Introduction
Radiotherapy is a critical component of breast cancer treatment, and nearly half of all breast cancer patients receive radiotherapy 1. During the course of external beam radiotherapy for breast cancer, the vast majority of patients (74%–100%) will report some degree of skin toxicity 2–5, which generally presents as erythema (redness, warmth, rash-like appearance), dry desquamation (dryness, itching, peeling), or moist desquamation (moist, oozing, tender, redness and exposure of the dermis) 6–9. Literature indicates that these skin changes can be associated with sensations of pain, burning, itching, pulling, tenderness, and increased sensitivity 10,11. Furthermore, skin toxicity is associated with impairments in quality of life including: fatigue, body image disturbance, sleep problems, emotional distress, reduced treatment satisfaction, and changes in day-to-day functioning 3,4,12.
During the course of radiotherapy, patients seek out and use a wide variety of self-care and symptom management strategies to cope with skin toxicity 12,13. Many of these patient-initiated approaches are associated with out-of-pocket expenditures. For example, patients have been found to: purchase items to reduce discomfort, buy more comfortable clothes/undergarments, replace clothes/bras which have been stained or otherwise damaged by prescribed skin care products, buy cosmetic products to conceal skin color changes, and use complementary/alternative medicine (CAM) approaches for symptom and side-effect control 12. Yet to date, we have no clear estimate of the economic burden associated with skin toxicity.
Published studies examining the “hidden costs” of cancer have not addressed the cost issues of breast cancer radiotherapy patients experiencing skin toxicity 14. Furthermore, a recent review of patient-rated measures of skin toxicity revealed no measures assessing out-of-pocket costs to patients 15. This gap in the literature is concerning because nonmedical out-of-pocket spending related to skin toxicity is not only important in and of itself, but could also be an important outcome variable in evaluating skin toxicity prevention and control interventions 16. For example, if a new cream were developed to help patients manage skin toxicity, which did not stain bras/clothes, it could lead to less out-of pocket spending, and consequently be more attractive to patients. Similarly, it will be important to note whether new accelerated radiotherapy regimens to the whole breast or partial breast radiotherapy 17,18 not only reduce the overall treatment time and incidence/duration of skin toxicity, but also reduce associated out-of-pocket spending.
To address this gap in the literature, our group developed a new scale, the “Skin Toxicity Costs” (STC) questionnaire, based on our previously published qualitative research 12. The STC assesses direct nonmedical out-of-pocket costs associated with skin toxicity in women undergoing breast cancer radiotherapy. Direct nonmedical costs are those expenses which occur as a result of breast cancer (including expenditures for symptom management), but do not include medical services. So for example, direct nonmedical costs might include CAM use, new clothing related to treatment side effects, or purchasing over the counter creams 19,20.
The primary aim of the present descriptive, exploratory study was to assess the feasibility of using the STC with breast cancer radiotherapy patients. Secondary aims were to: assess the utility of the STC in providing an estimate of the magnitude and range of nonmedical out-of-pocket costs associated with skin toxicity from the individual perspective; examine the specific nature of the costs associated with acute skin toxicity; explore potential background predictors of personal expenditures; and explore the relationship between patient-reported dermatologic quality of life and expenditures.
Methods
Design
This retrospective study was designed as a one-time survey of breast cancer radiotherapy patients who were in their fifth week of radiotherapy. The fifth week was chosen for three reasons: 1) literature suggests that 100% of patients will experience skin toxicity by this point in their treatment 3; 2) it is close to the end of treatment, which enables participants to reflect back over their entire radiotherapy experience; and 3) after week 5, patients’ treatment plans begin to differ – some patients will go on to receive a radiotherapy boost, some will not. Consequently, week 5 data collection allowed for the largest and most homogenous sample.
Participants
Participants were recruited from three radiation oncology clinics: at Mount Sinai Medical Center (n=31), at Roswell Park Cancer Institute (n=9), and at Weill Cornell Medical Center (n=10). In total, this convenience sample consisted of 50 participants. See Table 1 for descriptive information on the sample. All participants were treated between January 2009 and June 2010.
