weisberg R. A. An Escherichia coli mutant unable to support site-specific recombination of bacteriophage lambda. J Mol Biol. 1985 May 25;183(2):129–140. doi: 10.1016/0022-2836(85)90207-4. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Nash H. A. The bacteriophage lambda int gene product. A filter assay for genetic recombination, purification of int, and specific binding to DNA. J Biol Chem. 1978 Oct 25;253(20):7149–7157. [PubMed] [Google Scholar]
- Kikuchi Y., Nash H. Integrative recombination of bacteriophage lambda: requirement for supertwisted DNA in vivo and characterization of int. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1099–1109. doi: 10.1101/sqb.1979.043.01.122. [DOI] [PubMed] [Google Scholar]
- Kimura M., Kimura J., Davie P., Reinhardt R., Dijk J. The amino acid sequence of a small DNA binding protein from the archaebacterium Sulfolobus solfataricus. FEBS Lett. 1984 Oct 15;176(1):176–178. doi: 10.1016/0014-5793(84)80935-7. [DOI] [PubMed] [Google Scholar]
- Kimura M., Wilson K. S. On the DNA binding protein II from Bacillus stearothermophilus. II. The amino acid sequence and its relation to those of homologous proteins from other prokaryotes. J Biol Chem. 1983 Mar 25;258(6):4007–4011. [PubMed] [Google Scholar]
- Kmiec E. B., Worcel A. The positive transcription factor of the 5S RNA gene induces a 5S DNA-specific gyration in Xenopus oocyte extracts. Cell. 1985 Jul;41(3):945–953. doi: 10.1016/s0092-8674(85)80075-1. [DOI] [PubMed] [Google Scholar]
- Koepsel R. R., Khan S. A. Static and initiator protein-enhanced bending of DNA at a replication origin. Science. 1986 Sep 19;233(4770):1316–1318. doi: 10.1126/science.3749879. [DOI] [PubMed] [Google Scholar]
- Krause H. M., Higgins N. P. Positive and negative regulation of the Mu operator by Mu repressor and Escherichia coli integration host factor. J Biol Chem. 1986 Mar 15;261(8):3744–3752. [PubMed] [Google Scholar]
- Laine B., Belaiche D., Sautiere P., Biserte G. Characterization and structural study of the DNA-binding protein HRm From Rhizobium meliloti. Biochem Biophys Res Commun. 1982 May 14;106(1):101–107. doi: 10.1016/0006-291x(82)92063-0. [DOI] [PubMed] [Google Scholar]
- Laine B., Bélaïche D., Khanaka H., Sautière P. Primary structure of the DNA-binding protein HRm from Rhizobium meliloti. Eur J Biochem. 1983 Mar 15;131(2):325–331. doi: 10.1111/j.1432-1033.1983.tb07265.x. [DOI] [PubMed] [Google Scholar]
- Laine B., Kmiecik D., Sautiere P., Biserte G., Cohen-Solal M. Complete amino-acid sequences of DNA-binding proteins HU-1 and HU-2 from Escherichia coli. Eur J Biochem. 1980 Feb;103(3):447–461. doi: 10.1111/j.1432-1033.1980.tb05968.x. [DOI] [PubMed] [Google Scholar]
- Laine B., Sautiere P., Spassky A., Rimsky S. A DNA-binding protein from E. coli isolation, characterization and its relationship with proteins H1 and B1. Biochem Biophys Res Commun. 1984 Mar 30;119(3):1147–1153. doi: 10.1016/0006-291x(84)90895-7. [DOI] [PubMed] [Google Scholar]
- Laine B., Sautière P., Biserte G., Cohen-Solal M., Gros F., Rouvière-Yaniv J. The amino- and carboxy-terminal amino acid sequences of protein HU from Escherichia coli. FEBS Lett. 1978 May 1;89(1):116–120. doi: 10.1016/0014-5793(78)80535-3. [DOI] [PubMed] [Google Scholar]
- Lange-Gustafson B. J., Nash H. A. Purification and properties of Int-h, a variant protein involved in site-specific recombination of bacteriophage lambda. J Biol Chem. 1984 Oct 25;259(20):12724–12732. [PubMed] [Google Scholar]
