Abstract
In addition to lipid kinase activity, the class-I PI 3-kinases also function as protein kinases targeting regulatory autophosphorylation sites and exogenous substrates. The latter include a recently identified regulatory phosphorylation of the GM-CSF/IL-3 βc receptor contributing to survival of acute myeloid leukaemia cells. Previous studies suggested differences in the protein kinase activity of the 4 isoforms of class-I PI 3-kinase so we compared the ability of all class-I PI 3-kinases and 2 common oncogenic mutants to autophosphorylate, and to phosphorylate an intracellular fragment of the GM-CSF/IL-3 βc receptor (βic). We find p110α, p110β and p110γ all phosphorylate βic but p110δ is much less effective. The two most common oncogenic mutants of p110α, H1047R and E545K have stronger protein kinase activity than wildtype p110α, both in terms of autophosphorylation and towards βic. Importantly, the lipid kinase activity of the oncogenic mutants is still inhibited by autophosphorylation to a similar extent as wildtype p110α. Previous evidence indicates the protein kinase activity of p110α is Mn2+ dependent, casting doubt over its role in vivo. However, we show that the oncogenic mutants of p110α plus p110β and p110γ all display significant activity in the presence of Mg2+. Furthermore we demonstrate that some small molecule inhibitors of p110α lipid kinase activity (PIK-75 and A66) are equally effective against the protein kinase activity, but other inhibitors (e.g. wortmannin and TGX221) show different patterns of inhibition against the lipid and protein kinases activities. These findings have implications for the function of PI 3-kinase, especially in tumours carrying p110α mutations.
Introduction
The class 1 phosphoinositide 3-kinases (PI 3-kinases) play a critical role in pathways regulating functions such as cell metabolism, cell growth and survival, cytoskeletal rearrangements and cell movement [1], [2]. The class 1a PI 3-kinases are heterodimers consisting of a regulatory (or adapter) subunit (most commonly p85α, but also p85β, p55α, p50α or p55γ) coupled to a 110 kDa catalytic subunit (p110α, β, or δ). The class 1b PI3-K is also a dimer composed of a regulatory subunit (p101, p84 or p87PIKAP) coupled to the catalytic subunit (p110γ) [3]–[5]. Recently a range of oncogenic mutations have been identified in PIK3CA (p110α) and PIK3R1 (p85α) [6], [7] and these result in elevation of the lipid kinase activity [8], [9].
The PI 3-kinases are named for their lipid kinase activity, phosphorylating the 3′ position of the inositol ring in phosphatidylinositol (PtdIns) lipids and the consequences of this activity are well defined [10]–[12]. However PI 3-kinases are also known to possess protein kinase activity, with the ability to phosphorylate their own subunits [13], [14]. Evidence has been presented that the intrinsic phosphorylation of PI 3-kinase on Ser608 of the regulatory p85α subunit represents a form of negative feedback regulation [14]. This phosphorylation of Ser608 is stimulated by class 1a agonists [14], [15] and yet was observed to result in a dose-dependent decrease in PI 3-K lipid kinase activity [13], [14], an effect which was reversible by treatment with protein phosphatase 2A [13], [14] and alkaline phosphatase [13]. Studies with recombinant forms of PI 3-kinase indicate that there are differences in the protein kinase activity of different isoforms, for example 110α has a greater ability to phosphorylate p85 than p110β does [15]. Very little is known about the effect of oncogenic mutations in p110α on protein kinase activity, except that they retain the ability to phosphorylate Ser608 in p85α [8]. However, there has not been any detailed side-by-side comparison of the protein kinase activity of all PI 3-kinase isoforms, nor the susceptibility of this activity to different inhibitors. Interestingly some small molecules have been shown to differentially inhibit the lipid and protein kinase activities of PI 3-kinases [16], [17] raising the possibility that some of the new drugs being developed to target PI 3-kinases may do the same.
Exogenous targets of the protein kinase activity have also been identified. These include IRS-1 [18]–[20], MEK-1 [21], PDE3B [22] and 4EBP1 and H-Ras [15]. In most cases the functional consequences of these phosphorylation events have not been investigated but a recent study has provided evidence that phosphorylation of the GM-CSF/IL-3 βc receptor by p110α is functionally important in regulating cell survival in acute myeloid leukaemia cells [23]. This highlights the need to better understand the nature of the PI 3-kinase protein kinase activity towards exogenous substrates since it may play a role in normal cell regulation and/or tumourogenesis.
One argument against an in vivo role for the protein kinase activity of PI 3-kinase is that some studies to date have indicated it is manganese rather than magnesium dependent [13]–[15] and while magnesium is the most abundant divalent cation in cells [24], manganese is only present as a trace element [25].
Therefore to better understand the protein kinase activity of PI 3-kinase we have undertaken a comparison of the relative protein kinase activities of all the Class I PI 3-kinases as well as two common p110α oncogenic mutants (H1047R and E545K). These studies compared both the autophosphorylation and the exogenous kinase activity towards βic. Activities were determined in the presence of either Mn2+ or Mg2+ and we have also compared the effects on protein kinase activity of a range of known PI 3-kinase lipid kinase inhibitors.
