Abstract
Fibulin-3, originally identified in senescent and Werner syndrome fibroblasts, has been implicated in cell morphology, growth, adhesion and motility. Fibulin-3 exhibits both antitumor and oncogenic activities towards human cancers; however, the role of Fibulin-3 in hepatocellular carcinoma (HCC) remains elusive. In this study, we showed that both the mRNA and protein levels of Fibulin-3 were remarkably downregulated in HCC cell lines and fresh tissues. Immunohistochemical data revealed that Fibulin-3 was decreased in tumorous tissues in 67.1% (171/255) of cases compared to the corresponding adjacent nontumorous tissues. The results of statistical analysis indicated that low Fibulin-3 expression, defined by the receiver operating characteristic curve (ROC), was significantly associated with tumor differentiation (P = 0.008), clinical stage (P = 0.014) and serum AFP levels (P<0.01). Furthermore, Kaplan-Meier and multivariate analysis suggested that Fibulin-3 is an independent negative prognostic indicator for both overall (P<0.001) and recurrence-free (P = 0.036) survival. In addition, an in vitro study demonstrated that knockdown of Fibulin-3 by siRNA markedly increased cell viability and promoted cell invasion in HCC cells. Collectively, our data suggest that Fibulin-3 exhibits antitumor effects towards HCC and serves as a biomarker of unfavorable prognosis for this deadly disease.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most prevalent malignancy diagnosed worldwide [1]. In recent decades, its incidence has been increasing in economically developed regions, including Japan, Western Europe and the United States [2], [3]. Despite advances in chemotherapy, surgical management and the clinical implementation of numerous therapeutic strategies, the mortality rate of HCC remains very high (up to 94%), making HCC the third most common cause of cancer-related death [1], [4], [5]. Etiology studies indicate that infection with a hepatitis virus (HBV or HCV) and the dysregulation of genes involved in cell proliferation are major risk factors for hepatocarcinogenesis [6], [7]. However, the accuracy and reproducibility of markers currently used in the clinic to predict prognosis after surgical resection are unsatisfactory [4]. It is therefore urgent and important to search for potential prognostic biomarkers to improve clinical management of HCC.
Fibulins, a group of secreted glycoproteins, comprise seven members characterized by repeated epidermal growth-factor-like domains and a unique C-terminal fibulin-type module [8]. Fibulins have been implicated in cell morphology, growth, adhesion and motility [8], [9], [10]. The Fibulin-3 gene is located at chromosome 2p16, contains 11 exons, and encodes a 493-amino acid protein with a molecular mass of 54 kD [8]. Fibulin-3 was originally identified in senescent and Werner syndrome fibroblasts [11]. It is highly conserved among different species with 92–94% of amino acids identical in human, rat and mouse [11], [12], [13]. In cancer, Fibulin-3 is differently expressed. Plasma Fibulin-3 levels have been demonstrated to be upregulated in mesothelioma [14]. An increase of Fibulin-3 was also observed in pancreatic cancer [15], cervical carcinomas [16] and malignant gliomas [17]. On the other hand, Fibulin-3 was found to be downregulated in colorectal [18], lung [19], breast [20], prostate [21], and nasopharyngeal carcinomas [22]. Although Nomoto et al. reported that mRNA expression of Fibulin-3 was decreased in methylated HCC cases [23], the protein level and clinical significance of Fibulin-3 in HCC have not been elucidated.
In this study, Fibulin-3 expression was first examined in HCC cell lines and tissue samples. Correlation of Fibulin-3 expression and clinicopathological features of HCC patients was then investigated. The prognostic value of Fibulin-3 in HCC was also evaluated. Moreover, the effects of Fibulin-3 on HCC cell proliferation and invasion were determined in vitro. Our data suggest that Fibulin-3 associates with tumor progression and functions as a tumor suppressor in HCC.
Materials and Methods
Cell Culture and Transfection
Huh-7, PLC/PRF/5, HepG2 and SK-Hep-1 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA), and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Gaithersburg, MD, USA) containing 10% fetal bovine serum (FBS), 100 mg/ml penicillin, and 100 mg/ml streptomycin. L02, SMMC-7721, Bel-7404, Bel-7402, QGY-7701, and QSG-7703 cell lines were obtained from the Type Culture Collection Cell Bank, Chinese Academy of Science Committee (Shanghai, China) and were cultured in Roswell Park Memorial Institute (RPMI) 1640 with 10% fetal bovine serum (FBS), 100 U/ml of penicillin, and 100 U/ml of streptomycin. All of the cells were incubated in a humidified atmosphere of 5% CO2 and 95% air at 37°C. QSG-7703 cells were transfected with Fibulin-3 siRNA using lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions with the following target sequences: Fibulin-3 siRNA #1: CACAACGTGTGCCAAGACATA and Fibulin-3 siRNA #2: CACGCAATGCACTGACGGATA.
Patients and Tissue Specimens
Primary HCC specimens along with complete clinical and pathological data were obtained from 255 HCC patients who underwent surgical resection at Sun Yat-sen University Cancer Center (SYSUCC), Guangzhou, China, between Jan 2001 and Dec 2007. The cohort consisted of 227 (89.0%) males and 28 (11.0%) females. The mean age was 47.9, ranging from 14.0 to 78.0. Postsurgical survival data were available for all 255 patients. Another 18 fresh HCC resection tissues and the corresponding adjacent liver tissues were collected for quantitative real-time PCR and western blot analysis. None of the patients had received adjuvant therapies before surgery. Tumor stage was defined according to the tumor-node-metastasis (TNM) classification of the American Joint Committee on International Union against Cancer. Tumor differentiation was assessed according to the Edmonson and Steiner grading system. The use of tissues for this study was approved by the Institute Research Medical Ethics Committee of SYSUCC. No informed consent (written or verbal) was obtained for use of retrospective tissue samples from the patients within this study, given that this was not deemed necessary by the Ethics Committee who waived the need for consent. All of the samples were anonymous.
Tissue Microarray (TMA) Construction
TMA containing 255 HCC and adjacent nontumorous liver tissues were constructed. Briefly, all of the specimens were fixed in 4% formalin and embedded in paraffin. The corresponding histological H&E-stained sections were reviewed by a senior pathologist to mark out representative areas. Using a tissue array instrument (Beecher Instruments, Silver Spring, MD), each tissue core with a diameter of 0.6 mm was punched from the marked areas and re-embedded.
