Abstract
Aims
Elevated intra-abdominal pressure may be a potentially modifiable risk factor for pelvic floor disorders. However, limited evidence exists due to the lack of instruments suitable to measure abdominal pressures in real world settings. The aim of this study was to develop and test a vaginal sensor prototype to measure intra-abdominal pressure in women.
Methods
We developed a non-directional vaginal sensor by housing pressure-sensing circuit boards in 1.2 × 3cm radially symmetric silicon capsules. We characterized the response in a standardized pressure chamber. Eight women wore a sensor intra-vaginally while undergoing filling cystometry. We compared peak pressures during coughing, valsalva, squatting, and jumping to those obtained using a #10 french rectal balloon urodynamics catheter. We calculated Pearson’s correlation coefficients between rectal and vaginal sensors for each event.
Results
The vaginal sensors exhibited linear responses during initial bench testing. Each transducer correlated well with the rectal balloon catheter during coughing, valsalva, and squatting (r= 0.97, 0.94, and 0.97, respectively). However, the rectal balloon catheter recorded higher peak and lower, often negative, trough pressures during jumping. The vaginal sensor showed no such artifact.
Conclusions
This vaginal pressure sensor can be used as a surrogate for measuring intra-abdominal pressure in women without advanced prolapse. By measuring pressure at the physiological source, the vaginal sensor is less prone to extraneous noise and error than current transducers. Using this prototype, we will next develop a remote wireless version to capture a range of abdominal pressures experienced outside of the laboratory setting.
Keywords: pressure transducer, pressure sensor, intra-abdominal pressure, vaginal transducer, physical activity, pelvic organ prolapse, incontinence, pelvic floor disorders
INTRODUCTION
Elevated intra-abdominal pressure (IAP) has been implicated in a host of medical complications, including hernias, severe drops in cardiac output, and post-operative complications such as prolonged healing time and wound dehiscence.(1-3) Many also believe that elevated IAP may cause the onset or recurrence of stress urinary incontinence and pelvic organ prolapse.(4) However, there is little empirical evidence to support these assertions. Given that the lifetime risk of having surgery for either pelvic organ prolapse or urinary incontinence is11.1%(5), a recent National Institutes of Health State of the Science conference emphasized the need for future research to establish the mechanisms of pelvic floor dysfunction.(6) Previous investigations about the effect of elevated IAP on pelvic floor disorders have been hampered by a lack of instruments with which to assess IAP during daily activities.
Currently, IAP can be assessed by placing sensors directly into the abdominal compartment, inferior vena cava, stomach, uterus, bladder, rectum and vagina.(7, 8) These methods are limited by their invasiveness or requirement to maintain strict sensor orientation. Additionally, the sheer bulk of current sensing equipment can limit the natural/fluid movement of the participant. The most commonly used methods, cystometric transducers placed in the bladder, rectum or vagina, are further limited to assessing activities that can be performed within the confines of the laboratory setting. Consequently, the available data about IAP in women relate to activities that can be measured while tethered to a urodynamics machine. These limited data reveal that activities practitioners often restrict after gynecological surgery (e.g. lifting heavy objects) raise IAP less than activities that are not routinely contraindicated (e.g. coughing, valsalva).(9-11) Given the health benefits of exercise in women (12, 13), it is important to understand the impact of all types of physical activity and elevated IAP on the incidence, progression or regression of pelvic floor disorders such that women can be objectively counseled using clinical evidence. However, obtaining future evidence about the impact of physical stressors on pelvic floor disorders relies on our ability to measure the risk factor in question.
The aim of this study was to develop and validate the performance of a prototype for an abdominal pressure sensor that could fit comfortably in the upper vagina. The hypothesis was that by developing this type of transducer, IAP could be measured more effectively during physical activity than current technology permits.
