Abstract
Purpose
Cystathionine β-synthase (CBS), a key enzyme in the transsulfuration metabolic pathway, converts homocysteine to cystathionine, which is converted to cysteine required for synthesis of the major retinal antioxidant glutathione (GSH). Enzyme activity assays suggest that CBS is present in human and pig retina, however recent studies reported that CBS is not expressed in mouse retina. We found this species difference puzzling. Given the plethora of studies using mouse retina as a model system, coupled with the importance of GSH in retina, we investigated CBS expression in mouse retina at the molecular and cell biological level.
Methods
Wildtype (WT) mice or mice lacking the gene encoding CBS (cbs−/−) were used in these studies. RNA and protein were isolated from retinas and liver (positive control) for analysis of cbs gene expression by RT-PCR and CBS protein expression by Western blotting, respectively. CBS was analyzed by immunofluorescence in retinal cryosections and primary retinal cells (ganglion, Müller, RPE). CBS enzyme activity was measured in primary Müller cells.
Results
RT-PCR revealed robust cbs expression in WT liver, brain and retina. Western blotting detected CBS in retina, brain and liver of WT mice, but not in cbs−/− mice liver. In immunohistochemical studies, CBS was present abundantly in the ganglion cell layer of retina; it was detected also in primary isolations of Müller, RPE and ganglion cells. CBS activity was detected in Müller cells by fluorescent detection of H2S.
Conclusions
We have compelling molecular evidence that CBS is expressed in mouse retina at the gene and protein level. Our immunofluorescence data suggest that it is present in several retinal cell types and the data from the enzyme activity assay suggest activity in Müller cells. These findings set the stage to investigate the role of CBS and the transsulfuration pathway in generation of GSH in mouse retina.
Keywords: retinal ganglion cells, primary cell culture, retinal Müller cells, retinal pigment epithelial cells, hydrogen sulfide, glutathione, antioxidant
Introduction
In the transsulfuration metabolic pathway, homocysteine is converted in the presence of cystathionine-β-synthase (CBS) to cystathionine, which is further converted in the presence of cystathionine γ-lyase (CSE) to cysteine. Cysteine is necessary for synthesis of glutathione (GSH); hence the transsulfuration pathway is an important mechanism by which this critical antioxidant is generated. CBS and CSE are two cytoplasmic pyridoxal 5′-phosphate-dependent (PLP) enzymes that play a role also in the synthesis of hydrogen sulfide (H2S) [2, 3]. H2S mediates a wide range of physiological effects including neuromodulation, signaling and cytoprotection [4, 5]. It has been shown to increase GSH levels and to play a role in scavenging molecules implicated in oxidative stress such as hydrogen peroxide and superoxide anions.
CBS deficiency in humans is linked to several ocular disorders including ectopic lentis, myopia, retinal degeneration, retinal detachment, optic atrophy, glaucoma, corneal abnormalities, and cataracts [6, 7]. Recently systematic analysis of CBS was undertaken in the human eye [1]; it was detected in the anterior segment (cornea, conjunctiva and iris) as well as retina and optic nerve. Lens had less CBS and vitreous body had none. Analysis of CBS in porcine eye showed similar distribution patterns [1]. Immunohistochemical data were confirmed with molecular methods and enzymatic assays. This report suggested that a functional transsulfuration pathway is present in several ocular tissues, including retina. Interestingly a subsequent study utilizing salamander and mouse retinas reported robust activity of CBS and CSE in salamander retina, but not in mouse [18]. The investigators speculated that the species differences observed between salamander and mouse were due to avascularity of the salamander retina versus the highly vascularized mouse retina. They theorized that the vascularized mouse retina ‘would not have a strong need for the antioxidant effects of CBS or CSE under normal conditions.’ It is noteworthy that the human retina is well-vascularized. Recently, a second group investigated CBS and CSE using immunohistochemical methods in mouse retina and reported that these two enzymes were not present [8]. They hypothesized that two other enzymes 3-mercaptopyruvate sulfurtransferase (3MST) and cysteine aminotransferase (CAT) are the primary pathways present in mammalian retina for H2S synthesis.
These reports presented a conundrum: why would there be robust CBS activity in retinas of two mammalian species (human and porcine) and an amphibian model (salamander), but not mice? Given the abundance of experimentation using the mouse as a model of retinal degeneration [9], coupled with the critical role of protection of the retina against oxidative stress, we investigated the presence of CBS in mouse retina. We utilized wildtype C57BL/6 mice and compared expression in retina with mice that lack the gene encoding CBS (cystathionine-β-synthase knockout mice, cbs−/−) [10]. In addition, we isolated retinal neurons, glial cells and epithelial cells from wildtype animals to analyze CBS expression. Our data suggest that CBS is present in the intact mouse retina of wildtype mice and is particularly abundant in isolated retinal ganglion cells, Müller and epithelial cells. It is not detected in retinas of cbs−/− mice.
Materials and Methods
Animals and primary cell culture
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice deficient in cbs were generated by Watanabe et al [10] and distributed through Jackson Laboratories. Breeding pairs of cbs+/− mice (B6.129P2-Cbstm1Unc/J) were used to generate cbs −/− and cbs+/+ mice per our earlier reports [11, 12]. Three retinal cell types were isolated from wildtype (C57BL/6) mice: ganglion cells were isolated from mice at 3–4 days [13]; Müller glial cells at 5–7 days [14]; pigmented epithelial (RPE) cells at 3 weeks [15]. The purity of each of these primary cell types has been confirmed in previous studies. Care and use of the mice adhered to institutional policies governing appropriate care and use of animals in research.
