Abstract
Asparaginase is an antineoplastic agent used in combination therapy for acute lymphoblastic leukemia (ALL). The asparaginase activity measured in serum reflects the effectiveness of the drug. However, the wide inter-individual variability in the pharmacokinetics of asparaginase suggests that the serum activity should be closely monitored in patients during therapy. In order to identify patients with low asparaginase exposure during treatment, a fast, sensitive, and high-throughput assay is required for measuring asparaginase activity in patient sera. In this study, asparaginase activity was determined by monitoring the enzymatically-coupled oxidation of reduced nicotinamide adenine dinucleotide (NADH) to NAD+ in a 96-well format. The rate of disappearance of NADH (ΔmOD/minute) was directly proportional to the activity of asparaginase, and the linear range of the assay was established from 0.025 to 2.2 IU/mL (R2 = 0.998) with a reportable range that was extended to 4.0 IU/mL by dilution with serum albumin. Inter-assay precision was established (low control CV% = 8.8, high control CV% = 9.0), as was intra-assay precision (low control CV% = 3.3, high control CV% = 2.7). The method is high-throughput and provides a broader linear range of detection compared to previously described assays. The speed, ease, and accuracy of the assay make it suitable for assessing serum asparaginase activity after standard doses of native E. coli, Erwinia, and PEGylated E. coli asparaginase given to children during the treatment of leukemia.
Keywords: Asparaginase activity, asparaginase, acute lymphoblastic leukemia, high throughput, clinical assay
Introduction
Asparaginase is an antineoplastic agent used in combination therapy to treat acute lymphoblastic leukemia (ALL) and lymphoma [1]. Asparaginase is a bacterial enzyme that catalyzes the hydrolysis of asparagine to aspartic acid and ammonia [1-4]. Asparagine is a non-essential amino acid for normal cells; in leukemia cells, however, there is a relative requirement for exogenous asparagine [2,5,6]. Asparaginase causes a systemic depletion of asparagine in blood, ultimately leading to leukemic cell death [2,6].
Asparaginase activity in vivo is affected by the preparation administered and by immunologic factors [3,7,8]. The pharmacokinetic properties of asparaginase differ considerably by the formulation, where the half-life of Erwinia asparaginase (Erwinase) is less than a day, ~1.25 days for native E. coli asparaginase (Elspar), and about a week for the PEGylated form of native E. coli asparaginase (Oncaspar) [1,5,9]. Adverse drug reactions can limit the dosing of asparaginase, with up to two-thirds of patients developing allergies [10]. Similarly, it has been shown that patients who develop allergies have neutralizing antibodies to asparaginase, possibly leading to sub-therapeutic activity and faster drug clearance [1,6,11-13]. In some patients, these are “silent antibodies”, which affect the activity of asparaginase without causing any clinical allergic reaction [3,14]. Higher serum levels of asparaginase have been associated with greater anti-leukemic effect.
To characterize inter-patient differences in asparaginase activity during asparaginase therapy, it is necessary to employ a sensitive and high-throughput assay for use in serum samples. The purpose of the manuscript is to describe an asparaginase activity measurement assay that is practical for a clinical setting. The assay is high-throughput, it eliminates the need for any sample pretreatment, and it has a broader linear range of asparaginase activity detection compared to other published procedures [1,2,13,15-19]. The performance characteristics of this assay described herein suggest that it is suitable for monitoring asparaginase activity levels during the treatment of ALL in patient samples.
Materials and methods
Basic principle
Asparaginase activity is measured in patient serum samples by a series of coupled enzymatic reactions occurring in a 96-well UV transparent microplate. In the first reaction, L-asparaginase in the patient sample catalyzes the hydrolysis of L-asparagine to L-aspartate (Figure 1A). Glutamic oxaloacetic transaminase subsequently catalyzes the transamination of L-aspartate and α-ketoglutarate to oxaloacetate and L-glutamate (Figure 1B). Oxaloacetate is then reduced to malate in the presence of malic dehydrogenase with the concurrent oxidation of reduced β-nicotinamide adenine dinucleotide (NADH) to β-nicotinamide adenine dinucleotide (NAD+) (Figure 1C). The time-dependent change in absorbance of NADH at 340 nm is monitored on a microplate reader using the kinetic rate method. The change in absorbance over time is directly proportional to the rate at which the NADH is consumed and it is directly related to the asparaginase activity of the sample (Figure 2A).
Figure 1.
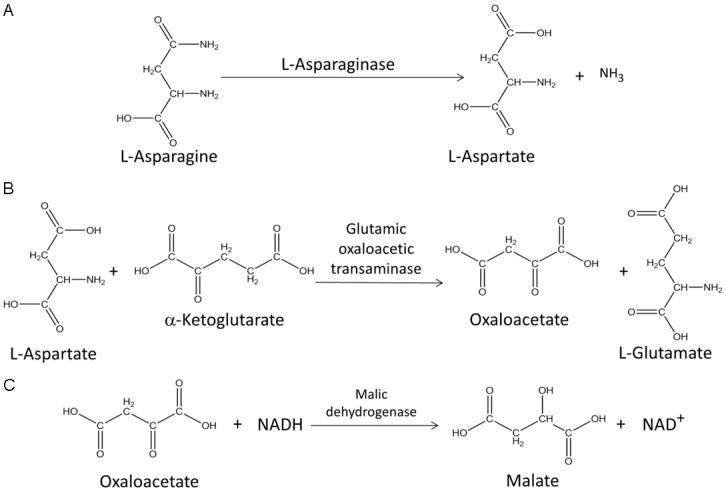
Quantifying asparaginase activity using an enzymed-coupled reaction. Asparaginase activity was determined spectrophotometrically in patient samples by a series of coupled enzymatic reactions (A-C). In order to quantify the release of L-aspartate from L-asparaginase, an enzyme reaction mixture containing asparagine, α-ketoglutarate, glutamic oxaloacetic transaminase, reduced β-nicotinamide adenine dinucleotide (NADH), and malic dehydrogenase is added to the samples and the change in absorbance over time observed is directly proportional to the rate at of NADH consumption and to the asparaginase activity of the sample.