MATERIALS AND METHODS
To develop the prototype the gynecology team (ER, IN) considered the physical design requirements of a pressure transducer, the intended physiologic location, and the available technology. The goal was to design a comfortable, accurate sensor that could be placed in the upper vagina to measure IAP. Accordingly, the following requirements were proposed to the engineering team (RH, PJ): 1) The sensor capsule would sustain supraphysiological pressures to 500 cmH20. 2) The sensor would be able to accurately measure IAPs under dynamic conditions in which pressure changes rapidly. 3) The sensor would be disposable and would not include any components that pose threats to biocompatibility. 4) The shape would be consistent with that of devices known to stay in the vagina without falling out with increased abdominal pressure. 5) The sensor would make accurate readings independent of orientation and the size would be as small as possible. 6) The device would be connected to a monofilament string to make removal as easy as removing a tampon. 7) The device would have a continuous exterior with no holes or crevasses. Finally, the device would be conducive to further development into a self-contained wireless sensor that will accurately measure real-world IAP outside of the laboratory setting for extended periods of time.
The bioengineering team fabricated eight working sensors that met the requirements. The final sensor design was cylindrical and measured 1.2cm in diameter and 3cm in length. (Figure 1) The vaginal sensor was then bench tested. Dynamic frequency responses, including natural frequencies and damping coefficients of both the vaginal sensor and standard rectal balloon catheter, were compared and recorded using a test setup in accordance with industry guidelines (ANSI/AAMI, BP22:1994/(R)2006).. The specific details of the sensor assembly and bench testing are outlined in a separate publication.(14)
Fig. 1.
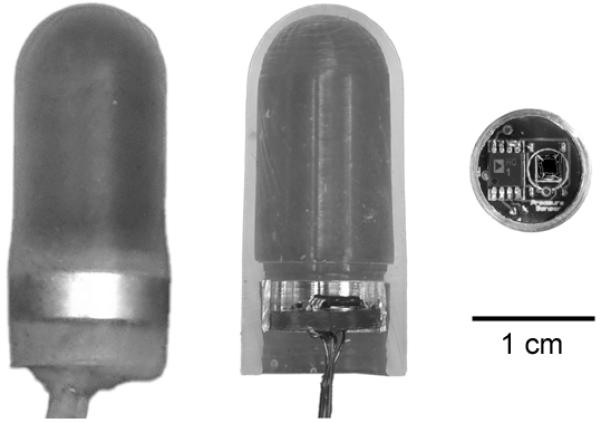
Vaginal pressure sensor. Left: complete sensor, Middle, in cross section, Right, circuit board
After developing the prototype, we designed this prospective study to develop and test the reproducibility of the intravaginal pressure sensor compared to rectal pressure measured during urodynamics testing. The Institutional Board Review at the University of Utah Health Sciences Center approved the study and all participants completed informed consent documents.
Women undergoing routine filling cystometry were recruited for the study. They were aged 21 years or older, with no known vaginal lesions, not menstruating, not pregnant, and had no pelvic organ prolapse beyond 1 cm below the hymeneal ring. In addition, they had no previous pelvic or vaginal surgery other than hysterectomy.
Prior to the study session, each disposable intra-vaginal sensor was characterized for linear response in a calibrated pressure chamber. After uroflowmetry, the urodynamics technician placed a LifeTech #6 french water-filled catheter into the bladder and a #10 french rectal balloon catheter past the internal sphincter to measure intra-abdominal pressure. The rectal catheter balloon was inflated with 5ml of sterile saline. These two catheters were connected per standard practice to a cystometry machine (Aquarius; Laborie Medical Technologies Corp USA, Williston, Vermont). The study physician then placed the intra-vaginal pressure sensor inside the participant’s vagina. If the patient had a uterus, the device was placed in the posterior fornix. If the patient did not have a uterus, the device was placed as proximally as possible at the vaginal cuff. The vaginal device was connected to an Agilent 34970A data acquisition unit which was controlled by a laptop running custom LabView 8.0 data collecting software. Data acquisition included initial voltage readings for zero (atmospheric) pressure that were used to establish sensor offset. The offset values were applied to the pressure transfer function to calculate pressure values.