Table 1.
Sample Characteristics
| Descriptive Information | |
|---|---|
| Age Range: 36–84 years | M(SD) |
| 54.88 (11.84) | |
|
| |
| Race | n (%) |
| White | 39 (78%) |
| Other | 8 (16%) Black, 3 (6%) Other |
|
| |
| Ethnicity | |
| Hispanic | 3 (6%) |
| Non-Hispanic | 47 (94%) |
|
| |
| Education | |
| College degree or post-graduate degree | 34 (68%) |
| < College degree | 16 (32%) |
|
| |
| Marital Status | |
| Currently married | 32 (64%) |
| Not currently married | 18 (36%) |
|
| |
| Employment | |
| Full-time | 26 (52%) |
| Less than full-time | 24 (48%) |
|
| |
| Income | |
| < $60,000 | 20 (40%) |
| ≥ $60,000 | 30 (60%) |
|
| |
| Previous breast cancer surgery | |
| Lumpectomy | 35 (70%) |
| Mastectomy | 15 (30%) |
|
| |
| Prior chemotherapy | |
| Yes | 28 (56%) |
| No | 22 (44%) |
|
| |
| Cancer Stage | |
| 0 | 13 (26%) |
| I | 19 (38%) |
| II or III | 18 (36%) |
Eligibility criteria for the present study included being: scheduled for a standard (5–7 week) course of external beam radiotherapy for breast cancer, able to speak and read English (as the measures were all in English); over age 18; female; willing to complete study assessments; and Stage 0, I, II or III breast cancer. Exclusion criteria were having any co-morbid major psychiatric diagnoses (e.g., any disorder with psychosis) or significant cognitive impairment (identified by physician) which could render women unable to follow the study procedures or give informed consent (as determined by medical chart review), or having metastatic disease.
This study was approved by the Institutional Review Board at each of the three sites, and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helskini. All participants gave their informed consent prior to their inclusion in the study.
Measures
Skin Toxicity Costs (STC) questionnaire
As noted above, no standardized or validated measure exists to assess out-of-pocket costs associated with acute skin toxicity in breast cancer radiotherapy patients. Therefore, we developed the STC, based on our previously published qualitative research 12, to evaluate such costs.
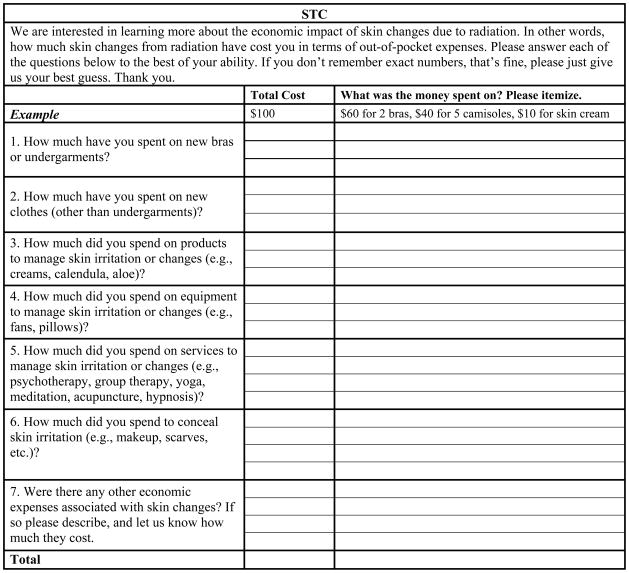
The STC is a 7-item, open-ended self-report survey (see Figure 1). For each item, patients are asked to report: a) amount spent in dollars, and b) nature of the expenditure (i.e., on what the money was spent). Items inquire about the purchase of new undergarments, new clothes, new products/equipment/services to manage skin toxicity, and items bought to conceal skin changes. There is a final question asking “Were there any other economic expenses associated with skin changes? If so please describe, and let us know how much they cost.” Patients’ responses are restricted to the active radiotherapy treatment period, and do not include costs associated with other breast cancer treatments (e.g., mastectomy bras and wigs are excluded).