- Lathe R., Buc H., Lecocq J. P., Bautz E. K. Prokaryotic histone-like protein interacting with RNA polymerase. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3548–3552. doi: 10.1073/pnas.77.6.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R. Fine-structure mapping of the firA gene, a locus involved in the phenotypic expression of rifampin resistance in Escherichia. J Bacteriol. 1977 Sep;131(3):1033–1036. doi: 10.1128/jb.131.3.1033-1036.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R., Lecocq J. P. The firA gene, a locus involved in the expression of rifampicin resistance in Escherichia coli. I. Characterisation of lambdafirA transducing phages constructed in vitro. Mol Gen Genet. 1977 Jul 7;154(1):43–51. doi: 10.1007/BF00265575. [DOI] [PubMed] [Google Scholar]
- Lathe R., Lecocq J. P. The firA gene, a locus involved in the expression of rifampicin resistance in Escherichia coli. II. Characterisation of bacterial proteins coded by lambdafirA transducing phages. Mol Gen Genet. 1977 Jul 7;154(1):53–60. doi: 10.1007/BF00265576. [DOI] [PubMed] [Google Scholar]
- Leong J. M., Nunes-Düby S., Lesser C. F., Youderian P., Susskind M. M., Landy A. The phi 80 and P22 attachment sites. Primary structure and interaction with Escherichia coli integration host factor. J Biol Chem. 1985 Apr 10;260(7):4468–4477. [PubMed] [Google Scholar]
- Lutz H., Von Meyenburg K., Hübscher U. Quantitation with monoclonal antibodies of Escherichia coli H protein suggests histone function. J Bacteriol. 1985 Jun;162(3):1005–1007. doi: 10.1128/jb.162.3.1005-1007.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajna J., Oppenheim A. B., Rattray A., Gottesman M. Translation initiation of bacteriophage lambda gene cII requires integration host factor. J Bacteriol. 1986 Jan;165(1):167–174. doi: 10.1128/jb.165.1.167-174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes S. H., Pruss G. J., Drlica K. Inhibition of RNA synthesis by oxolinic acid is unrelated to average DNA supercoiling. J Bacteriol. 1983 Jul;155(1):420–423. doi: 10.1128/jb.155.1.420-423.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materman E. C., Van Gool A. P. Compact Escherichia coli nucleoids in a highly supercoiled conformation. J Bacteriol. 1978 Aug;135(2):703–706. doi: 10.1128/jb.135.2.703-706.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- Mechulam Y., Fayat G., Blanquet S. Sequence of the Escherichia coli pheST operon and identification of the himA gene. J Bacteriol. 1985 Aug;163(2):787–791. doi: 10.1128/jb.163.2.787-791.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende L., Timm B., Subramanian R. Primary structures of two homologous ribosome-associated DNA-binding proteins of Escherichia coli. FEBS Lett. 1978 Dec 15;96(2):395–398. doi: 10.1016/0014-5793(78)80446-3. [DOI] [PubMed] [Google Scholar]
- Miller H. I., Kikuchi A., Nash H. A., Weisberg R. A., Friedman D. I. Site-specific recombination of bacteriophage lambda: the role of host gene products. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1121–1126. doi: 10.1101/sqb.1979.043.01.125. [DOI] [PubMed] [Google Scholar]
- Miller H. I., Kirk M., Echols H. SOS induction and autoregulation of the himA gene for site-specific recombination in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6754–6758. doi: 10.1073/pnas.78.11.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. I., Nash H. A. Direct role of the himA gene product in phage lambda integration. Nature. 1981 Apr 9;290(5806):523–526. doi: 10.1038/290523a0. [DOI] [PubMed] [Google Scholar]
- Miller H. I. Primary structure of the himA gene of Escherichia coli: homology with DNA-binding protein HU and association with the phenylalanyl-tRNA synthetase operon. Cold Spring Harb Symp Quant Biol. 1984;49:691–698. doi: 10.1101/sqb.1984.049.01.078. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., Gellert M., Nash H. A. Involement of supertwisted DNA in integrative recombination of bacteriophage lambda. J Mol Biol. 1978 May 25;121(3):375–392. doi: 10.1016/0022-2836(78)90370-4. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Patel I., Bastia D. Conformational changes in a replication origin induced by an initiator protein. Cell. 1985 Nov;43(1):189–197. doi: 10.1016/0092-8674(85)90023-6. [DOI] [PubMed] [Google Scholar]