Routine filling cystometry testing then ensued. The rectal and bladder catheters were zeroed to atmospheric pressure and the performance of the catheters was checked when participants performed a series of coughs. Discrepancies were corrected by either flushing or repositioning the catheters. Filling cystometry was done in the standing position. The bladder was filled with sterile room temperature water at 50ml/minute. Once the bladder was filled to 100ml, the participant was once again instructed to perform a series of coughs to ensure that the catheters were performing optimally. The standard urethral function test procedure then began. At a bladder volume of 200ml, the filling was stopped and the patient was asked to perform a Valsalva maneuver to reach a bladder pressure over 100cmH2O. This was repeated two more times. If no leakage occurred then the bladder was filled to 300ml and the patient was asked to perform three more Valsalva maneuvers. Following filling cystometry, participants were asked to perform three additional tasks with the rectal and vaginal catheters in place. While maintaining a straight back, subjects bent their legs to squat to the ground and return to a standing position. They repeated the squats while lifting 20 pounds. Finally, they jumped in place.
Peak pressures from the rectal and vaginal sensors were plotted using Microsoft Excel. Events were categorized as cough, valsalva, squatting, and jumping. Pearson’s correlation coefficients (r) were calculated between the rectal and vaginal sensors for each group of events. A coefficient of determination (R2) was calculated to explain the variance in the model for combined peak pressures by sensor type.
RESULTS
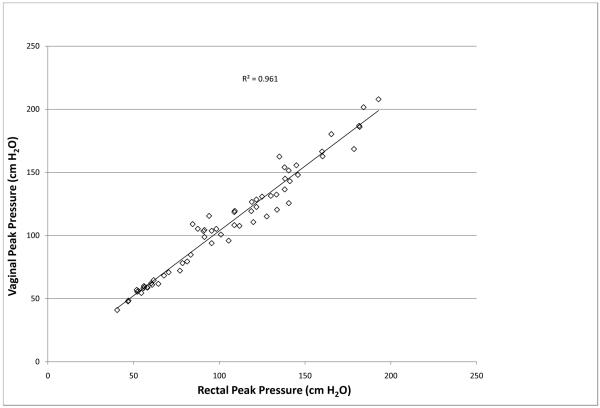
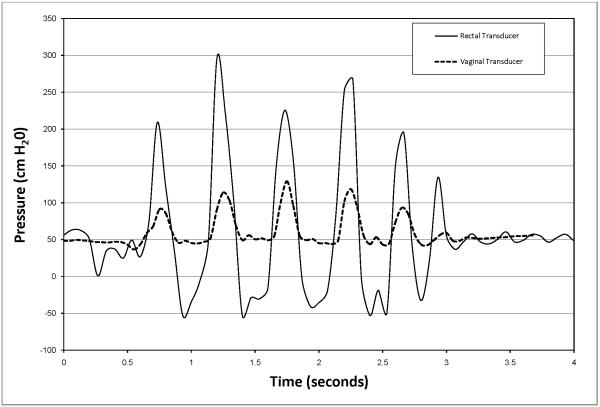
Prior to each clinical study the intra-vaginal pressure sensor displayed a linear response (R=.9996) to pressure changes throughout the range of 0 to 387cmH2O in the standardized pressure chamber. An overshoot of 107% was measured from the balloon catheter’s response to an impulse pressure input. The natural frequency, Fn, of the rectal transducer was calculated to be 17.6 Hz and the damping coefficient, D, was calculated to be 0.01 representing a highly underdamped system. Natural frequencies and damping coefficients for the newly designed vaginal pressure sensor and reference transducer were incalculable, demonstrating no measurable overshoot during both swept sine wave and impulse response tests. Clinically, the intra-vaginal pressure sensor measurements correlated with the urodynamic rectal balloon catheter during coughing (r=0.97), valsalva maneuver (r= 0.94) and squatting with and without weight (r= 0.97). An example of a tracing for an individual participant during valsalva is show in Figure 2. The combined R2 for peak pressures during coughing, valsalva and squatting was 0.961 (Figure 3).The transducers responded differently, however, when the subjects jumped in place. The rectal balloon catheter measured higher peak pressures and lower trough pressures, often decreasing to negative pressures. These differences were noted only during jumping which caused abrupt intra-abdominal pressure changes quicker than 450cmH2O/sec. However, the intravaginal pressure sensor demonstrated fewer artifacts, with positive values throughout events. An example of this difference is shown in Figure 4.
