Abstract
QUESTION
A 6-second spirometry test is easier than full exhalations. We compared the reliability of the ratio of the Forced expiratory volume in 1 second/Forced expiratory volume in 6 seconds (FEV1/FEV6) to the ratio of the FEV1/Forced vital capacity (FEV1/FVC) for the detection of airway obstruction.
METHODS
The PLATINO population-based survey in individuals aged 40 years and over designed to estimate the prevalence of post-Bronchodilator airway obstruction repeated for the same study participants after 5–9 years in three Latin-American cities.
RESULTS
Using the FEV1/FVC<Lower limit of normal (LLN) index, COPD prevalence apparently changed from 9.8 to 13.2% in Montevideo, from 9.7 to 6.0% in São Paulo and from 8.5 to 6.6% in Santiago, despite only slight declines in smoking prevalence (from 30.8% to 24.3%). These changes were associated with differences in Forced expiratory time (FET) between the two surveys. In contrast, by using the FEV1/FEV6 to define airway obstruction, the changes in prevalence were smaller: 9.7 to 10.6% in Montevideo, 8.6 to 9.0% in São Paulo, and 7.5 to 7.9% in Santiago. Changes in the prevalence of COPD with criteria based on FEV1/FVC correlated strongly with changes in the FET of the tests (R2 0.92) unlike the prevalence based on a low FEV1/FEV6 (R2 = 0.40).
CONCLUSION
The FEV1/FEV6 is a more reliable index than FEV1/FVC because FVC varies with the duration of the forced exhalation. Reporting FET and FEV1/FEV6<LLN helps to understand differences in prevalence of COPD obtained from FEV1/FVC-derived indices.
Introduction
Accurate determination of the prevalence of Chronic obstructive pulmonary disease (COPD) is needed so that the allocation of health care resources may have the desired impact. Further, to determine whether plans such as smoking cessation programs or therapy are effective in decreasing COPD incidence and prevalence it is necessary to have reliable spirometric indices that can accurately detect the disease state and its changes.
The ratio of Forced expiratory volume in one second (FEV1) to Forced vital capacity (FVC) has been the parameter-of-choice to define the presence of airflow limitation. However, its interpretation has been a matter of intense debate because its ultimate value depends not only on the degree of airflow obstruction but also on the value of the FVC, which in turn is heavily influenced by the duration of the expiratory time. With slow lung emptying, as occurs with aging and especially in individuals with airflow obstruction, FVC is sensitive to the expiratory time: the longer the expiratory time, the larger the FVC and the smaller the FEV1/FVC.
The six-second spirometry has been proposed as a simplified alternative to an FVC maneuver [1]–[7]. Indeed, the ratio of the FEV1/Forced expiratory volume in 6 seconds (FEV6) has been found nearly equivalent to FEV1/FVC for the diagnosis of COPD, but the former is simpler, causes less fatigue, and is possibly more specific than FEV1/FVC [8]. In addition, several inexpensive electronic devices measure FEV1 and FEV6, improving the Peak flow measurement and offering truly low-cost spirometry [9]–[11]. However, fewer reference values are available for FEV6 and FEV1/FEV6 compared with the gold standard FVC and FEV1/FVC [12]–[15].
The quality of spirometry varies among technicians and among centers participating in a collaborative study; thus, changes in personnel over time may influence repeated measurements of the same individuals. To correct for this, one of the components of spirometry quality is the duration of the expiratory maneuvers. Current American Thoracic Society (ATS)/European Respiratory Society (ERS) quality standards for spirometry [16] define a valid expiration as one lasting at least 6 seconds with an end-of-test volume (EOTV) of <25 mL during the final second. Many modern spirometers perform automatic checks for maneuver acceptability and repeatability and provide messages and quality grades. However the spirometer operator is free to ignore these messages. We hypothesized that if the expiratory flow were low, FVC and consequently FEV1/FVC, may differ if the expiration lasts 6, 8, 10, or more seconds especially if emptying has not been complete. Under these circumstances, a 6-second spirometry may be a more stable indicator because individual results are compared at fixed predetermined times.
The purpose of this work was to compare estimates of COPD prevalence based on different indices of airway obstruction across Latin American Project on Pulmonary Obstruction (PLATINO) Study centers and between baseline and follow-up spirometries performed at three of the PLATINO study sites.
Population and Methods
Ethics statement
The study protocol was approved by the Ethics Committee on Research, Pontificial Catholic University of Chile School of Medicine, by the Ethics Committee of the Maciel Hospital in Montevideo Uruguay, and by the Ethics Committee on Research of the Federal University of São Paulo/São Paulo Hospital. Study participants provided signed informed consent.
Details of the selection method and the population sample size of the PLATINO baseline have been previously published [17]. Multistage cluster sampling was used to obtain a representative sample of subjects aged 40 years or over from the metropolitan area of each of the following five large Latin American cities: Montevideo; São Paulo; Santiago; Mexico City, and Caracas.
Study questionnaires are available on the Internet (http://www.platino-alat.org) and a detailed description of the PLATINO study completed questionnaires has been published elsewhere [18]–[21].
