Abstract
Objective
Treatment with chronic corticosteroids has been associated with frequent significant adverse effects. We hypothesized that a long-term, low-dose prednisone regimen for OMG would have a low rate of major side effects.
Methods
Consecutive OMG patients from a single institution over a 16-year-period and treated with ≥1 month of daily prednisone were included. Steroid-related complications were defined as the development/worsening of conditions requiring alteration to medical therapy. Serious complications included conditions requiring emergency care, hospitalization, or surgery.
Results
83 patients with follow-up ranging from 1-271 months (median: 58 months) were included. Fifty-eight (70%) patients had follow-up ≥24 months. The maximum prednisone dose ranged from 10-60mg. Tapering to ≤10mg per day required ≤4 months for all but two patients. Median average daily dose following the initial course was 5mg daily (interquartile range: 4-7.5 mg). During the first two years, there were 24.5 complications per 100 person-years. Only one patient had a serious complication within the first two years (2 year cumulative risk: 1%), but this individual was not following the recommended regimen.
Conclusions
Low-dose prednisone for OMG has an acceptable side-effect profile and causes few serious complications (∼1% two year risk). However, patients need monitoring to detect the relatively common, but less serious, complications (∼39% two year risk) in order to adjust medical therapy in a timely fashion.
Keywords: Ocular Myasthenia Gravis, Prednisone, Steroid complication
Introduction
The use of corticosteroids in the immunologic treatment of ocular myasthenia gravis (OMG) is controversial primarily because of the potential for significant side effects (1). Some have suggested that steroids should be used only “when absolutely necessary” in the treatment of myasthenia gravis,(2) while others have shown favorable outcomes with long-term, lower dose regimens of prednisone (3). In addition, prednisone appears to reduce conversion from OMG to the generalized form (GMG) (4). The combination of prednisone with other therapies, such as azathioprine, may allow for lower dosing of prednisone, but it remains unclear whether these therapies are equivalent or superior to prednisone when used alone and whether they offer improved outcomes when used in combination with prednisone (5-11). Unlike corticosteroids, which are effective within weeks, these other immunosuppressive therapies often achieve their benefit only after several months.
We hypothesized that the systemic hypertension, diabetes mellitus, osteoporosis, gastrointestinal disorders, and infections that typically occur with long-term moderate to high dose therapy (3), may be minimized with long-term, low dose therapy (prednisone < 10 mg daily) especially if co-interventions are utilized (11,12). Our goal was to determine the side effect profile of a low dose regimen for the treatment of OMG and to determine the safety of systemic corticosteroid use in OMG.
Methods
Consecutive patients with OMG evaluated and managed at the Institute for Neurology and Neurosurgery at Roosevelt Hospital and New York Eye and Ear Infirmary by one of the investigators (MK) were considered for inclusion. The study was approved by the Institutional Review Board. Patients who were begun on prednisone between October 1984 and December 2010 and treated with a minimum of 1 month of daily prednisone were included. OMG was diagnosed on the basis of having ptosis, binocular diplopia, or extraocular motility limitation confirmed by ice test, edrophonium test, repetitive nerve stimulation electromyography, single fiber electromyography, or positive acetylcholine receptor antibody testing (13,14). Patients had their last clinical assessment between August 1996 and June 2011. In general, patients were evaluated monthly for the first 3 months and thereafter every 6 months.
Patients were not randomized to therapy. Those with diplopia or with ptosis that blocked the visual axis and were unresponsive to pyridostigmine were treated with prednisone. Patients were not given corticosteroids if they refused or had a contraindication including active infection, gastrointestinal ulcer, history of tuberculosis, difficult to control diabetes mellitus that was difficult to control, severe hypertension, or congestive heart failure. Patients with a history of a positive PPD or one or more calcified lesions, suggestive of healed tuberculosis, on the chest computed tomography received isoniazid 300 mg and pyridoxine 50 mg concomitantly with prednisone. Patients were prescribed a daily H2 blocker (ranitidine hydrochloride, nizatidine, or famotidine) and daily calcium 1000 mg to 1500 mg as long as they took prednisone. If baseline bone mineral density (not done in all patients) showed osteopenia of the lumbar spine or hips, biphosphonate therapy was also prescribed.
Complications of steroids were defined as the development of or worsening of any of the following conditions that required a change in management (e.g., addition of a medication): osteopenia, osteoporosis, bone fracture, hypertension, diabetes mellitus, gastrointestinal disturbance, psychosis, depression, dementia, infection (other than viral URI), elevated intraocular pressure, glaucoma, or cataract. Serious complications included conditions requiring emergency care, hospitalization, or surgery.