Our studies provide the first evidence that oncogenic mutations of the p110α isoform of PI 3-kinase cause an upregulation of its protein kinase activity under physiologically relevant conditions. We describe distinct differences between wildtype and mutant p110α in relation to both the levels of p85α and p110 phosphorylation in buffers containing physiologically relevant Mg2+ concentrations, and the resulting impact on lipid kinase. We go on to show that the oncogenic forms of p110α also have increased protein kinase activity towards an exogenous substrate (βic). We further describe the protein kinase activity of the remaining Class I isoforms, elucidating the effects that this phosphorylation has on lipid kinase activity. These studies provide evidence that the protein kinase activity of class-I PI 3-kinase is capable of playing an important regulatory role in the cell and may contribute to the oncogenic potential of mutant forms of PI 3-kinase.
Materials and Methods
Recombinant PI 3-Kinase Synthesis
All Class 1a isoforms and mutants were produced in-house by co-expressing full-length human p85α with the indicated human full-length catalytic subunit in Sf9 cells infected with a recombinant baculovirus containing coding sequences for both the p85α (p85α; Genbank accession NM_181523) and Class 1a p110 subunits (p110α, Genbank accession NM_006218; p110β, NM_006219; p110δ, NM_005026) or Class 1b p110 subunit only (p110γ, NM_002649).
Site directed mutagenesis of p110α to yield the oncogenic mutants was performed by using either complementary (overlapping sense and antisense) oligonucleotides containing sequence mismatches incorporating the desired point mutation, or back to back phosphorylated primers spanning the region to be mutated (with one primer containing the desired point mutation). For both methods resultant plasmids were sequenced to confirm the insertion of the desired mutations prior to generation of recombinant baculovirus. All p110 constructs (wildtype and mutant) contain an N-His6 rTEV tag used to purify the complex by IMAC before final purification by anion exchange on MonoQ column. The N-His6-tag was removed by overnight cleavage with rTEV at 4°C, as this has been previously shown to impact protein kinase activity [26], [27].
Recombinant βic Production
Production and purification of the histidine-tagged recombinant βic protein encompassing amino acids 445-881 of the intracellular domain of GM-CSF/IL-3 βc has been previously described [23], [28].
Inhibitors
Wortmannin and LY294002 were from Sigma-Aldrich (St Louis, USA); TGX-221 was from Symansis (Auckland, NZ); PIK-75, A66 and AS252424 were synthesized in-house as previously described [29], [30].
Protein Kinase Assays
Unless otherwise stated, protein kinase assays were carried out in a buffer containing 50 mM NaCl, 20 mM Tris/Cl (pH 7.4), 0.1 mM Na-orthovanadate, 12 µM ATP, 5 mM DTT, 2 µCi γ 33P-ATP, and either 5 mM MgCl2,or 5 mM MnCl2 or both (as stated); Each reaction tube contained 0.5 µg kinase, 0.5 µg βic and inhibitors at stated concentrations. Unless otherwise stated, incubations were allowed to proceed for 20 minutes at 32°C and terminated by the addition of 5x electrophoresis sample buffer before complete denaturation at 99°C for 5 min. Components were separated by SDS PAGE, Coomassie-stained, dried and analysed by autoradiography (Molecular Dynamics Storm 680 PhosphorImager and quantified using ImageQuantTL software).
PP2A Treatment
Where stated, kinases were dephosphorylated using the catalytic portion of PP2A (Sigma P1618 from bovine kidney); 1 μg of kinase was incubated with 0.3 U of PP2A for 15 minutes at 30°C in a buffer containing 4 mM CaCl2, 10 mM MgCl2, 5 mM MnCl2, 50 mM NaCl, 20 mM Tris/Cl (pH 7.4) and 5 mM DTT.
Lipid Kinase Assays
To verify the impact of phosphorylation on lipid kinase activity, kinases were either pretreated with ATP (phosphorylated) or PP2A (unphosphorylated) before determining lipid kinase activity using phosphoinositol (PI) as a substrate. More specifically, kinases were either treated according to the Protein Kinase Assay (for 1 hour at 37°C without γ33P-ATP) or PP2A Treatment methods outlined above before the addition of EDTA to a final concentration of 2 mM EDTA; 10 μL of kinase (equivalent to 0.5 μg) was mixed with 90 μL buffer containing 40 mM Tris/Cl, 200 mM NaCl, 1 mM EDTA (pH 7.4). Each reaction point consisted of 20 μL of this kinase mixed with 10 μL of 1 mg/mL PI (Lipid Products, Surrey UK) in 10 mM Tris/Cl, 1 mM EDTA (pH 7.4), and 30 μL of ATP mix (10 mM MgCl2, 200 μM ATP, 1 μCi γ33P-ATP). The reaction was allowed to proceed for 1 hour at room temperature and stopped with 100 μL of 1 M HCl, before chloroform lipid extraction as previous (Method 2 described in [31]) with minor alterations described here. Specifically the re-extraction buffer consisted of 50:50 methanol/1 M HCl and the dried lipid was resuspended in 30 μL of chloroform: methanol (4:1 v/v). TLC plates were pre-treated with a solution containing 8 mM oxalic acid and 1 mM EDTA (pH 8) in MQ H2O/ethanol (3:1 v/v), and allowed to dry at room temperature overnight. Lipids were separated on the TLC plates using propan-1-ol/glacial acetic acid/MQ H2O (65:4:31 v/v). Assay results were analysed by autoradiography (Molecular Dynamics Storm 680 PhosphorImager and quantified using ImageQuantTL software).
Lipid Kinase IC50 Determination
IC50 values were determined using the PI3K (human) HTRF Assay (Millipore, #33-016). All PI 3-K isoforms were made in-house and used in the range of their EC65–80 titration (18 ng/mL for H1047R, 6.5 ng/mL for E545K, 50 ng/mL for p110α, 400 ng/mL for p110β, 65 ng/ml for p110δ and 400 ng/mL for p110γ). Drugs were dissolved in DMSO and serially diluted in the same. Final DMSO concentration in assay was 2.5%.
