Abstract
(−)-Epigallocatechin gallate (EGCG) is the most abundant and biologically active polyphenol in green tea (Camellia sinensis) leaves and many of its cellular effects are consistent with its actions as a topoisomerase II poison. In contrast to genistein and several other related bioflavonoids that act as interfacial poisons, EGCG was the first bioflavonoid shown to act as a covalent topoisomerase II poison. Although studies routinely examine the effects of dietary phytochemicals on enzyme and cellular systems, they often fail to consider that many compounds are altered during cooking or cellular metabolism. To this point, the majority of EGCG (and related catechins) in green tea leaves is epimerized during the brewing process. Epimerization reverses the stereochemistry of the bond that bridges the B- and C-rings, and converts EGCG to (−)-gallocatechin gallate (GCG). Consequently, a significant proportion of EGCG that is ingested during the consumption of green tea is actually GCG. Therefore, the effects of GCG and related epimerized green tea catechins on human topoisomerase IIα and IIβ were characterized. GCG increased levels of DNA cleavage mediated by both enzyme isoforms with an activity that was similar to that of EGCG. GCG acted primarily by inhibiting the ability of topoisomerase IIα and IIβ to ligate cleaved DNA. Several lines of evidence indicate that GCG functions as a covalent topoisomerase II poison that adducts the enzyme. Finally, epimerization did not affect the reactivity of the chemical substituents (the three hydroxyl groups on the Bring) that were required for enzyme poisoning. Thus, the activity of covalent topoisomerase II poisons appears to be less sensitive to stereochemical changes than interfacial poisons.
INTRODUCTION
Phytochemicals are a rich source of compounds with anticancer and chemopreventive properties.1–3 A number of these chemicals, including bioflavonoids, curcumin, and isothionates, appear to exert at least some of their cellular effects through interactions with topoisomerase II,4–11 an essential enzyme that regulates DNA supercoiling and removes knots and tangles from the genetic material.12–17
Topoisomerase II-active phytochemicals alter enzyme activity by stabilizing covalent protein-cleaved DNA complexes (i.e., cleavage complexes) that are intermediates in the catalytic cycle of the enzyme.4–11 Because these compounds convert topoisomerase II to a cellular toxin that generates breaks in the genome, they are referred to as topoisomerase II poisons.14,18 This nomenclature is used to distinguish agents that increase levels of cleavage complexes from catalytic inhibitors, which function by impairing the overall enzymatic activity of type II topoisomerases.
Topoisomerase II poisons act by two distinct mechanisms and are categorized as either “interfacial” or “covalent” (previously know as redox-dependent).14,19,20 Interfacial topoisomerase II poisons, which include a number of widely prescribed anticancer drugs and several bioflavonoids, act at the protein-DNA interface in the vicinity of the active site tyrosine. They form non-covalent interactions with topoisomerase II and DNA within the ternary enzyme-DNA-poison complex.14,19,20 Most interfacial topoisomerase II poisons intercalate into the double helix at the cleaved scissile bond and increase levels of cleavage complexes by inhibiting the ability of the enzyme to ligate DNA.14,19,20
Covalent topoisomerase II poisons contain protein reactive groups, such as quinones (or hydroxyl substituents that can be converted to quinones), isothiocyanates, or maleimides.14,19,21,22 These chemicals originally were referred to as “redox-dependent topoisomerase II poisons” because many of the initial compounds that were identified underwent redox cycling (or oxidation/reduction reactions) as a prerequisite for activity.21,23 In contrast to interfacial compounds, covalent poisons adduct to the enzyme at amino acid residues (usually sulfhydryl residues) outside of the active site.11,24 Consequently, their ability to poison topoisomerase II can be abrogated by reducing agents such as dithiothreitol (DTT) or β-mercaptoethanol.21,23,25 Although covalent topoisomerase II poisons enhance enzyme-mediated DNA cleavage when added to the protein-DNA complex, they display the distinguishing feature of inhibiting topoisomerase II activity when incubated with the enzyme prior to the addition of DNA.21,23 It is notable that these latter two characteristics are not shared by interfacial topoisomerase II poisons.
The detailed mechanism by which covalent topoisomerase II poisons affect enzyme activity is not well understood. However, it appears to be related (at least in part) to the ability to close the N-terminal gate of the protein.24,26,27 In support of this hypothesis, quinones are believed to disrupt disulfide bridges that help keep the N-terminal gate of the enzyme open, and protein adduction results in a significant increase in gate closing.24,26,27 Closure of the N-terminal gate is known to enhance DNA bending and increase levels of DNA cleavage when the double helix is already complexed with topoisomerase II.28,29 In contrast, gate closure in the absence of DNA blocks the ability of plasmid molecules to enter the active site of the enzyme.26 Thus, the effects of quinones on the N-terminal gate provide a mechanistic basis for stabilizing pre-existing cleavage complexes (i.e., acting as a poison when added to enzyme-DNA complexes) while excluding DNA binding to unoccupied enzymes (i.e., acting as a catalytic inhibitor when added to topoisomerase II in the absence of DNA).
