Abstract
Both hypertrophic scars and keloid scars are caused by abnormal wound healing, the key feature of which is excess collagen fiber secretion by fibroblasts. Many different factors could affect the process of hypertrophic scar and keloid formation, but most have not been identified to date. We assume that, during wound healing, melanocytes from the stratum basale contact or interact with fibroblasts from the dermal layers after the basal membrane is damaged, which in turn facilitates fibroblast proliferation and the secretion and deposition of collagen. This plays a significant role in the generation of hypertrophic scars and keloids.
Keywords: Melanocyte, scar, wound healing, fibroblast, keloid
Introduction
One potential result of skin wound healing is the formation of a pathological scar, i.e., a hypertrophic scar or keloid. Pathological scars are one of the most difficult lesions for plastic surgeons to address, and multiple genetic factors (including race and complexion) and individual or environmental factors are considered to contribute to pathological scar formation. There is a relationship between pathological scar formation and skin color, as supported by the following phenomena: (1) the incidence of keloid formation in black people is much higher than that in other races, and five to fifteen times higher than that in white people; (2) patients who suffer from albinism rarely develop keloids; (3) the incidence of pathological scarring varies across different parts of the body even in the same individual; for example, fewer keloids develop in the palm and thenar eminence, where melanocytes are less common; and (4) adolescents and pregnant women, who are subjected to increased hormone secretion and skin pigmentation, are more susceptible to developing keloids [1]. All of these factors indicate that pathological scarring has a close relationship with melanin pigmentation, but only a few studies have addressed this association.
Under normal conditions, melanocytes, which are located in the basal layer of the skin, do not proliferate and do not express cytokines related with themselves. When wounds occur, the damaged epidermal tissue leads to changes in the microenvironment of local skin, inducing the proliferation of melanocytes and the formation of melanin. These cells migrate to the injured area due to the influence of many cytokines [2]. At the same time, the basement membrane is broken when the epidermal layer is injured, and neonatal granulation tissue and proliferative fibroblasts grow upwards to fill the injured area. During this process, fibroblasts contact the melanocytes that are migrating from the basement membrane of the injury border and subsequently begin to proliferate.
As a result, it can be assumed that contact between melanocytes from the epidermis and fibroblasts from the dermis play a significant role in the formation of pathological scars.
Hypothesis
We propose that, during wound healing, melanocytes from the basal layer of the epidermis migrate to the damaged area and encounter the proliferative fibroblasts from the dermis and that this encounter stimulates some action or interaction. Interactions between these cells promote fibroblast proliferation and increase collagen secretion, while activating transforming growth factor-β (TGF-β) signal transduction and enhancing its expression level. Consequently, pathological scarring is induced.
We further assume that the number and activity of melanocytes in the skin play an important role in pathological scar formation. It is well-known that the incidence rate of keloids in black individuals is much higher than that in white individuals. Furthermore, areas on the body surface that contain higher concentrations of melanocytes are much more susceptible to keloid formation than areas with fewer melanocytes. Both of these phenomena can be considered to be related to differences in the number, distribution and activity of the melanocytes in different parts of the skin.
Fibroblasts and TGF-β
It is well known that fibroblasts play a key role in the formation of pathological scars; these cells propagate in a large number, and they facilitate the excretion and deposition of collagen fiber [3,4]. However, the proliferative capacity of the fibroblasts and their collagen secretory activity are regulated by many cytokines, such as growth factors, lymphokines, monokines, colony-stimulating factor, interleukin, and interferon. Among these, transforming growth factor-β is the most significant factor in the literature.
TGF-β plays a crucial role in regulating the wound healing process. This molecule can promote the division and proliferation of fibroblasts, facilitate the synthesis of collagen protein and suppress collagenase activity. Simultaneously, it increases the secretion and deposition of extracellular matrix proteins [5-7]. A considerable number of studies have shown that fibroblasts in hypertrophic scars and keloids contain significantly more TGF-β and TGF-β receptors than normal skin and common fibroblasts [7-9]. The biological effects of TGF-β are regulated by a special intracellular signal transduction pathway. The levels of P-Smad2 and P-Smad3 (P-Smad2/3), which represent the activated pattern of Smad expression, can reflect the degree of TGF-β signal transduction directly [10]. During the formation of pathological scars, both TGF-β and other molecules in its signal transduction pathway are highly expressed and contribute to the formation of pathological scars.