Results
Characterisation of Lipid Kinase Activity in the Presence and Absence of PI 3-kinase Autophosphorylation
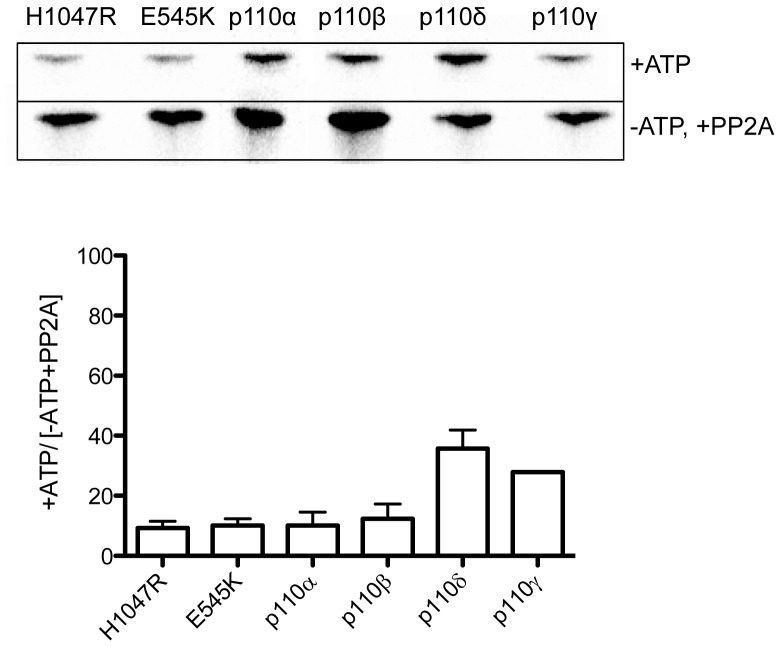
Previous studies using endogenous purified proteins have shown that phosphorylation of class-I PI 3-K reduces the lipid kinase activity of all isoforms except p110γ [13]–[15], [32], [33], although more recently a study comparing wildtype and mutant p110α reported that p85α phosphorylation had no impact on lipid kinase activity [34]. Here we have performed a comprehensive side-by-side comparison of all Class-I PI 3-kinases as well as two common oncogenic mutants of p110α ( Figure 1 ). Importantly the presence of epitope tags is known to affect the activity of recombinant PI 3-kinases [26], [27] so our recombinant proteins have the tag cleaved to minimize any such effects. Since it has been reported that there are pre-existing high levels of phosphorylation in the Sf9-produced recombinant protein [35], we first pre-treated the kinase with protein phosphatase 2A, and then carried out incubations in the presence and absence of ATP. These experiments showed a clear effect of protein phosphorylation on lipid kinase activity ( Figure 1 ), with wildtype and mutant p110α and p110β exhibiting >80% knockdown in lipid kinase activity with phosphorylation, while p110δ and γ were less impacted. While these results contrast to the recent report of Layton et al (2012) [34], the results for p110α and p110β are in agreement with previous findings [13], [14], [33]. The moderate 60% knockdown of p110δ lipid kinase activity varies somewhat from previous findings [32] which demonstrated >90% knockdown of lipid kinase activity with autophosphorylation. Moreover previous studies suggested there would be no change in lipid kinase activity for p110γ following autophosphorylation [36], [37], and while p110γ was less impacted than p110α and β, in our hands it was still reduced to less than 40% activity. Importantly we find that the lipid kinase activity of p110α mutants is reduced following autophosphorylation by the same degree as wildtype p110α ( Figure 1 ).
Figure 1. Effects of protein phosphorylation on lipid kinase conversion of PI to PI3P.
Representative autoradiographs and graphs showing the level of 33P incorporated into PI3P in the presence and absence of ATP and PP2A (N≥2).