Spirometry was performed utilizing the portable, battery-operated, ultrasound EasyOne spirometer (ndd Medical Technologies, Zurich, Switzerland). Spirometry tests were performed at baseline and 15 min after the administration of 200 µg of salbutamol post -Bronchodilation (post-BD), with the goal of meeting American Thoracic Society (ATS) acceptability and repeatability criteria [22]. We employed the definition and the severity stratification of airway obstruction proposed by the Global Initiative for Obstructive Lung Disease (GOLD): a ratio of the post-Bronchodilator (post-BD) FEV1 over FVC<0.70 [23]. We also applied the Lower limit of normal (LLN) criteria [8], [24], [25], defined as the lower 5th percentile for predicted post-BD FEV1/FEV6 and FEV1/FVC utilizing equations derived from the baseline examination of the healthy, never-smoking subset of our cohort [26].
Quality control of spirometry testing was described previously [27], [28], and procedures included in all centers identical spirometers, homogeneous training of the technicians, and review of all tests carried out by one expert (R-P) with weekly quality reports per technician and participating center. A six-category quality grade was assigned to each test according to the number of acceptable maneuvers and to the repeatability of FEV1 and FVC following the ATS criteria. Grade A quality tests had three acceptable maneuvers with the best two FEV1 and FVC within 150 mL [16], [22]; grade B was equivalent to the 1994 ATS criteria, with three acceptable maneuvers with the two best FEV1 and FVC matching within 200 mL; grade C tests had two or three acceptable maneuvers repeatable within 250 mL; grade D tests had 2–3 acceptable maneuvers with poor repeatability; grade E tests had only one acceptable test; and grade F tests had no acceptable maneuvers.
In three cities (Montevideo, Santiago, and São Paulo), a second survey was performed after approximately 5, 6, and 9 years, respectively, on the same individuals recruited for the first evaluation using the same spirometers and techniques. All technicians were urged to obtain grade A spirometries in both evaluations, but technicians differed among the three cities and there was technician turnover between the baseline and the follow-up.
Descriptive analyses included group comparisons using the Pearson χ2 test for nominal variables, the Mann-Whitney U test and ordered logistic regression for ordinal variables, and the Wald test for continuous variables. Linear and logistic regression models were employed to evaluate multivariable relationships.
All analyses were performed using a commercially available statistical software package (Stata v10.0) (StataCorp, College Station, TX, USA) with the survey (svy) commands that consider sampling strategy (cities and basic geostatistical areas).
Results
A total of 2,136 spirometries (2,064 post-BD) were obtained from the 2,201 individuals interviewed in Montevideo, São Paulo, and Santiago and used in the analysis along with 3,021 spirometries (2,942 post-BD) from 3,151 baseline participants. Main anthropometric and post-BD spirometric data, as well as smoking prevalence, history of self-reported asthma, and COPD of these subjects, are described in Table 1. (For the pre-BD spirometry results see Table S1).
Table 1. PLATINO study participants' characteristics and post-BD spirometry results by study center.
| Montevideo, Uruguay | São Paulo, Brazil | Santiago, Chile | ||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| N | 943 | 683 | 1000 | 612 | 1208 | 898 |