Fig 1.
Skin Toxicity Costs Questionnaire
In order to ensure that the scale was understandable to most patients, readability was evaluated. Specifically, we used the Tests Document Readability online utility (http://www.online-utility.org/english/readability_test_and_improve.jsp). This utility calculates the US grade level needed to understand the text. As recommended, indices were averaged to yield an overall readability statistic 21,22. Specifically, the Coleman Liau index, the Flesh Kincaid Grade Level, the ARI (Automated Readability Index) and the SMOG index were used. Based on these indices, the average grade level needed to understand the STC is 6.5. This result is consistent with guidelines suggesting that printed materials should be at or below an 8th grade reading level 23.
Background questionnaire
Patients were also asked to complete a ten-item background questionnaire inquiring about: age, cancer stage, chemotherapy history, surgical history, race, ethnicity, marital status, employment, education, and household income.
The Skindex 16 24 is a 16-item measure of dermatologic quality of life. This measure has demonstrated test-retest reliability (r=0.88–0.90), good internal consistency (Cronbach’s alpha = 0.86–0.93), and both content and construct validity. The Skindex 16 has 3 subscales – Emotion (e.g., worry about your skin condition), Symptoms (e.g., your skin condition itching), and Functioning (e.g., the effects of your skin condition on your daily activities). Each item is rated from 0=Never Bothered to 6=Always Bothered, “during the past week.” The measure has previously been used in studies of skin toxicity in breast cancer radiotherapy patients 25.
Procedure
Patients scheduled for radiotherapy for primary breast cancer were referred by their radiation oncologist. Patients who agreed to participate were told that they would be given the STC and the background questionnaire to complete on a day convenient for them in their fifth week of treatment. On that day, research assistants met the patients, distributed the questionnaires, and gave patients the option of completing the measures in a private room in the radiation oncology clinic or completing them at home and returning them the next day. Both research assistants and referring physicians were available to answer any participant questions.
Data analyses
All analyses were performed using SAS 9.2 26. If any cost item had missing data, we assigned it a dollar value of zero to be conservative in our estimates. In a preliminary step, we checked for between-site differences; that is, whether costs differed significantly between Mount Sinai, Weill Cornell, and Roswell Park. No significant between-site differences were found in terms of costs (F (2, 47) = .70; p = .50). Therefore, all further analyses were conducted on the combined sample.
Results
Primary Aim
Feasibility
In this preliminary study, the STC proved practical and feasible to administer. Participants were able to complete the questionnaire in less than 5 minutes, and no eligible patient refused to complete the measure.
Secondary Aims
Description of the magnitude and range of nonmedical out-of-pocket costs associated with skin toxicity
Table 2 presents descriptive statistics on the entire sample, as well as on the subsample of women who spent more than $0. Results indicate 94% reported some direct costs (e.g., money spent to manage skin toxicity). Mean direct costs in the entire sample were $131.64 (95% CI: 84.05–179.23).
Table 2.
Summary of Patient Expenditures in US Dollars.
| n | % total n | Mean $ | SD $ | Median $ | Min $ | Max $ | |
|---|---|---|---|---|---|---|---|
| All women | 50 | 100% | 131.64 | 167.44 | 75.00 | 0.00 | 770.00 |
| All women who spent >$0 | 47 | 94% | 140.04 | 169.30 | 75.00 | 1.00 | 770.00 |
Examination of the specific nature of the costs associated with acute skin toxicity
An item level analysis yielded information on distributions of spending, as well as on the nature of the spending (see Table 3). From the quantitative perspective, results indicate that the most frequently endorsed items were STC#1 (new undergarments) and STC#3 (products to manage skin toxicity). The least frequently endorsed items were STC#4 (equipment), STC#5 (CAM and other services), STC#6 (concealment), and STC#7 (other costs).