Divalent Cation Dependence
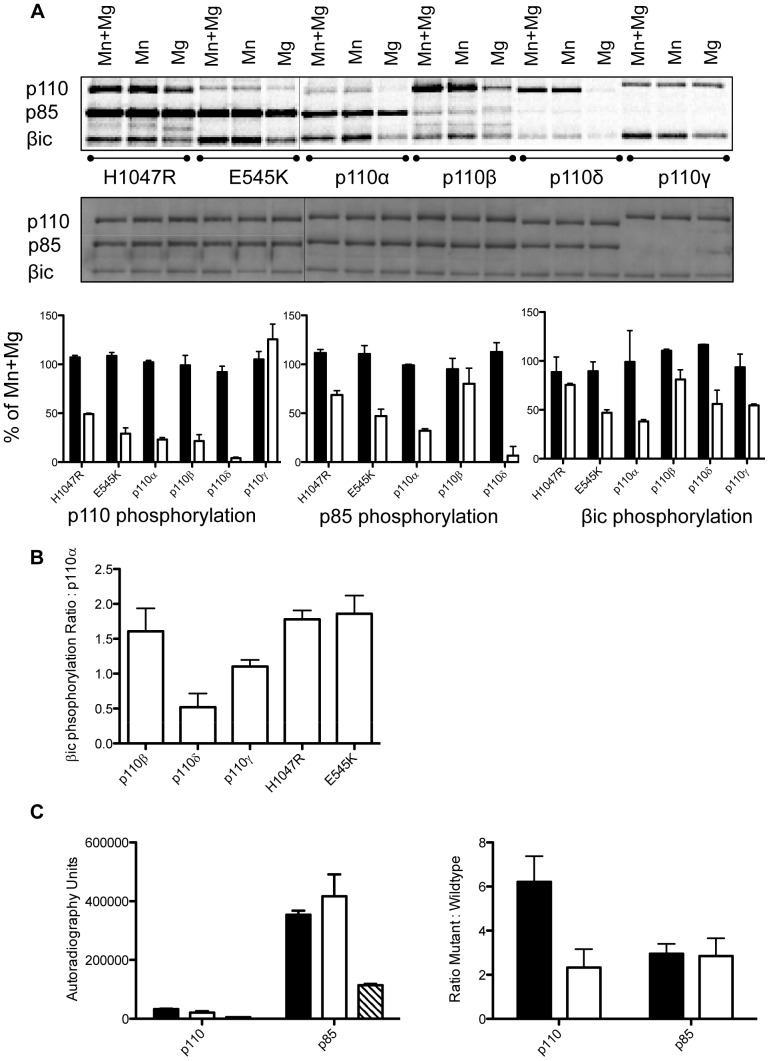
One of the earliest reports of the protein kinase activity of PI 3-kinase showed that this activity was dependent on Mn2+ [14], and this was reiterated by Dhand et al. (1994) [13]. While subsequent studies have been carried out comparing the Mn/Mg dependent autophosphorylation of p110α and p110δ [32] and p110α and p110β [33], a comprehensive investigation of all the isoforms has never been completed. Furthermore, no studies have been carried out to test the ability of different PI 3-kinase isoforms to phosphorylate the exogenous substrate βic. Therefore we compared the protein kinase activities of all the isoforms and the two oncogenic mutants in the presence of Mn2+, Mg2+ or both ( Figure 2A ).
Figure 2. Effects of divalent cation on protein kinase activity.
A) Representative autoradiograph scan showing relative intensity of phosphorylation in the presence of Mn2+ and Mg2+, Mn2+ alone, and Mg2+ alone. Graphs summarise the results of N = 5 individual experiments for Manganese (black bars) and Magnesium (white bars). B) Graph showing the abilities of different isoforms to phosphorylate βic, relative to p110α, N≥3 experiments. C) Graphs comparing the degree of autophosphorylation for the two oncogenic mutants, H1047R (black bars) and E545K (white bars) to p110α (hatched bars), N≥3 experiments.
We observe autophosphorylation of p110 subunit by p110α wildtype and mutants, and p110β in the presence of Mg2+, albeit much reduced in comparison to kinase incubated with Mn2+, while p110γ retained 100% activity in Mg2+ only buffer. We only observe significant p85 phosphorylation in the proteins containing wildtype or oncogenic p110α ( Figure 2A ). Of particular interest, phosphorylation of the exogenous substrate βic was observed in the presence of Mg2+ for all isoforms but with the relative level of phosphorylation being different for the different isoforms. p110β and the oncogenic mutants exhibited the most activity (approaching 2-fold more activity than p110α), while p110 γ and p110α had equal activity, and p110δ very little ( Figure 2A and 2B ). The largest observable difference between the two oncogenic forms of PI 3-kinase was that autophosphorylation of p110 remained strong in the presence of Mg2+ for H1047R mutant but was not present in the E545K mutant and wildtype p110α ( Figure 2C ). The significance of this remains to be elucidated.
Lipid Kinase Inhibitors also Inhibit the Protein Kinase Activity of PI 3-kinase
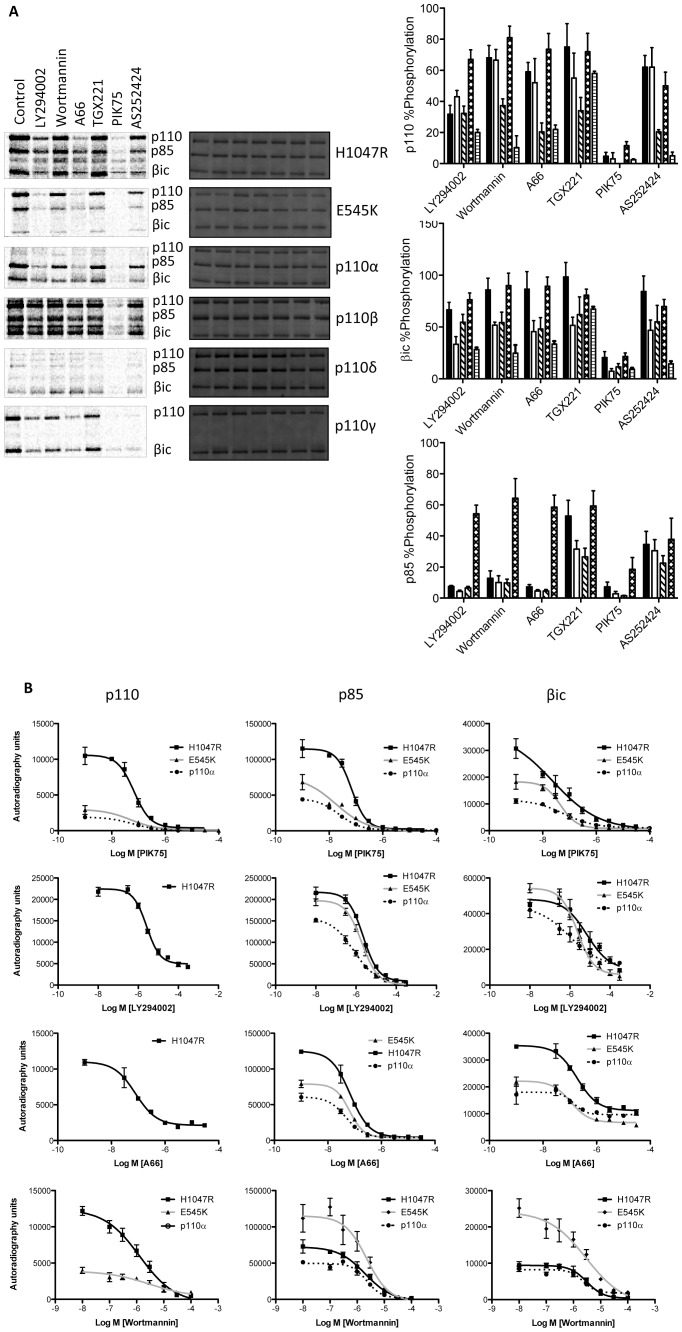
We also tested the effect of different small molecules known to inhibit the lipid kinase activity of PI 3-kinases. We first challenged all the isoforms and mutants with a fixed concentration of the pan-specific inhibitors LY294002 and wortmannin, p110α-specific inhibitors A66 and PIK-75, the p110β-specific inhibitor TGX221, and the p110γ inhibitor AS252424. All inhibitors were initially screened at a final concentration of 10 μM except LY294002, which was used at a final concentration of 100 μM. A66 and AS252424 follow the same pattern of isoform selectivity as seen in their lipid kinase activities ( Figure 3A ). Interestingly p110β protein kinase activity was largely resistant to all inhibitors except PIK-75, with TGX221 being notably ineffective despite it being known as a p110β selective lipid kinase inhibitor. Wortmannin was also surprisingly ineffective. Our results clearly showed that PIK-75 was the most effective inhibitor, knocking down the p110 and p85 phosphorylation of all kinases by ≥90% even though it was previously described as a p110α selective inhibitor ( Figure 3A ).