Figure 2.
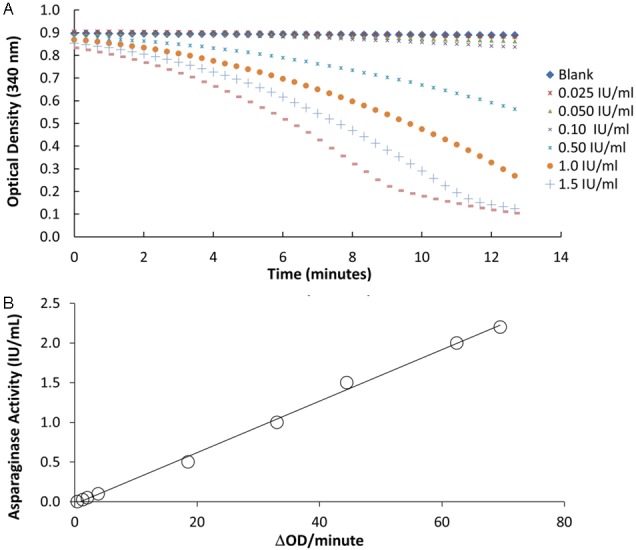
Asparaginase activity is measured spectrophotometrically using a calibration curve. A: Absorption readings are taken every 20 seconds for 15 minutes after initiating the reaction to capture changes in absorbance over time associated with asparaginase activity. Only the linear portions of the curve are used to estimate the reaction rate (ΔmOD/minute). B: A calibration curve is constructed by plotting the known asparaginase concentrations of the calibrators (0.025-2.2 IU/mL) versus the truncated reaction rates. The regression equation is used to determine the asparaginase activity of a sample with unknown activity. The coefficient of determination (R2) for the regression line was estimated to be 0.998.
Preparation of reagents and standards for asparaginase activity measurement in samples
The enzyme reaction mixture (ERM) was prepared by combining 200 mL of 10 x Tris buffered saline, 400 mL of glycerol, 1400 mL of double distilled water, 200 mg of α-ketoglutaric acid, 264 mg of asparagine, 200 mg of β-NADH, 1200 units of glutamic oxalacetic transaminase (suspension in 3.0 M (NH4)2SO4, 0.05 M Maleate, 2.5 mM α-ketoglutarate), and 200 units of malic dehydrogenase (solution in 50% glycerol containing 0.05 M potassium phosphate buffer, pH 7.5). The solution was mixed well, aliquotted at 23 mL per tube, and stored at -80°C. All ERM components were purchased from Sigma (St. Louis, MO). A solution of 5% bovine serum albumin (BSA) was prepared by dissolving five grams of BSA (Sigma, St. Louis, MO) in 100 mL of double distilled water, aliquotted at 2 mL per tube, and stored at -20°C. An E. coli asparaginase standard solution with 500 IU/mL activity was prepared by dissolving the contents of one vial of 500 IU E. coli L-asparaginase white lyophilized powder (BioVendor Laboratory Medicine, Inc., Candler, NC), with 1 mL of 50% glycerol (Sigma, St. Louis, MO) in 1 x phosphate buffered saline, pH 7.4 (Gibco, Carlsbad, CA). The standard solution was then aliquotted at 10 μL per tube, and stored at -80°C.
Calibrators and controls were prepared using the 500 IU/mL asparaginase standard solution and 5% BSA immediately prior to beginning the assay. Using the standard solution, a 10 IU/mL standard working solution was prepared along with further diluted calibrators with activities of 2.0, 1.5, 1.0, 0.5, 0.1, 0.05, 0.025, and 0 (blank) IU/mL. High activity controls (1.0 IU/mL) and low activity controls (0.10 IU/mL) were prepared in a similar fashion and used for quality control purposes.
Patient sample collection and storage
Specimens were collected from patients on the TOTAL XV [20] or TOTAL XVI [21] protocol at St. Jude Children’s Research Hospital using evacuated collection tubes for serum (BD Vacutainer®, Franlin Lakes, NJ), and the exact time the sample was drawn was recorded. The samples were allowed to clot for 30 minute at room temperature, centrifuged at 400 x g to separate the serum, and transferred to a clean tube. The sera were stored frozen at -80°C until the time of analysis. The study was approved by the St. Jude Children’s Research Hospital Institutional Review Board and was conducted within the US. Written informed consent was obtained from the patients themselves (if the patients were ≥18 years of age) or from the parents of the patients (for patients <18 years of age).