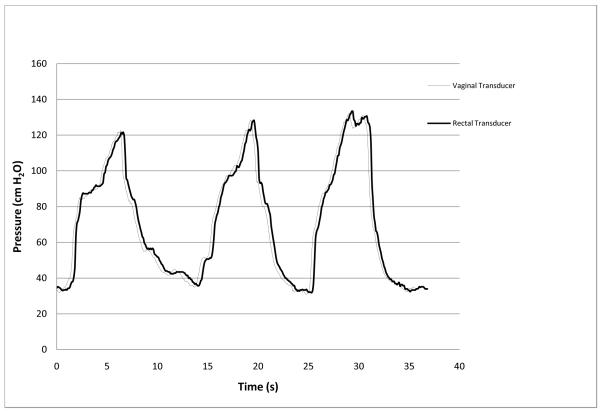
Fig. 2.
Response of vaginal pressure sensor and rectal balloon sensor during three consecutive valsalva maneuvers (from one subject)
Fig. 3.
Combined correlation of vaginal pressure sensor and rectal balloon sensor during coughing, valsalva, and squatting with and without weight
Fig. 4.
Response of vaginal pressure sensor and rectal balloon sensors during 5 consecutive jumps (from one subject)
The vaginal pressure sensor was easily placed in all subjects. The device was retained in participants with stage II or less pelvic organ prolapse. Two women expelled the devices with exertion and were noted to have greater prolapse (Stage III) than appreciated on initial evaluation. All participants stated that the device was comfortable to wear. They also stated that the vaginal sensor was more tolerable to be placed than either the rectal or bladder urodynamic catheters.
DISCUSSION
We have developed a novel intra-vaginal pressure sensor that can be used to monitor intra-abdominal pressure during a variety of physical activities. This vaginal device correlated with the standard rectal balloon catheter when recording activities that generated gradual pressure changes. However, there were significant differences between the two devices when measuring physical activities with abrupt and rapid pressure changes. The rectal transducer exhibited over- and under-shoot responses that are typical of underdamped second-order systems and other fluid-coupled devices.(15) The results of our frequency response testing demonstrate that the intravaginal sensor has superior dynamic response compared to a standard fluid-coupled rectal balloon catheter and does not require the use of a fluid filled catheter line which is known to cause system resonance and noise. (14). Location of the sensor cannot explain the clinical dynamic response differences because balloon catheters measure IAP equivalently when placed in either the rectum or vagina.(16) Placing the vaginal transducer’s microsensor at the physiological source eliminates the need for a fluid-coupled catheter. This reduces the resonance error and permits more accurate IAP readings during high impact activities.
Other commercially available sensors, such as the micro-tip transducer, also measure pressure at the physiological source. However, micro-tip transducers are designed to be placed in fluid filled surroundings. Therefore, IAP can be measured with a micro-tip transducer within the bladder, but the risk of infection and injury are too great to be used during vigorous activities outside a controlled setting. Additionally, micro-tip transducers need to maintain strict sensor orientation for accurate measurements.(17) This precludes IAP measurements during activities where continuous jostling would displace traditional sensors. The microsensor of the vaginal sensor we developed is encased by medical grade compliant silicon. The radially symmetric design allows placement in both fluid and non-fluid filled environments. This permits accurate and continuous IAP measurements within the vagina without concern for sensor orientation.