Table 3.
| STC Item and Reported Expenditures | Frequency by Cost | Total Cost Range | |
|---|---|---|---|
| 1. Undergarments: New bras (including sports bras, bras without underwire), New camisoles, New pajamas, Undershirts, Replacing bras stained by creams, Replacing bras stained by color treatment markers, Replacing bras which are uncomfortable | $0 | 42% | $0–$305 |
| $1–$100 | 34% | ||
| >$100 | 24% | ||
| 2. Clothes: New t-shirts, New cotton clothing (instead of wool) | $0 | 76% | $0–$400 |
| $1–$100 | 20% | ||
| >$100 | 4% | ||
| 3. Products to manage toxicity: Herbal/Mineral (Aloe, Vitamin E, Calendula, Arnica, French Green clay), Over the Counter (Eucerin, Vaseline, sunscreen, Medline remedy cream, baby shampoo, Cortaid, shea and honey moisturizer, Lubriderm, Noxema cold cream), “Natural” (natural soap, natural cream), Prescribed (Aquaphor, Xeroform bandages, non-adhesive gauze, Xclair, Cortisone) | $0 | 34% | $0–$400 |
| $1–$100 | 60% | ||
| >$100 | 6% | ||
| 4. Equipment to manage toxicity: Pillows to support the breast (including body pillows, u-shaped pillows, moist allergy pillows), Nursing Pads, Frozen Peas | $0 | 90% | $0–$45 |
| $1–$100 | 10% | ||
| >$100 | 0% | ||
| 5. CAM and other services: Acupuncture, Group therapy | $0 | 98% | $0–$450 |
| $1–$100 | 0% | ||
| >$100 | 2% | ||
| 6. Concealment: Scarves | $0 | 96% | $0–$50 |
| $1–$100 | 4% | ||
| >$100 | 0% | ||
| 7. Other expenses: Cab rides | $0 | 94% | $0–$81 |
| $1–$100 | 6% | ||
| >$100 | 0% | ||
Exploration of potential background predictors
Examination of the distribution of costs revealed that (as is typical of cost data) the data were positively skewed and leptokurtic. Therefore, we used the BOXCOX transformation in PROC TRANSREG in SAS 26 to transform the data. Box-Cox 27 transformations, one of the most common types of transformations, are used to identify optimal transformations of a dependent variable. However, in order for this procedure to produce successful results, all values of the dependent variable (i.e., costs) must be greater than zero. Consequently, we added a constant (0.0001) to all values. We then proceeded with running PROC TRANSREG, with the default LAMBDA= list of –3 TO 3 by increments of 0.25. Results revealed an optimal lambda of 0.25. Accordingly, we transformed the cost data by raising them to the power of .25. These transformations reduced skewness and kurtosis to within acceptable limits, and all following inferential statistics were performed on transformed data.
The relationship between background factors and transformed costs were examined using univariate and multiple linear regression analyses (note: ethnicity was excluded from these analyses as there were too few Hispanic women in the sample). Results of univariate analyses revealed that race, education, and household income were significantly related to costs (see Table 4) such that Whites spent more than other races, more educated women spent more, and those with higher household income spent more. Effect sizes for race and household income were in the small range 28. The effect size for education was in the medium range.
Table 4.
Univariate relationships between background variables and costs.
| COSTS | |||||
|---|---|---|---|---|---|
| F | df | p | R2 | f 2 | |
| Age | 0.81 | 1, 48 | .374 | .017 | .017 |
| Race | 5.78 | 1, 48 | .020 | .108 | .121 |
| Education | 7.97 | 1, 48 | .007 | .143 | .166 |
| Marital Status | 0.34 | 1, 48 | .563 | .007 | .007 |
| Employment | 0.31 | 1, 48 | .578 | .007 | .007 |
| Income | 4.33 | 1, 48 | .043 | .083 | .090 |
| Surgery Type | 0.93 | 1, 48 | .934 | .000 | .000 |
| Prior Chemotherapy | 0.01 | 1, 48 | .940 | .000 | .000 |
| Cancer Stage | 0.31 | 2, 47 | .732 | .013 | .013 |
Note: An f2 of .02 is considered small, .15 is considered medium, and .35 is considered large. Shaded cells in this table are significant at p < .05.