Figure 3. Effects of pan- and isoform-specific inhibitors on protein kinase activity.
A) Representative autoradiograph scans showing relative intensity of phosphorylation in the presence of pan-inhibitors LY294002 and wortmannin, and p110α-specific inhibitors A66 and PIK-75, p110β-specific inhibitor TGX221 and p110γ-specific inhibitor AS252424. Graphs summarise the results of N≥3 experiments for H1047R (black bars) and E545K (white bars) to p110α (hatched bars), p110β (cross-hatched bars) and p110γ (horizontal stripe bars) B) IC50 curves for selected inhibitors PIK-75, LY294002, A66 and wortmannin against p110α, H1047R and E545K.
To further understand these results we generated IC50 inhibition curves for PIK-75, LY294002, A66 and wortmannin against p110α and the oncogenic mutants ( Figure 3B and Table 1 ). Interestingly the IC50’s generated for the protein kinase and lipid kinase inhibition were broadly in the same range for PIK-75, LY294002 and A66 ( Table 1 and 2 ). However, the IC50 of wortmannin against the protein kinase activity was significantly higher than the lipid kinase activity in the conditions tested ( Figure 3B , Table 1 and 2 ).
Table 1. The IC50 of different small molecule inhibitors against the protein kinase activities of wildtype and mutant p110α.
| Protein Kinase IC50 (nM) | |||||||||
| p110 | p85 | βic | |||||||
| H1047R | E545K | p110α | H1047R | E545K | p110α | H1047R | E545K | p110α | |
| PIK-75 | 99 | 101 | 56 | 67 | 18 | 27 | 15 | 84 | 52 |
| LY294002 | 2584 | ND | ND | 2267 | 1903 | 757 | 6111 | 2413 | 1151 |
| A66 | 47 | ND | ND | 69 | 64 | 47 | 185 | 106 | 135 |
| Wortmannin | 4150 | 2320 | ND | 1960 | 2490 | 2220 | 3240 | 3480 | 2260 |
ND = no signal detected.
Table 2. The IC50 of different small molecule inhibitors against the lipid kinase activity of wildtype and mutant p110α.
| Lipid Kinase IC50 (nM) | |||
| H1047R | E545K | p110α | |
| PIK-75 | 6 | 24 | 7 |
| LY294002 | 1836 | 528 | 1986 |
| A66 | 41 | 30 | 34 |
| Wortmannin | 3 | 3 | 3 |
Discussion
PI 3-K has been recognized as a dual lipid/protein kinase since the seminal papers of Carpenter et al. (1993) and Dhand et al. (1994) [13], [14]. While the lipid kinase activity is acknowledged as critical for many cellular functions, the physiological significance of the protein kinase activity has long been questioned due to its Mn2+ dependency and lack of in vivo relevance. However, we have recently shown that PI 3-K protein kinase activity is deregulated in >80% of primary AML patient samples [23], [38] and that PI 3-K-mediated phosphorylation of the GM-CSF/IL-3 receptors regulates cell survival [23]. These findings have reinitiated interest in this area. Here we have systematically studied the in vitro protein kinase activity of all human class-1 PI 3-kinase isoforms as well as 2 common gain-of-function oncogenic mutants of p110α, the catalytic-domain H1047R mutant and the helical-domain mutant E545K. We further evaluate and discuss their ability to autophosphorylate as well as phosphorylate an exogenous substrate (GM-CSF/IL-3 βc receptor-derived βic peptide), in the presence and absence of Mn2+ and Mg2+, and the impact of a range of ATP-competitive inhibitors on their protein kinase activity.