| Age (years) | 60.3 (12.7) | 63.5 (12.1) | 55.2 (11.3) | 62.4 (9.9) | 57.0 (12.0) | 62.1 (11.0) |
| Male | 40.3% (37.2; 43.5) | 40.6% (36.9; 44.3) | 44.2% (41.1; 47.3) | 44.1% (40.2; 48.1) | 38.5% (35.7; 41.2) | 36.3% (33.2; 39.5) |
| Obese | 33.8% (30.8; 36.8) | 39.4% (35.7; 43.1) | 25.4% (22.7; 28.1) | 32.7% (29.0; 36.5) | 32.2% (29.5; 34.8) | 33.5% (30.4; 36.6) |
| Height (cm) | 161.0 (10.1) | 160.4 (10.3) | 160.1 (9.5) | 159.9 (10.3) | 159.2 (9.6) | 158.9 (9.5) |
| Weight (kg) | 73.5 (16.6) | 74.8 (15.8) | 70.0 (15.7) | 72.4 (15.6) | 72.2 (14.1) | 72.7 (14.4) |
| BMI (Kg/m2) | 28.3 (5.7) | 29.0 (5.4) | 27.3 (5.6) | 28.5 (8.1) | 28.5 (5.0) | 28.7 (5.2) |
| FEV1 (L) | 2.62 (0.82) | 2.54 (0.80) | 2.68 (0.79) | 2.43 (0.72) | 2.70 (0.79) | 2.52 (0.77) |
| FVC (L) | 3.43 (1.02) | 3.42 (1.05) | 3.44 (0.95) | 3.05 (0.84) | 3.52 (0.97) | 3.27 (0.93) |
| FEV1/FVC (%) | 76.4 (9.0) | 74.8 (9.6) | 78.0 (9.2) | 79.2 (6.9) | 76.6 (8.7) | 76.8 (8.1) |
| FEV6 (L) | 3.30 (0.97) | 3.22 (0.96) | 3.31 (0.90) | 3.02 (0.84) | 3.37 (0.93) | 3.18 (0.90) |
| FEV1 (% pred ) | 96.6 (17.6) | 101.4 (19.3) | 95.1 (18.3) | 95.3 (18.9) | 98.6 (16.5) | 100.6 (18.9) |
| FVC (% pred ) | 99.1 (15.5) | 103.1 (18.3) | 97.7 (17.3) | 91.7 (15.9) | 102.3 (14.1) | 100.2 (16.4) |
| FEV6 (% pred ) | 99.2 (15.5) | 100.9 (16.4) | 97.3 (16.3) | 93.8 (16.3) | 101.7 (14.2) | 100.5 (15.7) |
| FEV1/FEV6 (%) | 79.4 (6.9) | 78.8 (7.0) | 80.7 (7.3) | 80.2 (6.6) | 79.6 (6.7) | 78.9 (6.8) |
| Prevalence of current smoking | 28.0% (25.2; 30.9) | 22.0% (18.9; 25.1) | 23.9% (21.3; 26.6) | 14.8% (12.0; 17.7) | 38.5% (35.7; 41.2) | 32.5% (29.4; 35.6) |
| Pack-years of smoking | 15.8 (25.1) | 14.5 (22.4) | 11.8 (18.7) | 12.8 (20.8) | 9.4 (14.9) | 10.2 (16.9) |
| Self-reported asthma * | 14.0% (11.8; 16.2) | 15.7% (13.0; 18.5) | 10.1% (8.2; 12.0) | 10.4% (8.0; 12.9) | 20.9% (18.6; 23.2) | 19.1% (16.5; 21.6) |
| Self-reported asthma * with current asthma ** | 4.9 (3.5; 6.3) | 5.8 (4.1; 7.6) | 3.1 (2.0; 4.2) | 6.2 (4.3; 8.1) | 5.9 (4.5; 7.2) | 6.0 (4.5; 7.6) |
| Self-reported COPD * | 2.4 (1.5; 3.4) | 3.5 (2.2; 4.9) | 5.3 (3.9; 6.7) | 8.2 (6.0; 10.3) | 6.0 (4.6; 7;3) | 7.3 (5.6; 9.0) |
Asthma and Chronic obstructive pulmonary disease (COPD) diagnosed previously by a physician.
Asthma remains present at the survey time.
Post-BD = tests tests performed after bronchodilator use. Prevalences were estimated taking the study design into account. PLATINO = The Latin American Project of Research on Obstructive Lung Disease; SD = Standard deviation; L = Liters; BMI = Body mass index; FEV1 = Forced expiratory volume in 1 second; FEV6 = Forced expiratory volume in six seconds; FVC = Forced vital capacity. See Text S1 for pre bronchodilator tests.
The prevalence of COPD and post-BD airflow obstruction, by means of several definitions, is described in Table 2, along with spirometry quality criteria including mean Forced expiratory time (FET) and within-test Coefficient of variability (COV) for several spirometry indicators. Spirometry quality varied among the sites during both the baseline and follow-up surveys and decreased during the second survey. The within-test COV for FEV6 was lower than that for FVC, and the within-test COV for FEV1/FEV6 was considerably lower (60%) than that of FEV1/FVC both prior to (pre-BD) and after (post-BD) bronchodilator use.
Table 2. Prevalence (%) of post-BD airway obstruction and indices of spirometry quality by study site.
| Montevideo, Uruguay | São Paulo, Brazil | Santiago, Chile | ||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| FEV1/FVC<0.7 | 19.5 (17.1; 22.0) | 27.5 (24.0; 31.0) | 15.7 (13.4; 17.9) | 8.5 (6.4; 10.6) | 16.2 (14.1; 18.3) | 15.2 (12.6; 17.7) |