Immunohistochemistry (IHC)
Formalin-fixed and paraffin-embedded HCC sections with a thickness of 4 µm were dewaxed in xylene and graded alcohols, hydrated, and washed in phosphate-buffered saline (PBS). After pretreatment in a microwave oven, endogenous peroxidase was inhibited by 3% hydrogen peroxide in methanol for 20 min, followed by avidin-biotin blocking using a biotin-blocking kit (DAKO, Germany). Slides were then incubated with Fibulin-3 antibody (1∶400, sc-33722) overnight in a moist chamber at 4°C, washed in PBS, and incubated with biotinylated goat anti-rabbit/mouse antibodies. Slides were developed with the Dako Liquid 3, ’3-diaminobenzidine tetrahydrochloride (DAB)+Substrate Chromogen System and counterstained with hematoxylin.
Quantitative Real-time PCR (qRT-PCR)
Total RNA was extracted from paired HCC samples with Trizol reagent (BIOO Scientific Co., USA), following the manufacturer’s instructions. The mRNA was reverse transcribed to cDNA by M-MLV Reverse Transcriptase (Promega Inc., USA). The levels of Fibulin-3 and β-actin were measured by SYBR green-based real-time PCR using the Stratagene Mx3000P Real-Time PCR system. Primers were designed as follows: Fibulin-3, forward: 5′- CAGGACACCGAAGAAACCAT-3′ and reverse: 5′-GTTTCCTGCTGAGGCTGTTC-3′; and β-actin, forward: 5′-TGGCACCCAGCACAATGAA-3′ and reverse: 5′-CTAAGTCATAGTCCGCCTAGAAGC A-3′. Conditions were set as follows: one cycle of 95°C for 10 min, followed by 40 amplification cycles of denaturation at 95°C for 10 s, annealing at 60°C for 20 s and elongation at 72°C for 15 s. Using the comparative threshold cycle (2−ΔCt) method, the relative expressions of Fibulin-3 in HCC were normalized to endogenous β-actin.
Western Blot
Cell or tissue lysates were boiled with 6X sodium dodecyl sulfate (SDS) loading buffer and then fractionated by SDS-PAGE. The proteins were transferred to PVDF membranes, incubated with a primary specific antibody for Fibulin-3 (1∶1000, Santa-Cruz Company, sc-33722) in 5% non-fat milk, and then incubated with a horse radish peroxidase (HRP)-conjugated anti-mouse secondary antibody. ECL detection reagent (Amersham Life Science, Piscataway, NJ, USA) was used to show the results.
MTT
Cell viability was assessed by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazo-lium bromide (MTT) assay. Briefly, 8×103 cells transfected with scramble or Fibulin-3 siRNA were seeded into 96-well plates and incubated for 24 h. After adding 100 ml/well of MTT solution, the cells were incubated for another 2.5 h. Supernatants were then removed, and the formazan crystals were dissolved in 100 ml/well DMSO. The absorbance at 570/630 nm of each sample was measured using a multilabel plate reader (PerkinElmer). Three independent experiments were performed.
Matrigel Invasion Assay
Cell invasion was performed using Millipore Biocoat Matrigel Invasion Chambers with 8 µM pore size (Millipore, Darmstadt, Germany), according to the manufacturer’s instructions.
IHC Evaluation
Semi-quantitative IHC detection was used to determine the Fibulin-3 protein levels. Using the H-score method, we multiplied the percentage score by the staining intensity score. The percentage of positively stained cells was scored as “0” (0%), “1” (1%–25%), “2” (26%–50%), “3” (51%–75%), or “4” (76%–100%). Intensity was scored as “0” (negative staining), “1” (weak staining), “2” (moderate staining), or “3” (strong staining). For each case, 1000 cells were randomly selected and scored. The scores were independently decided by 2 pathologists (Dr. Y Cao and Dr. MY Cai).
Selection of Cutoff Score
Receiver operating characteristic (ROC) curve analysis was employed to determine the cutoff score for tumors with high Fibulin-3 expression using the 0,1-criterion. For the outcomes being studied in HCC patients, sensitivity and specificity were plotted against the Fibulin-3 scores to generate various ROC curves. The count closest to the points of maximum sensitivity and specificity was selected as the cutoff value. Cases defined as having high Fibulin-3 expression had scores below or equal to the cutoff value, while cases defined as having low Fibulin-3 expression had scores above the cutoff value. To perform ROC curve analysis, clinicopathological features were dichotomized as follows: tumor multiplicity (single vs. multiple), tumor size (<5 cm vs. ≥5 cm), AFP level (<20 ng/ml vs. ≥20 ng/ml), tumor differentiation (well-moderate vs. poor-undifferentiated), stage (I+II vs. III+IV), vascular invasion (yes vs. no), relapse (yes vs. no) and survival status (dead vs. alive).
Statistical Analysis
The statistical analyses were performed using the SPSS 16.0 software (SPSS, Chicago, IL, USA). ROC curve analysis was applied to determine the cutoff value for high expression of Fibulin-3 by the 0,1-criterion, and the areas under the curve (AUCs) were calculated. The Mann-Whitney U test was used for comparison between groups. The Wilcoxon matched paired test was used to determine the significance of Fibulin-3 expression in fresh HCC and normal liver tissues. The χ2 test was performed to analyze the correlation between Fibulin-3 expression and clinicopathological parameters. The Kaplan-Meier method (the log-rank test) was utilized for survival analysis and univariate analysis. Different independent analyses were performed depending on the selected population (overall population and various morphological and pathological subgroups). The Cox proportional hazards regression model was used to identify independent prognostic factors. P<0.05 (two-tailed) was considered to be statistically significant.
Results
Fibulin-3 Expression in HCC Cell Lines and Tissues by qRT-PCR and Western Blot
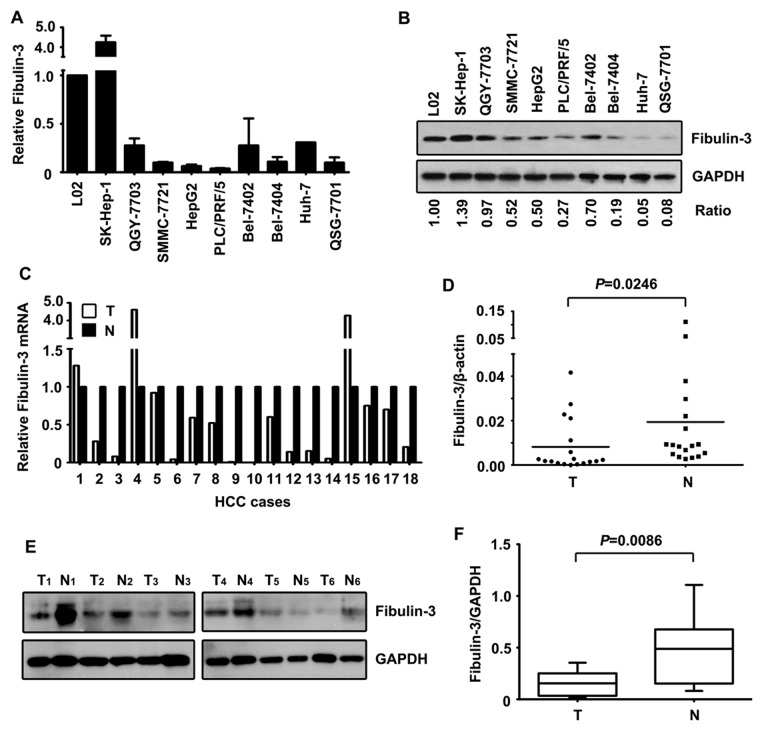
First, we determined the expression of Fibulin-3 in HCC cell lines, using qRT-PCR and western blot. The results showed that Fibulin-3 was markedly decreased in HCC cell lines at both the mRNA (Fig. 1A) and protein (Fig. 1B) levels, compared with the expression in the immortalized liver cell line L-02.