Green tea (Camellia sinensis) is one of the most widely consumed beverages in the world and is a rich source of bioflavonoids.1,30–33 Because of the antioxidant and reactive properties of dietary bioflavonoids, it is believed that they provide a number of health benefits to adults.1,30–34 To this point, green tea has been suggested to reduce the incidence of cardiovascular disease and cancers, such as breast, prostate, colorectal, and lung, in humans.1,30–34
(−)-Epigallocatechin gallate (EGCG) (Figure 1) is the most abundant and biologically active polyphenol in green tea leaves, and many of the chemopreventative properties of the beverage have been attributed to this compound.1,30–34 High concentrations of EGCG are cytotoxic to cultured mammalian cells and can trigger genotoxic events.35–38 In addition, this catechin inhibits cell proliferation, induces apoptosis, inhibits a number of protein kinases, and blocks the activation of several transcription factors.30,39 EGCG also enhances DNA cleavage mediated by topoisomerase II and many of its cellular effects are consistent with its actions as a topoisomerase II poison.4,8 In contrast to genistein and several related bioflavonoids that act as interfacial poisons, EGCG was the first bioflavonoid shown to act as a covalent topoisomerase II poison.8 The activity of EGCG against human type II topoisomerases is dependent on the presence of the three hydroxyl groups on the B-ring (Figure 1).8,9 Presumably, as the compound undergoes redox reactions, the phenolic B-ring is converted to a reactive quinone.
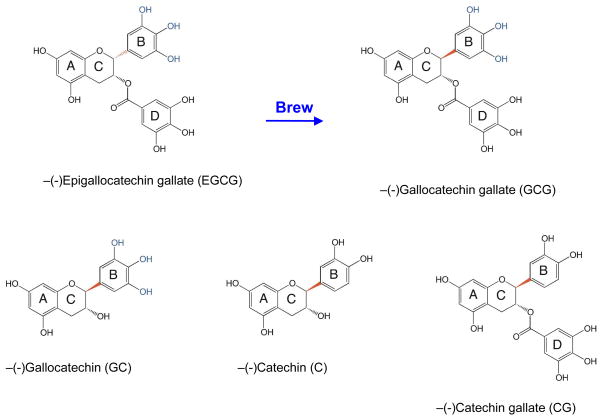
Figure 1.
Brewing epimerizes EGCG to GCG. The structures of additional epimerized green tea catechins (GC, CG, and C) formed during brewing are shown. The bond (shown in red) that changes orientation during brewing bridges the B- and C-rings. The three hydroxyl groups on the B-ring that are essential for activity as a covalent topoisomerase II poison are highlighted in blue.
Although studies routinely examine the effects of dietary phytochemicals on enzyme and cellular systems, they often fail to consider that many compounds are altered during cooking or cellular metabolism. For example, curcumin, the principal flavor and color component of turmeric (a common spice used in curries and a variety of other Asian cuisines), has no effect on human type II topoisomerases.40 However, the oxidative metabolites of curcumin are potent topoisomerase II poisons.40
Although EGCG is abundant in dried green tea leaves, ~50–63% of this compound (and related catechins) is epimerized during a 20 min brewing process.41,42 Epimerization reverses the stereochemistry of the bond that bridges the B- and C-rings (indicated in red in Figure 1), and converts EGCG to (−)-gallocatechin gallate (GCG).33,41,42 Consequently, a significant proportion of EGCG that is ingested during the consumption of green tea is actually GCG.
Therefore, the effects of GCG and related catechins on human topoisomerase IIα and IIβ were characterized. GCG enhanced DNA cleavage mediated by both enzyme isoforms with an activity that was similar to that of EGCG. Furthermore, GCG acted as a covalent topoisomerase II poison and epimerization did not affect the reactivity of the chemical substituents that are required for enzyme poisoning. Thus, the activity of covalent topoisomerase II poisons appears to be less sensitive to stereochemical changes than interfacial poisons.
EXPERIMENTAL PROCEDURES
Enzymes and Materials
Recombinant wild-type human topoisomerase IIα and IIβ were expressed in Saccharomyces cerevisiae and purified as described previously.43 Negatively supercoiled pBR322 DNA was prepared from Escherichia coli using a Plasmid Mega Kit (Qiagen) as described by the manufacturer. EGCG, GCG, (−)-gallocatechin (GC), (−)-catechin gallate (CG), and (−)-catechin (C) were purchased from LKT. 1,4-Benzoquinone, etoposide, and DTT were obtained from Sigma. All compounds were prepared as 20 or 50 mM stock solutions in 100% DMSO and stored at −20 °C.
DNA Cleavage
DNA cleavage reactions were performed using the procedure of Fortune and Osheroff.44 Assay mixtures contained 150 nM topoisomerase IIα or IIβ, 7.5 nM negatively supercoiled pBR322 DNA, 0–300 μM EGCG, GCG, GC, CG or C in 20 μL of DNA cleavage buffer [10 mM Tris-HCl, pH 7.9, 5 mM MgCl2, 100 mM KCl, 0.1 mM EDTA, and 2.5% (v/v) glycerol]. Assay buffer contained ~2 μM residual DTT that was carried over from the topoisomerase II storage buffer. Reactions were incubated for 6 min at 37 °C. In some cases, 0–15 min time courses were monitored. Enzyme-DNA cleavage intermediates were trapped by adding 2 μL of 5% SDS and 1 μL of 375 mM EDTA, pH 8.0. Proteinase K was added (2 μL of a 0.8 mg/mL solution), and reaction mixtures were incubated for 30 min at 45 °C to digest topoisomerase II. Samples were mixed with 2 μL of 60% sucrose in 10 mM Tris-HCl, pH 7.9, 0.5% bromophenol blue, and 0.5% xylene cyanol FF, heated for 2 min at 45 °C, and subjected to electrophoresis in 1% agarose gels in 40 mM Tris-acetate, pH 8.3, and 2 mM EDTA containing 0.5 μg/mL ethidium bromide. DNA cleavage was monitored by the conversion of negatively supercoiled plasmid DNA to linear molecules. DNA bands were visualized by ultraviolet light and quantified using an Alpha Innotech digital imaging system.