We then entered all three significant predictors of costs (race, household income, education) into the same model. Initially, we assessed for multicollinearity by examining tolerance and the Variance Inflation Factor (VIF). Generally, a tolerance value of less than 0.1 and a VIF of greater than 10 are considered to suggest that multicollinearity may be an issue. In our case, all tolerance values were greater than 0.1 (race=.87, education=.59, salary=.66), and all VIF values were less than 10 (race=1.15, education=1.69, salary=1.51) indicating that multicollinearity was not a concern. The model revealed that together, race, household income, and education accounted for nearly 26% of the variance in direct costs [F(3, 46) = 5.33, p =.003; R2 = .258]. When examining the unique contributions of each variable, neither race [F(1,46)=1.74, p=.193] nor household income [F(1,46)=0.04, p=.850] contributed to direct costs. Education did uniquely contribute to the prediction of direct costs [F(1, 46)=5.79, p=.020]. Results revealed that more educated women (with at least a college degree) spent more money related to their skin toxicity. This analysis controlled for other variables in the model.
Exploration of potential relationship between dermatologic quality of life and expenditures
Using the transformed cost data, we correlated direct costs with the three subscales of the Skindex-16: Emotion, Symptoms, and Functioning. We found that direct costs were significantly associated with Skindex-16 Functioning (r=.27, p=.050), but were not significantly associated with Skindex-16 Emotion (r=.02, p=.891) or Skindex-16 Symptoms (r=.21, p=.114). Greater impairment in Functioning was associated with greater costs.
Discussion
Results of the present study reveal the hidden, nonmedical, out-of-pocket costs associated with acute skin toxicity in the context of a traditional course of breast cancer radiotherapy. Patient out-of-pocket costs are an important component of patients’ experiences 19, yet these costs are rarely included in breast cancer cost-of-illness studies 29. To our knowledge, this study is the first to quantify individual costs associated with this particular treatment side-effect, as well as the first to present a scale specifically designed to assess such costs. Broadly speaking, results indicate that: 1) the STC is a practical, brief, easy-to-administer measure; 2) skin toxicity is associated with patient financial burden; 3) the STC is a useful measure of skin toxicity-related costs, and can indicate specific areas of patient expenditures and need; 4) education significantly predicts patient expenditures; and 5) impaired functioning due to skin toxicity was significantly associated with increased direct costs. Each point and associated implications will be discussed below.
First, the STC adds to the extant scales to assess nonmedical costs in cancer (e.g., COIN form 19, Economic Impact of Breast Cancer measure 30) by uniquely assessing costs associated with skin toxicity in breast cancer radiotherapy patients. The feasibility results show that the STC is brief, acceptable to patients, readable, that it yields valuable information on nonmedical costs, and that it is sensitive to individual differences in spending.
Second, nearly all women (94%) reported at least some out-of-pocket costs associated with skin toxicity. This result is consistent with reports indicating that nearly all women report skin toxicity during radiotherapy 2–5.
Third, results demonstrate that the types of patient spending most greatly affected by skin toxicity are spending on new undergarments (specifically new bras) and spending on products to directly manage skin toxicity. In terms of undergarments, the “type of expenditure” responses to STC item 1 suggest that patients are generally buying new bras either to be more comfortable (e.g., bras without underwire are less irritating) or to replace old ones which have been stained/damaged by prescribed creams or treatment markers. These data suggest that patients could potentially benefit from skin toxicity management approaches which are less damaging to clothing. In terms of products, as can be seen in Table 3, patients reported using 21 different products to try to manage skin changes on their own. This data is consistent with qualitative reports 12, and suggests both that patients are actively searching for ways to ameliorate this side effect and that current skin-toxicity management approaches may be insufficient to meet all of patients’ needs.