Figure 1. Expression of Fibulin-3 in HCC cell lines and fresh tissue samples by qRT-PCR and western blot.
A. The mRNA levels of Fibulin-3 in immobilized liver cell line (L02) and HCC cell lines were determined using qRT-PCR. B. Protein levels of Fibulin-3 in HCC cell lines were detected by western blot. The ratio of Fibulin-3/GAPDH was indicated below. C. The mRNA levels of Fibulin-3 in 18 pairs of HCC and corresponding adjacent liver samples were examined. Relative Fibulin-3 mRNA was presented. D. Significance of alteration of Fibulin-3 mRNA was revealed by Wilcoxon matched paired test. E. Expressions of Fibulin-3 protein in 18 paired tissues were examined by western blot. Representative images of Fibulin-3 expression were presented. F. Relative intensity of Fibulin-3 normalized to GAPDH was calculated (n = 18).
This decrease in Fibulin-3 was further confirmed in 18 pairs of HCC fresh tissues. In 77.8% (14/18) of the samples, Fibulin-3 mRNA levels were much lower in HCC than normal tissues (Fig. 1C&D). Additionally, protein levels of Fibulin-3 were significantly reduced in tumorous tissues compared to those in adjacent nontumorous samples (Fig. 1E&F).
Determination of Cutoff Value for Low Fibulin-3 Expression in HCC
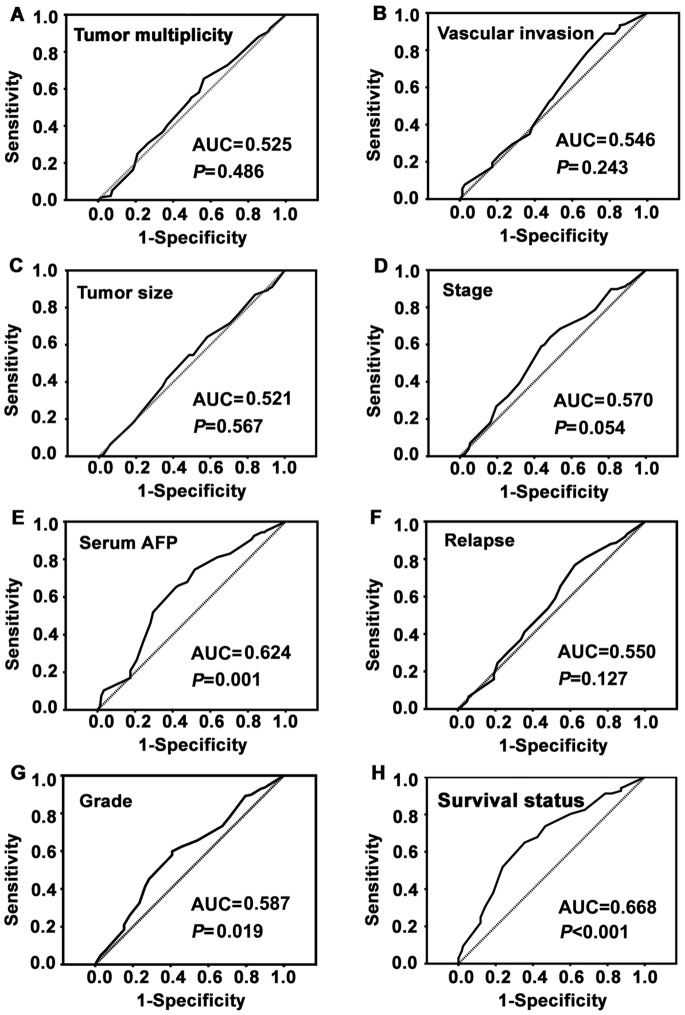
To determine an optimal cutoff value to identify low Fibulin-3 expression, an ROC curve was utilized according to the results of the IHC evaluation. The ROC curve for survival status possessed the smallest distance (0.0, 1.0), indicating that Fibulin-3 expression has the greatest prognostic ability (maximum sensitivity and specificity) for survival status (Fig. 2). Therefore, the score of 6.75 was chosen as the cutoff value for low Fibulin-3 expression.
Figure 2. Determination of the cutoff value of low Fibulin-3 expression in HCC tissues by ROC curves.
The sensitivity and 1-specificity were plotted for each clinical feature, such as tumor multiplicity, tumor size, serum AFP, pathological grade, clinical stage, vascular invasion, relapse and survival status. The areas under the curve (AUCs) and the P values were indicated.
Association between Fibulin-3 Expression and Clinicopathological Features
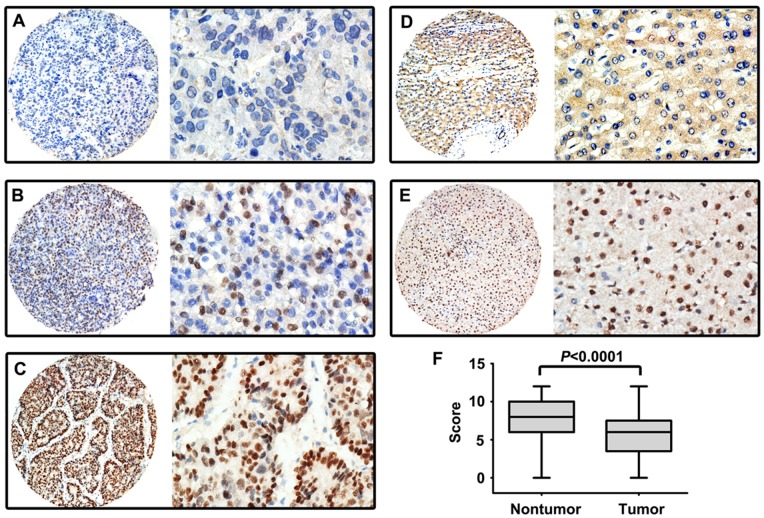
A cohort comprising 255 HCC cases was collected to construct a TMA to determine the expression pattern of Fibulin-3 in HCC. The results of the TMA-based IHC showed that Fibulin-3 was mainly expressed in the nucleus of tumor (Fig. 3A–C) and normal liver cells (Fig. 3D&E). The overall expression of Fibulin-3 was revealed to be significantly downregulated in HCC tissues compared to nontumorous tissues (Fig. 3F). Furthermore, Fibulin-3 was reduced in tumorous tissues in 67.1% (171/255) of cases compared to the corresponding adjacent nontumorous tissues.