The effect of melanocytes on fibroblasts in wound healing
As mentioned above, the basal membrane of the epidermis is damaged during the wound healing process. After the inflammatory exudation, hyperplasia and renovation stages, fibroblasts proliferate abundantly, and granulation tissue fills the wound and grows upwards. Therefore, fibroblasts could come into contact with melanocytes from the basal layer of the undamaged area, and these cells may affect each other to some extent. This action or interaction could result in scarring, changes in pigmentation, and other phenomena.
How can this Intercellular action and its concrete effects be confirmed? Muriel et al showed that active factors secreted by fibroblasts play a dominant role in the proliferation of melanocytes and in the synthesis and degradation of melanosomes [11]. Meanwhile, Taylor et al found that α-melanocyte-stimulating hormone (α-MSH), which is produced by melanocytes in the skin, could increase the secretion of TGF-β and inhibit the production of IFN-α by activating T cells, thus stimulating the multiplication of fibroblasts [12,13]. Moreover, in vitro studies have confirmed that α-MSH could promote the growth and generation of fibroblasts in a keloid cultured in vitro and stimulate the secretion of TGF-β1.
Taken together, the results of these studies both indicate that melanocytes have a direct impact on fibroblasts. Previous experiments showed that the supernatant of in vitro cultured proliferating melanocytes significantly stimulated proliferation and DNA synthesis in hypertrophic scar fibroblasts [2]. In our experiment, we co-cultured melanocytes and fibroblasts in vitro, keeping the cells in separate chambers of a Millicell culture plate. The base of Millicell culture plate was PET film, which has a the pore size of 1 μm, which allowed a variety of protein molecules and cytokines to be exchanged freely between compartments. The upper chamber contained melanocytes, and the lower chamber contained fibroblasts. We observed the cells after 2, 5, and 8 days in the co-culture system and found that the fibroblasts in co-culture grew and proliferated more quickly than the fibroblasts in the control group. There were some overlaps between cell synapses, and cells typically reached up to 80% confluence within 5 or 8 days. The MTT test showed that the growth and proliferative capacity of co-cultured fibroblasts was higher than that of the control group (Figure 1); i.e., the melanocytes stimulated the growth of the fibroblasts. Furthermore, we collected the co-cultured fibroblasts on the 8th day and measured the mRNA expression of type I collagen and TGF-β1 by RT-PCR. The type I collagen and TGF-β1 levels were significantly higher in co-cultured fibroblasts than in the control group. We also tested the expression of P-Smad2/3 proteins in the co-cultured fibroblasts by immunohistochemical staining (Figure 2). P-Smad2/3 protein staining was significantly enhanced in co-cultured fibroblasts compared to controls (Figure 3). All of these results proved that melanocytes could stimulate fibroblast growth, proliferation, and collagen synthesis, leading to the emergence and development of pathological scarring. The activation of the TGF-β signaling pathway may be the main mechanism underlying this phenomenon.
Figure 1.
