Abstract
Objective: This study aimed to investigate neuronal apoptosis and expression of apoptosis related proteins (Fas, Caspase-3 and Bcl-2) in the brain of rates with morphine addiction. Methods: A total of 48 adult male Sprague-Dawley rats weighing 190-210 g were randomly divided into 3 groups (n=16 per group): morphine addiction group, morphine abstinence group and control group. Rats in the addiction group and the abstinence group were intraperitoneally treated with morphine for 13 days to induce morphine addiction. In abstinence group, rats were then intraperitoneally treated with naloxone at 5 mg/kg to induce abstinence for 30 min. Rats in the control group were injected with normal saline. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) was employed to detect apoptotic cells. Immunohistochemistry and Western blot assay were performed to determine the expressions of Fas, Bcl-2 and Caspase-3 in the hippocampus. Results: When compared with the control group, the proportion of apoptotic neurons increased significantly in the addiction group and the abstinence group (P<0.01), accompanied by significantly increased expressions of Fas and Caspase-3 (P<0.01) and markedly decreased Bcl-2 expression (P<0.01) in the hippocampuse. However, no significant differences were observed between the addiction and the abstinence group (P>0.05). Conclusion: Long term use of morphine can induce neuronal apoptosis in the brain by increasing the expressions of pro-apoptotic Fas and Caspase-3 and decreasing the anti-apoptotic Bcl-2 expression, which might be one of mechanisms underlying the opiate-induced neuronal damage.
Keywords: Morphine addiction, hippocampus, apoptosis, Fas, Bcl-2, Caspase-3
Introduction
Opioids as potent analgesics have been used in pain treatment for more than 100 years, but long term use of opioids may induce addiction resulting in physical dependence and psychological dependence. The chronic effect of morphine on cerebral neurons suggests that long term use of opioids may induce the structural alteration of neurons [1,2]. The neuronal apoptosis and its mechanisms have been investigated in vitro, but few studies are conducted to investigate these in vivo. Recently, Hassanzadeh et al [3,4] investigated the neuronal apoptosis in the spinal cord and cerebral cortex in vivo in a rat model. In the present study, immunohistochemistry and western blot assay were employed to evaluate the neuronal apoptosis in the hippocampus of rats with morphine addiction, and the mechanisms underlying the opioids induced neuronal apoptosis were also investigated.
Materials and methods
Animals and grouping
A total of 48 adult male Sprague-Dawley (SD) rats weighing 190-210 g were purchased from the Experimental Animal Center of the Second Military Medical University. These rats were randomly assigned into 3 groups: morphine addiction group, morphine abstinence group and control group (n=16 per group).
Preparation of morphine addiction and morphine abstinence models
Rats in the addiction and the abstinence group were intraperitoneally injected with morphine (Lot number: 90902; Shenyang First Pharmaceutical Factory) thrice daily (8:00, 12:00 and 20:00) in a daily dose increment manner. On the first day, the dose was 5 mg/kg. In the first 6 days, the daily increment of dose was 15 mg/kg. Since day 7, the daily increment of dose was 30 mg/kg. On the thirteen day, the dose was 290 mg/kg [5]. In control group, rats were intraperitoneally treated with normal saline of equal volume. In morphine abstinence group, rats were then intrapeitoneally treated with naloxone (Lot number: 010705; Beijing Sihuan Pharmaceutical Factory) at 5 mg/kg to induce abstinence for 30 min. The recognizable abstinence symptoms were observed including standing (1, 1-5 times; 2, 6-10 times; 3, >11 times), wet-dog shaking, stretching, teeth chatter, jumping, cunnilingus (1, 1-3 times; 2, 4-6 times; 3, >7 times). The abstinence symptoms were scored. The score of rats with touching-induced screaming increased by 2.
Sample collection
Eight rats were randomly selected from each group, and sacrificed. The muscle and fascia were removed from the skull and foramina magnum was exposed. The skull was opened along the sagittal plane via the foramina magnum. The dura mater was carefull removed, and the whole brain was obtained. The hippocampus was separated according to previously described [6] and then stored in liquid nitrogen for western blot assay. The remaining rats were perfused and fixed. The brain was collected followed by dehydration and transparentization. After embedding in paraffin, sections were obtained for histological examination and immunohistochmistry. Coronal sections (4 μm) were cut and adherent to slides.