Figure 3. Expression of Fibulin-3 in HCC tissues by IHC.
Micrographs showed weak (A), moderate (B), and strong (C) staining of Fibulin-3 in HCC, as well as low (D) and high (E) expression of Fibulin-3 in normal liver tissues. (Left panel: magnification ×100; Right panel: magnification ×400.) F. Reproducibility of the measurement in all 255 patients was calculated using the Wilcoxon matched paired test.
We next investigated the relationship between Fibulin-3 expression and clinicopathological variables of HCC patients. The statistical analysis showed that low Fibulin-3 expression was significantly correlated with poor differentiation (P = 0.008), advanced stage (P = 0.014), and high AFP serum levels (P<0.001). However, there were no statistical connections between Fibulin-3 expression and the other clinicopathological parameters, including age, gender, HBsAg, cirrhosis, tumor multiplicity, tumor size, vascular invasion, and disease relapse (P>0.05, Table 1).
Table 1. Correlation between the clinicopathologic variables and Fibulin-3 expression in HCC.
| Variable | Fibulin-3 protein | ||||
| All cases | Low expression | High expression | ?2 | P valuea | |
| Age (years)b | 1.177 | 0.278 | |||
| <47.9 | 133 | 69 (51.9%) | 64 (48.1%) | ||
| ≥47.9 | 122 | 55 (45.1%) | 67 (54.9%) | ||
| Gender | 0.024 | 0.878 | |||
| Male | 227 | 110 (48.5%) | 117 (51.5%) | ||
| Female | 28 | 14 (50%) | 14 (50%) | ||
| HBsAg | 0.127 | 0.722 | |||
| Positive | 222 | 105 (48.2%) | 115 (51.8%) | ||
| Negative | 33 | 17 (51.5%) | 16 (48.5%) | ||
| AFP (ng/ml) | 13.673 | <0.001 | |||
| <20 | 106 | 37 (34.9%) | 69 (65.1%) | ||
| ≥20 | 149 | 87 (58.4%) | 62 (41.6%) | ||
| Cirrhosis | 0.170 | 0.680 | |||
| Yes | 184 | 88 (47.8%) | 96 (52.2%) | ||
| No | 71 | 36 (50.7%) | 35 (49.3%) | ||
| Tumor size (cm) | 0.913 | 0.339 | |||
| <5 | 123 | 56 (45.5%) | 67 (54.5%) | ||
| ≥5 | 132 | 68 (51.5%) | 64 (48.5%) | ||
| Tumor multiplicity | 0.619 | 0.431 | |||
| Single | 136 | 63 (46.3%) | 73 (53.7%) | ||
| Multiple | 119 | 61 (51.3%) | 58 (48.7%) | ||
| Differentiation | 7.100 | 0.008 | |||
| Well-Moderate | 157 | 66 (42.0%) | 91 (58.0%) | ||
| Poor-Undifferentiated | 98 | 58 (59.2%) | 40 (40.8%) | ||
| Stage | 5.978 | 0.014 | |||
| I–II | 127 | 52 (40.9%) | 75 (59.1%) | ||
| III–IV | 128 | 72 (56.3%) | 56 (43.7%) | ||
| Vascular invasion | 0.484 | 0.487 | |||
| Yes | 75 | 39 (52.0%) | 36 (48.0%) | ||
| No | 180 | 85 (47.2%) | 95 (52.8%) | ||
| Relapse | 0.764 | 0.382 | |||
| Yes | 104 | 54 (51.9%) | 50 (48.1%) | ||
| No | 151 | 70 (46.4%) | 81 (53.6%) | ||
Chi-squared test;
Mean age; AFP, alpha-fetoprotein; HBsAg, hepatitis B surface antigen.
Correlation of Fibulin-3 Expression with Prognosis of HCC Patients
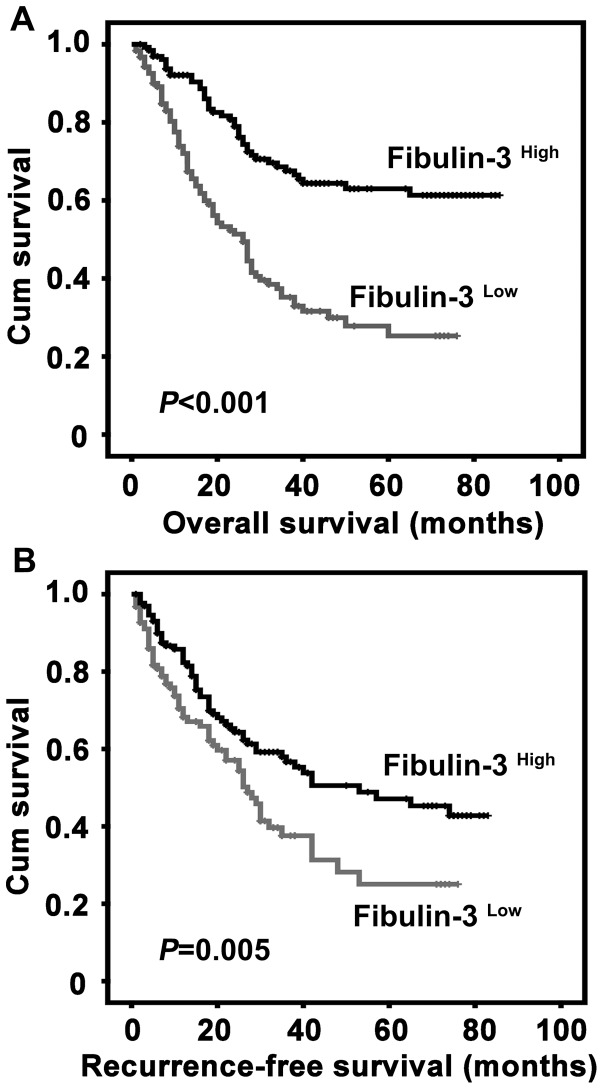
To determine the value of Fibulin-3 for the prognosis of postsurgical HCC patients, Kaplan-Meier survival analyses were conducted. Survival data were available for all 255 patients. The average survival time was 24.5 months for patients with low Fibulin-3 expression and 40.5 months for patients with high Fibulin-3 expression. The results indicated that patients with low Fibulin-3 expression had much shorter survival times (P<0.001) (Fig. 4A) and had a higher tendency of disease recurrence (P = 0.005) (Fig. 4B).