Instrumentation
A BioTek EL x 808 IU microplate reader (Winooski, VT) with an internal incubator set at 37°C, and a 340 nm filter was used to analyze all samples, controls, and calibrators. Gen 5 software (BioTeK Inc., Winooski, VT) was used for data collection and analysis. After the enzyme reaction mixture was added to the plate, the microplate was shaken for ten seconds to initiate the reaction. The kinetic data acquisition began at the end of the shaking period, and a twenty-second interval between absorbance readings was included.
Assay procedure
Ten microliters of control, calibrator, or of a patient’s sample that received no asparaginase, native E. coil asparaginase, Erwinia asparaginase, or PEGylated E. coli asparaginase was pipetted to the wells of a flat bottom UV-transparent 96-well microplate (Corning, Lowell, MA). All samples, standards, and controls were run in quadruplicate, with the high activity control, low activity control, and blank included on each plate. Samples with activities higher than the upper range of the calibration curve were re-assayed after dilution with 5% BSA. The enzyme activity for all samples was measured using the native E. coli asparaginase calibration curve regardless of the preparation (E. coli, Erwinia, or PEGylated E. coli asparaginase) used during treatment.
After temperature equilibration to 37°C, ERM was added to each well using a 12-channel electronic pipette. A volume of 190 μL was dispensed into each well, one row at a time, with a twenty-second interval between each addition. The kinetic data acquisition began upon the final ERM dispensing. Due to the time required to add ERM to the microplate, there is a lag time in the reaction initiation between the first and last rows on the plate. Nevertheless, the timed addition of ERM allows for more accurate readings, as data are read at a twenty-second intervals and the time of analysis are truncated to account for the varying reaction start times.
Assay validation
Accuracy, precision, and recovery
Intra-assay precision was evaluated by assaying 24 replicates of the low activity (0.10 IU/mL) and high activity (1.0 IU/mL) controls. Inter-assay precision was similarly evaluated with high activity (1.0 IU/mL) and low activity (0.10 IU/mL) controls, and measurements were repeated daily for twenty days. The accuracy is reported as the percent error of the measurement and the precision is reported as coefficient of variation (CV%). Recovery was evaluated by comparing paired sample sets: one with added asparaginase and a second with an equal volume of added blank. The recovery was evaluated either in normal human serum (NHS) or in randomly selected patient samples. Each set was run at three different asparaginase spiked activities (1 μL of 1.0, 5.0 or 10.0 IU/mL of asparaginase or BSA was added to 9 μL of samples).
Linearity, sensitivity, and reportable range
A calibration curve was constructed using calibrators with activities of 2.2, 2.0, 1.5, 1.0, 0.5, 0.1, 0.05, 0.025, and 0 (blank) IU/mL to determine the linear range. The coefficient of determination (R2), slope, and y-intercept were used to evaluate the linearity of the calibration curve. The lower limit of detection, the biological limit of detection, and the functional sensitivity were determined by assaying twelve replicates of four different concentrations of asparaginase: blank, 0.0125, 0.0250, and 0.050 IU/mL. From these results, the lower limit of detection was estimated by the mean of blank sample plus two times the standard deviation. Additionally, the biological limit of detection was calculated by adding the lower limit of detection to two times the standard deviation of the sample with the lowest activity (0.0125 IU/mL). Similarly, the functional sensitivity was determined by plotting the coefficient of variation (CV) versus concentration and determining the concentration where the CV is equal to 20%. The analytical measurement range was established as the asparaginase concentrations between the functional sensitivity and the highest calibrator. The direct analytical measurement range was extended by diluting samples with activities above that measured for the highest calibrator.
Sample stability
Serum sample stability was assessed by spiking normal human serum with 0.1 and 1.0 IU/mL of asparaginase. The test samples were aliquotted and stored at -80°C for long term stability studies, and at room temperature and 4°C for short-term stability studies. Furthermore, concentrated asparaginase stock solutions (500 IU/mL in BSA) were also stored at -80°C. The stability of the concentrated asparaginase solution was determined by diluting the stock solution to 0.1 and 1.0 IU/mL immediately before determining the activity. The measured activity of all stability samples was compared to the activity of freshly prepared asparaginase controls.
Interference
The possible interferences in patient samples by bilirubin, hemoglobin, and lipids at different levels were investigated. Sets of 0.10, 1.0, and 1.5 IU/mL asparaginase in NHS were prepared. One set was spiked with interferent and the other set was spiked with the equal volume of blank. The percent error caused by interference was calculated by comparing the difference between the results in each set.
Modeling asparaginase activity
To estimate the pharmacokinetics of asparaginase, a one-compartment pharmacokinetic model with first-order absorption and Michaelis-Menten elimination was used [3,22]. Population-based pharmacokinetics and individual activity time courses were estimated using nonlinear mixed effects modeling via NONMEM (version 7.2) [23].
Results
Data analysis
The OD readings as a function of time at different asparaginase concentrations are illustrated in Figure 2A. The absorbance of the 2.0 and 1.5 IU/mL asparaginase calibrator began plateauing at 9 and 11 minutes, respectively. The linearity of the calibration curves (concentration vs. ΔmOD/minute) was determined by averaging the reaction rates at different time ranges along the ΔmOD/minute vs. time plot, and the average slope between 0 and 9 minutes gave the best linearity for the resulting calibration curves (Table 1 and Figure 2B).