Fourth, the results revealed that education level was the only variable which was uniquely and significantly associated with patient spending. These results are consistent with research which has shown increased education to be significantly related to increased alternative or complementary healthcare use 31,32. Past literature has hypothesized that increased education may increase the chances that people will: 1) be exposed to various types of healthcare through their own reading on the subject; 2) educate themselves about their illnesses and the variety of possible treatments; and/or 3) question the authority of conventional practitioners. Future research, with larger sample sizes, should focus on better understanding the education-spending relationship 31.
Fifth, the results revealed that higher levels of participant spending were associated with greater functional impairment due to skin toxicity. Interestingly, spending was not associated with either emotional reactions to skin changes or with skin symptoms. This suggests that patients are spending money more to help minimize the effects of skin toxicity on daily living, and less to feel better (either emotionally or physically). Future research should explore this issue further, and work to identify which aspects of functional impairments lead to increased spending.
The present study is not without its limitations. First, this study used a relatively small sample size. This sample size was sufficient to achieve our primary aim of testing the feasibility of using the STC to describe nonmedical costs associated with skin toxicity feasibility. However, future research should use larger samples to more precisely specify the cost estimates obtained here. Second, the STC was only administered to women undergoing a traditional course of breast cancer radiotherapy (in the fifth week of treatment), and excluded women who were treated with hypofractionated regimens. As recent randomized trials support the regular use of hypofractionated regimens for adjuvant whole-breast radiotherapy in some women with early breast cancer 33,34, future research should consider including both women undergoing standard and hypofractionated regimens. In this way, the STC could be used to compare out-of-pocket costs between the two radiotherapy regimens. Such research should include assessment points both during radiotherapy as well as in the weeks afterwards. Third, the present study relied entirely on patient self-report data. We recognize that a more stringent approach may have been to ask patients to save and return receipts to verify expenditures. However, we agree with other authors 30 that such an approach has the potential to be overly burdensome for patients, as well as the potential to lead to inadvertent disclosure of personal financial information (e.g., credit card account information). Fourth, the data were collected in a retrospective fashion, and results were based on patient recall. Future research should consider collecting STC data prospectively (e.g., starting at the beginning of treatment and on a weekly basis afterwards) to limit any potential recall bias 35. Fifth, the sample is unique in that each of the three institutions from which the sample was drawn has implemented some cost-saving measures for patients (e.g., some creams are provided to patients free of charge, coupons are provided in the clinic for some of the creams). Therefore, it should be noted that the present results may actually underestimate out-of-pocket costs associated with skin toxicity due to these clinic programs. It is not clear whether such procedures are standard practice across institutions, and therefore generalizability of the present findings should be examined in future research. Sixth, future studies may wish to expand the use of the STC beyond the radiotherapy treatment period in order to capture additional expenditures associated with late effects of radiotherapy.
After future replication studies, we anticipate that the STC could be used as an outcome variable (e.g., to facilitate cost-effectiveness analyses, to allow for cost comparisons across different treatment or skin management regimens 30,35), as a behavioral indicator of symptom burden (e.g., perhaps those women who suffer more functional impairments spend more to ameliorate such impairments), as one component of quality of life (e.g., a component of “economic well-being”), or as part of a needs assessment (e.g., do patients need help paying for bras, for creams, etc?). Additionally, the information provided by the STC may help patients plan more accurately for their radiation treatment experience, and may help healthcare professionals inform their patients about what costs to expect 30,35,35.
Acknowledgments
Preparation of this manuscript was supported by the National Cancer Institute (K07 CA131473) and by the American Cancer Society (RSGPB-04-213-01-CPPB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, National Institutes of Health, or American Cancer Society. We would like to express our sincere gratitude to Ms. Bari Scheckner, Ms. Bianca Love, and Ms. Frances Harfouche for their invaluable help in the data collection. Most importantly, we would like to thank all of the study participants for so graciously sharing their experiences.
Footnotes
Conflict of Interest
None of the authors have a financial relationship with the NCI which sponsored the research. Authors have full control of all primary data, and we agree to allow the journal to review the data if requested.