RT-PCR to detect cystathionine-β-synthase in mouse retina
To determine whether or not cbs was expressed in mouse retina, RNA was isolated from neural retinas pooled from 4 mice using TRIzol (Invitrogen, Carlsbad, CA). RNA was isolated also from liver as a positive control [16]. RNA was converted to cDNA using SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA). PCR was performed using three different primer pairs specific for mouse cbs (Table 1). PCR was performed at 35 cycles of 95°C for 30 s, 60°C for 45 s, and 72°C for 45 s. 18S (Quantum RNA TM) was used as internal control. PCR products were analyzed on a 2% agarose gel by electrophoresis. To determine the relative expression of cbs in mouse retina as compared to liver quantitative real time PCR was performed as described [13] using Absolute QPCR SYBR Green Fluorescein Mix from ABgene (Thermo Scientific, Surrey, United Kingdom) and the BioRad icycler (Hercules, CA). cbs gene expression was also analyzed in primary ganglion and Müller cells. The primers used to detect cbs were the same as above. PCR was performed for 40 cycles of 95°C for 30 s, 60°C for 45 s, and 72°C for 45 s; melt curve analysis confirmed the purity of the end products. Resulting CT values were normalized to 18S and analyzed using the comparative CT method to obtain fold-changes in gene expression [19]. The analysis was performed in duplicate.
Table 1.
Sequences of primers for cbs gene expression studies
| Gene | NCBI Accession Number | Primer Sequence | Predicted band size |
|---|---|---|---|
| Mouse Primers | |||
| CBS | NM_144855 | Forward: 5’- AGG GCT ATC GCT GCA TTA TCG TGA-3’ Reverse: 5’- AGC TTC CAC CAC ATA GCA GTC CTT −3’ |
567 |
| CBS | NM_144855 | Forward: 5’- CCTATGAGGTGGAAGGGATT −3’ Reverse: 5’- TGTAGTTCCGCACAGAGTCA −3’ |
246 |
| CBS | NM_144855 | Forward: 5’- GTAGCTTACAGGGCCTTTCC −3’ Reverse: 5’- CTAACCAGGTCCCTGAGGAT −3’ |
186 |
Western blot analysis to detect CBS in mouse retina
Liver, brain and neural retinal tissue isolated from C57BL/6 mice were used for immunoblot analysis of the CBS protein (Mr ~ 63 kDa). Liver from homozygous knockout (cbs−/−) mice served as a negative control. 20 µg of protein from liver, brain and 60 µg of protein from retina were subjected to SDS PAGE at 90 volts for 90 minutes and transferred to nitrocellulose membranes at 200 mA for 1 h per our method [13]. Membranes were blocked in 5% milk in TBST for 1 h at 25°C (room temperature, RT) followed by incubation in CBS antibody (1:400, Santa Cruz, Santa Cruz, CA) in 5% milk in TBST buffer at 4°C overnight. Membranes were washed in TBST and incubated with HRP-conjugated rabbit anti-goat IgG antibody (1:3000) for 2 h at RT. The ECL Western blot detection system (Thermo Scientific, Waltham, MA) was used to visualize the protein bands. Membranes were stripped and reprobed with β-actin, which served as the loading control [13].
Immunodetection of CBS in intact retinal tissue and isolated retinal cells
Eyes were removed from C57BL/6 (WT) and cbs−/− mice, placed immediately in OCT embedding compound and flash frozen without fixation; 10 µm thick cryosections were prepared as described [17]. Retinal cryosections were fixed in 4% paraformaldehyde in PBS, washed thrice with PBS Triton X-100 and blocked with PowerBlock (Biogenx, San Ramon, CA) for 1 h. Sections were incubated overnight in a humidified chamber at 4°C with a rabbit anti-mouse polyclonal antibody against CBS (1:250) (Aviva, SanDiego, CA) followed by PBS Triton X-100 washes. Alexa Fluor 555 conjugated donkey anti-rabbit IgG (Invitrogen, Carlsbad, CA) (1:1000) was used as secondary antibody. Slides were again washed with PBS Triton X-100 and coverslipped with Flourosheild with DAPI (Sigma Chem. Co., St. Louis, MO) to stain nuclei. Sections were viewed by epifluorescence using a Zeiss Axioplan–2 microscope (Carl Zeiss, Göttingen, Germany), equipped with the Axiovision program (version 4.7), and an HRM camera.
To detect CBS in retinal cells, immunocytochemical methods were used in primary ganglion, Müller and RPE cells isolated from C57BL/6 mice per our methods [13–15]. Cells were cultured on microscope coverslips, fixed in 4% paraformaldehyde for ~5 min, washed with PBS-Triton–X-100, incubated with primary antibodies specific for each cell type (Neu-N verifying neuronal origin of ganglion cells, vimentin verifying glial origin for Müller cells, anti-RPE65 verifying RPE cells) in co-immunolocalization experiments with anti-body against CBS. Following overnight incubation at 4°C, cells were washed in PBS-Triton X-100 and incubated with the appropriate secondary antibody, washed again and counterstained with DAPI to visualize nuclei. Sections were examined by epifluorescence as described for retinal sections. Table 2 provides a list of the primary and secondary antibodies used in these studies.