Detection of neuronal apoptosis
Cell apoptosis detection kit and TUNEL detection kit (RD, USA) were employed for the detection of neuronal apoptosis. In brief, paraffin-embedded sections were deparaffinized and dehydrated. After washing in PBS, sections were treated with 20 μg/mL Proteinase K for 20 min. After washing in PBS thrice (3 min for each), sections were rinsed with 0.3% Triton X-100 for 10 min followed by washing in PBS. These sections were incubated with TUNEL reaction mixture at 37°C for 1 h. Following washing in PBS thrice (3 min for each), sections were treated with HRP conjugated streptavidin (1:200; Beijing Zhongshan Biotech Co., Ltd) at 37°C for 30 min. After washing in PBS thrice (3 min for each), sections were treated with 0.04% DAB and 0.03% H2O2 at room temperature for visualization for 8-12 min. After washing in water, counterstaining was done with hematoxylin followed by mounting with resin. In the negative control, TUNEL reaction mixture was replaced with PBS. The positive control sections were pre-treated with DNase I for 10 min followed by TUNEL staining. Cells with blue granules in the nucleus were regarded as positive for TUNEL. A total of 100 cells were counted at a high magnification, and the percentage of TUNEL positive cells was calculated.
Immunohistochemistry for Fas, Bcl-2 and Caspase-3
Immunohistochemistry for Fas, Bcl-2 and Caspase-3 was done with detection kit (Dako, Danmark). Paraffin-embedded sections were deparaffinized and dehydrated. After washing in PBS thrice (3 min for each), sections were treated with 3% H2O2 at room temperature for 20 min to inactivate endogenous peroxidase. After washing in PBS thrice (3 min for each), antigen retrieval was performed at 98°C twice (10 min for each). Sections were allowed to cool to temperature. After washing in PBS thrice (3 min for each), sections were incubated with normal goat serum at room temperature for 30 min. Then, these sections were indenpendently treated with primary antibodies (Fas: 1:60; Bcl-2: 1:80; caspase: 1:30) at room temperature for 8 h. Following washing in PBS thrice (3 min for each), sections were incubated with HRP conjugated streptavidin at 37°C for 30 min. Following washing in PBS thrice (3 min for each), visualization was done with DAB. After washing in water, counterstaining was done with hematoxylin followed by mounting with resin. In blank control, primary antibody was replaced with PBS. In alternative control, primary antibody was replaced with normal serum. The known positive control served as a positive control. Two sections were randomly selected from each rat, and three fields were randomly selected at a high magnification. The proportion of positive cells was calculated.
Western blot assay of Fas, Bcl-2 and Caspase-3
The hippocampus was taken out of the liquid nitrogen and homogenized with a buffer (1:10) followed by centrifugation at 800xg at 4°C for 10 min. The supernatant was obtained and protein quantification was done with Lowry method. The proteins were stored at -80°C. Then, 40 μg of total proteins were subjected to 10% SDS-PAGE and then transferred onto membranes which were then blocked in 5% non-fat milk at 4°C overnight. The membranes were treated with rabbit anti-Fas or Bcl-2 polyclonal antibody (1:200; Dako, Danmark). After washing in buffer A thrice for 10 min, membranes were treated with goat anti-rabbit IgG (Beijing Zhongshan Biotech Co., Ltd). After washing, these membranes were incubated with HRP conjugated streptavidin at 1:200. After washing thrice, the membranes were underwent visualization with DAB. Representative photographs were captured and the bands on membranes were analyzed with Gel Doc 2000 system (BioRad, USA).
Statistical analysis
Statistical analysis was done with SAA. Quantitative data were expressed as mean ± standard deviation (x̅±s). One way analysis of variance was employed for comparisons among groups. A value of P<0.05 was considered statistically significant.
Results
Abstinence symptoms in rats with morphine addiction
In morphine addiction group, the abstinence score was 4.24±0.40, which was comparable to that in control group (3.99±0.42), but markedly lower than that in morphine abstinence group (20.63±3.65; P<0.01). This suggests that morphine addiction was induced in these rats.