References
- 1.National Cancer Database [database online] Chicago, IL: American College of Surgeons; 2006. [Google Scholar]
- 2.Wengstrom Y, Haggmark C, Strander H, Forsberg C. Perceived symptoms and quality of life in women with breast cancer receiving radiation therapy. Eur J Oncol Nurs. 2000;4(2):78–88. doi: 10.1054/ejon.1999.0052. [DOI] [PubMed] [Google Scholar]
- 3.Knobf MT, Sun Y. A longitudinal study of symptoms and self-care activities in women treated with primary radiotherapy for breast cancer. Cancer Nurs. 2005;28(3):210–218. doi: 10.1097/00002820-200505000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Berthelet E, Truong PT, Musso K, et al. Preliminary reliability and validity testing of a new Skin Toxicity Assessment Tool (STAT) in breast cancer patients undergoing radiotherapy. Am J Clin Oncol. 2004;27(6):626–631. doi: 10.1097/01.coc.0000138965.97476.0f. [DOI] [PubMed] [Google Scholar]
- 5.Freedman GM, Li T, Nicolaou N, Chen Y, Ma CC, Anderson PR. Breast intensity-modulated radiation therapy reduces time spent with acute dermatitis for women of all breast sizes during radiation. Int J Radiat Oncol Biol Phys. 2009;74(3):689–694. doi: 10.1016/j.ijrobp.2008.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 7.Harper JL, Franklin LE, Jenrette JM, Aguero EG. Skin toxicity during breast irradiation: pathophysiology and management. South Med J. 2004;97(10):989–993. doi: 10.1097/01.SMJ.0000140866.97278.87. [DOI] [PubMed] [Google Scholar]
- 8.Mendelsohn FA, Divino CM, Reis ED, Kerstein MD. Wound care after radiation therapy. Adv Skin Wound Care. 2001;15(5):216–224. doi: 10.1097/00129334-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 9.McQuestion M. Evidence-based skin care management in radiation therapy. Semin Oncol Nurs. 2006;22(3):163–173. doi: 10.1016/j.soncn.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28–46. doi: 10.1016/j.jaad.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 11.Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31(5):1171–1185. doi: 10.1016/0360-3016(94)00423-I. [DOI] [PubMed] [Google Scholar]
- 12.Schnur JB, Ouellette SC, DiLorenzo TA, Green S, Montgomery GH. A qualitative analysis of acute skin toxicity among breast cancer radiotherapy patients. Psycho-Oncology. 2011;20(3):260–268. doi: 10.1002/pon.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wengstrom Y, Haggmark C, Forsberg C. Coping with radiation therapy: strategies used by women with breast cancer. Cancer Nurs. 2001;24(4):264–271. doi: 10.1097/00002820-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Wagner L, Lacey MD. The hidden costs of cancer care: an overview with implications and referral resources for oncology nurses. Clin J Oncol Nurs. 2004;8(3):279–287. doi: 10.1188/04.CJON.279-287. [DOI] [PubMed] [Google Scholar]
- 15.Schnur JB, Love B, Scheckner BL, Green S, Wernicke AG, Montgomery GH. A systematic review of patient-rated measures of radiodermatitis in breast cancer radiotherapy. American Journal of Clinical Oncology. doi: 10.1097/COC.0b013e3181e84b36. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konski A, Watkins-Bruner D. The RTOG Outcomes Model: economic end points and measures. Expert Opin Pharmacother. 2004;5(3):513–519. doi: 10.1517/14656566.5.3.513. [DOI] [PubMed] [Google Scholar]
- 17.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 18.Hopwood P, Haviland JS, Sumo G, Mills J, Bliss JM, Yarnold JR. Comparison of patient-reported breast, arm, and shoulder symptoms and body image after radiotherapy for early breast cancer: 5-year follow-up in the randomised Standardisation of Breast Radiotherapy (START) trials. Lancet Oncol. 2010;11(3):231–240. doi: 10.1016/S1470-2045(09)70382-1. [DOI] [PubMed] [Google Scholar]