Table 2.
List of antibodies and suppliers for immunodetection of CBS in mouse retina
| Primary Antibody | Dilution | Supplier |
| Rabbit anti-CBS | (1:250) | Aviva, SanDiego, CA |
| Goat anti-Vimentin | (1:250) | Millipore, Temecula, CA |
| Rabbit anti-Neu N | (1:200) | Millipore, Temecula, CA |
| Rabbit RPE 65 | (1:250) | AbCam, Cambridge, MA |
| Mouse anti-β-actin | (1:5000) | Sigma, St. Louis, MO |
| Goat anti-CBS | (1:400) | Santa Cruz Corp, Santa Cruz , CA |
| Secondary Antibody | Dilution | Supplier |
| HRP-conjugated rabbit anti-goat IgG | (1:3000) | Sigma, St. Louis, MO |
| HRP-conjugated goat anti-mouse IgG | (1:5000) | Sigma, St. Louis, MO |
| Alexa Fluor 555 Donkey anti-rabbit IgG | (1:1000) | Invitrogen, Eugene,OR |
| Alexa Fluor 488 Donkey anti-goat IgG | (1:1000) | Invitrogen, Eugene,OR |
| Alexa Fluor 488 Donkey anti-mouse IgG | (1:1000) | Invitrogen, Eugene,OR |
Detection of CBS enzyme activity in primary Müller cells
To determine the CBS enzyme activity in mouse retina, an assay that is based on detection of H2S was used. Primary Müller cells were isolated from C57BL/6 mice per our method [14] and cultured on glass chamber slides (Lab-Tek II, IL; Cat # 154941,) in DMEM (GIBCO,NY; Cat. 12320). On the day of the experiment, the regular media was removed and cells were cultured in DMEM (minus cysteine) (GIBCO; cat # 21013-024) for 6 h after which they were incubated with a green fluorescent probe to detect H2S [23] for 2 h. This was followed by four incubation conditions: (1) control: media containing no cysteine; (2) Cys + Hcy: media containing L-cysteine (50 µM) + homocysteine (100 µM); (3) Cys: media containing L-cysteine (50 µM); (4) Cys + Hcy + AOAA: media containing L-cysteine (50 µM) + homocysteine (100 µM) + AOAA (100 µM, Sigma-Aldrich). (Cells were pre-treated with AOAA 12 h prior to the experiment.) After 1 h, media was removed to halt the enzyme activity. The cells were gently washed with PBS and stained with DAPI. The fluorescence was detected using the Zeiss Axioplan–2 microscope as described for immunocytochemistry.
Results
Expression of CBS in mouse retina
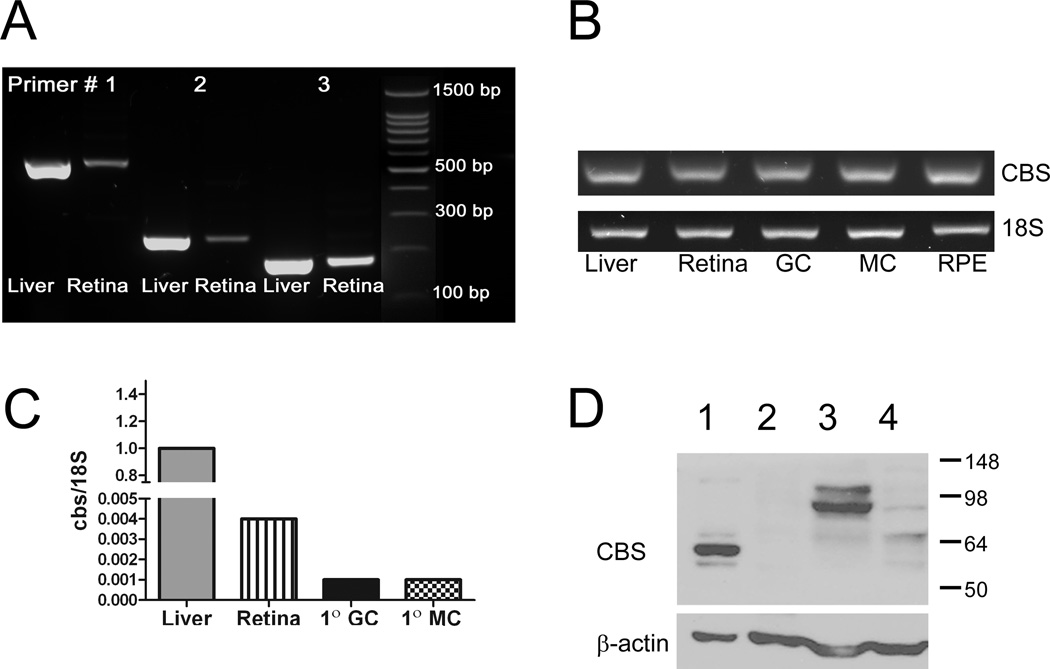
There have been no reports published on the expression in mouse retina of the gene encoding CBS. To address this, primers specific for mouse cbs were designed for RT-PCR, which was performed using RNA isolated from mouse neural retina and freshly isolated retinal cells. RNA from liver, which has abundant expression of cbs [16], was used as a positive control. We first tested three primer pairs for cbs using liver and retinal samples. As shown in Fig. 1A, bands of the expected size were detected regardless which primer set was used (567 bp, 246 bp, 186 bp (Table 1)). These data provide strong evidence that cbs is expressed in neural retina. Additionally, cbs was expressed also in three primary retinal cell preparations (Fig. 1B). Ganglion cells were isolated from neonatal mice, Müller cells were harvested from 5–7 day old mouse pups and RPE cells were harvested from 3 week old mice [13–15]. In adult retina, as well as the three retina cell types, cbs was expressed. The RT-PCR method provides information about the presence or absence of a gene; it is not quantitative. To determine the relative expression, qRT-PCR was performed using neural retina, primary ganglion, Müller cells and liver; the expression of cbs (normalize to 18S) in liver was ~400 fold greater than in retina, respectively (Fig. 1C).