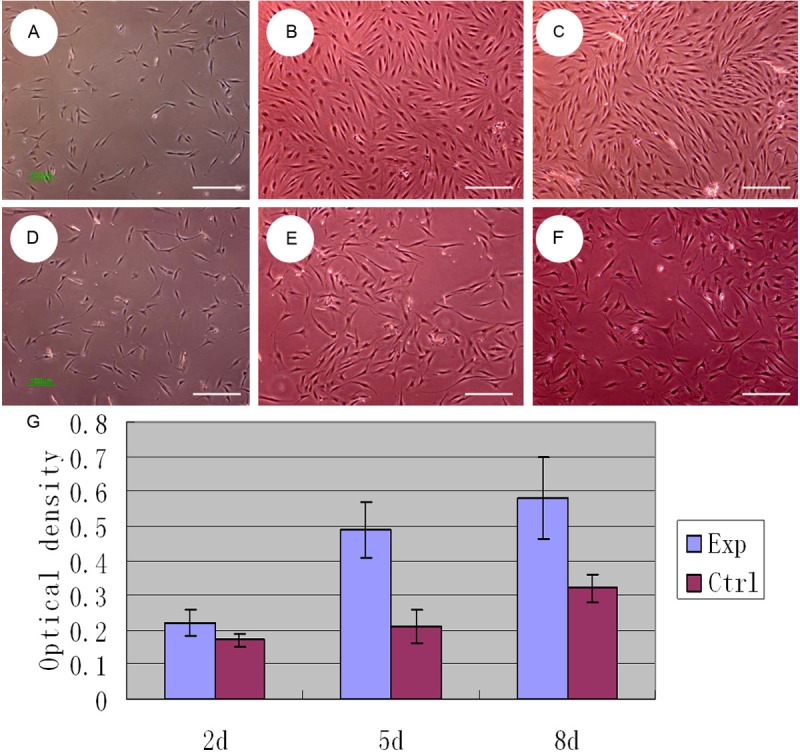
Microscope observation (40×) of fibroblasts co-cultured with melanocytes at 2, 5, 8 days (A-C) and its control group (D-F). G. MTT test showed that the growth and proliferative capacity of co-cultured fibroblasts was higher than that of the control group (fibroblasts alone).
Figure 2.
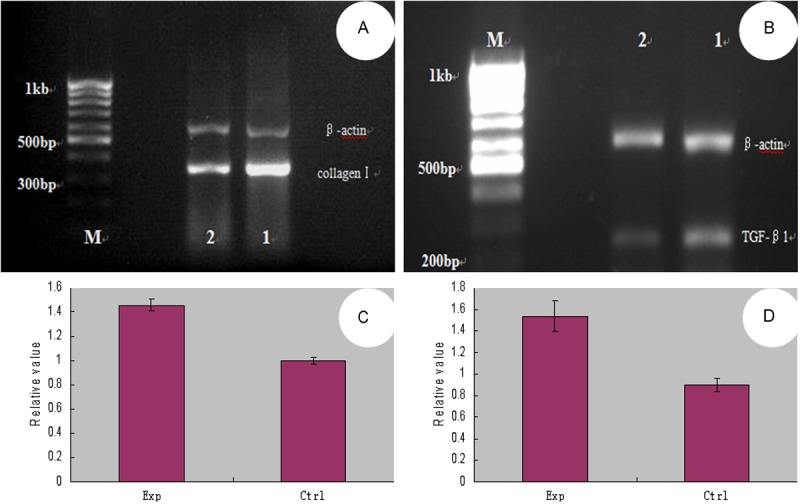
PCR for Collagen I (A) and TGF-β1 (B) mRNA of (M: DNA marker; 1: experimental group; 2: control group); Relative gray scale values of Collagen I (C) and TGF-β1 (D) mRNA showed the growth and proliferative capacity of co-cultured fibroblasts was higher than that of the control group (fibroblasts alone).
Figure 3.
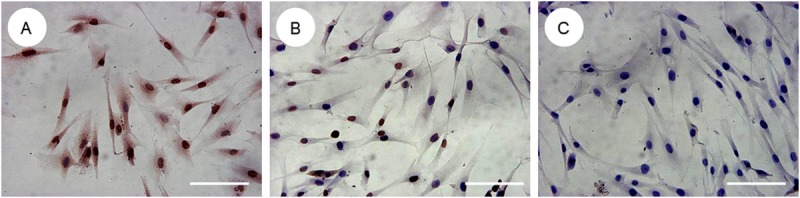
Immunohistochemical study of P-Smad2/3 expression in co-culture fibroblasts (A: Experimental group; B: Control group; C: Blank group). P-Smad2/3 protein staining was significantly enhanced in co-cultured fibroblasts compared to control group (fibroblasts alone).
Conclusions
Melanocytes can stimulate the growth and proliferation of fibroblasts, increase collagen synthesis and extracellular matrix deposition, activate the TGF-β signaling pathway, and promote the development of pathological scarring, dependent on melanocyte number, distribution in the skin, activation status, and other factors. However, more detailed and in-depth experiments will be necessary to clarify and confirm the specific roles played by these factors during the process of scar development.