| GOLD stages 2–4 | 7.8 (6.0; 9.6) | 8.4 (6.7; 10.0) | 6.1 (4.7; 7.4) | 5.3 (3.4; 7.2) | 5.8 (4.4; 7.2) | 6.0 (4.4; 7.7) |
| FEV1/FVC<LLN | 9.8 (7.9; 11.8) | 13.2 (10.7; 15.6) | 9.7 (7.8; 11.6) | 6.0 (4.0; 7.9) | 8.5 (7.1; 10.1) | 6.6 (5.1; 8.2) |
| FEV1/FEV6<LLN | 9.7 (7.8; 11.7) | 10.6 (8.8; 12.5) | 8.6 (6.9; 10.3) | 9.0 (6.7; 11.2) | 7.5 (6.1; 9.0) | 7.9 (6.3; 9.6) |
| FET [mean (SD)] | 10.4 (3.8) | 11.7 (5.1) | 9.5 (3.3) | 7.2 (1.0) | 10.8 (3.4) | 8.9 (2.1) |
| FET (median, IQR) | 9.7 (7.8; 12.1) | 10.3 (8.2; 13.9) | 9.3 (7.2; 10.8) | 7.0 (6.6; 7.7) | 10.2 (8.4; 12.4) | 8.8 (7.5; 9.9) |
| Quality grade A,B,C post-BD (% of tests) | 97.4 (96.3; 98.5) | 93.9 (91.6; 96.9) | 96.2 (94.8, 97.5) | 92.9 (90.9; 95.0) | 98.9 (98.2; 99.5) | 95.3 (93.7 96.9) |
| Quality grade A, post-BD (% of tests) | 93.0 (91.2; 94.3) | 84.0 (80.8; 87.2) | 83.1 (80.4; 85.6) | 68.8 (65.3; 72.3) | 92.8 (91.2; 94.4) | 81.3 (78.2; 84.3) |
| FET≥6 s (% of tests) | 95.1 (93.7; 96.6) | 95.2 (93.6; 96.8) | 90.5 (88.6; 92.4) | 96.9 (95.5; 98.4) | 98.9 (98.4; 99.5) | 96.3 (95.1; 97.6) |
| COV for FVC, mean (SD) | 1.4 (1.6) | 2.0 (2.0) | 1.9 (2.3) | 1.7 (2.9) | 1.3 (1.5) | 1.4 (3.9) |
| COV for FEV6 ,mean (SD) | 1.4 (1.6) | 1.6 (1.7) | 1.7 (2.1) | 1.6 (2.8) | 1.3 (1.5) | 1.2 (1.1) |
| COV for FEV1/FVC ,mean (SD) | 1.0 (1.3) | 1.3 (1.5) | 1.2 (1.6) | 1.0 (1.4) | 0.9 (0.8) | 1.0 (1.6) |
| COV for FEV1/FEV6 mean (SD) | 0.6 (0.9) | 0.7 (0.8) | 0.7 (0.9) | 0.7 (0.9) | 0.5 (0.5) | 0.6 (2.3) |
FET = Forced expiratory time; Grade A = fulfilling quality criteria by American Thoracic Society/European Respiratory Society (ATS-ERS 2005), three acceptable maneuvers with Forced expiratory volume in one second (FEV)1 and Forced vital capacity (FVC) reproducible to 150 mL; Grade A,B,C = Adequate quality tests by (ATS-ERS 2005): two or three acceptable maneuvers with FEV1 and FVC reproducible to 250 mL. COV = within-test coefficient of variability. LLN = Lower limit of normal according to PLATINO without bronchodilator reference values. See Text S1 for pre-BD results.
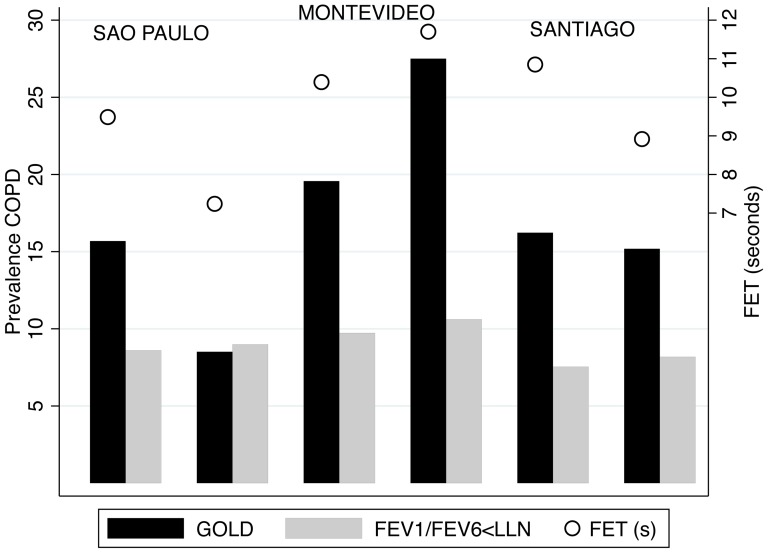
The prevalence of COPD among cities participating in the baseline and longitudinal PLATINO evaluation is shown in Table 2. Using definitions based solely on FEV1/FVC there was an apparently large increase in prevalence in Montevideo, whereas the prevalence appeared to be lower in São Paulo and Santiago. Prevalence correlated well with a longer average Forced expiratory time (FET) in the tests, with shortest mean FET in Sao Paulo and longest in Montevideo (Table 2) (Figure 1).
Figure 1. Prevalence of Chronic obstructive lung disease (COPD) by Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (black bars) based on Forced expiratory volume in one second (FEV1)/Forced vital capacity (FVC)<0.7, and by FEV1/FEV6<Lower limit of normal (LNN) (<5th percentile for FEV1/FEV6, gray bars) in the three cities during baseline and follow-up.
In addition, mean Forced expiratory time (FET) is plotted (right scale, empty circles) for the post-Bronchodilator tests (post-BD). Prevalence of COPD by FEV1/FVC criteria (GOLD) varies directly with Forced expiratory time (FET), unlike prevalence based on FEV1/FEV6. Prevalences based on a FEV1/FVC<LLN also depend to a great extent on FET (not shown).
Analysis restricted to patients in GOLD stages 2–4 shows that prevalence in the first and second evaluations were very similar, whereas employing a FEV1/FEV6<LLN definition, prevalence increased slightly in all three cities and the measurement was independent of mean FET (see Figure 1).
