The extracellular matrix (ECM) is the three-dimensional (3D) environment in which cells grow in vivo. For cancer research, cancer cells, grown in the 3D ECM-like scaffolds in a way as in real tumor in vivo, can serve as tumor models to study important problems in cancer treatment, such as multi-drug resistance (MDR) to anti-cancer therapeutics, without using animals.[1–3] Thus the development of 3D scaffolds that can mimic ECM in tumor is important to generate in vitro tumor models. The scaffolds should allow the cancer cells to grow in 3D environment because the 2D cultured cancer cells have exhibited significantly less malignant phenotype in comparison to cells growing in vivo.[4,5] In addition, many aspects of cell behaviors, such as morphogenesis, adhesion, migration and level of gene expression, are changed when cells are cultured on the 2D substrates.[6–8]
Recently, cancer cell culture on 3D scaffolds has attracted intensive research efforts. The 3D scaffolds can mimic the structure of ECM better and therefore cells growing on them show characteristics closer to those growing under the physiological conditions. In the literature, several different types of scaffolds, including hydrogel, matrigel and macroporous polymeric materials, have been employed to culture cancer cells in 3D.[9–19] These synthetic scaffolds have the advantages of high structural reproducibility, ease of mass production and stiffness controllability; however, there are some drawbacks that still need to be further improved. In the hydrogel and matrigel based 3D cell culture, the seeding of cells was done by suspending cells into fluidic gel precursor solution and thus after the gelation process, the cells are embedded inside the gel.[20] The gel based 3D scaffolds have been widely employed to study the cellular gene expression regulation by applying various types of external stimulus.[21–25] However, since the cancer cells are confined inside the gel, sometimes they cannot be treated in the same way as 2D cultured cells. For example, they cannot be detached off the scaffolds as free cells, consequently, no passage can be obtained from such 3D culture. Besides, the diffusion of chemicals into the gel is much slower than that in the fluidic state, which may introduce remarkable error in testing the efficacy of anti-cancer drugs. Porous scaffolds made from synthetic or biological polymers could be very costly, and in addition they do not mimic the fibrous structures of the natural ECMs.[1,26]
Here we used our recently developed biomimetic scaffold[27] made of branched hollow silica microfibers to culture cancer cells in 3D. As a result, the cancer cells can grow in the scaffold freely to form tumor-like multicellular spheroids in vitro and also promote tumor growth in vivo. The resultant scaffold mimics the fibrous ECM in real tumor, which is rich in leaky branch-like blood vessel and fibrous proteins,[28] in that it is fibrous open network structure, allowing nutrients to be delivered to the cells efficiently. The scaffold was prepared without employing any instrument but simply by aging a polyvinylpyrrolidone (PVP) aqueous solution under room temperature followed by a silica coating.[27] Scanning electron microscopy (SEM), flow cytometry, hematoxylin and eosin (HE) staining, and in vivo implantation results have demonstrated that MCF-7 cancer cells cultured on the current 3D silica scaffold retained significantly more oncological characters than those cultured on the conventional 2D substrate.
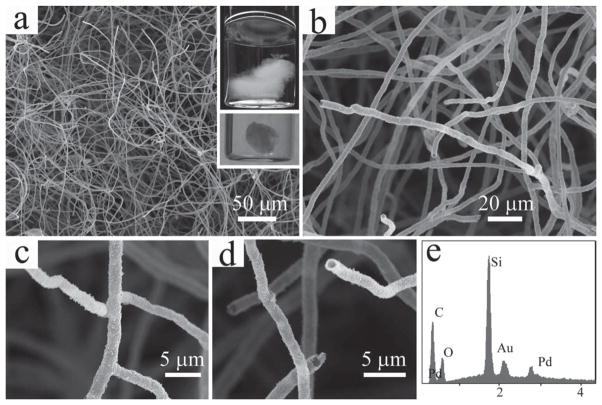
In our previous work, we discovered that the PVP molecules can self-assemble in their homogeneous aqueous solution to form centimeter-scale jellyfish like aggregates.[27] The macroscopic aggregates are composed of a large number of highly branched hollow polymer microfibers. After coating the PVP aggregates with a thick layer of silica through base catalyzed hydrolysis of tetraethyl orthosilicate (TEOS), branched hollow microfibrous silica scaffold can be obtained. From a digital photograph of the silica coated PVP aggregate shown in Figure 1a inset, it can be seen clearly that the overall structure of the aggregate is three-dimensional both when suspended in water and after dried out by critical point drying (CPD) treatment. The fibers that constitute the 3D structure are fairly uniform and have a diameter of around 2–3 μm (Figures 1a,1b). Both the branched and hollow characteristics of the fibers remain intact after the silica coating (Figures 1c,1d). An intensive silicon peak in the Energy Dispersive X-ray Spectrum (EDS) confirms that these fibers are mainly made of silica (Figure 1e).