In assays that determined whether cleaved DNA was protein-linked, proteinase K treatment was omitted. To determine the reversibility of topoisomerase II-mediated DNA cleavage induced by GCG, EDTA (final concentration of 18 mM) was added to reaction mixtures prior to treatment with SDS. To examine the effects of a reducing agent on the actions of EGCG against human topoisomerase IIα or IIβ, 500 μM dithiothreitol (DTT) was incubated with 200 μM GCG, 25 μM 1,4-benzoquinone, or 50 μM etoposide for 5 min prior to initiating DNA cleavage reactions. Alternatively, DTT was added to reaction mixtures for 5 min following a 6 min DNA cleavage reaction.
To examine the effects of GCG on human topoisomerase IIα or IIβ in the absence of DNA, 200 μM GCG was incubated with 150 nM enzyme for 0–10 min at 37 °C in 15 μL of DNA cleavage buffer. Cleavage was initiated by the addition of 7.5 nM negatively supercoiled pBR322 DNA to the reaction mixture.
To determine whether GCG-enhanced topoisomerase II-mediated DNA cleavage was induced by the formation of DNA lesions, 200 μM GCG was incubated with 7.5 nM negatively supercoiled pBR322 DNA for 5 min at 37 °C in DNA cleavage buffer. The mixture was passed through a Bio-spin 6 column to remove the bioflavonoid and isolate the DNA. Cleavage was initiated by adding 150 nM enzyme to the DNA-containing reaction mixture in the absence or presence of an additional 200 μM GCG. Samples were incubated for 6 min at 37 °C and were processed and analyzed as described above.
Ligation of Cleaved DNA by Human Topoisomerase II
DNA ligation mediated by human topoisomerase IIα or IIβ was monitored according to the procedure of Byl et al.45 DNA cleavage/ligation equilibria were established for 10 min at 37 °C as described above for DNA cleavage assays in the absence of compound or in the presence of 200 μM GCG. DNA ligation was initiated by shifting samples from 37 to 0 °C. The shift to low temperature allows enzyme-mediated ligation but prevents new rounds of DNA cleavage from occurring. Thus, it results in a unidirectional sealing of the cleaved DNA. Reactions were stopped at time points up to 40 s by the addition of 2 μL of 5% SDS followed by 1 μL of 375 mM EDTA, pH 8.0. Samples were processed and analyzed as above. Ligation was monitored by the loss of linear DNA.
RESULTS AND DISCUSSION
Enhancement of Topoisomerase II-mediated DNA Cleavage by GCG
EGCG represents ~40–60% of the bioflavonoids in green tea leaves. However, the majority of the compound is converted to GCG during the brewing process (Figure 1).41,42 The only difference between EGCG and GCG is an epimerization of the bond (in red) that bridges the B- and C-rings. The Bring houses the three hydroxyl groups that are essential to the actions of EGCG as a topoisomerase II poison.8,9 Therefore, this epimerization has the potential to alter the spatial orientation of the B-ring and, hence, the activity of the bioflavonoid against the type II enzyme.
A number of studies have examined the effects of “minor” structural changes on the activities of interfacial topoisomerase II poisons. In some cases, movement of a single oxygen atom in etoposide or methoxy group in amsacrine by even one carbon can have major consequences and abolish activity.46,47 In other cases, the change in stereochemistry about a single bond in etoposide or in a tricyclic quinolone completely eliminates drug activity or even converts the interfacial poison into a catalytic inhibitor of topoisomerase II.48,49
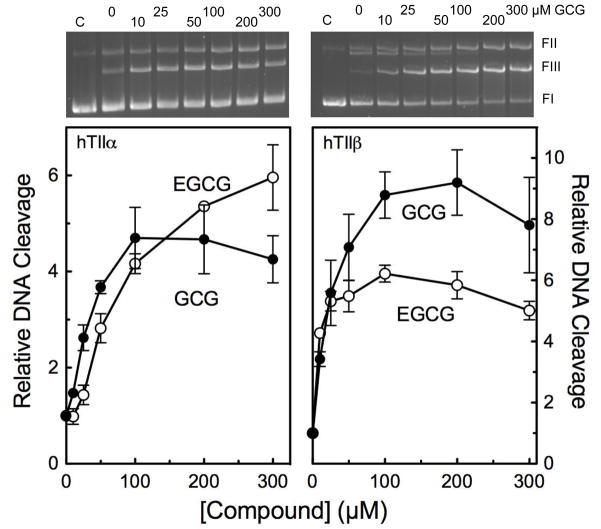
Corresponding stereochemistry studies with covalent topoisomerase II poisons have not been performed. Therefore, the effects of GCG on DNA cleavage mediated by human topoisomerase IIα and IIβ were examined (Figure 2). GCG increased levels of scission with both isoforms (~6- and 9-fold, respectively) over a wide range of concentrations. Results were similar to those seen with EGCG.