Figure 1. RT PCR and western blot analysis of CBS in mouse retina.
(A) Equal amounts of mRNA were reverse transcribed from liver and neural retina. The synthesized cDNA was amplified by PCR to quantify the cbs mRNA using three sets of primers specific for mouse cbs (Table 1). The size of the expected PCR products were 567, 246 and 186 bp. (B) Equal amounts of mRNA were reverse transcribed from liver, neural retina, primary mouse ganglion cells (GC), Müller cells (MC) and retinal pigment epithelial cells (RPE). The synthesized cDNA was amplified by PCR to quantify the cbs mRNA, the size of the expected PCR product was 186 bp. (C) cbs gene expression in neural retina, primary ganglion and Müller cells was compared with the liver by real time PCR. 18S gene served as housekeeping gene. (D) Western blot analysis of CBS in liver harvested from cbs+/+ (WT) mouse (lane 1), liver of cbs−/− mouse (lane 2) brain of cbs+/+ (WT) (lane 3), and neural retina of cbs+/+ (WT) mouse (lane 4). The liver isolated from the cbs −/− mice (lane 2) served as negative control. β-actin (Mr~ 42 kDa) served as a loading control.
The gene expression experiments were followed by western blot analysis using a commercially available antibody. There have been a number of reports of CBS in various tissues and the expected molecular size ranges from 63–72 kD [20]. The predominant band in liver has a molecular weight of ~63 kD (Fig. 1D, lane 1). To confirm the specificity of the antibody, we isolated protein from liver of cbs−/− mice. There was no band detected using our antibody, although the β-actin loading control provides clear evidence that an equivalent amount of protein was loaded onto the gel (Fig. 1D, lane 2). In WT mouse retina and brain (Fig. 1D, lanes 3 and 4); a strong band with a slightly higher molecular weight (~67 KD) was detected, which falls within the expected size range for CBS. Taken collectively, the data from these experiments provide compelling evidence that the gene encoding CBS and the protein itself are present in mouse retina.
Detection of CBS in intact mouse retina
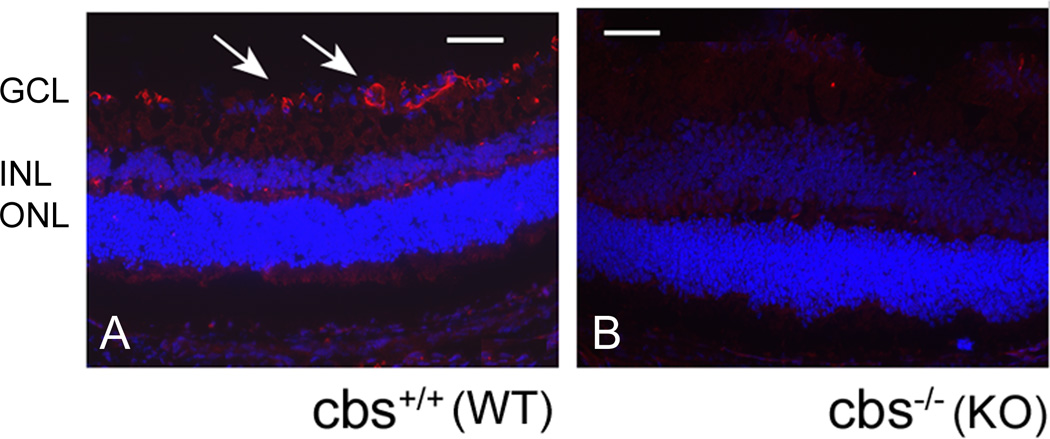
Given that CBS was detected at the gene and protein level in isolated retinal tissue, we next asked which retinal cell layers were positive for CBS. Immunohistochemical studies were performed in intact mouse retinal cryosections obtained from 3 week old C57BL/6 (WT) and cbs−/−mice. Sections stained with anti-CBS antibody (red fluorescence); nuclei were stained with DAPI. Evaluation of sections by immunofluorescence detected CBS in several retinal layers, with predominant expression observed in the ganglion cell layer (Fig. 2, A). When retinal cryosections of cbs−/− mice (subjected to immunodetection using anti-CBS) were examined, no immunopositive staining was observed. These findings provide information about the specificity of the antibody in addition to providing information as to which cellular layers are positive for CBS (Fig 2B).
Figure 2. Immunohistochemical analysis of CBS in intact mouse retina.