Table 1.
Linearity of calibration curve from 0.0-2.2 IU/mL asparaginase generated from different truncations of the ΔmOD/minute curves of calibrators
| Region (min) | R2 | Y-intercept | Slope |
|---|---|---|---|
| 0-9 | 0.9984 | 0.0011 | 0.0311 |
| 0-10 | 0.9982 | 0.0164 | 0.0310 |
| 0-11 | 0.9938 | 0.0566 | 0.0319 |
| 0-12 | 0.9754 | 0.0816 | 0.0330 |
| 1-10 | 0.9983 | 0.0335 | 0.0296 |
| 1-11 | 0.9900 | 0.0504 | 0.0304 |
| 1-12 | 0.9659 | 0.0880 | 0.0319 |
| 2-10 | 0.9979 | 0.0410 | 0.0284 |
| 2-11 | 0.9853 | 0.0730 | 0.0297 |
| 2-12 | 0.9494 | 0.0716 | 0.0307 |
Table 2 shows that appropriate truncation of ΔmOD/minute data is an effective way to compensate for different reaction times of wells in a plate. Asparaginase high and low controls (0.10 and 1.0 IU/mL), were added to wells and the asparaginase activities were measured. With data truncation, the percent error was less than 8% among measured asparaginase concentrations, whereas the percent error was as high as 31.3% without truncation.
Table 2.
Illustration that truncation of ΔmOD/minute versus asparaginase concentration curves minimizes the variation and error in concentration estimation for low and high controls
| Sample | With data truncation | Without data truncation | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 0.10 IU/mL (Low Control) | 1.0 IU/mL (High Control) | 0.10 IU/mL (Low Control) | 1.0 IU/mL (High Control) | |||||
|
| ||||||||
| read IU/mL | % error | read IU/mL | % error | read IU/mL | % error | read IU/mL | % error | |
| A | 0.106 | 6.1 | 1.02 | 1.8 | 0.106 | 6.1 | 1.02 | 1.8 |
| B | 0.098 | -1.8 | 1.00 | 0.1 | 0.094 | -6.1 | 0.95 | -4.9 |
| C | 0.100 | 0.1 | 1.01 | 0.6 | 0.089 | -10.7 | 0.90 | -9.6 |
| D | 0.106 | 6.4 | 1.04 | 4.3 | 0.090 | -10.4 | 0.89 | -11.3 |
| E | 0.096 | -3.8 | 1.03 | 3.0 | 0.076 | -24.3 | 0.82 | -17.7 |
| F | 0.100 | 0.1 | 0.98 | -2.3 | 0.074 | -26.4 | 0.73 | -26.8 |
| G | 0.100 | -0.1 | 1.04 | 4.5 | 0.070 | -30.2 | 0.74 | -26.5 |
| H | 0.107 | 7.3 | 1.05 | 5.1 | 0.070 | -30.2 | 0.69 | -31.3 |
Accuracy, precision, and recovery
Accuracy, as percent error, and precision, as coefficient of variation (CV%), in low and high activity controls are reported on Table 3. Intra- and inter-assay accuracy ranged from 0.2 to 8.2% and the precision was ≤9.0% CV. Recovery was determined by the activity difference between samples with a known added amount of asparaginase (Table 4) compared to the same sample with no added asparaginase (blank). The average recoveries of asparaginase were within 96% to 108% in NHS and 111% to 124% in a randomly selected spi-ked patient sample (Table 4).
Table 3.
Intra- and inter-assay precision and accuracy
| Asparaginase Control (IU/mL) | Intra-assay (n = 24) | Inter-assay (n = 20) | ||
|---|---|---|---|---|
|
| ||||
| % CV | % Error | % CV | % Error | |
| 0.10 (Low Control) | 3.3 | 8.2 | 8.8 | 1.3 |
| 1.0 (High Control) | 2.7 | 0.2 | 9.0 | 2.0 |
Table 4.
Assay recovery
| Matrix | Spiked concentrations | Total activity measured | Activity recovered | Recovery |
|---|---|---|---|---|
|
| ||||
| IU/mL | IU/mL | IU/mL | % | |
| Normal human serum (NHS) | 0 | -0.003 | - | - |
| 0.100 | 0.105 | 0.108 | 108% | |
| 0.500 | 0.501 | 0.504 | 101% | |
| 1.00 | 0.959 | 0.962 | 96% | |
| Patient sample | 0 | 0.691 | - | - |
| 0.100 | 0.815 | 0.124 | 124% | |
| 0.500 | 1.25 | 0.556 | 111% | |
| 1.00 | 1.80 | 1.11 | 111% | |
Linearity, sensitivity, and reportable range
Linearity between 0.025 and 2.2 IU/mL (R2 = 0.998) was es-tablished (Figure 2B and Table 1) with adequate accuracy (≤7.3% error) of target values at both the high and low controls (Table 2). The mean measurement of the blank sample was 0.0014 IU/mL with a standard deviation of 0.0005 IU/mL, resulting in a lower limit of detection of 0.0024 IU/mL. The standard deviation of the lowest calibrator (0.0125 IU/mL) was 3.1x10-5 IU/mL, resulting in a biological limit of detection of 0.0025 IU/mL. The functional sensitivity was determined as the concentration of asparaginase with a CV equal to 20% and it was estimated to be 0.011 IU/mL. The analytical measurement range was therefore from 0.011 to 2.2 IU/mL. Ultimately, it eliminated the need for sample pretreatment, as the activity levels of the majority of clinical samples fell within this range. However, the upper limit can be extended to 4.0 IU/mL by diluting specimens with BSA for specimens with higher concentrations of asparaginase.