Figure 2.
GCG enhances DNA cleavage mediated by human topoisomerase IIα (hTIIα; left) and IIβ(hTIIβ; right) with an activity that is similar to that of its epimer, EGCG. DNA cleavage reactions were carried out in the presence of 0–300 μM EGCG (open circles) or GCG (closed circles). Levels of DNA cleavage were quantified from the band of linear DNA and expressed relative to reactions carried out in the absence of catechin. Error bars represent standard deviations for three or four independent experiments. Representative agarose gels of DNA cleavage reactions carried out in the presence GCG are shown at the top for the respective topoisomerase II isoform. FI represents negatively supercoiled circular DNA, FII represents nicked DNA, and FIII represents linear DNA.
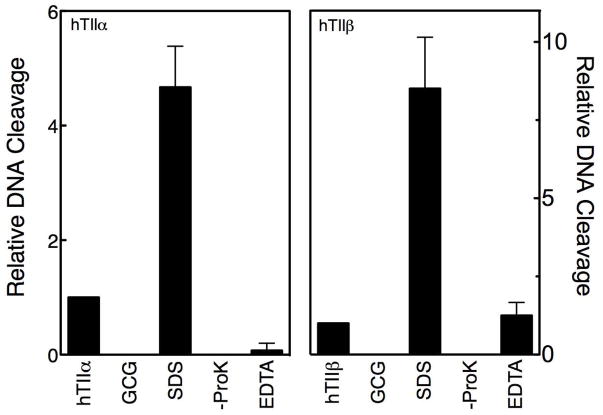
A series of control reactions was carried out to ensure that the DNA cleavage enhancement observed with GCG was mediated by topoisomerase II (Figure 3). No GCG-induced DNA scission was seen in the absence of enzyme. Furthermore, in the absence of proteinase K, the linear DNA band disappeared and was replaced by a band that remained at the origin of the gel. This latter experiment demonstrates that cleaved plasmids are covalently linked to the type II enzymes. Finally, with both topoisomerase IIα and IIβ, GCG-induced cleavage was reversed when reactions were incubated with EDTA (which chelates the essential active site Mg2+ ions and blocks re-cleavage of the DNA following ligation) prior to trapping cleavage complexes with SDS. This reversibility is inconsistent with an enzyme-independent reaction.
Figure 3.
GCG-induced DNA cleavage is mediated by topoisomerase II. Data for human topoisomerase IIα (hTIIα; left) and IIβ(hTIIβ; right) are shown. DNA cleavage control reactions were carried out in the absence of catechin (hTIIα or hTIIβ) or in the presence of 200 μM GCG but in the absence of enzyme (GCG). All other reactions contained topoisomerase II and 200 μM GCG. DNA cleavage reactions were terminated by the addition of SDS (SDS). To determine whether cleaved DNA was protein-linked, proteinase K treatment was omitted (-ProK). The reversibility of DNA cleavage was examined by adding EDTA prior to SDS (EDTA). The level of DNA cleavage in the absence of GCG was set to 1 and all other reactions were expressed relative to that value. Error bars represent standard deviations for three independent experiments.
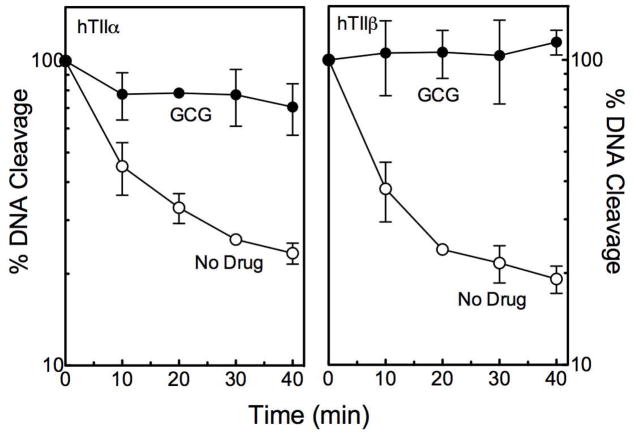
The DNA cleavage and ligation reactions of topoisomerase II exist in a tightly coupled equilibrium.12–14,16,17,50 Most interfacial topoisomerase II poisons, such as etoposide, increase levels of cleavage complexes primarily by inhibiting the DNA ligation activity of the enzyme.14,50,51 In contrast, bioflavonoid-based topoisomerase II poisons have variable effects on DNA ligation, ranging from modest (suggesting that the compound affects both the cleavage and ligation reactions) to strong.6,8 As seen in Figure 4, GCG strongly inhibits the ability of topoisomerase IIα and IIβ to ligate cleaved DNA.
Figure 4.
GCG inhibits topoisomerase II-mediated DNA ligation. Data for DNA ligation mediated by human topoisomerase IIα (hTIIα; left) and IIβ(hTIIβ; right) are shown. Reactions were carried out in the absence (open circles) or presence (closed circles) of 200 μM GCG. Ligation is expressed as the percent loss of linear DNA. Levels of cleaved DNA at the start of the reaction were set to 100%. Error bars represent standard deviations for three independent experiments.