Retinal cryosections from (A) wt and (B) cbs −/− mice were stained with antibody against CBS (1:250) (Aviva Systems Biology, CA).CBS (red fluorescence) was detected in all layers of the retina of wt mouse especially in gcl; CBS was not detected in cbs −/−retina. DAPI was used to stain nuclei. (Scale bar: 60 µm; gcl: ganglion cell layer; inl: inner nuclear layer; onl: outer nuclear layer)
Detection of CBS in isolated retinal cells
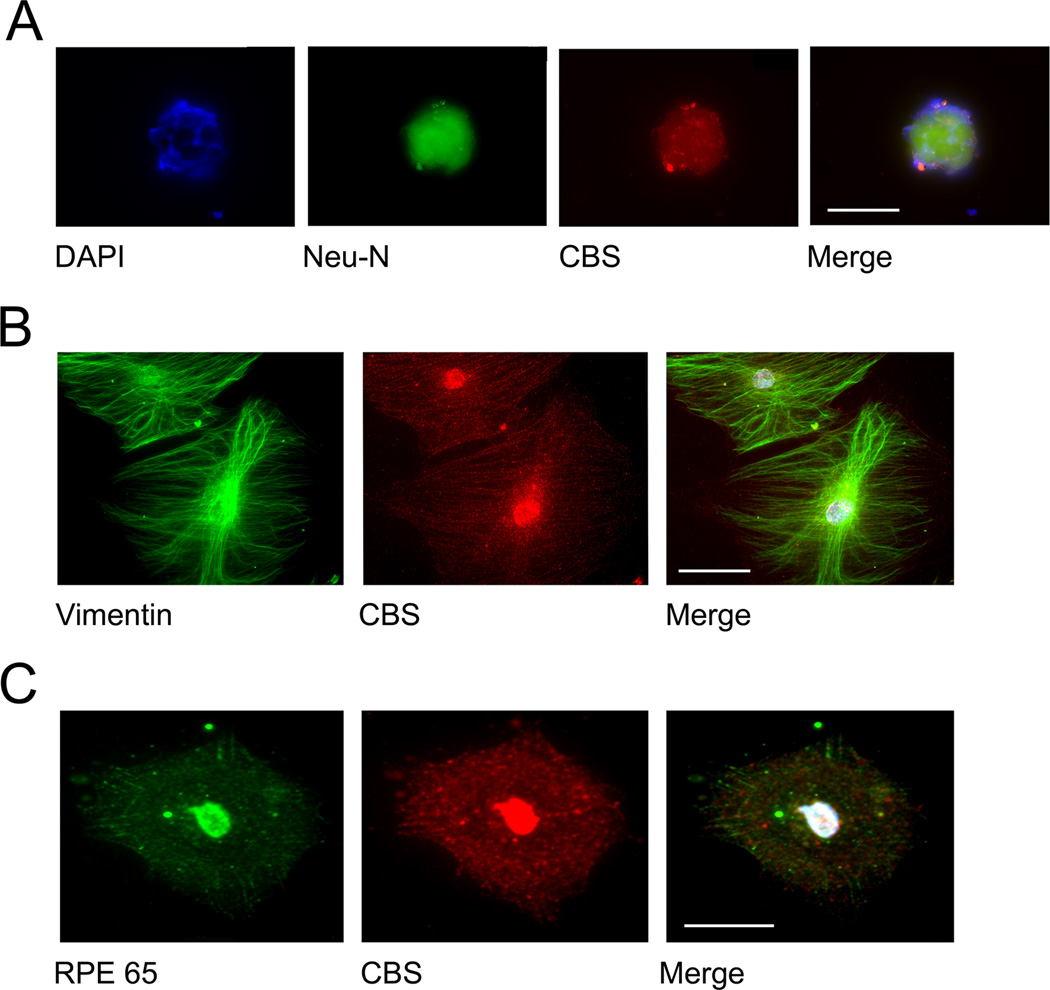
To confirm the immunofluorescent studies performed in retinal cryosections, we isolated three retinal cell types from mouse. Retinal ganglion cells were isolated from neonatal mice by immunopanning and subjected to immunocytochemistry. These cells are positive for Thy1.2, as shown in earlier studies [13] and for the neuronal marker Neu-N (Fig. 2A). There is abundant expression of CBS in these isolated neurons (Fig. 3A). In studies using slightly older mice, retinal Müller glial cells were harvested and subjected to immunocytochemistry. Vimentin was used to verify the glial origin of these cells. CBS was abundantly present in these cells (Fig. 3B). RPE cells were harvested from mice at ~3 weeks of age; their origin was verified using anti-RPE65 antibody. CBS was present in these cells as well (Figure 3, red fluorescence). CBS is a nuclear and cytoplasmic protein [21]; interestingly CBS appeared to be localized primarily to the nucleus in ganglion cells, but was present in the cytoplasm and nucleus of Müller and RPE cells (Figure 3, panels B and C).
Figure 3. Immunocytochemical analysis of CBS and cell purity markers in isolated retinal cells.
Primary ganglion cells (A); primary Müller cells (B); primary RPE cells (C) were grown on coverslips and subjected to immunofluorescent detection of CBS (red fluorescence) and appropriate cell markers (green fluorescence). Neuronal origin of ganglion cells was confirmed using Neu-N (green fluorescence, panel A). Vimentin, an intermediate filament protein (green fluorescence, panel B) was used as a marker for Müller glial cells. RPE 65 (green fluorescence, panel C) was used as positive marker for RPE cells. All cells expressed CBS abundantly. (Scale bar: 20 µm (A); 30 µm (B); 30 µm (C)).