Study participants universally favored wearing the vaginal device over the rectal and bladder catheters, as others have also found.(18) The vaginal sensor is smaller than standard menstrual tampons. The minimal invasiveness and comfort of this device will allow IAP measurements without hindering participation in daily physical activity. The vaginal sensor has been designed to measure intraabdominal pressure coupled across the vaginal wall and thus necessitates placement in the upper vagina above the pelvic floor. A possible limitation of this study is the assumption that close coupling between the sensor and the inner wall of the vagina is achieved and leads to an accurate reflection of intraabdominal pressure. However, our goal was to compare the new sensor to the current laboratory standard both on the bench and the clinic. Future work on this sensor application will focus on placement and coupling errors.
This study was limited by precluding subjects with advanced pelvic organ prolapse. The device must remain above the pelvic floor musculature for retention. However, this limitation is not felt to hinder the ultimate goal of understanding the impact of strenuous physical activity on the pelvic floor. The sensor is small and situated in the upper vagina and therefore is unlikely to impact continence in any way; we did not test whether leak point pressures differed with or without the sensor in place.
This feasibility study utilized a connecting cable for data acquisition. The tethered design limits range of motion during IAP measurements. However, the results of this study have encouraged us to proceed with designing a wireless version of the implant. Our current sensor capsule can accommodate a small radio frequency transmitter. A Holter-type receiver worn by the subject will replace the current data acquisition unit. This wireless design will permit unencumbered movement during physical activity.
Conclusion
We have successfully designed and tested a novel vaginal pressure sensor that can be used as a surrogate for measuring intra-abdominal pressure in women with normal vaginal support. The vaginal device correlates well with the current standard rectal balloon catheter for measuring IAP during filling cystometry and performs more reliably than the rectal urodynamics catheter during activities that produce rapid IAP changes. By measuring pressure at the physiological source, and without regard to orientation, this sensor overcomes the limitations of standard fluid-filled transducer catheters. The results have encouraged us to begin development of a wireless version of the vaginal sensor which will help to characterize pressures generated during real world activities. We anticipate that this device will ultimately improve our understanding of the role that strenuous physical activity plays in the incidence, progression and recurrence of pelvic floor disorders.
Contributor Information
Evan M Rosenbluth, Department of Obstetrics and Gynecology, University of Utah School of Medicine.
Paul J Johnson, Combined Graduate Student, Department of Mechanical Engineering, University of Utah.
Robert W Hitchcock, Department of Bioengineering, University of Utah.
Ingrid E Nygaard, Department of Obstetrics and Gynecology Urogynecology Division, University of Utah School of Medicine.
References
- 1.Cobb WS, Burns JM, Kercher KW, Matthews BD, James Norton H, Todd Heniford B. Normal intraabdominal pressure in healthy adults. J Surg Res. 2005 Dec;129(2):231–5. doi: 10.1016/j.jss.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Mendoza CB, Jr., Postlethwait RW, Johnson WD. Veterans Administration cooperative study of surgery for duodenal ulcer. II. Incidence of wound disruption following operation. Arch Surg. 1970 Sep;101(3):396–8. doi: 10.1001/archsurg.1970.01340270044012. [DOI] [PubMed] [Google Scholar]