Figure 1.
SEM images of the microfibrous silica scaffold. (a,b) Low and high magnification images of the silica fibers that constitute the 3D scaffold; (c,d) Images showing the branched and hollow nature of the silica fibers; (e) EDS spectrum showing the elemental composition of the scaffold, Au and Pt are from the sputtered coating formed during SEM sample preparation. Inset in (a): photographs of the silica scaffold suspended in water (top) and dried by CPD (bottom, the scaffold was cut to fit the size of the sample holder for the CPD device).
It has been found that silica, as a biocompatible material, favors cell adhesion,[29] thus the silica microfibers in the 3D scaffolds were directly used without any surface modification. After being seeded onto the 3D microfibrous silica scaffold, the MCF-7 breast cancer cells can grow and proliferate freely in the culture medium. The existence of branched structure creates plenty of space among the fibers, and enables the cancer cells to be seeded deeply into the scaffold, rather than just sitting on its surface. This is evidenced by the fact that, when changing the focal depth of the optical microscope, cells are seen clearly at the different levels of the central area of the scaffold. The overall structure of the MCF-7 cells on a silica scaffold after three days of culture is shown in Figure 2a. At a higher magnification, one can find that there are numerous cells settling onto the silica microfibers throughout the whole scaffold. Unlike on the 2D plate where cells can grow in individual form, on the fibrous scaffold, most of them exist in the form of multicellular spheroids (Figure 2b) similar to those that can be found in real tumor. The morphology of cancer cells in the spheroids is primarily spherical, rather than flat as typically seen on 2D culture (Figure 2c). It seems that the silica microfibers are not acting simply like a bird’s nest to hold the eggs, but rather they have intervened into the cell clumps (Figure 2d). When a single cell is seen occasionally on the scaffold, interestingly, it has anchored itself firmly onto the junction of several fibers (Figure 2e). The silica scaffold introduced in this work is different from the scaffolds prepared through electrospinning, although both of them are composed of fibers. The electrospun scaffolds can be better described as 2D mats with nanoscale roughness, thus cells can only be seeded on their surface and spread out in a manner similar to the behavior on a 2D petri dish.[30–32] However, cells can be seeded deeply inside the ECM-like 3D microfibrous silica scaffold, and thus their morphology is totally different from that in the 2D culture.
Figure 2.
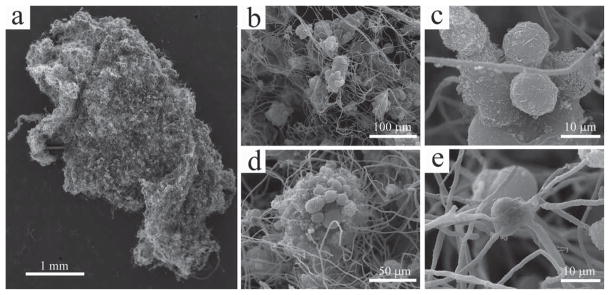
SEM images of the MCF-7 cancer cells on the silica scaffold. (a) An overall image of the cell-scaffold construct; (b) the cancer cells grow in the form of multicellular spheroids on the scaffold; (c) the cells exhibit spherical morphology on the scaffold; (d) the multicellular spheroids have been intervened by (rather than simply sitting onto) the silica fibers; (e) a single cancer cell that is anchored firmly to several fibers around it.