Acknowledgements
This research is supported by Shanghai Education Funding Council (06BZ021) and National Natural Science Foundation of China (81101438, 81201476).
Disclosure of conflict of interest
The authors have no financial interest in any products, devices, or drugs mentioned in this article.
References
- 1.Ketchum LD, Cohen IK, Maters FW. Hypertrophic scars and keloids: A collective review. Plast Reconstr Surg. 1974;53:140–154. doi: 10.1097/00006534-197402000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Zhang LX, Guo SZ, Wang Z. Biological effects of supernatant from melanocytes culture on proliferation of hypertrophic scar fibroblasts. J Fourth Mil Med Univ. 2000;21:669–670. [Google Scholar]
- 3.Zhang Z, Garron TM, Li XJ, Liu Y, Zhang X, Li YY, Xu WS. Recombinant human decorin inhibits TGF-beta1-induced contraction of collagen lattice by hypertrophic scar fibroblasts. Burns. 2009;35:527–537. doi: 10.1016/j.burns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Qian YL, Yang J, Wang DR. Pingyangmycin in treatment of keloids: a clinical and experimental study. Chinese Journal of Medical Aesthetics and Cosmetology. 2009;15:110–113. [Google Scholar]
- 5.Gao FL, Zhang YG, Wang DR. Advance in blocking TGF-β signaling pathway in different levels. Chinese Journal of Injury Repair and Wound Healing. 2007;2:181–183. [Google Scholar]
- 6.Zhu Q, Li J. A combined candidate therapy for the scar-free repair of cleft lip based on inhibitors of TGF-β. Med Hypotheses. 2011;76:86–88. doi: 10.1016/j.mehy.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Zhang ZF, Zhang YG, Hu DH, Shi JH, Liu JQ, Zhao ZT, Wang HT, Bai XZ, Cai WX, Zhu HY, Tang CW. Smad interacting protein 1 as a regulator of skin fibrosis in pathological scars. Burns. 2011;37:665–672. doi: 10.1016/j.burns.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Wang R, Ghahary A, Shen Q, Scott PG, Roy K, Tredget EE. Hypertrophic scar tissues and fibroblasts produce more transforming growth factor-beta1 mRNA and protein than normal skin and cells. Wound Repair Regen. 2000;8:128–137. doi: 10.1046/j.1524-475x.2000.00128.x. [DOI] [PubMed] [Google Scholar]
- 9.Chin GS, Liu W, Peled Z, Lee TY, Steinbrech DS, Hsu M, Longaker MT. Differential expression of transforming growth factor-beta receptors I and II and activation of Smad 3 in keloid fibroblasts. Plast Reconstr Surg. 2001;108:423–429. doi: 10.1097/00006534-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Lin X, Duan X, Liang YY, Su Y, Wrighton KH, Long J, Hu M, Davis CM, Wang J, Brunicardi FC, Shi Y, Chen YG, Meng A, Feng XH. PPM1A functions as a Smad Phosphatase to Terminate TGF-beta Signaling. Cell. 2006;125:915–928. doi: 10.1016/j.cell.2006.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cario-André M, Pain C, Gauthier Y, Casoli V, Taieb A. In vivo and in vitro evidence of dermal fibroblasts influence on human epidermal pigmentation. Pigment Cell Res. 2006;19:434–442. doi: 10.1111/j.1600-0749.2006.00326.x. [DOI] [PubMed] [Google Scholar]
- 12.Taylor AW, Yee DG, Nishida T, Namba K. Neuropeptide regulation of immunity. The immunosuppressive activity of alpha-melanocyte-stimulating hormone(alpha-MSH) Ann N Y Acad Sci. 2000;917:239–247. doi: 10.1111/j.1749-6632.2000.tb05389.x. [DOI] [PubMed] [Google Scholar]
- 13.Zheng JS, Xing X, Zheng QY, Xue CY. Expression of α-melanocyte-stimulating hormone in keloid-derived fibroblasts and its effects on keloid-derived fibroblasts. Chinese Journal of Practical Aesthetic and Plastic Surgery. 2005;16:68–70. [Google Scholar]