With the exception of p110δ, our results show that all the forms of PI 3-K investigated retain some level of protein kinase activity in the presence of Mg2+ alone, with p110γ and the H1047R mutant retaining significantly more protein kinase activity than the other forms. Divalent cations such as Mg2+ and Mn2+ are required to coordinate the phosphates in ATP to give it the correct conformation for catalytic reactions [39], and while magnesium is the most abundant divalent cation in cells [24], manganese is only present as a trace element at μM concentrations, with free cytoplasmic concentrations further reduced due to intracellular compartmentalization [25]. Previous evidence indicated that the protein kinase activity of PI 3-K was Mn2+ dependent [13]–[15], which cast doubt over the in vivo role of this enzyme activity. Therefore our observation of protein kinase activity in the presence of only Mg2+ (especially the oncogenic mutants of p110α) provides evidence that this activity could be important in vivo.
All the forms of PI 3-K, again excepting p110δ, were effective to different degrees with regards to phosphorylating the exogenous substrate, βic. The oncogenic forms of PI 3-K have increased protein kinase activity compared to wildtype p110α, and these mutants, together with p110β and p110γ showed strong phosphorylation of βic. The increased protein kinase activity against an exogenous substrate indicates that these forms could possibly phosphorylate other substrates, including those contributing to oncogenesis. This could in part explain the oncogenic effects seen with overexpression of p110β and p110γ in cell models [40], [41], and why cellular overexpression of these alone can contribute to tumourogenesis [42], [43]. It is important to note that while we have shown that PI 3-K-mediated phosphorylation of the GM-CSF/IL-3 receptors regulates cell survival [23], it seems likely that other p110-mediated phosphorylation events also occur, since evidence has been presented that PI 3-kinase phosphorylates a range of signaling molecules including IRS-1 [18]–[20], 4EBP1 and H-Ras [15]. To date these phosphorylation events have been poorly characterized but it remains possible that these might contribute to activation of signaling pathways in cancer.
In general p110α and the E545K mutant showed strong p85 phosphorylation with little p110 autophosphorylation, while p110β and p110γ showed high p110 phosphorylation. The H1047R mutant maintained the strong p85 phosphorylation as seen with the wildtype PI 3-Kα isoform, but also showed elevated levels of p110 phosphorylation, approaching those of p110β. The significance of this increase in p110 phosphorylation in the H1047R mutant is not clear, especially as the lipid kinase activity of both oncogenic isoforms and wildtype p110α are inhibited by pre-phosphorylation to a similar extent. However since the level of reduction in the lipid kinase activity is similar in all three forms of p110α and p110β, it demonstrates, in agreement with Layton et al (2012) [34], that the increased lipid kinase activity in H1047R and E545K are not due to any reduction in auto-regulatory protein kinase activity. This further reinforces the hypothesis that the area where the increased protein kinase activity might have the most impact on cellular function is in the phosphorylation of exogenous substrates.
Our observations regarding the potency of PIK-75 relative to other inhibitors (TGX221 and AS252424) support our previous findings showing PIK-75 preferential inhibition of Ser585 phosphorylation in the GM-CSF/IL-3 receptors [23]. The fact that the inhibition of lipid and protein kinase activity by small molecules does not always directly correlate is also interesting and has potential implications for the use of PI 3-K inhibitors in the clinic, as it may explain the functional differences between different inhibitors in some contexts. For example, our finding that LY294002 is more effective at blocking the protein kinase activity of PI 3-K relative to wortmannin supports the unexpected observation that LY294002 was more effective than wortmannin at blocking the anti-apoptotic effect of GM-CSF [44]. However detailed explanation for the observed differences will require in depth structural studies to define how protein substrates interact with the kinase domain.
In summary, we compared the in vitro protein kinase activity of all human class-1 PI 3-kinase isoforms as well as 2 common gain-of-function oncogenic mutants of p110α, H1047R and E545K. We found that the oncogenic mutants have stronger protein kinase activity as measured by autophosphorylation, as well as phosphorylation of the exogenous substrate, βic. Furthermore we show that the oncogenic mutants (especially H1047R) are not as reliant on Mn2+ and retain significantly more protein kinase activity in the presence of Mg2+ alone. We demonstrate that the protein kinase activity of the wildtype and oncogenic forms of p110α are all inhibited by small molecules known to inhibit the lipid kinase activity of p110α; including LY294002, A66 and to a lesser degree wortmannin. However, inhibition was particularly pronounced with PIK-75. In addition we showed that p110β and p110γ exogenous and auto-phosphorylation was retained in the presence of Mg2+, and these isoforms were relatively resistant to inhibition by all inhibitors except PIK-75 (and AS252424 for p110γ). In contrast p110δ was Mn2+ dependent, only weakly phosphorylated βic and was fully suppressed by all inhibitors used. Since PI 3-K phosphorylates exogenous substrates and has been linked to changes in lipid kinase activity and possible activation of alternate signaling pathways [45], this increased protein kinase activity of E545K and especially H1047R in the presence of Mg2+ could have implications for the physiological activity of PI 3-K, especially in tumours carrying these mutations.
Acknowledgments
We thank Associate Professor Gordon Rewcastle for assistance with inhibitor production.