Data analysis by technician and by city shows that the mean FET explained a higher percentage of variability of FEV1/FVC than that of FEV1/FEV6 (complete analysis at baseline, by city and by technician is provided in Tables S2 and S3).
Variability in COPD Prevalence Among Cities
Using definitions based on FEV1/FVC<0.7 leads to considerable variations in COPD prevalence among cities in the baseline study (from 15.7% in São Paulo to 19.5% in Montevideo) and more marked in the follow-up (from 8.5% in São Paulo to 27.5% in Montevideo), similar to what was observed with the FEV1/FVC<LLN (from 8.5% in Santiago to 9.8% in Montevideo at baseline and from 6.0% in São Paulo to 13.2% in Montevideo during follow-up). Variations in prevalence were lower utilizing a GOLD stages 2–4 definition (from 5.8% in Santiago to 7.8% in Montevideo at baseline and from 5.3% in São Paulo to 8.4% in Montevideo during follow-up). The differences were even narrower with the use of the FEV1/FEV6<LLN (from 7.5% in Santiago to 9.7% in Montevideo at baseline and from 7.9% in Santiago to 10.6% in Montevideo) (see Table 2).
Discussion
The PLATINO longitudinal study on the prevalence of COPD in three Latin American cities possessed two important findings: first, it showed that the use of a ratio that fixes the time of exhalation (FEV1/FEV6) is more robust in providing comparisons on COPD prevalence than the fixed FEV1/FVC and the FEV1/FVC using LLN. This is due to the decrease in the variability of results introduced by differences in the duration of expiration after the minimal value of 6 seconds recommended by the ATS/ERS guidelines is reached. Second, using the FEV1/FEV6 criteria there is a stabilization or slight increase in the prevalence of airflow obstruction in the three cities surveyed.
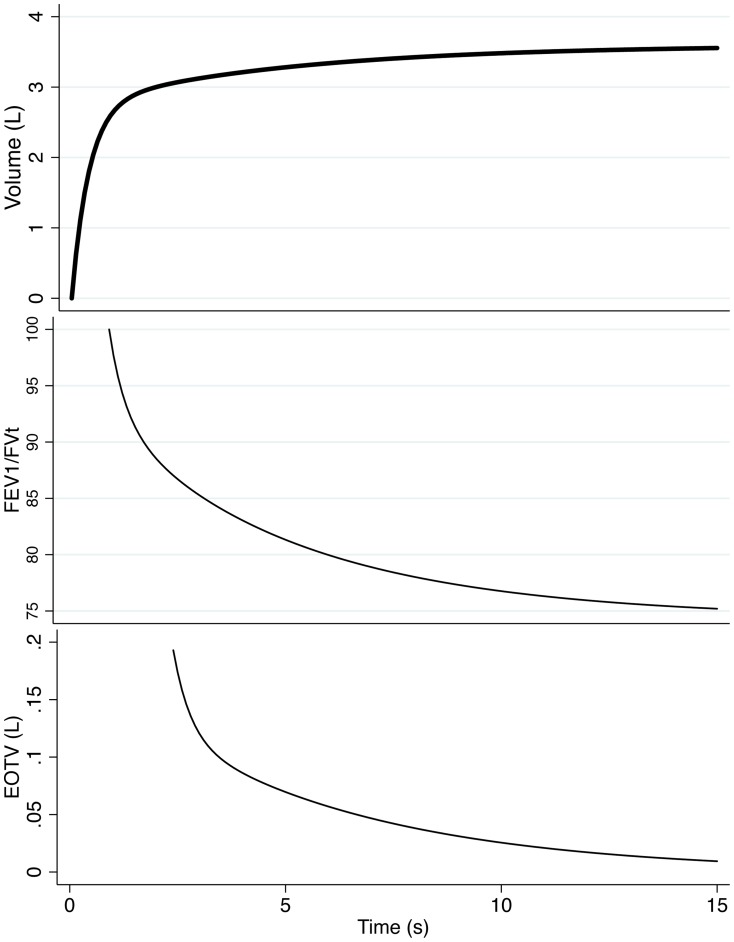
In this longitudinal population study using the same cohort of subjects, we found conflicting prevalence data using criteria derived from FEV1/FVC, the gold standard, with that derived from FEV1/FEV6. Prevalence of COPD based on the fixed ratio (FEV1/FVC<0.7) criteria was higher than that estimated by the FEV1/FVC<LLN or the FEV1/FEV6<LLN criteria, but in addition, during the follow-up survey, this increased significantly in Montevideo (from 19.5 to 27.5%), whereas prevalence in São Paulo apparently decreased from 15.7 to 8.5% and in Santiago, from 16.2 to 15.2%. In a relatively short time, such as that between the two evaluations in the PLATINO Study, the changes in COPD prevalence based on the fixed ratio criteria were unusual and unlikely, and even more so on observing a significant decrease in smoking prevalence in both cities (Montevideo and São Paulo). This discrepancy in results persisted even when using the more specific FEV1/FVC<LLN criteria (see Table 2). As such large changes in the prevalence of a chronic disease are unlikely; heterogeneity in mean FET across participating cities and along time by varying spirometric technique may have caused a spurious change in the recorded prevalence, despite the overall good quality of the spirometric tests (quality grades A,B,C, see Table 2). Several lines of evidence support this explanation: first, by restriction of the analysis to GOLD stages 2–4, a criterion requiring not only a low FEV1/FVC but also a low FEV1. As observed in Table 2, the results did not exhibit the same pattern, but rather tended to decrease the differences observed in the larger sample. When the analysis was repeated using the FEV1/FEV6<LLN criteria for airflow obstruction the prevalence in Montevideo increased from 9.7% to 10.6%, in Santiago from 7.5% to 7.9%, and from 8.6% to 9.0% in São Paulo. In addition, there were no significant changes in self-reported asthma or COPD, which could have accounted for the huge variations in airflow obstruction as estimated by the fixed ratio criteria and FEV1/FVC<LLN (see Table 1). The second evidence is that the rise in prevalence in Montevideo was associated with a significantly longer mean FET in that city, and the decrease in prevalence in Santiago and São Paulo, with a decrease in the FET. A healthy and young lung empties quickly, and in children, the vital capacity is usually expelled in <3 