Figure 4. Association of low Fibulin-3 expression in HCC tissue and unfavorable overall survival and recurrence-free survival by Kaplan-Meier survival analysis.
Probabilities of overall survival (A) and recurrence-free survival (B) of 255 total HCC patients were analyzed using Kaplan-Meier survival analysis (log-rank test).
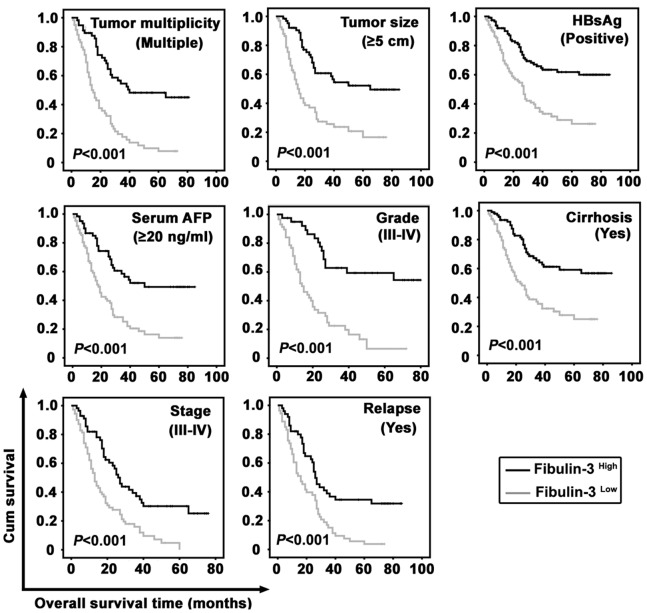
The prognostic value of Fibulin-3 was further confirmed by stratified survival analysis. Results showed that low expression of Fibulin-3 was closely connected with poor overall survival after surgical resection in 8 subgroups classified by the factors contributing to poorer outcome in HCC patients (P<0.001 for all of the groups) (Fig. 5). The effect of Fibulin-3 on the recurrence-free survival rates of the 8 subgroups was also determined (Fig. S1).
Figure 5. Correlation of Fibulin-3 expression with overall survival in morphologic and pathological HCC subgroups.
Survival analyses were performed in subgroups divided by the factors that contribute to poorer outcomes of HCC patients, using Kaplan-Meier survival analysis (log-rank test).
Univariate and Multivariate Analyses of Prognostic Variables in HCC Patients
To test whether the cohort in the present study was representative, univariate analysis was performed. Results showed that Fibulin-3, as well as tumor size, serum AFP level, tumor multiplicity, clinical stage, vascular invasion, tumor differentiation and relapse, were responsible for the overall and recurrence-free survival of HCC patients (Table 2&3).
Table 2. Univariate and multivariate analyses of prognostic factors of overall survival.
| Variable | All cases | Univariate | Multivariate | |||
| Mean | Median | P value | HR (95% CI) | P value | ||
| Age (years)a | 0.804 | |||||
| <47.9 | 133 | 50.2 | 39.0 | |||
| ≥47.9 | 122 | 48.6 | 38.0 | |||
| Gender | 0.408 | |||||
| Male | 227 | 49.2 | 38.0 | |||
| Female | 28 | 52.5 | NR | |||
| HBsAg | 0.644 | |||||
| Positive | 222 | 50.2 | 40.0 | |||
| Negative | 33 | 44.8 | 38.0 | |||
| Cirrhosis | 0.477 | |||||
| Yes | 184 | 48.7 | 38.0 | |||
| No | 71 | 52.3 | NR | |||
| AFP (ng/ml) | <0.001 | 1.753 (1.122-2.739) | 0.014 | |||
| <20 | 106 | 65.3 | NR | |||
| ≥20 | 149 | 38.9 | 27.0 | |||
| Tumor size (cm) | <0.001 | 1.121 (0.752-1.671) | 0.575 | |||
| <5 | 123 | 59.6 | NR | |||
| ≥5 | 132 | 41.6 | 27.0 | |||
| Tumor multiplicity | <0.001 | 1.074 (0.668-1.727) | 0.767 | |||
| Single | 136 | 63.1 | NR | |||
| Multiple | 119 | 35.6 | 24.0 | |||
| Differentiation | <0.001 | 1.059 (0.723-1.549) | 0.770 | |||
| Well-Moderate | 157 | 57.1 | NR | |||
| Poor-Undifferentiated | 98 | 36.9 | 25.0 | |||
| Stage | <0.001 | 5.199 (2.837-9.527) | <0.001 | |||
| I–II | 127 | 73.8 | NR | |||
| III–IV | 128 | 26.7 | 18.0 | |||
| Vascular invasion | <0.001 | 1.197 (0.785-1.826) | 0.402 | |||
| Yes | 75 | 28.4 | 18.0 | |||
| No | 180 | 58.6 | NR | |||
| Relapse | <0.001 | 2.250 (1.464-3.457) | <0.001 | |||
| Yes | 104 | 30.9 | 24.0 | |||
| No | 151 | 65.1 | NR | |||
| Fibulin-3 | <0.001 | 0.468 (0.315-0.695) | <0.001 | |||
| Low expression | 131 | 34.1 | 26.0 | |||
| High expression | 124 | 62.0 | NR | |||
Mean age; NR, not reached; HbsAg, hepatitis B surface antigen; AFP, alpha-fetoprotein; HR, hazard ratio; CI, confident interval.