GCG Is a Covalent Topoisomerase II Poison
GCG contains the same trihydroxy-B-ring that is critical for the actions of EGCG as a covalent topoisomerase II poison.6,8 In addition, GCG contains the same non-aromatic C-ring, which precludes binding in the protein-DNA pocket used by genistein and other interfacial topoisomerase II poisons.9 Thus, it is likely that GCG functions as a covalent topoisomerase II poison. However, several experiments were carried out to address this critical mechanistic issue.
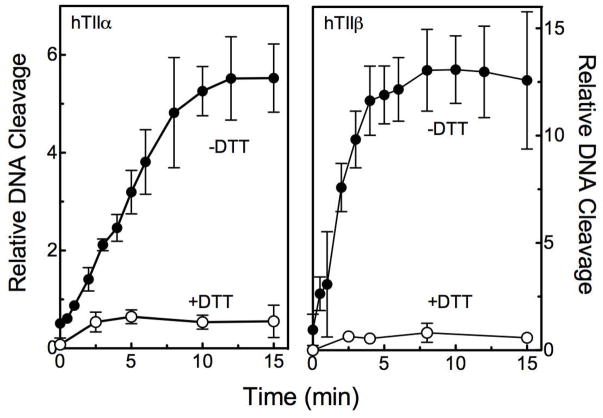
First, the effect of DTT on GCG activity was determined. This reducing agent should block the ability of the trihydroxy-B-ring to be converted to a quinone via redox cycling. Alternatively, if a quinone is formed, DTT could interact with the compound and deplete it from the reaction mixture. Thus, if GCG acts as a covalent poison, DTT should abrogate its ability to enhance topoisomerase II-mediated DNA cleavage. Time courses for GCG-induced DNA cleavage by topoisomerase IIα and IIβ in the absence and presence of DTT are shown in Figure 5. No DNA cleavage enhancement was seen for either enzyme isoform when reaction mixtures contained a molar excess of DTT over GCG. A similar lack of DNA cleavage enhancement was observed when 1,4-benzoquinone (a prototypical covalent topoisomerase II poison)23 was added to the DNA cleavage complex in the presence of DTT (Figure 6, top panels). In a parallel experiment with etoposide (an interfacial topoisomerase II poison), DTT did not affect levels of DNA cleavage (Figure 6, top panels).
Figure 5.
GCG is a covalent topoisomerase II poison. Time courses for DNA cleavage mediated by human topoisomerase IIα (hTIIα; left) and IIβ(hTIIβ; right) are shown. Reactions were carried out in the absence (closed circles) or presence (open circles) of 500 μM DTT. DNA cleavage values were quantified relative to reactions carried out in the absence of GCG for 6 min. Error bars represent the standard deviations for three independent experiments.
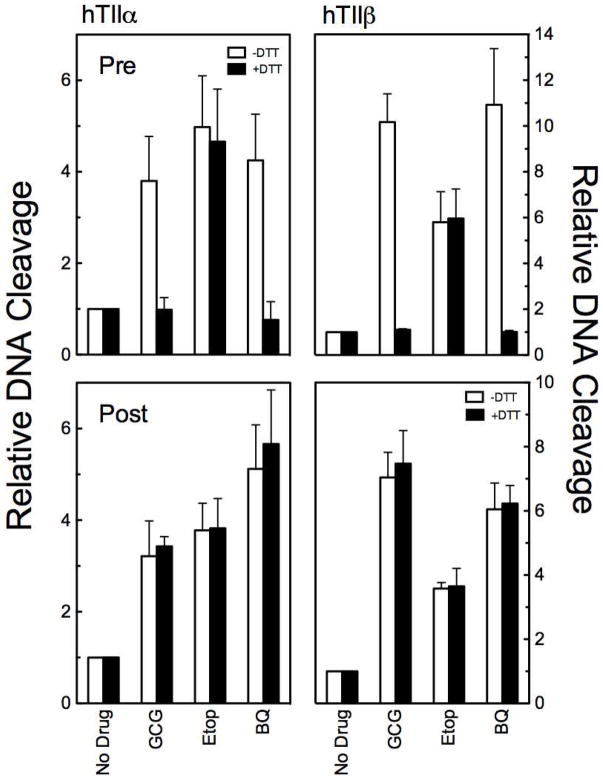
Figure 6.
GCG is a covalent topoisomerase II poison. DNA cleavage data is shown for human topoisomerase IIα (hTIIα; left) and IIβ(hTIIβ; right). Reactions were carried out in the absence (open bars) or presence (closed bars) of 500 μM DTT. Assays in the top panels (Pre) included DTT in reactions mixtures. Assays in the bottom panels (Post) included DTT, but the reducing agent was not added to the reaction mixtures until DNA cleavage-ligation equilibria were established after 5 min. Reactions were carried out in the absence of drug (No Drug) or in the presence of 200 μM GCG (GCG), 50 μM etoposide (Etop), or 25 μM 1,4-benzoquinone (BQ). Error bars represent standard deviations for three independent experiments.
Second, once covalent topoisomerase II poisons form protein adducts, their oxidation state appears to be irrelevant.23 As a result, if a reducing agent is added to assay mixtures after DNA cleavage-ligation equilibria have been established in the presence of a covalent poison, they are unable to reverse the cleavage enhancement.23 As seen in Figure 6 (bottom panels) DTT had no effect on DNA cleavage once GCG-topoisomerase II adducts were formed. Similar results were seen with 1,4-benzoquinone.