Statistical analysis was performed using R: A language and environment for statistical computing (R Foundation for Statistical Computing, http://www.R-project.org). We performed univariate analyses to produce summary measures for continuous (medians, interquartile ranges, and ranges) and proportions. Significance was defined as the 0.05 level. Person-time for incidence rates was determined using time of prednisone initiation as the start of follow-up and using either the number of weeks to last follow-up (censored) or the development of the first complication (event) as the end of person-time. Kaplan-Meier plots were used for graphical presentation of the survival curves. Confidence limits were calculated using the normal approximation of the Poisson distribution.
Results
83 patients with confirmed OMG were included. Median age at diagnosis was 61 years (interquartile range: 46.5-73 years; range: 16-87 years). 60 (72%) patients were men. Follow-up ranged from 1-271 months with a median of 58 months. Fifty-eight (70%) patients had follow-up of at least 24 months. Patients were on a maximum dose of prednisone ranging from 10-60 mg (all but 3 patients were on a maximum dose of 40-60 mg). Tapering from this dose to 10 mg per day required 4 months or less for all but two of the patients and a significant majority of the patients 58/83 (70%) required exactly 3 months to taper to 10 mg. The median average daily dose following the initial course was 5 mg daily (interquartile range: 4-7.5 mg).
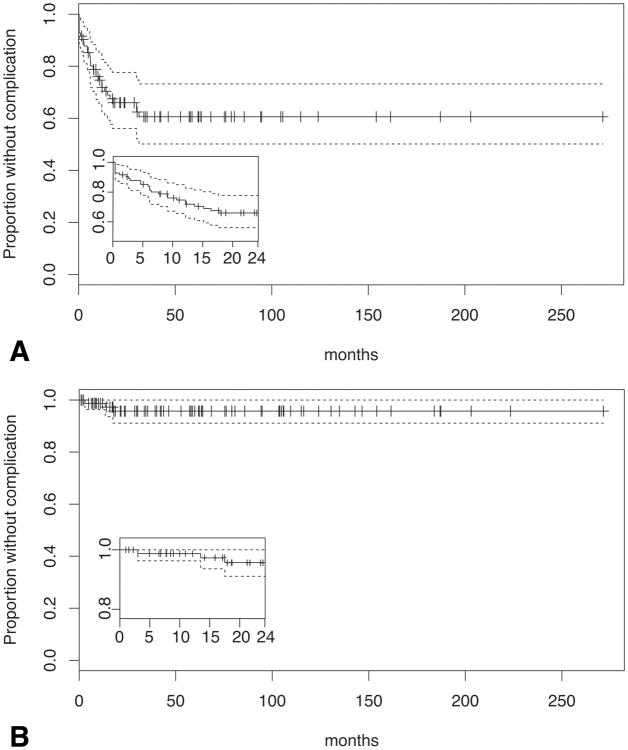
Twenty-nine (35%) patients developed a complication (Table 1) that could potentially be related to steroid use within the total period of follow-up (439 person-years) corresponding to a rate of 6.6 complications per 100 person-years (95%CI: 4.2-9.0, Fig 1A). Nine of these 29 patients (31%) developed more than one complication during the total period of follow-up. Within the first two years, the compilcation rate was 24.5 per 100 person-years (26 complications during the 106 person years accumulated during the first two years of study; 95%CI:15.0-33.9, Fig 1A, inset). There were 10 complications in the first year (1 year cumulative risk: 12%), and three during the second year (2 year cumulative risk: 16%).
Table 1.
Summary of complications possibly related to predinsone. Serious complications indicated in bold.