Stability
The stability of asparaginase stored at -80°C, 4°C, or at room temperature was determined in NHS, and at -80°C for the concentrated stock solution in BSA by comparing the activity of the stability samples to the activity of freshly prepared asparaginase controls. NHS samples spiked with 0.1 IU/mL of asparaginase (Tables 5 and 6) were stable for 390 days at -80°C, 72 hours at 4°C, and 8 hours at room temperature. Similarly, NHS samples spiked with 1.0 IU/mL of asparaginase (Tables 5 and 6) were stable for 390 days at -80°C, 72 hours at 4°C, and 72 hours at room temperature. Asparaginase concentrated stock solutions stored at -80°C were stable for 120 days (Table 5) and were used to prepare asparaginase solutions with a final concentration of 0.1 or 1.0 IU/mL of asparaginase. Both the 0.1 IU/mL and the 1.0 IU/mL BSA solutions (Table 6) were stable for 72 hours at 4°C, and 72 hours at room temperature.
Table 5.
Stability of asparaginase at -80°C
| Time (day) | % Difference from freshly prepared sample | |||
|---|---|---|---|---|
|
| ||||
| Asparaginase in NHS | Asparaginase in Concentrated Stock | |||
|
| ||||
| 0.1 IU/mL (Low Control) | 1.0 IU/mL (High Control) | 0.1 IU/mL (Low Control) | 1.0 IU/mL (High Control) | |
| 5 | -4.2 | -5.1 | -4.5 | -0.8 |
| 8 | 0.2 | -3.6 | -5.3 | -6.3 |
| 10 | -6.2 | 3.5 | -8.4 | -5.1 |
| 15 | -12.5 | -5.9 | -7.2 | -7.7 |
| 22 | -9.4 | 1.3 | -5.2 | -4.2 |
| 29 | -12.9 | -8.2 | -6.2 | -1.2 |
| 37 | -0.8 | -6.3 | -10.9 | -12.3 |
| 40 | -16.5 | -8.1 | -0.5 | -6.1 |
| 48 | -4.5 | -0.9 | 9.3 | 2.8 |
| 73 | -2.0 | -1.1 | -9.2 | -9.6 |
| 120 | -15.8 | -10.6 | -7.0 | -10.2 |
| 210 | 1.7 | 4.2 | -24.4 | -20.8 |
| 270 | -16.6 | -7.5 | -23.0 | -22.8 |
| 390 | 2.8 | 11.8 | -11.0 | -5.1 |
NHS: normal human serum.
Table 6.
Stability of asparaginase at 4°C and at room temperature
| Time (hour) | % Difference from fresh prepared sample | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 4°C | Room Temperature | |||||||
|
| ||||||||
| NHS | Asparaginase in BSA | NHS | Asparaginase in BSA | |||||
|
| ||||||||
| 0.10 IU/mL Low Control | 1.0 IU/mL High Control | 0.10 IU/mL Low Control | 1.0 IU/mL High Control | 0.10 IU/mL Low Control | 1.0 IU/mL High Control | 0.10 IU/mL Low Control | 1.0 IU/mL High Control | |
| 1.5 | 4.9 | 1.9 | 3.7 | -2.6 | 8.3 | -1.4 | 8.6 | 1.5 |
| 3 | -13.9 | -9.1 | 2.7 | -7.1 | -12.1 | -8.7 | 3.6 | -6.9 |
| 4.5 | -4.9 | -10.0 | 0.2 | -8.6 | 4.4 | -7.3 | 3.8 | -5.7 |
| 8 | 4.3 | -3.2 | 0.5 | -3.2 | 10.4 | -3.8 | 15.1 | -2.8 |
| 24 | 0.3 | -10.9 | -2.7 | -12.2 | 47.2 | -3.4 | -1.5 | -6.3 |
| 72 | -4.0 | -10.2 | -3.3 | -9.6 | 27.1 | -1.0 | -5.0 | -11.6 |
NHS: normal human serum.
Interference
Hemoglobin interference was negligible when asparaginase concentration was high or when the interferent concentration was low (Table 7). At 1.5 IU/mL asparaginase, the percent errors in asparaginase activity readings were less than or equal to ± 3.3% when hemoglobin present in the specimen ranged from 100 to 1000 mg/dL (Table 7). The percent errors caused by 100 and 200 mg/dL of hemoglobin were less than or equal to ± 11.7% at all levels of asparaginase tested. However, the interference became significant as asparaginase concentration became lower. At an asparaginase activity of 0.10 IU/mL, the percent error was as high as -59% when hemoglobin was present at 1000 mg/dL (Table 7). Additionally, the interferences of lipids and bilirubin were more severe than that of hemoglobin. There was a directly proportional relationship between interferent concentration and percent error at all levels of asparaginase activity tested (Table 7). Lipids caused the asparaginase activity measurement to be lower than the expected value, while bilirubin causes the asparaginase activity measurements to be higher than the expected value.