Table 3. Univariate and multivariate analyses of prognostic factors of recurrence-free survival.
| Variable | All cases | Univariate | Multivariate | |||
| Mean | Median | P value | HR (95% CI) | P value | ||
| Age (years)a | 0.725 | |||||
| <47.9 | 133 | 43.2 | 30.0 | |||
| ≥47.9 | 122 | 42.9 | 36.0 | |||
| Gender | 0.459 | |||||
| Male | 227 | 44.7 | 35.0 | |||
| Female | 28 | 33.2 | 42.0 | |||
| HBsAg | 0.291 | |||||
| Positive | 222 | 43.0 | 35.0 | |||
| Negative | 33 | 47.9 | 42.0 | |||
| Cirrhosis | 0.379 | |||||
| Yes | 184 | 41.6 | 30.0 | |||
| No | 71 | 48.5 | 42.0 | |||
| AFP (ng/ml) | <0.001 | 1.444 (0.974-2.142) | 0.067 | |||
| <20 | 106 | 53.0 | 57.0 | |||
| ≥20 | 149 | 36.0 | 26.0 | |||
| Tumor size (cm) | <0.001 | 1.276 (0.857-1.899) | 0.230 | |||
| <5 | 123 | 53.2 | NR | |||
| ≥5 | 132 | 35.7 | 22.0 | |||
| Tumor multiplicity | 0.007 | 1.122 (0.748-1.682) | 0.579 | |||
| Single | 136 | 49.3 | 42.0 | |||
| Multiple | 119 | 36.7 | 26.0 | |||
| Differentiation | 0.001 | 1.202 (0.819-1.763) | 0.348 | |||
| Well-Moderate | 157 | 49.6 | 53.0 | |||
| Poor-Undifferentiated | 98 | 32.1 | 26.0 | |||
| Stage | <0.001 | 2.262 (1.319-3.879) | 0.003 | |||
| 127 | 61.5 | NR | ||||
| III–IV | 128 | 24.5 | 18.0 | |||
| Vascular invasion | <0.001 | 2.665 (1.718-4.133) | <0.001 | |||
| Yes | 75 | 19.6 | 15.0 | |||
| No | 180 | 57.2 | NR | |||
| Fibulin-3 | 0.005 | 0.676 (0.466-0.979) | 0.038 | |||
| Low expression | 131 | 34.3 | 27.0 | |||
| High expression | 124 | 49.5 | 53.0 | |||
Mean age; NR, not reached; HbsAg, hepatitis B surface antigen; AFP, alpha-fetoprotein; HR, hazard ratio; CI, confident interval.
Multiple Cox regression analysis was further utilized to evaluate the independent prognostic value of Fibulin-3. Results revealed that Fibulin-3 was an independent prognostic marker for overall survival (Hazard Ratio (HR) 0.468, P<0.001) and recurrence-free survival (Hazard Ratio (HR) 0.676, P = 0.036) (Table 2&3).
Increase of Cell Proliferation and Invasion by Fibulin-3 siRNA
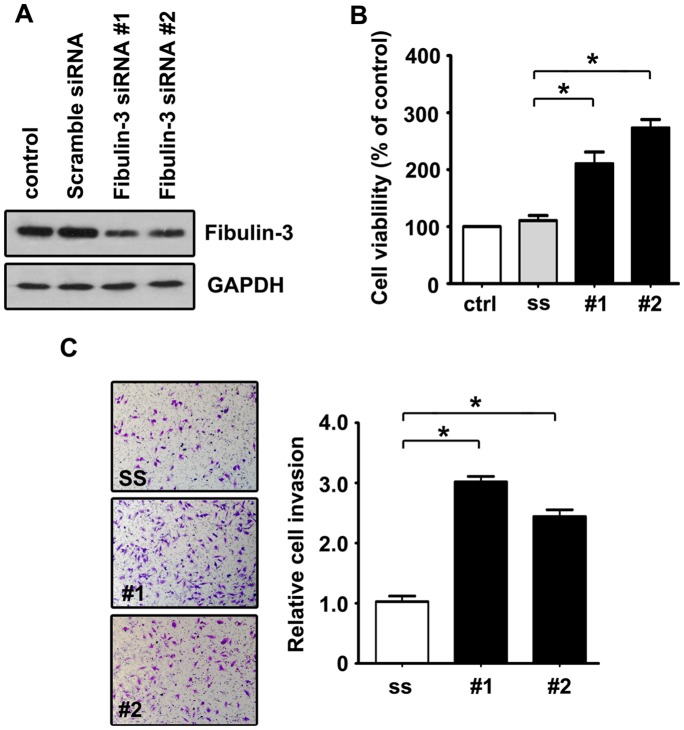
We next examined whether a decrease in Fibulin-3 affected HCC progression. Two Fibulin-3 siRNAs were proven to effectively downregulate the expression of Fibulin-3 (Fig. 6A). The knockdown of Fibulin-3 in QGY-7703 cells led to a reduction in cell viability (Fig. 6B), as indicated by MTT assay. The effect of Fibulin-3 on cell invasion was further investigated using a Matrigel invasion assay. Results showed that Fibulin-3 inhibition significantly increased serum-induced transwell migration of QGY-7703 cells by 3.04 and 2.45-fold in siRNA #1 and siRNA#2 groups, respectively (Fig. 6C).
Figure 6. Promotions of cell proliferation and invasion by Fibulin-3 siRNA in QGY-7703 cells.
A. Fibulin-3 siRNA noticeably downregulated expression of Fibulin-3 protein. Scramble and Fibulin-3 siRNA were transfected into QGY-7703 cells for 24 h. Relative Fibulin-3 expressions were detected by western blot. B. Fibulin-3 siRNA significantly increased cell viability in HCC cells. Cells transfected with scramble siRNA (ss) and Fibulin-3 siRNA (#1 and #2) were seeded into 96-well plates and cultured for 24 h. The cell viabilities were determined using MTT assays. The data are means ± SD of three independent experiments, *P<0.05. C. Fibulin-3 siRNA promoted cell invasion in vitro. QGY-7703 cells were seeded into transwell chambers coated with Matrigel. After 24 h, invaded cells stained by 0.1% crystal violet were counted. The data are means ± SD of three independent experiments, *P<0.05.
Discussion
HCC is a heterogeneous cancer with very high mortality. Searching for valuable biomarkers for HCC diagnosis and prognostic prediction has been attracting an increasing amount of interest. Plenty of proteins, such as SIRT3 [24], DKK1 [25] and PLK4 [26], have been shown to have clinical significance for predicting HCC prognosis. In addition, other signatures of cancer, including DNA methylation, circulating tumor cells and histone modification, have been attracting the attention of researchers for use in determining the nature of HCC. In this study, we aimed to investigate the expression and the prognostic value of Fibulin-3 in a large cohort of HCC patients.
Our data showed that the Fibulin-3 protein level was decreased in HCC patients and was associated with unfavorable prognosis. It is noteworthy that Fibulin-3 acts as an independent prognostic biomarker in HCC. Fibulin-3 expression was inversely correlated with both overall and recurrence-free survival of HCC patients. Examination of Fibulin-3 expression in HCC may aid in the development of new therapeutic strategies. In agreement with our data, decreased levels of Fibulin-3 were observed in other human cancers. For example, Tong et al. reported that Fibulin-3 was downregulated in colorectal cancer and was associated with poor prognosis [18]. Hwang et al. demonstrated that reduction of Fibulin-3 was associated with tumor progression and poor prognosis in nasopharyngeal carcinomas [22]. Sadr-Nabavi et al. showed that reduction of Fibulin-3 in sporadic breast cancer was correlated with poor prognosis [20]. Surprisingly, Fibulin-3 was also found to be upregulated in other cancers. Song et al. showed that the overexpression of Fibulin-3 in cervical carcinoma was an indicator of poor survival [16]. Increased expression of Fibulin-3 was observed in malignant glioma [17]. Recently, the upregulation of plasma Fibulin-3 was proven to be of clinical significance for the diagnosis of pleural mesothelioma [14]. In the present study, low Fibulin-3 expression was related to poor differentiation and advanced stages of HCC, suggesting Fibulin-3 may be involved in HCC progression. Although promoter methylation contributes to Fibulin-3 downregulation in human cancers, including colorectal cancer, lung cancer and HCC, the detailed mechanism by which Fibulin-3 is downregulated in HCC requires future investigation.