| Patient # | Onset (months) | Comments |
|---|---|---|
| Osteopenia | ||
| 10 | 120 | |
| 22 | 18 | |
| 26 | 10 | |
| 28 | 9 | Rx alendronate |
| 30 | 125 | Rx calcium + vitamin D |
| 43 | 30 | Rx risedronate |
| 44* | 12 | |
| 49 | 24 | |
| 69* | 42 | Rx alendronate |
| 70 | 18 | |
| Osteoporosis | ||
| 3* | 11 | Rx alendronate |
| 25 | 24 | Rx calcium + vitamin D (improved later) |
| 38 | 25 | Rx alendronate |
| 53* | 72 | Rx risedronate |
| 76 | 70 | Baseline osteoporosis, on alendronate, complicated by fracture |
| 80* | 23 | Rx alendronate |
| Diabetes mellitus | ||
| 2 | 60 | 2 years after D/C prednisone |
| 3* | 50 | Rx pioglitazone |
| 15 | 120 | Rx metformin + insulin |
| 19 | 48 | Rx 4 oral agents |
| 44* | 1 | Transient, no Rx |
| 60 | 48 | Rx oral agent (4 oral agents by year 8) |
| 61 | 4 | Rx metformin + pioglitazone |
| 62* | 12 | Rx glyburide + insulin |
| Hypertension | ||
| 4 | 1 | Rx 2 agents |
| 27* | 36 | Rx additional oral agent |
| 35 | 1 | Increased propranolol |
| 62* | 1 | Rx metoprolol + captopril, complicated by myocardial infarction |
| 63 | 1 | Rx lisinopril |
| 78* | 1 | Rx valsartan |
| 80* | 24 | Rx oral agent |
| Psychological | ||
| 78* | 1 | Agitation, no Rx |
| Cataract | ||
| 53* | 65 | Nuclear sclerosis only |
| 69* | 72 | Nuclear sclerosis only |
| Intraocular pressure | ||
| 27* | 36 | Ocular hypertension |
| 69* | 48 | Ocular hypertension |
| Infection | ||
| 12 | 54 | Lid cellulitis requiring hospitalization |
patients with ≥2 complications
Figure 1.
Kaplan-Meier survival curves for all (A) and major (B) complications (solid lines) during the study, with 95% confidence intervals (dashed lines). Hashes on solid line represent censored observations. Inset: complications during first two years.
Three patients had serious complications during the total period of follow-up corresponding to a rate of 0.7 serious complications per 100 person-years (95%CI:0.0-1.5, Fig 1B). None occurred during the first year (1 year cumulative risk: 0%) and only one occurred during the second year (2 year cumulative risk: 1%). The rate within the first two years was 2.2 (95%CI:0.0-4.7, Fig 1B, inset) serious complications per 100 person-years. The one patient who had a serious complication during this time period developed severe hypertension complicated by myocardial infarction during month 12. However, this patient was not following the recommended prednisone regimen, instead taking 20-40 mg daily throughout the time prior to his associated complication by obtaining prednisone from multiple practitioners.
Discussion
Our retrospective study demonstrated a low rate of serious complications related to a low dose steroid regimen for OMG. 70% of our patients had more than 2 years of follow-up. Assuming a constant rate of 2.2 serious complications per 100 person-years during a two year study, we have extrapolated our results to a larger patient cohort. If we assume a sample size of 231 patients, we would expect 4.3% (10 of 231, 95%CI: 0-9%) of the patient treated with steroids to develop a serious complication that could be related to steroids. However, because the one patient in our study with a serious complication during the first two years was not following the low dose regimen, we believe that the lower rate of 0.7 serious complications per 100 person-years seen over the entire study period represents a more accurate estimate. Given these assumptions, we would expect only 1.4% of subjects (3 of 231) to develop a serious complication.
Our data suggest that the use of a low dose corticosteroid regimen has an acceptable side-effect profile for the treatment of ocular myasthenia gravis. This is in contrast to the frequent adverse effects seen with the relatively high dose, long term corticosteroid regimens (15,16). Many clinicians are wary of the adverse effects of chronic corticosteroid use and frequently prescribe only pyridostigmine. While this can be helpful for ptosis, it is rarely completely successful in relieving diplopia and fails to alter the underlying autoimmune process that leads to GMG in patients who first present with OMG (6,17).
Our study was limited because it was retrospective and represented a single center's experience. There may have been patient selection bias, although we included patients without regard to having detectable acetylcholine receptor binding antibody. Finally, the use of retrospective data from standard office visits, rather than systematic data collection, limits our ability to detect all other adverse effects of prednisone.
In conclusion, it appears that a low-dose prednisone regimen for the treatment of OMG causes few serious complications. Patients need monitoring for relatively common, but less serious complications, to ensure that these issues are detected early and allow for timely adjustment of medical therapy.
Acknowledgments
This study was supported in part by an unrestricted departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc., New York, and by NIH/NEI core grant P30-EY06360 (Department of Ophthalmology). Dr. Bruce receives research support from the NIH/NEI (K23-EY019341).
Footnotes
Conflict of Interest: The authors report no conflict of interest.