Table 7.
Validation of interferences in asparaginase activity assay
| Interferent | Interferent conc. (mg/dL) | Asparaginase Levels (IU/mL) | ||
|---|---|---|---|---|
|
| ||||
| 0.10 | 1.0 | 1.5 | ||
|
| ||||
| % Error | ||||
| Hemoglobin | 100 | 8.7 | 4.9 | 3.3 |
| 200 | -4.0 | 11.7 | 2.0 | |
| 400 | -14.3 | 13.5 | 2.4 | |
| 1000 | -59.0 | -8.2 | -2.9 | |
| Intralipid | 150 | -11.1 | -29.3 | -17.7 |
| 450 | -32.8 | -47.9 | -43.1 | |
| 750 | -52.5 | -84.1 | -76.0 | |
| Bilirubin | 1 | 17.2 | 11.4 | 17.0 |
| 10 | 11.9 | 20.3 | 15.2 | |
| 20 | 57.6 | 29.4 | 34.4 | |
Application of assay to patient samples
Asparaginase activity in patient sera will be used to determine the pharmacokinetics of PEGylated E. coli asparaginase at doses of 2,500 and 3,500 IU/m2 given intravenously for patients on the TOTAL XVI protocol at St. Jude Children’s Research Hospital (Figure 3). The assay can also be used to measure asparaginase in serum after native E. coli asparaginase and Erwinia asparaginase administration, where serum activities measured 1 or 7 days after intramuscular native E. coli asparaginase administration ranged from 2.313 to 0.002 IU/mL in patients given 25,000 IU/m2. Similarly, activities measured 1 or 3 days after intramuscular Erwinia asparaginase administration ranged from 1.032 to 0.019 U/mL in patients given 20,000-25,000 U/m2.
Figure 3.
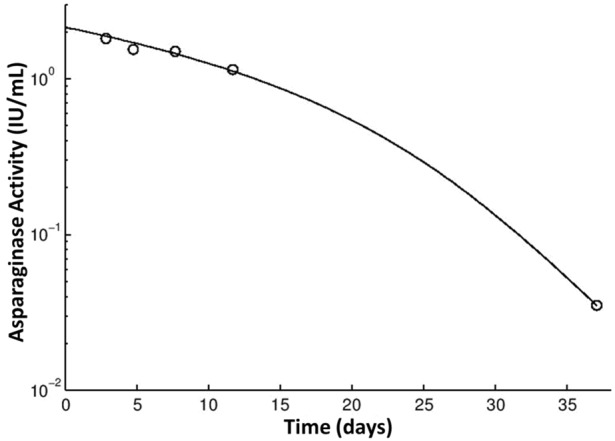
Measurement and modeling of asparaginase activity in patient samples. The asparaginase activity of an acute lymphoblastic (ALL) patient enrolled in the TXVI protocol was measured before treatment and at 3, 5, 8, 12, and 37 days after a single dose of PEGylated E. coli asparaginase at 3,000 units/m2 during induction (○). The solid black line represents the best fit from the pharmacokinetic modelling.
Discussion
This assay will allow for the effective analysis of activity levels of patients receiving all forms of clinically used asparaginase [1,8,24]. There is increasing interest in performing therapeutic drug monitoring for asparaginase, due to the possible influence of asparaginase-specific antibodies on the systemic exposure of asparaginase [25]. As asparaginase is a non-human protein, the presence of antibodies may result in the inactivation of the drug [6,26]. This is not an infrequent occurrence as 0% to 63% of patients experience hypersensitivity reactions during treatment and 15% to 80% of patients have been reported to form antibodies [8,10,14,27-30]. Similarly, not all patients that have antibodies will develop an allergic reaction. This phenomenon, known as “silent hypersensitivity”, occurs in anywhere from 5% to 46% of patients [3,8,31]. Patients that develop antibodies without any clinical symptoms may have increased asparaginase cl-earance that contributes to a poor treatment outcome.
Few of the previously des-cribed asparaginase activity measurement assays are high-throughput, and most require laborious sample preparation [1,2,13,15-19]. Furthermore, similar high-throughput assays based on the detection of ammonia require meticulous care during testing and have a narrower linear range of detection compared to the method described in this manuscript (linear over an 88-fold range compared to a 24-fold range) [15,18]. Our method utilizes 96-well plates and is able to measure 21 samples, along with controls, in one run. The assay compensates for the differing reaction times by timed addition of reagents and by data truncation to ensure linearity in the enzymatic reactions used for estimating the activity of each calibrator and control. These assay parameters are designed to improve the linearity range and to eliminate the need for sample pretreatment. Our high-throughput assay offers a fast, accurate, and sensitive method that has been successfully used to determine the asparaginase pharmacokinetic properties of patient sera from children with acute lymphoblastic leukemia.
Acknowledgements
This work was supported by grants CA 21765, LLS 6168-12 from the National Institutes of Health and the American Lebanese Syrian Associated Charities and by CA142665 from the National Cancer Institute and by an investigator-initiated grant from Sigma Tau Pharmaceuticals Inc.