The growth and proliferation of MCF-7 breast cancer cells on the ECM-like microfibrous silica scaffolds can be observed easily by the increase in the size of the tumor-like cell spheroids over time. When the cells are first seeded onto the scaffold, only small spheroids (less than 30 μm in diameter) are seen under the optical microscope (Figure 3a), and the SEM observation reveals that the number of cells in the individual spheroids is generally less than 20 (Figure 3d). After consecutive culture for 7 days, there is a significant increase in the size of the tumor-like clumps (Figure 3b). More importantly, from the SEM image, it can be identified that the expansion of the cell spheroids takes place in a 3D manner, supported by the fact that both the diameter of the spheroids and the stacking layers of cells are multiplied (Figure 3e). The proliferation of cells on the silica scaffold results in the formation of many large spheroids (more than 100 μm in diameter) within 10 days of culture. Due to the greater thickness, the cell spheroids can only be imaged as dark shadows under the optical microscope (Figure 3c). However, it can be confirmed by SEM that the number of cells in each clump has been increased remarkably (Figure 3f). It should also be noted that the morphology of cells has remained spherical throughout the whole culture process.
Figure 3.
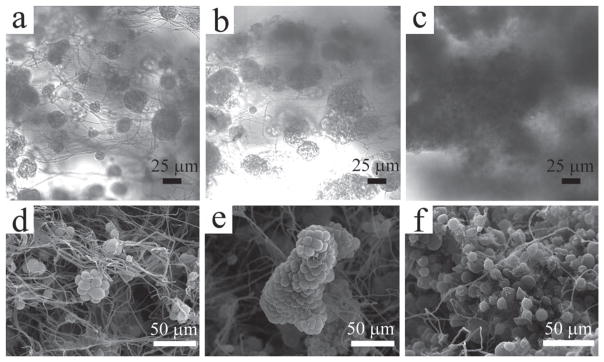
Characterization of the multicellular spheroids formed on the 3D scaffolds. (a–c) Optical images taken by the inverted light microscope showing the growth of multicellular spheroids in the culture medium. The size of the spheroids increased over the time (from left to right: day 4, day 7 and day 10, respectively); (d–f) SEM images corresponding to the multicellular spheroids shown in a–c. More number of cells can be seen along with the increase in the spheroids sizes.
In order to observe the structure at the core area of the cell spheroids, the HE staining process was carried out. From the cross-sectioned sample, it can be seen that there are many MCF-7 cells inside individual spheroids (Figure S1-a). At a higher magnification, one can find that the silica microfibers, typically marked by black arrows, are presented throughout the section and have been intertwined with the cells (Figure S1-b). The HE staining images (Figure S1) show multicellular spheroids formed as a result of 3-D culture on the ECM-like silica scaffold, comparable to those found in the images of in vivo tumors.[33] The spatial relationship between cells and fibers revealed from the HE staining is consistent with that observed from the SEM study in Figure 2d.
The cell division cycle is composed of four distinct sequential phases: gap 1 (G1) phase (where cell size is increased), synthesis (S) phase (when DNA is synthesized), gap 2 (G2) phase (where a cell continues to grow), and mitosis (M) phase (where a cell stops growing but divides into two daughter cells). G1, S and G2 phase are collectively called interphase. Each daughter cell will begin a new cycle after cell division. Gap 0 (G0) phase is a state of quiescence where cells leave the division cycle to make cell division temporarily or reversibly stopped. Nonproliferative cells usually enter the G0 phase from G1. The percentage of cells in different phases of a cell cycle such as G0/G1 and S phases could be determined using flow cytometry because cells at those phases have different DNA content and the cellular DNA content could be measured when a dye is used to label the cellular DNA.[34] The flow cytometry results showed that about 57% of the conventional 2D cultured cells were in G0/G1 phase (Figure 4a), while this number reached up to 80% when the cells were cultured on the 3D scaffold (Figure 4b). The percentage of cells that were in S phase is 37% and 17% for 2D and 3D cultured cells, respectively. The flow cytometry measurement is consistent with what has been reported in the literature, that the formation of multicellular spheroids in the in vitro cell culture will result in an increased percentage of cells in the G0/G1 phase.[35–37] As is also the case in a fast-growing tumor, where new cancer cells become further away from the blood vessels, they tend to arrest in the G0/G1 phase due to the increasing hypoxic environment.[38–40] And this has been considered as a key cause of MDR in the clinical treatments of tumors, as most anti-cancer drugs are mainly effective to the actively dividing tumor cells.[40–42] Compared to the 2D substrate, the percentage of G0/G1 phase cells generated by our 3D microfibrous silica scaffold is higher, and thus is closer to the case in a real tumor. Therefore, the highly reproducible 3D silica scaffold could potentially be applied as a platform to study the MDR of chemotherapy medicines in vitro.[1–3]
Figure 4.