Paradoxical effects of Fibulin-3 on tumor progression have been reported. The overexpression of Fibulin-3 in Hela cells promotes angiogenesis, proliferation and invasion by increasing the expression of VEGF [27]. In pancreatic adenocarcinomas, Fibulin-3 binds EGFR (competitive to EGF) causing autophosphorylation of EGFR at Tyr-992 and Tyr-1068 and the subsequent phosphorylation of AKT at Thr-308 and ERK at Thr-202 and Tyr-204 and, thus, accelerates pancreatic adenocarcinoma growth [28]. Fibulin-3 promoted glioma growth by promoting Notch-1 cleavage and upregulating the active Notch-1 intracellular domain (NICD) to reduce apoptosis [29]. Interestingly, an opposite effect of Fibulin-3 in glioma was also shown; overexpression of Fibulin-3 inhibited malignant glioma growth by suppressing EGFR-AKT signaling [30]. Antitumor activity of Fibulin-3 was also reported in other cancers. For example, Albig et al. showed that Fibulin-3 abolished angiogenic activities and sprouting in MB114 cells by decreasing the expression of MMP-2 and MMP-3 and increasing the expression of TIMP-1 and TIMP-3. [31]. Kim and colleagues observed that enforced expression of Fibulin-3 in A549 non-small cell lung cancer (NSCLC) cells attenuated cell invasion by reducing expressions of MMP-2 and MMP-7 [32]. Hwang et al. reported that Fibulin-3 inhibited nasopharyngeal carcinoma cell migration and invasion by decreasing the phosphorylation of AKT at Ser-473 [22]. Furthermore, Fibulin-3 sensitized pancreatic cancer cells to a PI3K/mTOR inhibitor by interacting with p27Kip1 [33]. In the present study, Fibulin-3 was found to be decreased in HCC cell lines and tissue samples. Low Fibulin-3 expression was associated with malignant parameters and unfavorable prognosis. In vitro experiments demonstrated that knockdown of Fibulin-3 resulted in an increase in cell viability and invasion. Collectively, our data suggest Fibulin-3 as a tumor suppressor in HCC.
In most cases, the biological functions of proteins depend on their cellular localization. Fibulin-3 is a member of the fibulin family of extracellular glycoproteins, suggesting Fibulin-3 should be localized to the cytoplasm. It has been reported that Fibulin-3 interacts with extracellular matrix protein 1 (ECM1) [34] and tissue inhibitor of metalloproteinase 3 (TIMP-3) [35] to localize to the basement membrane. However, nuclear Fibulin-3 was also depicted in malignant gliomas [17], pleural mesothelioma [14] and nasopharyngeal carcinomas [22]. In the present study, Fibulin-3 was observed in both the cytoplasm and nucleus of HCC cells. As Fibulin-3 lacks a nuclear localization signal, the mechanism of translocation of Fibulin-3 into the nucleus and its nuclear function remain to be determined.
In summary, our data provide comprehensive understanding that Fibulin-3 is remarkably downregulated in HCC. Reduction of Fibulin-3 was associated with tumor differentiation, clinical stage and serum AFP level. Low Fibulin-3 expression predicts lower overall survival and recurrence-free survival. The knockdown of Fibulin-3 in HCC cells resulted in cell proliferation and invasion. Taken together, although the detailed mechanism remains unclear, Fibulin-3 functions as a tumor suppressor in HCC and is of clinical significance in predicting the postsurgical prognosis of patients who suffer from this deadly disease.
Supporting Information
(TIF)
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (No. 81172345) and China Postdoctoral Science Foundation (No. 2012M511867). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A, Bray F (2011) Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Bosch FX, Ribes J, Diaz M, Cleries R (2004) Primary liver cancer: worldwide incidence and trends. Gastroenterology 127: S5–S16. [DOI] [PubMed] [Google Scholar]
- 3. Erichsen R, Jepsen P, Jacobsen J, Norgaard M, Vilstrup H, et al. (2008) Time trends in incidence and prognosis of primary liver cancer and liver metastases of unknown origin in a Danish region, 1985–2004. Eur J Gastroenterol Hepatol 20: 104–110. [DOI] [PubMed] [Google Scholar]
- 4. Marquardt JU, Galle PR, Teufel A (2012) Molecular diagnosis and therapy of hepatocellular carcinoma (HCC): an emerging field for advanced technologies. J Hepatol 56: 267–275. [DOI] [PubMed] [Google Scholar]
- 5. Breuhahn K, Gores G, Schirmacher P (2011) Strategies for hepatocellular carcinoma therapy and diagnostics: lessons learned from high throughput and profiling approaches. Hepatology 53: 2112–2121. [DOI] [PubMed] [Google Scholar]
- 6. Wong DK, Huang FY, Lai CL, Poon RT, Seto WK, et al. (2011) Occult hepatitis B infection and HBV replicative activity in patients with cryptogenic cause of hepatocellular carcinoma. Hepatology 54: 829–836. [DOI] [PubMed] [Google Scholar]
- 7. Riordan JD, Keng VW, Tschida BR, Scheetz TE, Bell JB, et al. (2013) Identification of rtl1, a retrotransposon-derived imprinted gene, as a novel driver of hepatocarcinogenesis. PLoS Genet 9: e1003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Timpl R, Sasaki T, Kostka G, Chu ML (2003) Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol 4: 479–489. [DOI] [PubMed] [Google Scholar]
- 9. de Vega S, Iwamoto T, Yamada Y (2009) Fibulins: multiple roles in matrix structures and tissue functions. Cell Mol Life Sci 66: 1890–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Argraves WS, Greene LM, Cooley MA, Gallagher WM (2003) Fibulins: physiological and disease perspectives. EMBO Rep 4: 1127–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ehlermann J, Weber S, Pfisterer P, Schorle H (2003) Cloning, expression and characterization of the murine Efemp1, a gene mutated in Doyne-Honeycomb retinal dystrophy. Gene Expr Patterns 3: 441–447. [DOI] [PubMed] [Google Scholar]