References
- 1.Asselin BL, Whitin JC, Coppola DJ, Rupp IP, Sallan SE, Cohen HJ. Comparative pharmacokinetic studies of three asparaginase preparations. J. Clin. Oncol. 1993;11:1780–1786. doi: 10.1200/JCO.1993.11.9.1780. [DOI] [PubMed] [Google Scholar]
- 2.Lanvers C, Vieira Pinheiro JP, Hempel G, Wuerthwein G, Boos J. Analytical validation of a microplate reader-based method for the therapeutic drug monitoring of L-asparaginase in human serum. Anal Biochem. 2002;309:117–126. doi: 10.1016/s0003-2697(02)00232-4. [DOI] [PubMed] [Google Scholar]
- 3.Panetta JC, Gajjar A, Hijiya N, Hak LJ, Cheng C, Liu W, Pui CH, Relling MV. Comparison of native E. coli and PEG asparaginase pharmacokinetics and pharmacodynamics in pediatric acute lymphoblastic leukemia. Clin Pharmacol Ther. 2009;86:651–658. doi: 10.1038/clpt.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pieters R, Appel I, Kuehnel HJ, Tetzlaff-Fohr I, Pichlmeier U, van der Vaart I, Visser E, Stigter R. Pharmacokinetics, pharmacodynamics, efficacy, and safety of a new recombinant asparaginase preparation in children with previously untreated acute lymphoblastic leukemia: a randomized phase 2 clinical trial. Blood. 2008;112:4832–4838. doi: 10.1182/blood-2008-04-149443. [DOI] [PubMed] [Google Scholar]
- 5.Dinndorf PA, Gootenberg J, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL) Oncologist. 2007;12:991–998. doi: 10.1634/theoncologist.12-8-991. [DOI] [PubMed] [Google Scholar]
- 6.Raetz EA, Salzer WL. Tolerability and efficacy of L-asparaginase therapy in pediatric patients with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010;32:554–563. doi: 10.1097/MPH.0b013e3181e6f003. [DOI] [PubMed] [Google Scholar]
- 7.Rizzari C, Zucchetti M, Conter V, Diomede L, Bruno A, Gavazzi L, Paganini M, Sparano P, Lo NL, Arico M, Milani M, D’Incalci M. L-asparagine depletion and L-asparaginase activity in children with acute lymphoblastic leukemia receiving i. m. or i.v. Erwinia C. or E. coli L-asparaginase as first exposure. Ann Oncol. 2000 Feb;11:189–93. doi: 10.1023/a:1008368916800. [DOI] [PubMed] [Google Scholar]
- 8.Panosyan EH, Seibel NL, Martin-Aragon S, Gaynon PS, Avramis IA, Sather H, Franklin J, Nachman J, Ettinger LJ, La M, Steinherz P, Cohen LJ, Siegel SE, Avramis VI. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children’s Cancer Group Study CCG-1961. J Pediatr Hematol Oncol. 2004;26:217–226. doi: 10.1097/00043426-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Muller HJ, Boos J. Use of L-asparaginase in childhood ALL. Crit Rev Oncol Hematol. 1998;28:97–113. doi: 10.1016/s1040-8428(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 10.Oettgen HF, Stephenson PA, Schwartz MK, Leeper RD, Tallai L, Tan CC, Clarkson BD, Golbey RB, Krakoff IH, Karnofsky DA, Murphy ML, Burchenal JH. Toxicity of E. coli L-asparaginase in man. Cancer. 1970;25:253–278. doi: 10.1002/1097-0142(197002)25:2<253::aid-cncr2820250204>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Avramis VI, Avramis EV, Hunter W, Long MC. Immunogenicity of native or pegylated E. coli and Erwinia asparaginases assessed by ELISA and surface plasmon resonance (SPR-biacore) assays of IgG antibodies (Ab) in sera from patients with acute lymphoblastic leukemia (ALL) Anticancer Res. 2009;29:299–302. [PubMed] [Google Scholar]
- 12.Ebeid EN, Kamel MM, Ali BA. Detection of anti-asparaginase antibodies during therapy with E. coli asparaginase in children with newly diagnosed acute lymphoblastic leukemia and lymphoma. J Egypt Natl Canc Inst. 2008;20:127–133. [PubMed] [Google Scholar]
- 13.Vrooman LM, Supko JG, Neuberg DS, Asselin BL, Athale UH, Clavell L, Kelly KM, Laverdiere C, Michon B, Schorin M, Cohen HJ, Sallan SE, Silverman LB. Erwinia asparaginase after allergy to E. coli asparaginase in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;54:199–205. doi: 10.1002/pbc.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avramis VI, Sencer S, Periclou AP, Sather H, Bostrom BC, Cohen LJ, Ettinger AG, Ettinger LJ, Franklin J, Gaynon PS, Hilden JM, Lange B, Majlessipour F, Mathew P, Needle M, Neglia J, Reaman G, Holcenberg JS, Stork L. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood. 2002;99:1986–1994. doi: 10.1182/blood.v99.6.1986. [DOI] [PubMed] [Google Scholar]
- 15.Ylikangas P, Mononen I. A fluorometric assay for L-asparaginase activity and monitoring of L-asparaginase therapy. Anal Biochem. 2000;280:42–45. doi: 10.1006/abio.2000.4500. [DOI] [PubMed] [Google Scholar]