Detection of CBS enzyme activity in primary retinal Müller cells
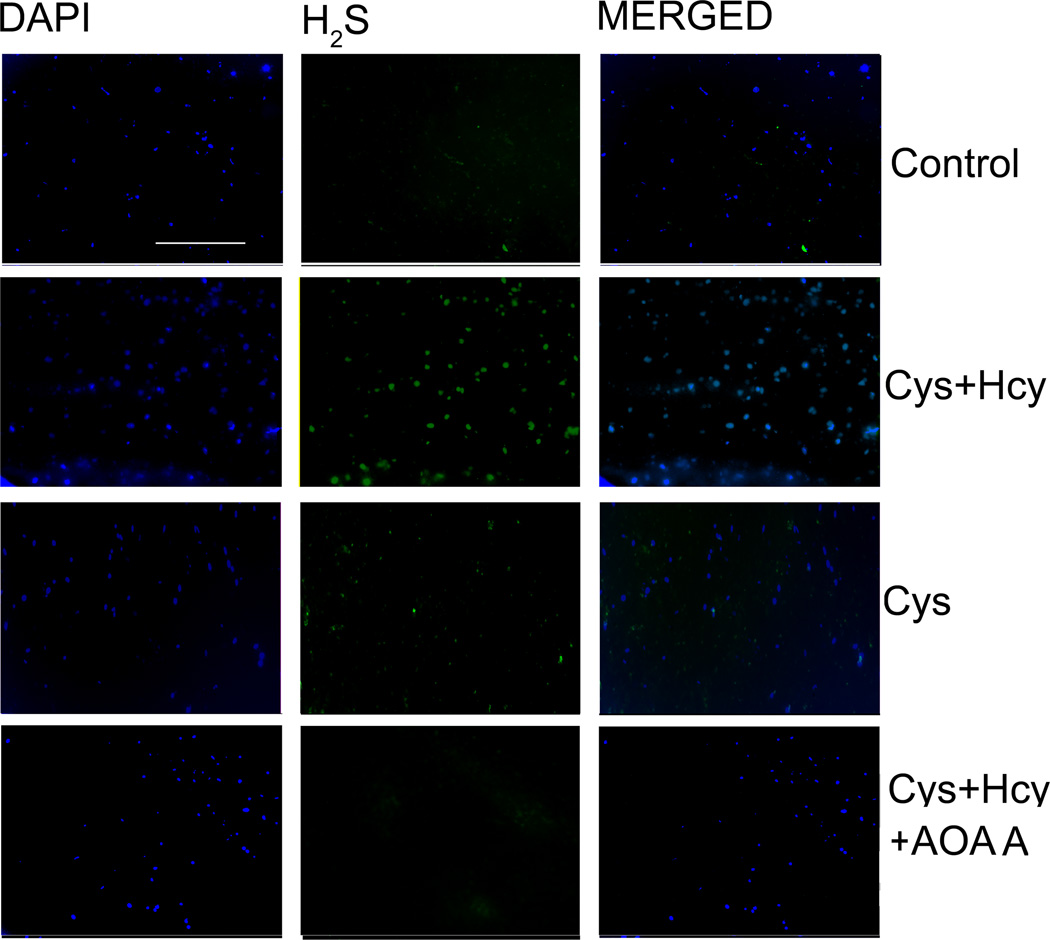
After confirming CBS protein expression in mouse Müller cells, we used these cells to assay CBS enzyme activity based on H2S production. H2S is synthesized by three enzymes: CBS, CSE and MST. Cysteine is used as a substrate by both CBS and CSE and is the precursor of H2S; homocysteine is a specific substrate for CBS. AOAA is a chemical inhibitor of the CBS enzyme. By incubating cells in media devoid of cysteine, baseline production of H2S can be detected using a fluorescent probe [23]. When cysteine and homocysteine are added to the incubation media, the amount of H2S produced can be detected and finally, by incubating cells with AOAA in the presence of cysteine + homocysteine the contribution of CBS to H2S production can be determined. As shown in Fig. 4, there was minimal detection of H2S in cells incubated in media containing no cysteine (baseline control). Cells treated with cysteine and homocysteine revealed robust H2S production as indicated by marked increase in green fluorescence (Fig. 4 Cys + Hcy), while cells treated only with cysteine showed a moderate increase in H2S compared with control (Fig. 4 Cys). Interestingly, in cells incubated with cysteine and homocysteine in the presence of the CBS inhibitor AOAA, the production of H2S was comparable to that of control cells. These data provide strong evidence of cbs enzyme activity in retinal Müller cells.
Figure 4. Detection of CBS enzyme activity in primary retinal Müller cells.