Third, in contrast to interfacial topoisomerase II poisons, covalent poisons inactivate enzyme function when they are incubated with topoisomerase II prior to the addition of DNA.21,23 When GCG was added to reaction mixtures that contained topoisomerase IIα or IIβ before the addition of plasmid, levels of cleavage dropped to zero within 3–5 min, with t1/2 values of less than 1 min (Figure 7).
Figure 7.
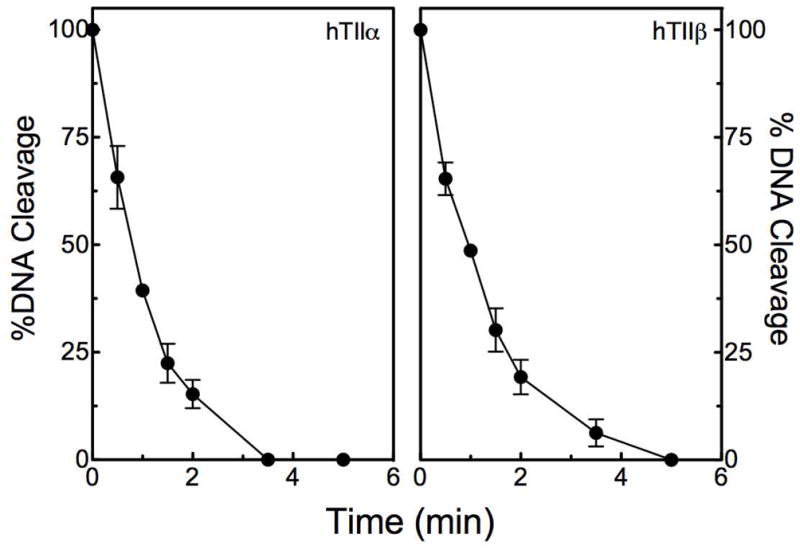
GCG inactivates DNA cleavage mediated by human topoisomerase IIα (hTIIα; left) and IIβ(hTIIβ; right) when incubated with the enzyme prior to the addition of DNA. Error bars represent standard deviations for three independent experiments.
Taken together, the data shown in Figures 5–7 provide strong evidence that GCG acts as a covalent topoisomerase II poison.
Finally, activated polyphenols can adduct a variety of biomolecules, including DNA bases.52 A number of studies have demonstrated that covalent DNA adducts are capable of acting as topoisomerase II poisons.53–56 Therefore, we determined whether any portion of the DNA cleavage enhancement induced by GCG was due to the adduction of DNA. To this end, the plasmid substrate was incubated with 200 μM GCG for 5 min, the catechin was removed by exclusion chromatography, and the purified DNA was incubated with topoisomerase IIα or IIβ (Figure 8). No changes in the levels of baseline DNA cleavage were observed. Moreover, DNA scission remained responsive to the further addition of 200 μM GCG in cleavage assays. These results indicate that the ability of GCG to enhance topoisomerase II-mediated DNA cleavage is not due to an adduction of the DNA substrate.
Figure 8.
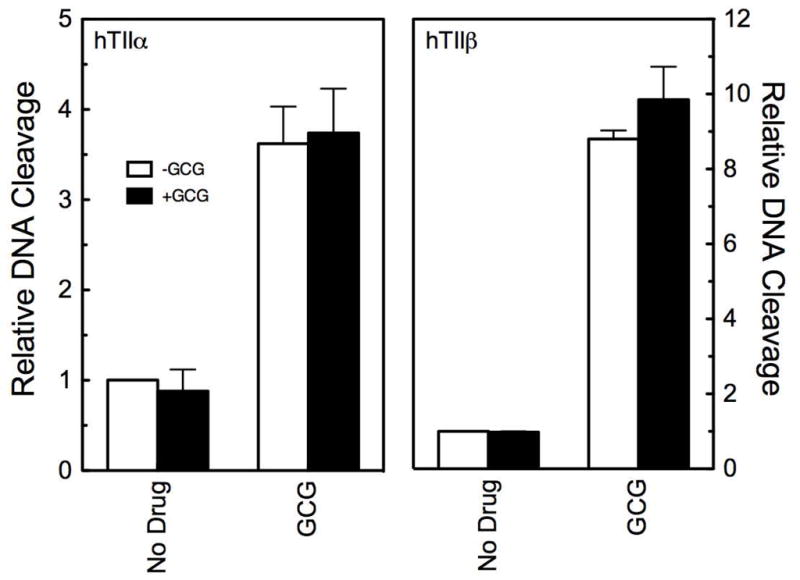
GCG does not enhance DNA cleavage mediated by human topoisomerase IIα (hTIIα; left) and IIβ(hTIIβ; right) by adducting DNA. DNA was incubated in the absence (open bars) or presence (closed bars) of 200 μM GCG for 5 min, centrifuged through a spin column to remove the bioflavonoid, and then used in DNA cleavage reactions. DNA cleavage was carried out in the absence (No Drug) or presence (GCG) of 200 μM GCG. Error bars represent standard deviations for three or four independent experiments.