Funding Statement
This work was funded by The Maurice Wilkins Centre for Molecular Biodiscovery; http://cmb1.auckland.ac.nz/. Dr Buchanan’s salary is funded by the Maurice Wilkins Centre for Molecular Biodiscovery, and the salaries of Dr Dickson and Ms Lee are fully funded by a grant from the Ministry of Business, Innovation and Employment; http://www.msi.govt.nz/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Shepherd PR, Withers DJ, Siddle K (1998) Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J 333 (Pt 3): 471–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shepherd PR, Kahn BB (1999) Glucose transporters and insulin action - implications for insulin resistance and diabetes mellitus. The New England journal of medicine 341: 248–257. [DOI] [PubMed] [Google Scholar]
- 3. Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, et al. (2001) Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol 17: 615–675. [DOI] [PubMed] [Google Scholar]
- 4. Suire S, Coadwell J, Ferguson GJ, Davidson K, Hawkins P, et al. (2005) p84, a new Gbetagamma-activated regulatory subunit of the type IB phosphoinositide 3-kinase p110gamma. Curr Biol 15: 566–570. [DOI] [PubMed] [Google Scholar]
- 5. Voigt P, Dorner MB, Schaefer M (2006) Characterization of p87PIKAP, a novel regulatory subunit of phosphoinositide 3-kinase gamma that is highly expressed in heart and interacts with PDE3B. J Biol Chem 281: 9977–9986. [DOI] [PubMed] [Google Scholar]
- 6. Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, et al. (2004) High frequency of mutations of the PIK3CA gene in human cancers. Science 304: 554. [DOI] [PubMed] [Google Scholar]
- 7. Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, et al. (2004) Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res 64: 7678–7681. [DOI] [PubMed] [Google Scholar]
- 8. Chaussade C, Cho K, Mawson C, Rewcastle GW, Shepherd PR (2009) Functional differences between two classes of oncogenic mutation in the PIK3CA gene. Biochem Biophys Res Commun 381: 577–581. [DOI] [PubMed] [Google Scholar]
- 9. Bader AG, Kang S, Vogt PK (2006) Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proceedings of the National Academy of Sciences of the United States of America 103: 1475–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fruman DA, Meyers RE, Cantley LC (1998) Phosphoinositide kinases. Annual review of biochemistry 67: 481–507. [DOI] [PubMed] [Google Scholar]
- 11. Wymann MP, Pirola L (1998) Structure and function of phosphoinositide 3-kinases. Biochimica et biophysica acta 1436: 127–150. [DOI] [PubMed] [Google Scholar]
- 12. Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, et al. (2006) Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature 441: 366–370. [DOI] [PubMed] [Google Scholar]
- 13. Dhand R, Hiles I, Panayotou G, Roche S, Fry MJ, et al. (1994) PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J 13: 522–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carpenter CL, Auger KR, Duckworth BC, Hou WM, Schaffhausen B, et al. (1993) A tightly associated serine/threonine protein kinase regulates phosphoinositide 3-kinase activity. Mol Cell Biol 13: 1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foukas LC, Beeton CA, Jensen J, Phillips WA, Shepherd PR (2004) Regulation of phosphoinositide 3-kinase by its intrinsic serine kinase activity in vivo. Mol Cell Biol 24: 966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Foukas LC, Daniele N, Ktori C, Anderson KE, Jensen J, et al. (2002) Direct effects of caffeine and theophylline on p110 delta and other phosphoinositide 3-kinases. Differential effects on lipid kinase and protein kinase activities. The Journal of biological chemistry 277: 37124–37130. [DOI] [PubMed] [Google Scholar]
- 17. Geltz NR, Augustine JA (1998) The p85 and p110 subunits of phosphatidylinositol 3-kinase-alpha are substrates, in vitro, for a constitutively associated protein tyrosine kinase in platelets. Blood 91: 930–939. [PubMed] [Google Scholar]
- 18. Lam K, Carpenter CL, Ruderman NB, Friel JC, Kelly KL (1994) The phosphatidylinositol 3-kinase serine kinase phosphorylates IRS-1. Stimulation by insulin and inhibition by Wortmannin. The Journal of biological chemistry 269: 20648–20652. [PubMed] [Google Scholar]
- 19. Tanti JF, Gremeaux T, Van Obberghen E, Le Marchand-Brustel Y (1994) Insulin receptor substrate 1 is phosphorylated by the serine kinase activity of phosphatidylinositol 3-kinase. The Biochemical journal 304 (Pt 1): 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uddin S, Fish EN, Sher DA, Gardziola C, White MF, et al. (1997) Activation of the phosphatidylinositol 3-kinase serine kinase by IFN-alpha. Journal of immunology 158: 2390–2397. [PubMed] [Google Scholar]
- 21. Bondev A, Rubio I, Wetzker R (1999) Differential regulation of lipid and protein kinase activities of phosphoinositide 3-kinase gamma in vitro. Biological chemistry 380: 1337–1340. [DOI] [PubMed] [Google Scholar]
- 22. Rondinone CM, Carvalho E, Rahn T, Manganiello VC, Degerman E, et al. (2000) Phosphorylation of PDE3B by phosphatidylinositol 3-kinase associated with the insulin receptor. The Journal of biological chemistry 275: 10093–10098. [DOI] [PubMed] [Google Scholar]