- 12. Narendran N, Guymer RH, Cain M, Baird PN (2005) Analysis of the EFEMP1 gene in individuals and families with early onset drusen. Eye (Lond) 19: 11–15. [DOI] [PubMed] [Google Scholar]
- 13. Blackburn J, Tarttelin EE, Gregory-Evans CY, Moosajee M, Gregory-Evans K (2003) Transcriptional regulation and expression of the dominant drusen gene FBLN3 (EFEMP1) in mammalian retina. Invest Ophthalmol Vis Sci 44: 4613–4621. [DOI] [PubMed] [Google Scholar]
- 14. Pass HI, Levin SM, Harbut MR, Melamed J, Chiriboga L, et al. (2012) Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med 367: 1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seeliger H, Camaj P, Ischenko I, Kleespies A, De Toni EN, et al. (2009) EFEMP1 expression promotes in vivo tumor growth in human pancreatic adenocarcinoma. Mol Cancer Res 7: 189–198. [DOI] [PubMed] [Google Scholar]
- 16. En-lin S, Sheng-guo C, Hua-qiao W (2010) The expression of EFEMP1 in cervical carcinoma and its relationship with prognosis. Gynecol Oncol 117: 417–422. [DOI] [PubMed] [Google Scholar]
- 17. Hu B, Thirtamara-Rajamani KK, Sim H, Viapiano MS (2009) Fibulin-3 is uniquely upregulated in malignant gliomas and promotes tumor cell motility and invasion. Mol Cancer Res 7: 1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tong JD, Jiao NL, Wang YX, Zhang YW, Han F (2011) Downregulation of fibulin-3 gene by promoter methylation in colorectal cancer predicts adverse prognosis. Neoplasma 58: 441–448. [DOI] [PubMed] [Google Scholar]
- 19. Yue W, Dacic S, Sun Q, Landreneau R, Guo M, et al. (2007) Frequent inactivation of RAMP2, EFEMP1 and Dutt1 in lung cancer by promoter hypermethylation. Clin Cancer Res 13: 4336–4344. [DOI] [PubMed] [Google Scholar]
- 20. Sadr-Nabavi A, Ramser J, Volkmann J, Naehrig J, Wiesmann F, et al. (2009) Decreased expression of angiogenesis antagonist EFEMP1 in sporadic breast cancer is caused by aberrant promoter methylation and points to an impact of EFEMP1 as molecular biomarker. Int J Cancer 124: 1727–1735. [DOI] [PubMed] [Google Scholar]
- 21. Kim YJ, Yoon HY, Kim SK, Kim YW, Kim EJ, et al. (2011) EFEMP1 as a novel DNA methylation marker for prostate cancer: array-based DNA methylation and expression profiling. Clin Cancer Res 17: 4523–4530. [DOI] [PubMed] [Google Scholar]
- 22. Hwang CF, Chien CY, Huang SC, Yin YF, Huang CC, et al. (2010) Fibulin-3 is associated with tumour progression and a poor prognosis in nasopharyngeal carcinomas and inhibits cell migration and invasion via suppressed AKT activity. J Pathol 222: 367–379. [DOI] [PubMed] [Google Scholar]
- 23. Nomoto S, Kanda M, Okamura Y, Nishikawa Y, Qiyong L, et al. (2010) Epidermal growth factor-containing fibulin-like extracellular matrix protein 1, EFEMP1, a novel tumor-suppressor gene detected in hepatocellular carcinoma using double combination array analysis. Ann Surg Oncol 17: 923–932. [DOI] [PubMed] [Google Scholar]
- 24. Zhang CZ, Liu L, Cai M, Pan Y, Fu J, et al. (2012) Low SIRT3 expression correlates with poor differentiation and unfavorable prognosis in primary hepatocellular carcinoma. PLoS One 7: e51703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shen Q, Fan J, Yang XR, Tan Y, Zhao W, et al. (2012) Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol 13: 817–826. [DOI] [PubMed] [Google Scholar]
- 26. Liu L, Zhang CZ, Cai M, Fu J, Chen GG, et al. (2012) Downregulation of polo-like kinase 4 in hepatocellular carcinoma associates with poor prognosis. PLoS One 7: e41293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Song EL, Hou YP, Yu SP, Chen SG, Huang JT, et al. (2011) EFEMP1 expression promotes angiogenesis and accelerates the growth of cervical cancer in vivo. Gynecol Oncol 121: 174–180. [DOI] [PubMed] [Google Scholar]
- 28. Camaj P, Seeliger H, Ischenko I, Krebs S, Blum H, et al. (2009) EFEMP1 binds the EGF receptor and activates MAPK and Akt pathways in pancreatic carcinoma cells. Biol Chem 390: 1293–1302. [DOI] [PubMed] [Google Scholar]
- 29. Hu B, Nandhu MS, Sim H, Agudelo-Garcia PA, Saldivar JC, et al. (2012) Fibulin-3 promotes glioma growth and resistance through a novel paracrine regulation of Notch signaling. Cancer Res 72: 3873–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu Y, Pioli PD, Siegel E, Zhang Q, Nelson J, et al. (2011) EFEMP1 suppresses malignant glioma growth and exerts its action within the tumor extracellular compartment. Mol Cancer 10: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Albig AR, Neil JR, Schiemann WP (2006) Fibulins 3 and 5 antagonize tumor angiogenesis in vivo. Cancer Res 66: 2621–2629. [DOI] [PubMed] [Google Scholar]
- 32. Kim EJ, Lee SY, Woo MK, Choi SI, Kim TR, et al. (2012) Fibulin-3 promoter methylation alters the invasive behavior of non-small cell lung cancer cell lines via MMP-7 and MMP-2 regulation. Int J Oncol 40: 402–408. [DOI] [PubMed] [Google Scholar]
- 33. Diersch S, Wenzel P, Szameitat M, Eser P, Paul MC, et al. (2013) Efemp1 and p27(Kip1) modulate responsiveness of pancreatic cancer cells towards a dual PI3K/mTOR inhibitor in preclinical models. Oncotarget 4: 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sercu S, Lambeir AM, Steenackers E, El Ghalbzouri A, Geentjens K, et al. (2009) ECM1 interacts with fibulin-3 and the beta 3 chain of laminin 332 through its serum albumin subdomain-like 2 domain. Matrix Biol 28: 160–169. [DOI] [PubMed] [Google Scholar]
- 35. Klenotic PA, Munier FL, Marmorstein LY, Anand-Apte B (2004) Tissue inhibitor of metalloproteinases-3 (TIMP-3) is a binding partner of epithelial growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1). Implications for macular degenerations. J Biol Chem 279: 30469–30473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)