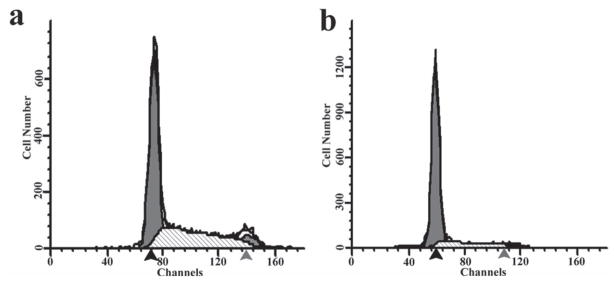
Cell phase inspection by flow cytometry for MCF-7 cells after one week culture on 2D (a) and 3D (b) substrates. a, about 57% and 37% of total inspected cells from 2D culturing were in G0/G1 phase and S phase, respectively. b, about 80% and 17% of total inspected cells from 3D culturing were in G0/G1 and S phases, respectively. Black arrow: G0/G1 peak; Gray arrow: G2-M peak.
To study the in vivo proliferation rate, an equal number of MCF-7 breast cancer cells cultured on the commercial 2D plate and 3D ECM-like microfibrous silica scaffold were injected into the opposite armpits of a nude mouse respectively (Figure 5a). The mice were sacrificed after 10 and 20 days of in vivo tumor growth, and the tumors were carefully removed and weighed. The digital photographs of a typical tumor resulting from the in vivo growth are shown in Figures 5b (10 days) and 5c (20 days). It can be seen that at both time points, tumors generated by cancer cells from the 3D ECM-like scaffold were significantly larger than those generated by cancer cells cultured on 2D substrates. And the average weight of the tumors (Figure 5d) was about 220 mg (3D, 10 days), 155 mg (2D, 10 days), 410 mg (3D, 20 days), and 300 mg (2D, 20 days), respectively. The ECM in real tumors rich in leaky blood vessels is a microfibrous 3D network mainly composed of collagen and elastin fibers. Our 3D scaffolds made of branched hollow silica microfibers mimic the structure of ECM in real tumors. As a result, before being implanted into the animals for inducing tumor formation, the cancer cells cultured in the silica scaffold actually grew in an ECM-like 3D environment (Figures 2 and 3), which is in contrast to the flat 2D environment in the conventional cell culture. Consequently, cells cultured on a 3D scaffold have been shown to mimic those in tumor better than the 2D cultured cells (Figures 2 and 3) in terms of both the morphological features (multicellular spheroids) and the gene expression levels of the in vivo tumor.[2,43–46] And such an advantage will allow the 3D cultured cells to establish the microenvironment (such as the blood vessel formation) and restore their physiological functions faster once implanted in vivo.[45] As a result, the growth of tumors originated from 3D in vitro cultured cells will be accelerated.
Figure 5.
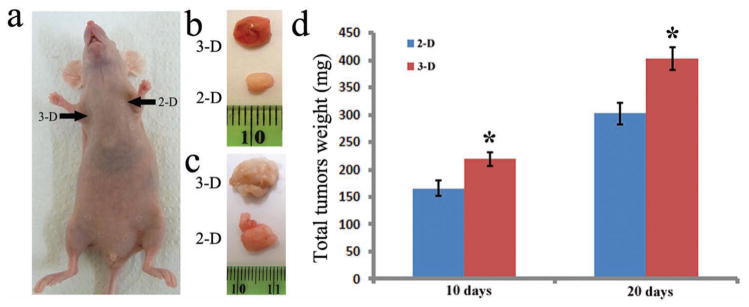
Comparison of the in vivo growth of MCF-7 breast tumors induced by 2D and 3D pre-cultured cells under in vitro conditions. a, The same number of MCF-7 breast cancer cells pre-cultured by 2D and 3D methods were injected into the left and right armpits of the same athymic nude mice, respectively. (b,c) Typical tumors that grew for 10 days (b) and 20 days (c) after exoimplantation. (d) Average weights of tumors induced by MCF-7 breast cancer cells pre-cultured by 2D and 3D methods after having grown for 10 days and 20 days. “*”: p < 0.05, compared to 2D groups.