Effects of Epimerization on the Activity of Other Green Tea Catechins on Topoisomerase II-Mediated DNA Cleavage
In addition to EGCG, green tea leaves contain three other major catechins, (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG), and (−)-epicatechin (EC).1,30–33 EGC acts as a covalent topoisomerase II poison, but ECG and EC do not induce topoisomerase II-mediated DNA cleavage.6,8 These findings indicate that the presence of the third hydroxyl moiety on the B-ring is essential to catechin activity against the type II enzymes, but that the D-ring is dispensable for activity.8,9
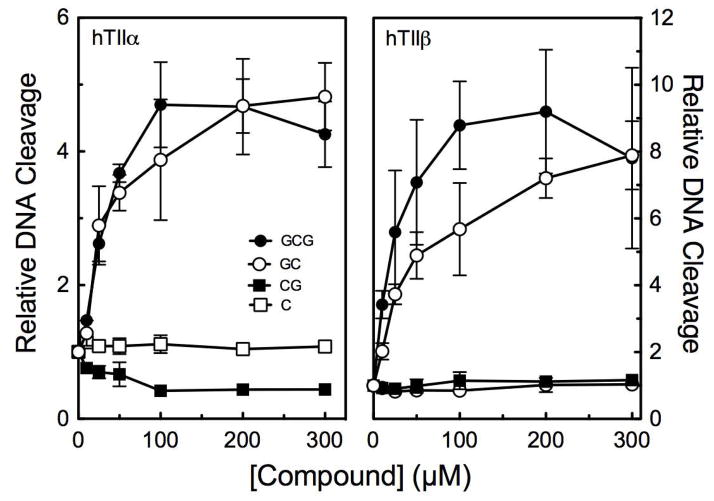
During the brewing process, EGC, ECG, and EC are epimerized to GC, CG, and C. (see Figure 1).41,42 Therefore, the effects of these latter compounds on DNA cleavage mediated by topoisomerase IIα and IIβ were assessed to determine whether the “rules” for catechin activity still apply following epimerization (Figure 9). GC (which contains three B-ring hydroxyl groups but lacks the D-ring) displayed activity similar to that of GCG against both enzyme isoforms. In control experiments, GC-induced DNA cleavage was abrogated by the addition of DTT (data not shown), indicating that it also is a covalent topoisomerase II poison. In contrast, CG and C (which contain only two hydroxyl groups on the B-ring) showed no ability to induce DNA cleavage. These results indicate that the same “rules” for the activity of green tea catechins against topoisomerase II appear apply to the epimerized compounds.
Figure 9.
GC (open circles), but not CG (closed squares) or C (open squares), enhances DNA cleavage mediated by human topoisomerase IIα (hTIIα; left) and IIβ(hTIIβ; right). Data for GCG (closed circles) are shown for comparison. Levels of DNA cleavage were quantified from the band of linear DNA and expressed relative to reactions carried out in the absence of catechin. Error bars represent standard deviations for three independent experiments.
SUMMARY
Dietary phytochemicals are routinely assessed for their abilities to alter cellular and purified systems. However, many studies do not take into account the fact that phytochemicals often are altered by cooking processes or by cellular metabolism. Our findings indicate that brewing, which epimerizes green tea catechins, has little effect on the ability of these compounds to function as covalent topoisomerase II poisons. Furthermore, results suggest (at least in the case of green tea catechins) that covalent poisons display greater structural latitude in their abilities to alter the activity of type II topoisomerases.
Acknowledgments
Funding
This research was supported by Grant GM033944 from the National Institutes of Health.
We are grateful to Adam C. Ketron, MaryJean Campbell, and R. Hunter Lindsey for critical reading of the manuscript.
ABBREVIATIONS
- EGCG
(−)-Epigallocatechin gallate
- EGC
(−)-epigallocatechin
- ECG
(−)-epicatechin gallate
- EC
(−)-epicatechin
- GCG
(−)-gallocatechin gallate
- GC
(−)-gallocatechin
- CG
(−)-catechin gallate
- C
(−)-catechin
- DTT
dithiothreitol
Footnotes
The authors declare no competing financial interests.