- 23. Thomas D, Powell JA, Green BD, Barry EF, Ma Y, et al. (2013) Protein kinase activity of phosphoinositide 3-kinase regulates cytokine-dependent cell survival. PLoS Biol 11: e1001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maguire ME, Cowan JA (2002) Magnesium chemistry and biochemistry. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine 15: 203–210. [DOI] [PubMed] [Google Scholar]
- 25. Wedler FC, Ley BW, Grippo AA (1989) Manganese(II) dynamics and distribution in glial cells cultured from chick cerebral cortex. Neurochem Res 14: 1129–1135. [DOI] [PubMed] [Google Scholar]
- 26. Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr GA, et al. (1998) Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Molecular and cellular biology 18: 1379–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun M, Hart JR, Hillmann P, Gymnopoulos M, Vogt PK (2011) Addition of N-terminal peptide sequences activates the oncogenic and signaling potentials of the catalytic subunit p110alpha of phosphoinositide-3-kinase. Cell Cycle 10: 3731–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guthridge MA, Stomski FC, Barry EF, Winnall W, Woodcock JM, et al. (2000) Site-specific serine phosphorylation of the IL-3 receptor is required for hemopoietic cell survival. Mol Cell 6: 99–108. [PubMed] [Google Scholar]
- 29. Jamieson SM, Flanagan JU, Kolekar S, Buchanan C, Kendall JA, et al. (2011) A drug targeting only p110alpha can block PI 3-kinase signalling and tumour growth in certain cell types. Biochem J 438: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pomel V, Klicic J, Covini D, Church DD, Shaw JP, et al. (2006) Furan-2-ylmethylene thiazolidinediones as novel, potent, and selective inhibitors of phosphoinositide 3-kinase gamma. Journal of medicinal chemistry 49: 3857–3871. [DOI] [PubMed] [Google Scholar]
- 31. Beeton CA, Chance EM, Foukas LC, Shepherd PR (2000) Comparison of the kinetic properties of the lipid- and protein-kinase activities of the p110alpha and p110beta catalytic subunits of class-Ia phosphoinositide 3-kinases. Biochem J 350 Pt 2: 353–359. [PMC free article] [PubMed] [Google Scholar]
- 32. Vanhaesebroeck B, Higashi K, Raven C, Welham M, Anderson S, et al. (1999) Autophosphorylation of p110delta phosphoinositide 3-kinase: a new paradigm for the regulation of lipid kinases in vitro and in vivo. EMBO J 18: 1292–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Czupalla C, Culo M, Muller EC, Brock C, Reusch HP, et al. (2003) Identification and characterization of the autophosphorylation sites of phosphoinositide 3-kinase isoforms beta and gamma. J Biol Chem 278: 11536–11545. [DOI] [PubMed] [Google Scholar]
- 34. Layton MJ, Saad M, Church NL, Pearson RB, Mitchell CA, et al. (2012) Autophosphorylation of serine 608 in the p85 regulatory subunit of wild type or cancer-associated mutants of phosphoinositide 3-kinase does not affect its lipid kinase activity. BMC Biochem 13: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Woscholski R, Dhand R, Fry MJ, Waterfield MD, Parker PJ (1994) Biochemical characterization of the free catalytic p110 alpha and the complexed heterodimeric p110 alpha.p85 alpha forms of the mammalian phosphatidylinositol 3-kinase. J Biol Chem 269: 25067–25072. [PubMed] [Google Scholar]
- 36. Stoyanova S, Bulgarelli-Leva G, Kirsch C, Hanck T, Klinger R, et al. (1997) Lipid kinase and protein kinase activities of G-protein-coupled phosphoinositide 3-kinase gamma: structure-activity analysis and interactions with wortmannin. The Biochemical journal 324 (Pt 2): 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maier U, Babich A, Nurnberg B (1999) Roles of non-catalytic subunits in gbetagamma-induced activation of class I phosphoinositide 3-kinase isoforms beta and gamma. J Biol Chem 274: 29311–29317. [DOI] [PubMed] [Google Scholar]
- 38. Powell JA, Thomas D, Barry EF, Kok CH, McClure BJ, et al. (2009) Expression profiling of a hemopoietic cell survival transcriptome implicates osteopontin as a functional prognostic factor in AML. Blood 114: 4859–4870. [DOI] [PubMed] [Google Scholar]
- 39. Cook PF, Neville ME Jr, Vrana KE, Hartl FT, Roskoski R Jr (1982) Adenosine cyclic 3′,5′-monophosphate dependent protein kinase: kinetic mechanism for the bovine skeletal muscle catalytic subunit. Biochemistry 21: 5794–5799. [DOI] [PubMed] [Google Scholar]
- 40. Vogt PK, Hart JR, Gymnopoulos M, Jiang H, Kang S, et al. (2010) Phosphatidylinositol 3-kinase: the oncoprotein. Curr Top Microbiol Immunol 347: 79–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vogt PK, Gymnopoulos M, Hart JR (2009) PI 3-kinase and cancer: changing accents. Curr Opin Genet Dev 19: 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Phillips WA, St Clair F, Munday AD, Thomas RJ, Mitchell CA (1998) Increased levels of phosphatidylinositol 3-kinase activity in colorectal tumors. Cancer 83: 41–47. [DOI] [PubMed] [Google Scholar]
- 43. Kang S, Denley A, Vanhaesebroeck B, Vogt PK (2006) Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci U S A 103: 1289–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Juss JK, Hayhoe RP, Owen CE, Bruce I, Walmsley SR, et al. (2012) Functional redundancy of class I phosphoinositide 3-kinase (PI3K) isoforms in signaling growth factor-mediated human neutrophil survival. PLoS One 7: e45933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bondeva T, Pirola L, Bulgarelli-Leva G, Rubio I, Wetzker R, et al. (1998) Bifurcation of lipid and protein kinase signals of PI3Kgamma to the protein kinases PKB and MAPK. Science 282: 293–296. [DOI] [PubMed] [Google Scholar]