To study the biocompatibility of the 3D ECM-like scaffold, the 3D microfibrous silica scaffold in a nearly spherical shape (~5 mm) without any cells were implanted into the C1D2F3 mice and then taken out after three weeks. A piece of loose connective tissue was also collected from nuchal region of another mouse and used as control. The pathological inspection results of a normal tissue and scaffold implanted tissue processed by HE staining are shown in Figure S2. It can be seen from Figure S2-c that the scaffolds were completely embedded into the host ECM (Figures S2-a,c). The fibers of the scaffold could hardly be discerned from the complicated background because silica has a relatively low contrast under the optical microscope. One of the biggest differences in the HE staining images of the scaffold implantation and the loose connective tissue is that the adipose cells (white bubbles) in the former have been segmented into many compartments while in the latter they appear to be very homogeneous (Figures S2-b,d). This is likely because the presence of silica microfibers has divided the space into many small segments and adipose cells can only grow inside each segment. In addition, the lymphocyte infiltration observed in the scaffold implantation showed no significant difference compared to that in the normal tissue (blue arrows in Figures S2-b,d). Overall, after being implanted for 3 weeks, the silica scaffold has been integrated well into the physiological environment of the mice, which has resulted in a histological outline that is very similar to that of the control tissues. The body weight of the mice did increase gradually during the whole implantation. All these results suggest that the microfibrous silica scaffold is indeed biocompatible.
In summary, this work described a novel 3D fibrous scaffold composed of highly biocompatible branched hollow silica micro-fibers, which were prepared simply in glass vials in a two-step process. The cancer cells can grow and proliferate on the scaffold in the form of multicellular spheroids. The HE staining results indicated that the silica microfibers had intertwined with cells inside the multicellular spheroids. Compared to the 2D culture, a higher percentage of cells cultured on the 3D silica scaffold are in the G0/G1 phase. And tumors induced by subcutaneous implantation of 3D cultured cells grow faster than those originated from 2D cultured cells. These results suggested that cancer cells cultured on the ECM-like 3D silica scaffold retained significantly more oncological character than those cultured on the conventional 2D substrates. The multicellular spheroids in the ECM-like scaffolds can find potential use as in vitro tumor models for studying cancer treatment without the use of animals.
Supplementary Material
Acknowledgments
This work was in part supported by National Institutes of Health (5R21EB009909-02 and 1R21EB015190-01A1) and National Science Foundation (DMR-0847758). We also would like to thank the financial support from National Science Foundation (CBET-0854414 and CBET-0854465), National Institutes of Health (5R01HL092526-02 and 4R03AR056848-03), Oklahoma Center for the Advancement of Science and Technology (HR11-006) and Oklahoma Center for Adult Stem Cell Research (434003), and Department of Defense Peer Reviewed Medical Research Program (W81XWH-12-1-0384). We also thank Drs. Zipeng Zhen, Haibao Zhu and Ziwei Deng for their kind help during experiments.
Footnotes
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Penghe Qiu, Department of Chemistry and Biochemistry, Stephenson Life Sciences Research Center, University of Oklahoma, Norman, OK, 73019, USA.
Xuewei Qu, Department of Chemistry and Biochemistry, Stephenson Life Sciences Research Center, University of Oklahoma, Norman, OK, 73019, USA.
Daniel J. Brackett, Health Science Center, University of Oklahoma and Veterans Research and Education Foundation, Oklahoma City, OK 73104, USA
Megan R. Lerner, Health Science Center, University of Oklahoma and Veterans Research and Education Foundation, Oklahoma City, OK 73104, USA
Dong Li, Department of Chemistry and Biochemistry, Stephenson Life Sciences Research Center, University of Oklahoma, Norman, OK, 73019, USA.