- Nash H. A., Robertson C. A. Purification and properties of the Escherichia coli protein factor required for lambda integrative recombination. J Biol Chem. 1981 Sep 10;256(17):9246–9253. [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Pedersen S., Reeh S. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J Bacteriol. 1977 Jan;129(1):378–387. doi: 10.1128/jb.129.1.378-387.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Baker T. A., van der Ende A., Kornberg A. Initiation of enzymatic replication at the origin of the Escherichia coli chromosome: contributions of RNA polymerase and primase. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3562–3566. doi: 10.1073/pnas.82.11.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim A. B., Gottesman S., Gottesman M. Regulation of bacteriophage lambda int gene expression. J Mol Biol. 1982 Jul 5;158(3):327–346. doi: 10.1016/0022-2836(82)90201-7. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Paci M., Pon C. L., Losso M. A., Gualerzi C. Proteins from the prokaryotic nucleoid. High-resolution 1H NMR spectroscopic study of Escherichia coli DNA-binding proteins NS1 and NS2. Eur J Biochem. 1984 Jan 2;138(1):193–200. doi: 10.1111/j.1432-1033.1984.tb07899.x. [DOI] [PubMed] [Google Scholar]
- Peacock S., Weissbach H., Nash H. A. In vitro regulation of phage lambda cII gene expression by Escherichia coli integration host factor. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6009–6013. doi: 10.1073/pnas.81.19.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettijohn D. E., Pfenninger O. Supercoils in prokaryotic DNA restrained in vivo. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1331–1335. doi: 10.1073/pnas.77.3.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettijohn D. E. Structure and properties of the bacterial nucleoid. Cell. 1982 Oct;30(3):667–669. doi: 10.1016/0092-8674(82)90269-0. [DOI] [PubMed] [Google Scholar]
- Pingoud A., Urbanke C., Alves J., Ehbrecht H. J., Zabeau M., Gualerzi C. Effect of polyamines and basic proteins on cleavage of DNA by restriction endonucleases. Biochemistry. 1984 Nov 20;23(24):5697–5703. doi: 10.1021/bi00319a006. [DOI] [PubMed] [Google Scholar]
- Pollock T. J., Nash H. A. Knotting of DNA caused by a genetic rearrangement. Evidence for a nucleosome-like structure in site-specific recombination of bacteriophage lambda. J Mol Biol. 1983 Oct 15;170(1):1–18. doi: 10.1016/s0022-2836(83)80224-1. [DOI] [PubMed] [Google Scholar]
- Pruss G. J., Manes S. H., Drlica K. Escherichia coli DNA topoisomerase I mutants: increased supercoiling is corrected by mutations near gyrase genes. Cell. 1982 Nov;31(1):35–42. doi: 10.1016/0092-8674(82)90402-0. [DOI] [PubMed] [Google Scholar]
- Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986 Aug 21;322(6081):697–701. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- Richardson S. M., Higgins C. F., Lilley D. M. The genetic control of DNA supercoiling in Salmonella typhimurium. EMBO J. 1984 Aug;3(8):1745–1752. doi: 10.1002/j.1460-2075.1984.tb02041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo P. J., Burghardt R. C. Chromatin structure in the unicellular algae Olisthodiscus luteus, Crypthecodinium cohnii and Peridiniun balticum. Chromosoma. 1980;76(1):91–99. doi: 10.1007/BF00292229. [DOI] [PubMed] [Google Scholar]
- Rizzo P. J., Cox E. R. Histone occurrence in chromatin from Peridinium balticum, a binucleate dinoflagellate. Science. 1977 Dec 23;198(4323):1258–1260. doi: 10.1126/science.563104. [DOI] [PubMed] [Google Scholar]