- 16.Tagami S, Matsuda K. An enzymatic method for the kinetic measurement of L-asparaginase activity and L-asparagine with an ammonia gas-sensing electrode. Chem Pharm Bull (Tokyo) 1990;38:153–155. doi: 10.1248/cpb.38.153. [DOI] [PubMed] [Google Scholar]
- 17.Nath CE, Dallapozza L, Eslick AE, Misra A, Carr D, Earl JW. An isocratic fluorescence HPLC assay for the monitoring of l-asparaginase activity and l-asparagine depletion in children receiving E. colil-asparaginase for the treatment of acute lymphoblastic leukaemia. Biomed Chromatogr. 2009;23:152–159. doi: 10.1002/bmc.1096. [DOI] [PubMed] [Google Scholar]
- 18.Avramis VI, Martin-Aragon S, Avramis EV, Asselin BL. Pharmacoanalytical assays of Erwinia asparaginase (erwinase) and pharmacokinetic results in high-risk acute lymphoblastic leukemia (HR ALL) patients: simulations of erwinase population PK-PD models. Anticancer Res. 2007;27:2561–2572. [PubMed] [Google Scholar]
- 19.Kojima Y, Wacker WE. An enzymatic method for the measurement of asparagine and a new assay of asparaginase activity. J Lab Clin Med. 1969;74:521–526. [PubMed] [Google Scholar]
- 20.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, Ribeiro RC, Rubnitz JE, Raimondi SC, Onciu M, Coustan-Smith E, Kun LE, Jeha S, Cheng C, Howard SC, Simmons V, Bayles A, Metzger ML, Boyett JM, Leung W, Handgretinger R, Downing JR, Evans WE, Relling MV. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pui CH CD, Sandlund JT, Bhojwani D, Evans WE, Relling MV, Jeha S. Treatment of childhood acute lymphoblastic leukemia without cranial irradiation. Ann Hematol. 2011;90:S61–63. [Google Scholar]
- 22.Muller HJ, Beier R, da Palma JC, Lanvers C, Ahlke E, von Schutz V, Gunkel M, Horn A, Schrappe M, Henze G, Kranz K, Boos J. PEG-asparaginase (Oncaspar) 2500 U/m(2) BSA in reinduction and relapse treatment in the ALL/NHL-BFM protocols. Cancer Chemother Pharmacol. 2002;49:149–154. doi: 10.1007/s00280-001-0391-5. [DOI] [PubMed] [Google Scholar]
- 23.Beal S, Sheiner L, Boeckmann A. NONMEM user’s guides. Ellicott City, MD, USA: Icon Development Solutions; 1989-2011. [Google Scholar]
- 24.van den Berg H. Asparaginase revisited. Leuk Lymphoma. 2011;52:168–178. doi: 10.3109/10428194.2010.537796. [DOI] [PubMed] [Google Scholar]
- 25.Rizzari C, Conter V, Stary J, Colombini A, Moericke A, Schrappe M. Optimizing asparaginase therapy for acute lymphoblastic leukemia. Curr Opin Oncol. 2013;25(Suppl 1):S1–9. doi: 10.1097/CCO.0b013e32835d7d85. [DOI] [PubMed] [Google Scholar]
- 26.Zalewska-Szewczyk B, Andrzejewski W, Mlynarski W, Jedrychowska-Danska K, Witas H, Bodalski J. The anti-asparagines antibodies correlate with L-asparagines activity and may affect clinical outcome of childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2007;48:931–936. doi: 10.1080/10428190701292049. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Kawedia JD, Cheng C, Pei D, Fernandez CA, Cai X, Crews KR, Kaste SC, Panetta JC, Bowman WP, Jeha S, Sandlund JT, Evans WE, Pui CH, Relling MV. Clinical utility and implications of asparaginase antibodies in acute lymphoblastic leukemia. Leukemia. 2012;26:2303–9. doi: 10.1038/leu.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woo MH, Hak LJ, Storm MC, Evans WE, Sandlund JT, Rivera GK, Wang B, Pui CH, Relling MV. Anti-asparaginase antibodies following E. coli asparaginase therapy in pediatric acute lymphoblastic leukemia. Leukemia. 1998;12:1527–1533. doi: 10.1038/sj.leu.2401162. [DOI] [PubMed] [Google Scholar]
- 29.Woo MH, Hak LJ, Storm MC, Sandlund JT, Ribeiro RC, Rivera GK, Rubnitz JE, Harrison PL, Wang B, Evans WE, Pui CH, Relling MV. Hypersensitivity or development of antibodies to asparaginase does not impact treatment outcome of childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2000;18:1525–1532. doi: 10.1200/JCO.2000.18.7.1525. [DOI] [PubMed] [Google Scholar]
- 30.Peterson RG, Handschumacher RE, Mitchell MS. Immunological responses to L-asparaginase. J Clin Invest. 1971;50:1080–1090. doi: 10.1172/JCI106579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strullu M, Corradini N, Audrain M, Orsonneau JL, Bouige D, Thomare P, Vermot-Desroches C, Mansuy A, Legrand A, Roze JC, Mohty M, Mechinaud F. Silent hypersensitivity to Escherichia coli asparaginase in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2010;51:1464–1472. doi: 10.3109/10428194.2010.494316. [DOI] [PubMed] [Google Scholar]