Primary retinal Müller cells were grown 6 h in media devoid of cysteine (control), media containing L-cysteine (50 µM) + homocysteine (100 µM) (Cys + Hcy), media containing L-cysteine (50 µM) (Cys) or media containing L-cysteine (50 µM) + homocysteine (100 µM) + AOAA (Cys + Hcy + AOAA) followed by 2 h incubation with a fluorescent probe to detect H2S, which is produced via the enzymatic activity of CBS. The green fluorescence represents H2S production and blue fluorescence represents DAPI staining of nuclei. (Scale bar: 300 µm)
Discussion
CBS is a key enzyme in the transsulfuration pathway. CBS deficiency is implicated in various ocular diseases, e.g. diabetic retinopathy, glaucoma, lens dislocation and retinal detachment [6,7]. Reports suggest that CBS is expressed in human, porcine and salamander retina; studies of CBS in mouse retina have been limited. The retina is exposed to high levels of oxidative stress and hence the presence of a robust transsulfuration pathway could serve as a source of glutathione, the major antioxidant in the retina, whereas absence of CBS could increase susceptibility of retina to oxidative damage. The present work evaluated CBS and the gene encoding this enzyme at the molecular level. Tools used included qRT-PCR, semi-quantitative RT-PCR, western blot analysis, immunohistochemistry in retinal tissues, immunocytochemistry in isolated retinal cells and an assay to detect CBSB enzyme activity in primary Müller cells. In addition, we took advantage of the availability of mice that lack the gene encoding CBS (cbs−/− mice) to confirm studies to detect this protein in retina.
RT-PCR analysis using primers specific for mouse revealed cbs expression in liver (positive control), and in retina and isolated primary retinal cells (ganglion, Müller and RPE) cells. These data provide the first evidence that cbs is expressed at the gene level in mouse retina. Immunoblotting experiments performed in liver, a rich source of CBS, detected this protein in abundance with the expected molecular weight (~63 kD). When livers harvested from cbs−/−mice were subjected to immunoblotting, no corresponding band was detected. These findings suggest the specificity of the antibody used in these studies. When we examined CBS in mouse retina and brain, a band of slightly higher molecular weight was observed. In the immunoblotting experiments using an affinity purified human CBS antiserum reported by Pong et al [18], the antibody did not recognize a band of the anticipated molecular size, although robust bands with higher molecular weight were readily detected (data not shown in their report). In our experiments, we used a commercially produced antibody, which was different than that used by Pong’s group. It is possible that the difference in our results from theirs reflects differences in the reagents used for the two studies. These are the only two studies utilizing immunoblotting to investigate CBS expression in mouse retina. Interestingly, we found the antibody used for western blotting was not effective for immunohistochemical analysis, which was the reason why we used two different anti-CBS antibodies in our experiments.
Immunofluorescence methods have been used previously to evaluate CBS levels in mouse retina [8, 18], with the suggestion that CBS was not present in retinas from this species. We investigated this using retinal cryosections of wildtype mice, as well as retinas of cbs−/− mice. In our hands, the antibodies we used detected CBS at a concentration of 1:250 in the wildtype retina, particularly in the ganglion cell layer. There was low level detection also in other retinal layers. It is noteworthy that there was no detection in retinas of the cbs−/− mice. The studies by Mikami et al [8] suggested no CBS in the mouse retina by immunofluorescence. The discrepancy between our findings and theirs may be due to the exceedingly low concentration of CBS antibody (1:3000) used in their studies of the retina. Interestingly, earlier reports from this lab analyzed brain using a more concentrated formulation of the antibody (1:500) and CBS was detected [22]. The source of the antibody used by Mikami’s group was not specified.
Our immunohistochemical analysis of CBS detected this protein in mouse retina, most notably in the ganglion cell layer. There was faint CBS staining in other retinal layers, which prompted us to explore CBS in isolated retinal cells by immunocytochemistry. Our laboratory has considerable expertise in isolating several primary cell types from mouse retina [13–15]. Using enriched populations of ganglion, Müller and RPE cells we were able to detect CBS in these three cell types. Cell purity was verified using appropriate markers.
In addition to establishing that the CBS gene and protein were present in mouse retina, we were interested also in assessing CBS enzyme activity. Earlier reports by Pong suggested that there was no CBS activity in mouse retina. Their method was based on production of cystathionine in a radioactivity assay in which 14C-labeled serine was the co-factor [18]. They did not add excess amounts of the substrates homocysteine or cysteine in their assay. We used a different approach to assay CBS. First, we studied the enzyme activity in an enriched population of retinal Müller cells because our immunocytochemical data (Fig. 3) showed considerable levels of the protein in these cells. Secondly, we enhanced the chances of detection of CBS by adding excess levels of substrate. The difference in our results from those of Pong and co-workers may be due to differences in the sensitivity of the assays, the availability of substrates or the use of an enriched homogenous group of cells rather than retinal lysates. Our data provide compelling evidence that retinal Müller cells have CBS enzyme activity and may play a role in endogenous production of H2S opening avenues for future studies.
Our molecular, biochemical, immunohistochemical and enzymatic assay data are in keeping with the experiments performed in human and porcine tissue that demonstrated that CBS is present in retina [1]. Our data differ from the earlier investigations of CBS in mouse retinas [8, 18] perhaps because of technical differences such as antibody concentrations in immunofluorescence studies and the use of isolated retinal cell types in enzyme activity assays. Taken collectively, our studies provide strong evidence that CBS is present in mouse retina at the gene and the protein level. Future studies will investigate the roles of CBS and the transsulfuration pathway in mouse retina.
Acknowledgments
Supported by: NIH R01 EY012830 and EY014560
Footnotes
Declaration of interest
There are no conflicts of interest to declare for any of the authors.