- Rizzo P. J. Electrophoretic study of histones in the unicellular alga Olisthodiscus luteus. Biochim Biophys Acta. 1980 Jul 24;624(1):66–77. doi: 10.1016/0005-2795(80)90226-3. [DOI] [PubMed] [Google Scholar]
- Rizzo P. J., Noodén L. D. Partial characterization of dinoflagellate chromosomal proteins. Biochim Biophys Acta. 1974 May 31;349(3):415–427. doi: 10.1016/0005-2787(74)90127-0. [DOI] [PubMed] [Google Scholar]
- Ross W., Landy A. Anomalous electrophoretic mobility of restriction fragments containing the att region. J Mol Biol. 1982 Apr 15;156(3):523–529. doi: 10.1016/0022-2836(82)90264-9. [DOI] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Gros F. Characterization of a novel, low-molecular-weight DNA-binding protein from Escherichia coli. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3428–3432. doi: 10.1073/pnas.72.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Kjeldgaard N. O. Native Escherichia coli HU protein is a heterotypic dimer. FEBS Lett. 1979 Oct 15;106(2):297–300. doi: 10.1016/0014-5793(79)80518-9. [DOI] [PubMed] [Google Scholar]
- Rouvière-Yaniv J. Localization of the HU protein on the Escherichia coli nucleoid. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):439–447. doi: 10.1101/sqb.1978.042.01.047. [DOI] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Yaniv M., Germond J. E. E. coli DNA binding protein HU forms nucleosomelike structure with circular double-stranded DNA. Cell. 1979 Jun;17(2):265–274. doi: 10.1016/0092-8674(79)90152-1. [DOI] [PubMed] [Google Scholar]
- Ryder K., Silver S., DeLucia A. L., Fanning E., Tegtmeyer P. An altered DNA conformation in origin region I is a determinant for the binding of SV40 large T antigen. Cell. 1986 Mar 14;44(5):719–725. doi: 10.1016/0092-8674(86)90838-x. [DOI] [PubMed] [Google Scholar]
- Ryter A., Chang A. Localization of transcribing genes in the bacterial cell by means of high resolution autoradiography. J Mol Biol. 1975 Nov 15;98(4):797–810. doi: 10.1016/s0022-2836(75)80011-8. [DOI] [PubMed] [Google Scholar]
- Searcy D. G., Delange R. J. Thermoplasma acidophilum histone-like protein. Partial amino acid sequence suggestive of homology to eukaryotic histones. Biochim Biophys Acta. 1980 Aug 26;609(1):197–200. doi: 10.1016/0005-2787(80)90212-9. [DOI] [PubMed] [Google Scholar]
- Searcy D. G. Histone-like protein in the prokaryote Thermoplasma acidophilum. Biochim Biophys Acta. 1975 Jul 23;395(4):535–547. doi: 10.1016/0005-2787(75)90076-3. [DOI] [PubMed] [Google Scholar]
- Searcy D. G., Stein D. B. Nucleoprotein subunit structure in an unusual prokaryotic organism: Thermoplasma acidophilum. Biochim Biophys Acta. 1980 Aug 26;609(1):180–195. doi: 10.1016/0005-2787(80)90211-7. [DOI] [PubMed] [Google Scholar]
- Searcy D. G. Thermoplasma acidophilum: intracellular pH and potassium concentration. Biochim Biophys Acta. 1976 Nov 18;451(1):278–286. doi: 10.1016/0304-4165(76)90278-6. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Carlson J. O., Pettijohn D. E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980 Oct;21(3):773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Sjåstad K., Fadnes P., Krüger P. G., Lossius I., Kleppe K. Isolation, properties and nucleolytic degradation of chromatin from Escherichia coli. J Gen Microbiol. 1982 Dec;128(12):3037–3050. doi: 10.1099/00221287-128-12-3037. [DOI] [PubMed] [Google Scholar]
- Spassky A., Buc H. C. Physico-chemical properties of a DNA binding protein: Escherichia coli factor H1. Eur J Biochem. 1977 Nov 15;81(1):79–90. doi: 10.1111/j.1432-1033.1977.tb11929.x. [DOI] [PubMed] [Google Scholar]