sec. On the other hand, in older individuals and especially in patients with airflow obstruction, complete emptying takes longer and cannot be achieved in a reasonable expiratory time. Therefore, although a longer FET may be the consequence of airflow obstruction, in the same individuals if FET is shorter, FVC would be underestimated, and because airflow obstruction is usually defined by a low FEV1/FVC, obstruction may even disappear spuriously. In the same individuals, if the FET is prolonged due to increased encouragement by technicians during testing, FVC will increase; thus. FEV1/FVC would decrease, leading to more individuals with “airflow obstruction” (see Figure 2).
Figure 2. Volume exhaled as a function of expiratory time (upper graph), FEV1/FEVt (middle graph) and End-of-test volume EOTV (lower graph).
The spirogram is a composite of the PLATINO baseline study based on a bi-exponential fit on individual data (see Text S1). As expiration is prolonged, EOTV decreases as well as the observed FEV1/FVC which increases the likelihood of diagnosing airflow obstruction by any FEV1/FVC-based criteria.
Further support for the use of the FEV1/FEV6 to harmonize results that could be confounded by technical differences is provided by the within-test COV for FEV1/FEV6 and FEV6. These were lower than those for FEV1/FVC and FVC, allowing detection of smaller changes that are important in follow-up studies. FEV1/FVC and FVC are more influenced by the FET than FEV1/FEV6 and FEV6, not only at the individual level but also when the analysis is extended to individual technicians and to one city (see Table R3). In fact, the variability of the mean FEV1/FVC at the city level, critical for estimating prevalence, depends much more on FET than the FEV1/FEV6.
In the first PLATINO evaluation in 93.9% of the post-BD tests, FVC was larger than FEV6, (at follow-up, similar numbers were 96.5%), demonstrating slow emptying due to age and disease. In other words, with an older population, variations in mean FET among technicians and cities, as expected even with good quality control, would produce spurious variations in airflow obstruction prevalence if based on FEV1/FVC to a much greater extent than if based on FEV1/FEV6. Current spirometric end-of testing criteria require a minimum 6-sec expiration, a <25-mL change in volume in the last second or incapacity to exhale further [16]. Even complying with these criteria, thus having a test of good quality, a different FVC would result if expiration were to last for 8 or 11 or more seconds, as observed in Montevideo compared with São Paulo. It is more practical and technically easier and more reliable to compare volume at a fixed expiratory time, as in the 6-sec spirometry.
Individuals with discrepant spirometric airflow obstruction diagnosis, that is having a low FEV1/FVC but normal FEV1/FEV6, often have a high FVC (>120% of predicted) without a low FEV1, unlikely those with a low FEV1/FEV6 and normal FEV1/FVC (see Table S4). This suggests a higher false-positive rate of COPD by low FEV1/FVC than by low FEV1/FEV6.
As shown previously, a clinical diagnosis of COPD has a low sensitivity (20%) and a high rate (67%) of false positives compared to spirometric diagnosis (FEV1/FEV6<LLN), (see Table S5)
An important finding of this study is the relative stabilization of COPD prevalence in the three cities that were re-surveyed. There was a small increase of 0.9% in Montevideo, of 0.4% in Santiago de Chile, and in São Paulo. These results from the same cohort studied at baseline are likely to be true because although a number of patients with severe airflow obstruction died, survivors were now 5–9 years older and one quarter of these had continued to smoke, likely explaining the observed mild increase in prevalence. To our knowledge, this is the first longitudinal study evaluating the prevalence of COPD in a population of stable subjects and the results are interesting in that there appears to be a stabilization in COPD prevalence. On the other hand, these data also suggests that there continues to be a problem of highly prevalent airflow obstruction and that we must increase our efforts to control all of the factors leading to its genesis.