Neuronal apoptosis in hippocampus of rats with morphine addiction
The apoptotic cells present with cytoplasm contraction, reduction in cell volume and nuclear condensation. The apoptotic cells were detached from surrounding cells. TUNEL staining showed apoptotic signal was less found in the nucleus of neurons of control group. However, in morphine addiction group and morphine abstinence group, a large amount of apoptotic signals were observed in the nucleus of neurons (Figure 1). The proportion of apoptotic cells was 12.89±2.81%, 12.79±2.98% and 2.32±1.58 in morphine addiction group, morphine abstinence group and control group, respectively. The apoptotic rate in morphine addiction group and morphine abstinence group was markedly higher than that in control group, but there was no significant difference between former two groups (P>0.05). This suggests that long term use of morphine may cause in crease in apoptotic neurons in rats.
Figure 1.
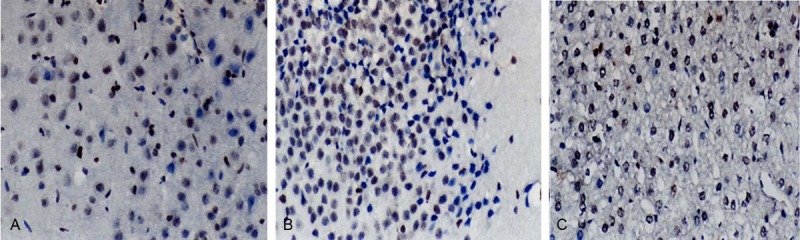
Detection of apoptotic cells in the hippocampus of rats with morphine addiction by immunohistochemistry. A: Control group; B: Morphine addiction group; C: Morphine abstinence group. Original magnification: ×800.
Protein expressions of Fas, Bcl-2 and caspase-3 in neurons of rats with morphine addiction
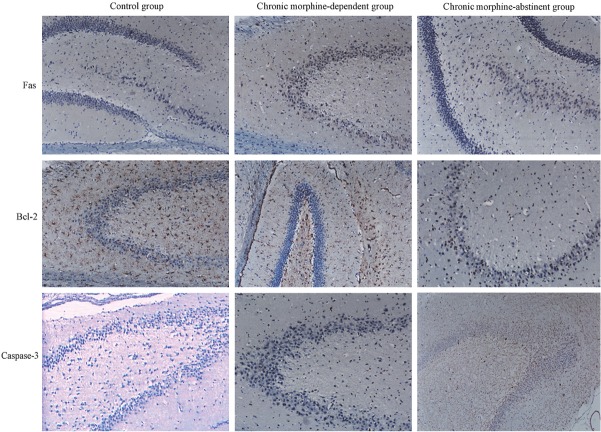
In the immunohistochemistry, cells with yellow-brown granules in the cytoplasm were regarded as positive (Figure 2). Results showed, when compared with control group, the expressions of Fas and Caspase-3 increased dramatically but Bcl-2 expression significantly reduced in morphine addiction group and morphine abstinence group (P<0.01). However, significant differences in protein expressions of Fas, Bcl-2 and caspase-3 were not observed between morphine addiction group and morphine abstinence group (P>0.05) (Table 1). Western blot assay also revealed that the expressions of Fas and Bcl-2 increased and the Bcl-2 expression reduced markedly in morphine addiction group and morphine abstinence group (P<0.05, Figure 3).
Figure 2.
Protein expressions of Fas, Bcl-2 and Caspase-3 in hippocampal cells of rats with morphine addiction. (Immunohistochemistry, Original magnification: ×200).
Table 1.
Percentage of hippocampal cells positive of Fas, Bcl-2 and Caspase-3 in rats with morphine addiction (n=8, x̅±s, %)
| Group | Fas | Bcl-2 | Caspase-3 |
|---|---|---|---|
| Morphine-dependent | 11.39±2.42** | 7.95±2.81** | 20.24±2.37** |
| Morphine-abstinent | 11.09±2.57* | 8.10±2.42* | 22.13±2.46* |
| Control | 7.07±2.24 | 10.96±2.18 | 8.21±2.33 |
P<0.05,
P<0.01 vs control group.
Figure 3.