Literature cited
- 1.Persa C, Osmotherly K, Chao-Wei Chen K, Moon S, Lou MF. The distribution of cystathionine beta-synthase (CBS) in the eye: implication of the presence of a transsulfuration pathway for oxidative stress defense. Exp Eye Res. 2006;4:817–823. doi: 10.1016/j.exer.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Kabil O, Banerjee R. The redox biochemistry of hydrogen sulfide. J. Biol. Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem. Biophys. Res. Commun. 2006;267:129–133. doi: 10.1006/bbrc.1999.1915. [DOI] [PubMed] [Google Scholar]
- 6.Kraus JP, Kozich V. Cystathionine b-synthase and its deficiency. In: Carmel R, Jacobsen DW, editors. Homocysteine in Health and Disease. Cambridge University Press; 2001. pp. 223–243. [Google Scholar]
- 7.Mudd SH. Hypermethioninemias of genetic and non-genetic origin: A review. Am J Med Genet C Semin Med Genet. 2011;157:3–32. doi: 10.1002/ajmg.c.30293. [DOI] [PubMed] [Google Scholar]
- 8.Mikami Y, Shibuya N, Kimura Y, Nagahara N, Yamada M, Kimura H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J Biol Chem. 2011;45:39379–86. doi: 10.1074/jbc.M111.298208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalupa LM, Williams RW. Eye Retina, and Visual System of the Mouse. MIT Press. 2008:477–581. [Google Scholar]
- 10.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocysteinemia. Proc. Natl. Acad. Sci. USA. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganapathy PS, Moister B, Roon P, Mysona BA, Duplantier J, Dun Y, Moister TK, Farley MJ, Prasad PD, Liu K, Smith SB. Endogenous elevation of homocysteine induces retinal neuron death in the cystathionine-beta-synthase mutant mouse. Invest. Ophthalmol. Vis. Sci. 2009;50:4460–4470. doi: 10.1167/iovs.09-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganapathy PS, Perry R, Tawfik A, Smith RM, Perry E, Roon P, Bozard BR, Ha Y, Smith SB. Homocysteine-mediated modulation of mitochondrial dynamics in retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2011;52:5551–5558. doi: 10.1167/iovs.11-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ha Y, Dun Y, Thangaraju M, Duplantier J, Dong Z, Liu K, Ganapathy V, Smith SB. Sigma receptor 1 modulates ER stress in retinal neurons. Invest. Ophthalmol. Vis. Sci. 2011;52:527–540. doi: 10.1167/iovs.10-5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozard BR, Chothe PP, Tawfik A, Williams C, Fulzele S, Prasad PD, Martin PM, Ganapathy V, Smith SB. Regulation of proton-coupled folate transporter in retinal Müller cells by the anti-psoriatic drug monomethylfumarate. Glia. 2012;3:333–342. doi: 10.1002/glia.22266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gnana-Prakasam JP, Martin PM, Zhang M, Atherton SS, Smith SB, Ganapathy V. Expression of the iron-regulatory protein haemojuvelin in retina and its regulation during cytomegalovirus infection. Biochem. J. 2009;419:533–543. doi: 10.1042/BJ20082240. [DOI] [PubMed] [Google Scholar]
- 16.Matthews RG, Elmore CL. Defects in homocysteine metabolism: diversity among hyperhomocyst(e)inemias. Clin Chem Lab Med. 2007;45:1700–1703. doi: 10.1515/CCLM.2007.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dun Y, Mysona B, Van Ells TK, Amarnath L, Ola MS, Ganapathy V, Smith SB. Expression of the glutamate-cysteine (xc-) exchanger in cultured retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tiss. Res. 2006;324:189–202. doi: 10.1007/s00441-005-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pong WW, Stouracova R, Frank N, Kraus JP, Eldred WD. Comparative localization of cystathionine beta-synthase and cystathionine gamma-lyase in retina: differences between amphibia. J Comp Neurol. 2007;505:158–165. doi: 10.1002/cne.21468. [DOI] [PubMed] [Google Scholar]
- 19.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C (T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 20.Regnier V, Billard J, Gupta S, Potier B, Woerner S, Paly E, Ledru AL, David S, Sabrina Luilier S4, Bizot JC, Vacano G, Kraus JP, Patterson D, Kruger W, Delabar JM. Brain Phenotype of Transgenic Mice Overexpressing Cystathionine b-Synthase. Plos one. 2012;7:e29056. doi: 10.1371/journal.pone.0029056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabil O, Zhou Y, Banerjee R. Human cystathionine beta-synthase is a target for sumoylation. Biochemistry. 2006;45:13528–13536. doi: 10.1021/bi0615644. [DOI] [PubMed] [Google Scholar]
- 22.Ichinohe A, Kanaumi T, Takashima S, Enokido Y, Nagai Y, Kimura H. Cystathionine b-synthase is enriched in the brains of Downs’s patients. Biochemical and Biophysical Research Communications. 2005;338:1547–1550. doi: 10.1016/j.bbrc.2005.10.118. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Peng B, Li S, Park CM, Whorton R, Xian M. Reaction based fluorescent probes for hydrogen sulfide. Organic letters. 2012;14:2184–2187. doi: 10.1021/ol3008183. [DOI] [PMC free article] [PubMed] [Google Scholar]