It is now clear that different criteria for confirmation of airflow obstruction lead to varying prevalences of COPD in cross-sectional studies [8], [25]. In addition, it is clear that GOLD stages 1–4 overestimate the real prevalence of COPD, and it is currently common to report GOLD stages 2–4 or to utilize <LLN criteria as a more specific alternative [8], [25]. However, criteria for airflow obstruction may exert an even greater impact on prevalence in longitudinal evaluations, as we show in this study. According to our data, FEV1/FEV6 is a better indicator of airflow obstruction, likely due to comparing volumes at fixed times of the expiratory maneuver and avoiding inconsistencies due to changes in the quality of the spirometries and especially in forced expiratory time across different technicians, centers, or along time. The BOLD and PLATINO studies have found a significant variation in the COPD prevalence in different cities that is even >3-fold, based on GOLD stages 1–4 criteria [29], [30], and part of this variation may be due to changes in the quality of spirometries across centers and especially to variations in mean expiratory time. Re-evaluation of the published prevalences using FEV1/FEV6-based definition or the even more restrictive definition requesting both a FEV1/FEV6<LLN and a FEV1<LLN [25] may result in a harmonization of these seemingly differences in prevalence. In other words, reporting the prevalence of FEV1/FEV6<LLN helps to better understand differences in COPD prevalence obtained from FEV1/FVC-derived indices, especially if mean FET is also reported, in addition to the percentage of spirometric tests fulfilling current ATS-ERS criteria (see also Table S4).
In summary, this longitudinal study shows that the FEV1/FEV6 is a more robust tool to evaluate differences in airflow obstruction prevalence across sites than FEV1/FVC, which has been favored to date and which uses either the <0.7 or LLN criteria. Employing this ratio, the prevalence of COPD appears to have increased slightly over the last 5–9 years in the three cities surveyed. Efforts to control the high prevalence of COPD require re-doubling efforts if we are to decrease the human and economic cost of this disease.
Supporting Information
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
We thank the Asociación Latinoamericana de Tórax (ALAT) for its support of the PLATINO Study. Sonia Buist and William Vollmer were advisors. The PLATINO Executive Committee included Carlos Torres, Juan Luna, Roberto Rodriguez-Roisin, Bartolome Celli, and Carmen Lisboa. The PLATINO team, in addition to main authors, includes María Márquez, María Blanco, Edilberto Pacheco, Fernanda Rosa, Aquiles Camelier, Francisco Franco-Marina, Alexander Corcho, Dolores Moreno, and Julio Pertuzé.
Funding Statement
Funding was provided by ALAT, Boehringer Ingelheim GmbH, Glaxo SmithKline, Novartis. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lundgren FL, Cabral MM, Climaco DC, de Macedo LG, Coelho Mde A, et al. (2007) Determination of the efficacy of FEV6 as a surrogate for FVC in the diagnostic screening for chronic obstructive pulmonary disease through the comparison of FEV1/FVC and FEV1/FEV6 ratios. J Bras Pneumol 33 2: 148–151. [DOI] [PubMed] [Google Scholar]
- 2. Enright RL, Connett JE, Bailey WC (2002) The FEV1/FEV6 predicts lung function decline in adult smokers. Respir Med 96 6: 444–449. [DOI] [PubMed] [Google Scholar]
- 3. Jing JY, Huang TC, Cui W, Xu F, Shen HH (2009) Should FEV1/FEV6 replace FEV1/FVC ratio to detect airway obstruction? A metaanalysis. Chest 135 4: 991–998. [DOI] [PubMed] [Google Scholar]
- 4. Lamprecht B, Schirnhofer L, Tiefenbacher F, Kaiser B, Buist SA, et al. (2007) Six-second spirometry for detection of airway obstruction: a population-based study in Austria. Am J Respir Crit Care Med 176 5: 460–464. [DOI] [PubMed] [Google Scholar]
- 5. Melbye H, Medbo A, Crockett A (2006) The FEV1/FEV6 ratio is a good substitute for the FEV1/FVC ratio in the elderly. Primary care respiratory journal : journal of the General Practice Airways Group 15 5: 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosa FW, Perez-Padilla R, Camelier A, Nascimento OA, Menezes AM, et al. (2007) Efficacy of the FEV1/FEV6 ratio compared to the FEV1/FVC ratio for the diagnosis of airway obstruction in subjects aged 40 years or over. Braz J Med Biol Res 40 12: 1615–1621. [DOI] [PubMed] [Google Scholar]
- 7. Vandevoorde J, Verbanck S, Schuermans D, Kartounian J, Vincken W (2005) FEV1/FEV6 and FEV6 as an alternative for FEV1/FVC and FVC in the spirometric detection of airway obstruction and restriction. Chest 127 5: 1560–1564. [DOI] [PubMed] [Google Scholar]
- 8. Perez-Padilla R, Hallal PC, Vazquez-Garcia JC, Muino A, Maquez M, et al. (2007) Impact of bronchodilator use on the prevalence of COPD in population-based samples. COPD 4 2: 113–120. [DOI] [PubMed] [Google Scholar]