- 3.Makela JT, Kiviniemi H, Juvonen T, Laitinen S. Factors influencing wound dehiscence after midline laparotomy. Am J Surg. 1995 Oct;170(4):387–90. doi: 10.1016/s0002-9610(99)80309-2. [DOI] [PubMed] [Google Scholar]
- 4.O’Dell KK, Morse AN. It’s not all about birth: biomechanics applied to pelvic organ prolapse prevention. J Midwifery Womens Health. 2008 Jan-Feb;53(1):28–36. doi: 10.1016/j.jmwh.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997 Apr;89(4):501–6. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 6.Landefeld CS, Bowers BJ, Feld AD, Hartmann KE, Hoffman E, Ingber MJ, et al. National Institutes of Health state-of-the-science conference statement: prevention of fecal and urinary incontinence in adults. Ann Intern Med. 2008 Mar 18;148(6):449–58. doi: 10.7326/0003-4819-148-6-200803180-00210. [DOI] [PubMed] [Google Scholar]
- 7.James ED, Niblett PG, MacNaughton JA, Shaldon C. The vagina as an alternative to the rectum in measuring abdominal pressure during urodynamic investigations. Br J Urol. 1987 Sep;60(3):212–6. doi: 10.1111/j.1464-410x.1987.tb05485.x. [DOI] [PubMed] [Google Scholar]
- 8.Malbrain ML. Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Intensive Care Med. 2004 Mar;30(3):357–71. doi: 10.1007/s00134-003-2107-2. [DOI] [PubMed] [Google Scholar]
- 9.Guttormson R, Tschirhart J, Boysen D, Martinson K. Are postoperative activity restrictions evidence-based? Am J Surg. 2008 Mar;195(3):401–3. doi: 10.1016/j.amjsurg.2007.12.014. discussion 3-4. [DOI] [PubMed] [Google Scholar]
- 10.O’Dell KK, Morse AN, Crawford SL, Howard A. Vaginal pressure during lifting, floor exercises, jogging, and use of hydraulic exercise machines. Int Urogynecol J Pelvic Floor Dysfunct. 2007 Dec;18(12):1481–9. doi: 10.1007/s00192-007-0387-8. [DOI] [PubMed] [Google Scholar]
- 11.Weir LF, Nygaard IE, Wilken J, Brandt D, Janz KF. Postoperative activity restrictions: any evidence? Obstet Gynecol. 2006 Feb;107(2 Pt 1):305–9. doi: 10.1097/01.AOG.0000197069.57873.d6. [DOI] [PubMed] [Google Scholar]
- 12.Parker ED, Schmitz KH, Jacobs DR, Jr., Dengel DR, Schreiner PJ. Physical activity in young adults and incident hypertension over 15 years of follow-up: the CARDIA study. Am J Public Health. 2007 Apr;97(4):703–9. doi: 10.2105/AJPH.2004.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wessel TR, Arant CB, Olson MB, Johnson BD, Reis SE, Sharaf BL, et al. Relationship of physical fitness vs body mass index with coronary artery disease and cardiovascular events in women. JAMA. 2004 Sep 8;292(10):1179–87. doi: 10.1001/jama.292.10.1179. [DOI] [PubMed] [Google Scholar]
- 14.Johnson P, Rosenbluth E, Nygaard I, Hitchcock R. Development of a novel intra-vaginal transducer with improved dynamic response. doi: 10.1007/s10544-009-9339-z. Submitted to Biomedical Microdevices, decision pending as of 04 30 09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkinson MB, Outram M. Principles of pressure transducers, resonance, damping and frequency response. Anaesthesia & Intensive Care Medicine. 2009;10(2):102–5. [Google Scholar]
- 16.Al-Taher H, Sutherst JR, Richmond DH, Brown MC. Vaginal pressure as an index of intra-abdominal pressure during urodynamic evaluation. Br J Urol. 1987 Jun;59(6):529–32. doi: 10.1111/j.1464-410x.1987.tb04870.x. [DOI] [PubMed] [Google Scholar]
- 17.Anderson RS, Shepherd AM, Feneley RC. Microtransducer urethral profile methodology: variations caused by transducer orientation. J Urol. 1983 Oct;130(4):727–8. doi: 10.1016/s0022-5347(17)51425-1. [DOI] [PubMed] [Google Scholar]
- 18.Dolan LM, Dixon WE, Brown K, Ord T, Hilton P. Randomized comparison of vaginal and rectal measurement of intra-abdominal pressure during subtracted dual-channel cystometry. Urology. 2005 Jun;65(6):1059–63. doi: 10.1016/j.urology.2004.12.025. [DOI] [PubMed] [Google Scholar]