The authors have no relevant financial disclosure.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peppa M, Krania M, Raptis SA. Hypertension and other morbidities with Cushing's syndrome associated with corticosteroids: a review. Integr Blood Press Control. 2011;4:7–16. doi: 10.2147/IBPC.S9486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaminski HJ, Daroff RB. Treatment of ocular myasthenia: steroids only when compelled. Arch Neurol. 2000;57:752–753. doi: 10.1001/archneur.57.5.752. [DOI] [PubMed] [Google Scholar]
- 3.Pascuzzi RM, Coslett HB, Johns TR. Long-term corticosteroid treatment of myasthenia gravis: report of 116 patients. Ann Neurol. 1984;15:291–298. doi: 10.1002/ana.410150316. [DOI] [PubMed] [Google Scholar]
- 4.Kupersmith MJ, Latkany R, Homel P. Development of generalized disease at 2 years in patients with ocular myasthenia gravis. Arch Neurol. 2003;60:243–248. doi: 10.1001/archneur.60.2.243. [DOI] [PubMed] [Google Scholar]
- 5.Sommer N, Sigg B, Melms A, Weller M, Schepelmann K, Herzau V, Dichgans J. Ocular myasthenia gravis: response to long-term immunosuppressive treatment. J Neurol Neurosurg Psychiatry. 1997;62:156–162. doi: 10.1136/jnnp.62.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mee J, Paine M, Byrne E, King J, Reardon K, O'Day J. Immunotherapy of ocular myasthenia gravis reduces conversion to generalized myasthenia gravis. J Neuroophthalmol. 2003;23:251–255. doi: 10.1097/00041327-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Rozsa C, Mikor A, Kasa K, Illes Z, Komoly S. Long-term effects of combined immunosuppressive treatment on myasthenic crisis. Eur J Neurol. 2009;16:796–800. doi: 10.1111/j.1468-1331.2009.02634.x. [DOI] [PubMed] [Google Scholar]
- 8.A trial of mycophenolate mofetil with prednisone as initial immunotherapy in myasthenia gravis. Neurology. 2008;71:394–399. doi: 10.1212/01.wnl.0000312373.67493.7f. [DOI] [PubMed] [Google Scholar]
- 9.Sathasivam S. Steroids and immunosuppressant drugs in myasthenia gravis. Nat Clin Pract Neurol. 2008;4:317–327. doi: 10.1038/ncpneuro0810. [DOI] [PubMed] [Google Scholar]
- 10.Benatar M, Kaminski HJ. Quality Standards Subcommittee of the American Academy of Neurology. Evidence report: the medical treatment of ocular myasthenia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2007;68:2144–2149. doi: 10.1212/01.wnl.0000263481.14289.90. [DOI] [PubMed] [Google Scholar]
- 11.Hatz HJ, Helmke K. Polymyalgia rheumatica and giant cell arteritis; diagnosis and side effects of low-dose long-term glucocorticoid therapy. Z Rheumatol. 1992;51:213–221. [PubMed] [Google Scholar]
- 12.Caldwell JR, Furst DE. The efficacy and safety of low-dose corticosteroids for rheumatoid arthritis. Semin Arthritis Rheum. 1991;21:1–11. doi: 10.1016/0049-0172(91)90051-z. [DOI] [PubMed] [Google Scholar]
- 13.Kupersmith MJ. Does early treatment of ocular myasthenia gravis with prednisone reduce progression to generalized disease? J Neurol Sci. 2004;217:123–124. doi: 10.1016/j.jns.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Kupersmith MJ. Does early immunotherapy reduce the conversion of ocular myasthenia gravis to generalized myasthenia gravis? J Neuroophthalmol. 2003;23:249–250. doi: 10.1097/00041327-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Fischer KC, Schwartzman RJ. Oral corticosteroids in the treatment of ocular myasthenia gravis. Ann N Y Acad Sci. 1976;274:652–658. doi: 10.1111/j.1749-6632.1976.tb47723.x. [DOI] [PubMed] [Google Scholar]
- 16.Fischer KC, Schwartzmann RJ. Oral corticosteroids in the treatment of ocular myasthenia gravis. Neurology. 1974;24:795–798. doi: 10.1212/wnl.24.8.795. [DOI] [PubMed] [Google Scholar]
- 17.Kupersmith MJ, Ying G. Ocular motor dysfunction and ptosis in ocular myasthenia gravis: effects of treatment. Br J Ophthalmol. 2005;89:1330–1334. doi: 10.1136/bjo.2004.063404. [DOI] [PMC free article] [PubMed] [Google Scholar]