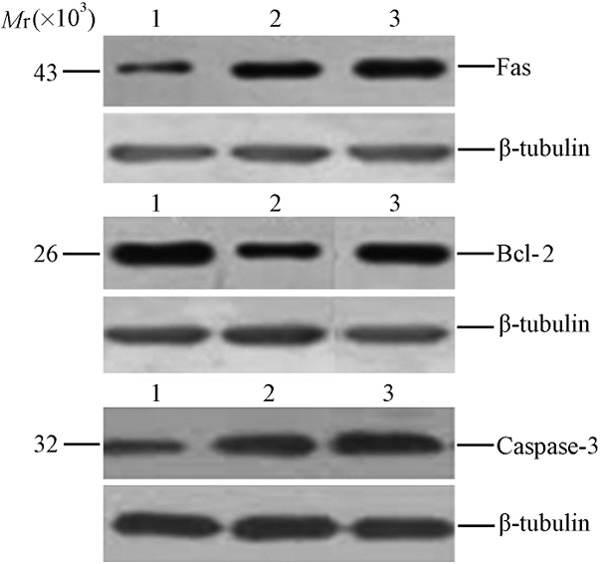
Western blot assay of Fas, Bcl-2 and Caspase-3 protein expression in rat hippocampus. 1: Control group; 2: Morphine addiction group; 3: Morphine abstinence group.
Discussion
Although several signaling pathways are involved in the process of apoptosis, the apoptosis related death signaling pathway is conservative. The mitochondrion dependent and death receptor dependent signaling pathways are two major pathways related to apoptosis. In the mitochondrion dependent signaling pathway, Bcl-2 may block the release of cytochrome C and the activation of specific proteases (caspase, a proteolylic enzyme which plays a key role in the nuclear fracture and apoptosis) resulting in inhibition of apoptosis. Death receptor Fas can specifically bind to Fas ligand (FasL) to induce apoptosis. Both signaling pathways converge at Caspase-3. The activation of caspase-3 then induce the activation of caspase activated deoxyribonuclease (CAD), resulting in DNA fracture and finally cell apoptosis.
In recent years, the multiple effects of opioids on neuronal structure (cytoskeleton) have been regarded as the markers of neuronal damage due to long term use of morphine and other opioids. Actually, cytoskeleton components (such as intermediate filaments and microtubules) are substrates of Fas ligand and caspases. The fracture of cytoskeleton may cause cytotoxicity and cell apoptosis. Studies have confirmed that, in rats with morphine or other opioids induced addiction, the expression of NF protein (a major component of intermediate filaments) reduced significantly [7,8]. In the new cortex of rats, long term use of morphine can reduce the number of neurons positive calcium binding protein D-28 000, a protein with neuroprotective effect [9]. This effect might be attributed to the opioids induced neuronal damage. Thus, these structural changes reflect the neuronal damage due to long term use of opioids, and this neuronal damage might be related to opioids induced alteration of NF protein, a component of cytoskeleton.
Studies on morphine addiction induced neuronal apoptosis usually focus on heroin addiction in vitro. Singhal et al [10] investigated the Jurkat cells and T lymphocytes of patients with heroin addiction. Their results showed 1×10-8 mol/L morphine resulted in the apoptosis rate of 20.1±0.4%, and 1×10-4 mol/L morphine caused the apoptosis rate of 29.1±2.2%. Morphine and opioid receptor specific agonist DAMGO (D-Ala2, N-Me-Phe4, Gly5-ol-enkephalin) may reduce Bcl-2 expression and increase Bax expression to induce apoptosis of lymphocytes and/or Jurkat cells. Morphine may also induce the mRNA expression of pro-apoptotic receptors in the lymphocytes and mouse spleen, lung and heart via activating opioid receptor [11]. In the spleen lymphocytes of stress treated mice (increase in endogenous opioid peptides) m the Fas mRNA expression increased significantly. This effect could be abolished by naltrexone or naloxone. This suggests that Fas mediated lymphocyte apoptosis is dependent endogenous opioid peptides [12]. These findings indicate that there might be endogenous opioid peptide mediated tension adjustment in stress treated mice. In the present study, the neuronal apoptosis and protein expression of Fas, Bcl-2 and caspase-3 were detected in rats with morphine addiction. Our results showed a large amount of apoptotic neurons were observed in the hippocampus of these rats, and the expressions of three apoptosis related proteins presented with alteration. When compared with control group, the expressions of Fas and caspase-3 increased markedly and Bcl-2 expression reduced significantly in morphine addication group and morphine abstinence group. These suggest that long term use of morphine may increase the apoptotic neurons via apoptosis related signaling pathway, which was consistent with findings in in vitro studies.