- Spassky A., Rimsky S., Garreau H., Buc H. H1a, an E. coli DNA-binding protein which accumulates in stationary phase, strongly compacts DNA in vitro. Nucleic Acids Res. 1984 Jul 11;12(13):5321–5340. doi: 10.1093/nar/12.13.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. R., Pruss G. J., Manes S. H., Burg L., Drlica K. DNA supercoiling in gyrase mutants. J Bacteriol. 1984 May;158(2):397–403. doi: 10.1128/jb.158.2.397-403.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. B., Searcy D. G. Physiologically important stabilization of DNA by a prokaryotic histone-like protein. Science. 1978 Oct 13;202(4364):219–221. doi: 10.1126/science.694528. [DOI] [PubMed] [Google Scholar]
- Sternglanz R., DiNardo S., Voelkel K. A., Nishimura Y., Hirota Y., Becherer K., Zumstein L., Wang J. C. Mutations in the gene coding for Escherichia coli DNA topoisomerase I affect transcription and transposition. Proc Natl Acad Sci U S A. 1981 May;78(5):2747–2751. doi: 10.1073/pnas.78.5.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanarayana T., Subramanian A. R. Specific association of two homologous DNA-binding proteins to the native 30-S ribosomal subunits of Escherichia coli. Biochim Biophys Acta. 1978 Sep 27;520(2):342–357. doi: 10.1016/0005-2787(78)90232-0. [DOI] [PubMed] [Google Scholar]
- Tanaka I., Appelt K., Dijk J., White S. W., Wilson K. S. 3-A resolution structure of a protein with histone-like properties in prokaryotes. Nature. 1984 Aug 2;310(5976):376–381. doi: 10.1038/310376a0. [DOI] [PubMed] [Google Scholar]
- Trucksis M., Depew R. E. Identification and localization of a gene that specifies production of Escherichia coli DNA topoisomerase I. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2164–2168. doi: 10.1073/pnas.78.4.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Nedospasov S. A., Georgiev G. P. On the structure of eukaryotic, prokaryotic, and viral chromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):457–473. doi: 10.1101/sqb.1978.042.01.049. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Nedospasov S. A., Bakayev V. V., Bakayeva T. G., Georgiev G. P. Histone-like proteins in the purified Escherichia coli deoxyribonucleoprotein. Nucleic Acids Res. 1977 Aug;4(8):2725–2745. doi: 10.1093/nar/4.8.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeponteau B., Lundell M., Martinson H. Torsional stress promotes the DNAase I sensitivity of active genes. Cell. 1984 Dec;39(3 Pt 2):469–478. doi: 10.1016/0092-8674(84)90454-9. [DOI] [PubMed] [Google Scholar]
- Wu F. Y., Kolb A., Buc H. A transcriptionally active plasmid-protein complex isolated from Escherichia coli. Biochim Biophys Acta. 1982 Mar 29;696(3):231–238. doi: 10.1016/0167-4781(82)90052-5. [DOI] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- Yamazaki K., Nagata A., Kano Y., Imamoto F. Isolation and characterization of nucleoid proteins from Escherichia coli. Mol Gen Genet. 1984;196(2):217–224. doi: 10.1007/BF00328053. [DOI] [PubMed] [Google Scholar]
- Zahn K., Blattner F. R. Sequence-induced DNA curvature at the bacteriophage lambda origin of replication. Nature. 1985 Oct 3;317(6036):451–453. doi: 10.1038/317451a0. [DOI] [PubMed] [Google Scholar]
- Zentgraf H., Berthold V., Geider K. Interaction of DNA with DNA binding proteins. II. Displacement of Escherichia coli DNA unwinding protein and the condensed structure of DNA complexed with protein HD. Biochim Biophys Acta. 1977 Feb 16;474(4):629–638. doi: 10.1016/0005-2787(77)90082-x. [DOI] [PubMed] [Google Scholar]
- van der Ende A., Baker T. A., Ogawa T., Kornberg A. Initiation of enzymatic replication at the origin of the Escherichia coli chromosome: primase as the sole priming enzyme. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3954–3958. doi: 10.1073/pnas.82.12.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]