- 9. Frith P, Crockett A, Beilby J, Marshall D, Attewell R, et al. (2011) Simplified COPD screening: validation of the PiKo-6(R) in primary care. Primary care respiratory journal : journal of the General Practice Airways Group 20 2: 190–198, 192 p following 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sichletidis L, Spyratos D, Papaioannou M, Chloros D, Tsiotsios A, et al. (2011) A combination of the IPAG questionnaire and PiKo-6(R) flow meter is a valuable screening tool for COPD in the primary care setting. Primary care respiratory journal : journal of the General Practice Airways Group 20 2: 184–189, 181 p following 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaufmann M, Hartl S, Geyer K, Breyer MK, Burghuber OC (2009) Measuring FEV(6) for detecting early airway obstruction in the primary care setting. Quality and utility of the new PiKo-6 device. Respiration 78 2: 161–167. [DOI] [PubMed] [Google Scholar]
- 12. Hankinson JL, Crapo RO, Jensen RL (2003) Spirometric reference values for the 6-s FVC maneuver. Chest 124 5: 1805–1811. [DOI] [PubMed] [Google Scholar]
- 13. Hankinson JL, Odencrantz JR, Fedan KB (1999) Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 159 1: 179–187. [DOI] [PubMed] [Google Scholar]
- 14. Pereira CA, Sato T, Rodrigues SC (2007) New reference values for forced spirometry in white adults in Brazil. J Bras Pneumol 33 4: 397–406. [DOI] [PubMed] [Google Scholar]
- 15. Perez-Padilla R, Valdivia G, Muino A, Lopez MV, Marquez MN, et al. (2006) Spirometric reference values in 5 large Latin American cities for subjects aged 40 years or over. Arch Bronconeumol 42 7: 317–325. [DOI] [PubMed] [Google Scholar]
- 16. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. (2005) Standardisation of spirometry. Eur Respir J 26 2: 319–338. [DOI] [PubMed] [Google Scholar]
- 17. Menezes AM, Victora CG, Perez-Padilla R (2004) The Platino project: methodology of a multicenter prevalence survey of chronic obstructive pulmonary disease in major Latin American cities. BMC Med Res Methodol 4 1: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montes de Oca M, Talamo C, Halbert RJ, Perez-Padilla R, Lopez MV, et al. (2009) Frequency of self-reported COPD exacerbation and airflow obstruction in five Latin American cities: the Proyecto Latinoamericano de Investigacion en Obstruccion Pulmonar (PLATINO) study. Chest 136 1: 71–78. [DOI] [PubMed] [Google Scholar]
- 19. Montes de Oca M, Talamo C, Halbert RJ, Perez-Padilla R, Lopez MV, et al. (2009) Health status perception and airflow obstruction in five Latin American cities: the PLATINO study. Respir Med 103 9: 1376–1382. [DOI] [PubMed] [Google Scholar]
- 20. Montes de Oca M, Halbert RJ, Lopez MV, Perez-Padilla R, Talamo C, et al. (2012) Chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J [DOI] [PubMed] [Google Scholar]
- 21. Lopez Varela MV, Montes de Oca M, Halbert RJ, Muino A, Perez-Padilla R, et al. (2010) Sex-related differences in COPD in five Latin American cities: the PLATINO study. Eur Respir J 36 5: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 22. American Thoracic Society (1995) Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med 152 3: 1107–1136. [DOI] [PubMed] [Google Scholar]
- 23. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, et al. (2007) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176 6: 532–555. [DOI] [PubMed] [Google Scholar]
- 24. Hardie JA, Buist AS, Vollmer WM, Ellingsen I, Bakke PS, et al. (2002) Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J 20 5: 1117–1122. [DOI] [PubMed] [Google Scholar]
- 25. Vollmer WM, Gislason T, Burney P, Enright PL, Gulsvik A, et al. (2009) Comparison of spirometry criteria for the diagnosis of COPD: results from the BOLD study. Eur Respir J 34 3: 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perez-Padilla R, Valdivia G, Muino A, Lopez MV, Marquez MN, et al. (2006) Spirometric Reference Values in 5 Large Latin American Cities for Subjects Aged 40 Years or Over. Arch Bronconeumol 42 7: 317–325. [DOI] [PubMed] [Google Scholar]
- 27. Perez-Padilla R, Vazquez-Garcia JC, Marquez MN, Menezes AM (2008) Spirometry quality-control strategies in a multinational study of the prevalence of chronic obstructive pulmonary disease. Respir Care 53 8: 1019–1026. [PubMed] [Google Scholar]
- 28. Perez-Padilla R, Vazquez-Garcia JC, Marquez MN, Jardim JR, Pertuze J, et al. (2006) The long-term stability of portable spirometers used in a multinational study of the prevalence of chronic obstructive pulmonary disease. Respir Care 51 10: 1167–1171. [PubMed] [Google Scholar]
- 29. Menezes AM, Perez-Padilla R, Jardim JR, Muino A, Lopez MV, et al. (2005) Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet 366 9500: 1875–1881. [DOI] [PubMed] [Google Scholar]
- 30. Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, et al. (2007) International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 370 9589: 741–750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)