Taken together, morphine may cause neuronal apoptosis via altering expressions of Fas, Bcl-2 and caspase-3. Our findings provide evidence for the mechanism and pathophysiology underlying the neuronal damage due to long term use of opioids.
References
- 1.Nestler EJ. Under siege: the brain on opiates. Neuron. 1996;16:897–900. doi: 10.1016/s0896-6273(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 2.Ferrer-Alcón M, García-Sevilla JA, Jaquet PE, La Harpe R, Riederer BM, Walzer C, Guimón J. Regulation of nonphosphorylated and phosphorylated forms of neurofilament proteins in the prefrontal cortex of human opioid addicts. J Neurosci Res. 2000;61:338–349. doi: 10.1002/1097-4547(20000801)61:3<338::AID-JNR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Hassanzadeh K, Habibi-asi B, Farajnia S, Roshangar L. Minocycline prevents morphine-induced apoptosis in rat cerebral cortex and lumbar spinal cord: a possible mechanism for attenuating morphine tolerance. Neurotox Res. 2011;19:649–659. doi: 10.1007/s12640-010-9212-0. [DOI] [PubMed] [Google Scholar]
- 4.Hassanzadeh K, Roshangar L, Habibi-asl B, Farajnia S, Izadpanah E, Nemati M, Arasteh M, Mohammadi S. Riluzole prevents morphine-induced apoptosis in rat cerebral cortex. Pharmacol Rep. 2011;63:697–707. doi: 10.1016/s1734-1140(11)70581-3. [DOI] [PubMed] [Google Scholar]
- 5.Ji JT, Wang XH, You ZD, Han TQ. To establish a morphine dependent rat model. Acad J Sec Mil Med Univ. 1997;18:81–82. [Google Scholar]
- 6.Bhargava HN, Gulati A. Modification of brain and spinal cord dopamine D1 receptors labeled with [3H] SCH 23390 after morphine withdrawal from tolerant and physically dependent rats. J Pharmacol Exp Ther. 1990;252:901–907. [PubMed] [Google Scholar]
- 7.Boronat MA, Olmos G, García-Sevilla JA. Attenuation of tolerance to opioid-induced antinociception and protection against morphine-induced decrease of neurofilament proteins by idazoxan and other I2-imidazoline ligands. Br J Pharmacol. 1998;125:175–185. doi: 10.1038/sj.bjp.0702031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaquet PE, Ferrer-Alcón M, Ventayol P, Guimón J, García-Sevilla JA. Acute and chronic effects of morphine and naloxone on the phosphorylation of neurofilament-H proteins in the rat brain. Neurosci Lett. 2001;304:37–40. doi: 10.1016/s0304-3940(01)01729-3. [DOI] [PubMed] [Google Scholar]
- 9.Maharajan P, Prencipe R, Falchetti R, Di Francesco P, Paino G, Maharajan V. Chronic morphine alters calbindin D-28k immunostaining patterns in mouse forebrain. Neurosci Lett. 1998;243:65–68. doi: 10.1016/s0304-3940(98)00065-2. [DOI] [PubMed] [Google Scholar]
- 10.Singhai PC, Kapasi AA, Reddy K, Franki N, Gibbons N, Ding G. Morphine promotes apoptosis in Jurkat cells. J Leukoc Biol. 1999;66:650–658. doi: 10.1002/jlb.66.4.650. [DOI] [PubMed] [Google Scholar]
- 11.Yin D, Mufson RA, Wang R, Shi Y. Fas-mediated cell death promoted by opioids. Nature. 1999;397:218. doi: 10.1038/16612. [DOI] [PubMed] [Google Scholar]
- 12.Yin D, Tuthill D, Mufson RA, Shi Y. Chronic restraint stress promotes lymphocyte apoptosis by modulating CD95 expression. J Exp Med. 2000;191:1423–1428. doi: 10.1084/jem.191.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]