Prof. Chuanbin Mao, Email: cbmao@ou.edu, Department of Chemistry and Biochemistry, Stephenson Life Sciences Research Center, University of Oklahoma, Norman, OK, 73019, USA
References
- 1.Horning JL, Sahoo SK, Vijayaraghavalu S, Dimitrijevic S, Vasir JK, Jain TK, Panda AK, Labhasetwar V. Mol Pharmaceut. 2008;5:849. doi: 10.1021/mp800047v. [DOI] [PubMed] [Google Scholar]
- 2.Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. J Biotechnol. 2010;148:3. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA, Rizzi SC. Biomaterials. 2010;31:8494. doi: 10.1016/j.biomaterials.2010.07.064. [DOI] [PubMed] [Google Scholar]
- 4.Kievit FM, Florczyk SJ, Leung MC, Veiseh O, Park JO, Disis ML, Zhang MQ. Biomaterials. 2010;31:5903. doi: 10.1016/j.biomaterials.2010.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Xiao ZF, Meng Y, Zhao YN, Han J, Su GN, Chen B, Dai JW. Biomaterials. 2012;33:1437. doi: 10.1016/j.biomaterials.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 6.Zhang SG. Nat Biotechnol. 2004;22:151. doi: 10.1038/nbt0204-151. [DOI] [PubMed] [Google Scholar]
- 7.Debnath J, Brugge JS. Nat Rev Cancer. 2005;5:675. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 8.Yamada KM, Cukierman E. Cell. 2007;130:601. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Kirshner J, Chen CJ, Liu PF, Huang J, Shively JE. Proc Natl Acad Sci USA. 2003;100:521. doi: 10.1073/pnas.232711199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J, Cuddihy MJ, Kotov NA. Tissue Eng Pt B - Rev. 2008;14:61. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 11.Benton G, George J, Kleinman HK, Arnaoutova IP. J Cell Physiol. 2009;221:18. doi: 10.1002/jcp.21832. [DOI] [PubMed] [Google Scholar]
- 12.Tibbitt MW, Anseth KS. Biotechnol Bioeng. 2009;103:655. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu YX, Chan-Park MB. Biomaterials. 2010;31:1158. doi: 10.1016/j.biomaterials.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 14.Ma PX, Choi JW. Tissue Eng. 2001;7:23. doi: 10.1089/107632701300003269. [DOI] [PubMed] [Google Scholar]
- 15.Malafaya PB, Silva GA, Reis RL. Adv Drug Delivery Rev. 2007;59:207. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Choi SW, Xie JW, Xia YN. Adv Mater. 2009;21:2997. doi: 10.1002/adma.200803504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geckil H, Xu F, Zhang XH, Moon S, Demirci U. Nanomedicine. 2010;5:469. doi: 10.2217/nnm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sempere LF, Gunn JR, Korc M. Cancer Biol Ther. 2011;12:198. doi: 10.4161/cbt.12.3.15979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina-Jimenez F, Benedicto I, Viet LDT, Gondar V, Lavillette D, Marin JJ, Briz O, Moreno-Otero R, Aldabe R, Baumert TF, Cosset FL, Lopez-Cabrera M, Majano PL. Virology. 2012;425:31. doi: 10.1016/j.virol.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Lee GY, Kenny PA, Lee EH, Bissell MJ. Nat Methods. 2007;4:359. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahn J, Mehraban F, Ingle G, Xin XH, Bryant JE, Vehar G, Schoenfeld J, Grimaldi CJ, Peale F, Draksharapu A, Lewin DA, Gerritsen ME. Am J Pathol. 2000;156:1887. doi: 10.1016/S0002-9440(10)65062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunert S, Jechlinger M, Beug H. Nat Rev Mol Cell Biol. 2003;4:657. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- 23.Xu JP, Wang W, Ludeman M, Cheng KV, Hayami T, Lotz JC, Kapila S. Tissue Eng Pt A. 2008;14:667. doi: 10.1089/tea.2007.0272. [DOI] [PubMed] [Google Scholar]