- 19.Sherman EJ, Pfister DG, Ruchlin HS, et al. The Collection of Indirect and Nonmedical Direct Costs (COIN) form: a new tool for collecting the invisible costs of androgen independent prostate carcinoma. Cancer. 2001;91(4):841–853. doi: 10.1002/1097-0142(20010215)91:4<841::AID-CNCR1072>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Moore KA. Breast cancer patients’ out-of-pocket expenses. Cancer Nurs. 1999;22(5):389–396. doi: 10.1097/00002820-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Friedman DB, Hoffman-Goetz L. A systematic review of readability and comprehension instruments used for print and web-based cancer information. Health Educ Behav. 2006;33(3):352–373. doi: 10.1177/1090198105277329. [DOI] [PubMed] [Google Scholar]
- 22.Jensen SA, Fabiano GA, Lopez-Williams A, Chacko A. The reading grade level of common measures in child and adolescent clinical psychology. Psychol Assess. 2006;18(3):346–352. doi: 10.1037/1040-3590.18.3.346. [DOI] [PubMed] [Google Scholar]
- 23.Cotugna N, Vickery CE, Carpenter-Haefele KM. Evaluation of literacy level of patient education pages in health-related journals. J Community Health. 2005;30(3):213–219. doi: 10.1007/s10900-004-1959-x. [DOI] [PubMed] [Google Scholar]
- 24.Chren MM, Lasek RJ, Sahay AP, Sands LP. Measurement properties of skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5(2):105–110. doi: 10.1007/BF02737863. [DOI] [PubMed] [Google Scholar]
- 25.Schmuth M, Wimmer MA, Hofer S, et al. Topical corticosteroid therapy for acute radiation dermatitis: a prospective, randomized, double-blind study. Br J Dermatol. 2002;146(6):983–991. doi: 10.1046/j.1365-2133.2002.04751.x. [DOI] [PubMed] [Google Scholar]
- 26.SAS (r) Proprietary Software 9.2 [computer program] Cary, N.C: SAS Institute Inc; 2009. [Google Scholar]
- 27.Box GEP, Cox DR. An Analysis of Transformations. Journal of the Royal Statistics Society, Series B. 1964;26:211–234. [Google Scholar]
- 28.Cohen JA. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 29.Campbell JD, Ramsey SD. The costs of treating breast cancer in the US: a synthesis of published evidence. Pharmacoeconomics. 2009;27(3):199–209. doi: 10.2165/00019053-200927030-00003. [DOI] [PubMed] [Google Scholar]
- 30.Gordon L, Scuffham P, Hayes S, Newman B. Exploring the economic impact of breast cancers during the 18 months following diagnosis. Psychooncology. 2007;16(12):1130–1139. doi: 10.1002/pon.1182. [DOI] [PubMed] [Google Scholar]
- 31.Astin JA. Why patients use alternative medicine: results of a national study. JAMA. 1998;279(19):1548–1553. doi: 10.1001/jama.279.19.1548. [DOI] [PubMed] [Google Scholar]
- 32.Patterson RE, Neuhouser ML, Hedderson MM, et al. Types of alternative medicine use by patients with breast, colon, or prostate cancer: Predictors, motives, and costs. The Journal of Alternative and Complementary Medicine. 2002;8:477–485. doi: 10.1089/107555302760253676.. [DOI] [PubMed] [Google Scholar]
- 33.Yarnold J, Bentzen SM, Coles C, Haviland J. Hypofractionated whole-breast radiotherapy for women with early breast cancer: myths and realities. Int J Radiat Oncol Biol Phys. 2011;79(1):1–9. doi: 10.1016/j.ijrobp.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 34.Smith BD, Bentzen SM, Correa CR, et al. Fractionation for Whole Breast Irradiation: An American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Int J Radiat Oncol Biol Phys. 2011;81(1):59–68. doi: 10.1016/j.ijrobp.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 35.Houts PS, Lipton A, Harvey HA, et al. Nonmedical costs to patients and their families associated with outpatient chemotherapy. Cancer. 1984;53(11):2388–2392. doi: 10.1002/1097-0142(19840601)53:11<2388::AID-CNCR2820531103>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]