- 24.Deans TL, Singh A, Gibson M, Elisseeff JH. Proc Natl Acad Sci USA. 2012;109:15217. doi: 10.1073/pnas.1204705109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heck JN, Ponik SM, Garcia-Mendoza MG, Pehlke CA, Inman DR, Eliceiri KW, Keely PJ. Mol Biol Cell. 2012;23:2583. doi: 10.1091/mbc.E11-10-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.a) Chen GP, Ushida T, Tateishi T. Macromol Biosci. 2002;2:67. [Google Scholar]; b) Mao CB, Wang F, Cao B. Angew Chem Int Ed. 2012;51:6411. doi: 10.1002/anie.201107824. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Cao B, Xu H, Mao CB. Angew Chem Int Ed. 2011;50:6264. doi: 10.1002/anie.201102052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu PH, Mao CB. ACS Nano. 2010;4:1573. doi: 10.1021/nn9009196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell NE, Kellenberger L, Greenaway J, Moorehead RA, Linnerth-Petrik NM, Petrik J. J Oncol. 2010;2010:586905. doi: 10.1155/2010/586905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Napierska D, Quarck R, Thomassen LC, Lison D, Martens JA, Delcroix M, Nemery B, Hoet PH. Small. 2013;9:430. doi: 10.1002/smll.201201033. [DOI] [PubMed] [Google Scholar]
- 30.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. J Biomed Mater Res. 2002;60:613. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimoto H, Shin YM, Terai H, Vacanti JP. Biomaterials. 2003;24:2077. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 32.Ma ZW, Kotaki M, Inai R, Ramakrishna S. Tissue Eng. 2005;11:101. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 33.Pathak AP, Artemov D, Neeman M, Bhujwalla ZM. Cancer Res. 2006;66:5151. doi: 10.1158/0008-5472.CAN-05-1788. [DOI] [PubMed] [Google Scholar]
- 34.Pozarowski P, Darzynkiewicz Z. Methods Mol Biol. 2004;281:301–311. doi: 10.1385/1-59259-811-0:301. [DOI] [PubMed] [Google Scholar]
- 35.Sutherland RM, Sordat B, Bamat J, Gabbert H, Bourrat B, Muellerklieser W. Cancer Res. 1986;46:5320. [PubMed] [Google Scholar]
- 36.Li CL, Tian T, Nan KJ, Zhao N, Guo YH, Cui J, Wang J, Zhang WG. Oncol Rep. 2008;20:1465. [PubMed] [Google Scholar]
- 37.Correa RJM, Peart T, Valdes YR, DiMattia GE, Shepherd TG. Carcinogenesis. 2012;33:49. doi: 10.1093/carcin/bgr241. [DOI] [PubMed] [Google Scholar]
- 38.Hammer S, To KKW, Yoo YG, Koshiji M, Huang LE. Cell Cycle. 2007;6:1919. doi: 10.4161/cc.6.15.4515. [DOI] [PubMed] [Google Scholar]
- 39.Horree N, Gort EH, van der Groep P, Heintz APM, Vooijs M, van Diest PJ. J Pathol. 2008;214:38. doi: 10.1002/path.2244. [DOI] [PubMed] [Google Scholar]
- 40.Huang L, Ao QL, Zhang QH, Yang XK, Xing H, Li F, Chen G, Zhou JF, Wang SX, Xu G, Meng L, Lu YP, Ma D. J Cancer Res Clin. 2010;136:447. doi: 10.1007/s00432-009-0675-4. [DOI] [PubMed] [Google Scholar]
- 41.Acker T, Plate KH. J Mol Med. 2002;80:562. doi: 10.1007/s00109-002-0355-1. [DOI] [PubMed] [Google Scholar]
- 42.Schnitzer SE, Schmid T, Zhou J, Brune B. Cell Death Differ. 2006;13:1611. doi: 10.1038/sj.cdd.4401864. [DOI] [PubMed] [Google Scholar]
- 43.Birgersdotter A, Sandberg R, Ernberg I. Semin Cancer Biol. 2005;15:405. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh S, Spagnoli GC, Martin I, Ploegert S, Demougin P, Heberer M, Reschner A. J Cell Physiol. 2005;204:522. doi: 10.1002/jcp.20320. [DOI] [PubMed] [Google Scholar]
- 45.Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, Mooney DJ. Nat Methods. 2007;4:855. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 46.Martin KJ, Patrick DR, Bissell MJ, Fournier MV. PLOS One. 2008:3. doi: 10.1371/journal.pone.0002994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.