References
- 1.Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butt MS, Sultan MT. Green tea: nature’s defense against malignancies. Crit Rev Food Sci Nutr. 2009;49:463–473. doi: 10.1080/10408390802145310. [DOI] [PubMed] [Google Scholar]
- 3.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin CA, Patel S, Ono K, Nakane H, Fisher LM. Site-specific DNA cleavage by mammalian DNA topoisomerase II induced by novel flavone and catechin derivatives. Biochem J. 1992;282:883–889. doi: 10.1042/bj2820883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strick R, Strissel PL, Borgers S, Smith SL, Rowley JD. Dietary bioflavonoids induce cleavage in the MLL gene and may contribute to infant leukemia. Proc Natl Acad Sci USA. 2000;97:4790–4795. doi: 10.1073/pnas.070061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandele OJ, Osheroff N. Bioflavonoids as poisons of human topoisomerase IIα and IIβ. Biochemistry. 2007;46:6097–6108. doi: 10.1021/bi7000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Lazaro M, Willmore E, Jobson A, Gilroy KL, Curtis H, Padget K, Austin CA. Curcumin induces high levels of topoisomerase I- and II-DNA complexes in K562 leukemia cells. J Nat Prod. 2007;70:1884–1888. doi: 10.1021/np070332i. [DOI] [PubMed] [Google Scholar]
- 8.Bandele OJ, Osheroff N. (−)-Epigallocatechin gallate, a major constituent of green tea, poisons human type II topoisomerases. Chem Res Toxicol. 2008;21:936–943. doi: 10.1021/tx700434v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandele OJ, Clawson SJ, Osheroff N. Dietary polyphenols as topoisomerase II poisons: B ring and C ring substituents determine the mechanism of enzyme-mediated DNA cleavage enhancement. Chem Res Toxicol. 2008;21:1253–1260. doi: 10.1021/tx8000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Lazaro M, Willmore E, Austin CA. The dietary flavonoids myricetin and fisetin act as dual inhibitors of DNA topoisomerases I and II in cells. Mutat Res. 2010;696:41–47. doi: 10.1016/j.mrgentox.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Lin RK, Zhou N, Lyu YL, Tsai YC, Lu CH, Kerrigan J, Chen YT, Guan Z, Hsieh TS, Liu LF. Dietary isothiocyanate-induced apoptosis via thiol modification of DNA topoisomerase IIα. J Biol Chem. 2011;286:33591–33600. doi: 10.1074/jbc.M111.258137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Champoux JJ. DNA topisomerases: structure, function, and mechanism. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 13.Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol. 2002;3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 14.Deweese JE, Osheroff N. The DNA cleavage reaction of topoisomerase II: wolf in sheep’s clothing. Nucleic Acids Res. 2009;37:738–748. doi: 10.1093/nar/gkn937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nitiss JL. DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer. 2009;9:327–337. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, Deibler RW, Chan HS, Zechiedrich L. The why and how of DNA unlinking. Nucleic Acids Res. 2009;37:661–671. doi: 10.1093/nar/gkp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vos SM, Tretter EM, Schmidt BH, Berger JM. All tangled up: how cells direct, manage and exploit topoisomerase function. Nat Rev Mol Cell Biol. 2011;12:827–841. doi: 10.1038/nrm3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bender RP, Osheroff N. DNA topoisomerases as targets for the chemotherapeutic treatment of cancer. In: Dai W, editor. Checkpoint Responses in Cancer Therapy. Humana Press; Totowa, New Jersey: 2008. pp. 57–91. [Google Scholar]
- 20.Pommier Y, Marchand C. Interfacial inhibitors: targeting macromolecular complexes. Nat Rev Drug Discov. 2012;11:25–36. doi: 10.1038/nrd3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Mao Y, Chen AY, Zhou N, LaVoie EJ, Liu LF. Stimulation of topoisomerase II-mediated DNA damage via a mechanism involving protein thiolation. Biochemistry. 2001;40:3316–3323. doi: 10.1021/bi002786j. [DOI] [PubMed] [Google Scholar]
- 22.Lindsey RH, Bender RP, Osheroff N. Stimulation of topoisomerase II-mediated DNA cleavage by benzene metabolites. Chem Biol Interact. 2005;153–154:197–205. doi: 10.1016/j.cbi.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 23.Lindsey RH, Jr, Bromberg KD, Felix CA, Osheroff N. 1,4-Benzoquinone is a topoisomerase II poison. Biochemistry. 2004;43:7563–7574. doi: 10.1021/bi049756r. [DOI] [PubMed] [Google Scholar]
- 24.Bender RP, Ham AJ, Osheroff N. Quinone-induced enhancement of DNA cleavage by human topoisomerase IIα: adduction of cysteine residues 392 and 405. Biochemistry. 2007;46:2856–2864. doi: 10.1021/bi062017l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindsey RH, Jr, Bender RP, Osheroff N. Effects of benzene metabolites on DNA cleavage mediated by human topoisomerase IIα: 1,4-hydroquinone is a topoisomerase II poison. Chem Res Toxicol. 2005;18:761–770. doi: 10.1021/tx049659z. [DOI] [PubMed] [Google Scholar]
- 26.Bender RP, Lehmler HJ, Robertson LW, Ludewig G, Osheroff N. Polychlorinated biphenyl quinone metabolites poison human topoisomerase IIα: altering enzyme function by blocking the N-terminal protein gate. Biochemistry. 2006;45:10140–10152. doi: 10.1021/bi0524666. [DOI] [PubMed] [Google Scholar]
- 27.Bender RP, Osheroff N. Mutation of cysteine residue 455 to alanine in human topoisomerase IIα confers hypersensitivity to quinones: enhancing DNA scission by closing the N-terminal protein gate. Chem Res Toxicol. 2007;20:975–981. doi: 10.1021/tx700062t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson MJ, Osheroff N. Effects of antineoplastic drugs on the post-strand-passage DNA cleavage/religation equilibrium of topoisomerase II. Biochemistry. 1991;30:1807–1813. doi: 10.1021/bi00221a012. [DOI] [PubMed] [Google Scholar]
- 29.Lee S, Jung SR, Heo K, Byl JA, Deweese JE, Osheroff N, Hohng S. DNA cleavage and opening reactions of human topoisomerase IIalpha are regulated via Mg2+-mediated dynamic bending of gate-DNA. Proc Natl Acad Sci USA. 2012;109:2925–2930. doi: 10.1073/pnas.1115704109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sang S, Hou Z, Lambert JD, Yang CS. Redox properties of tea polyphenols and related biological activities. Antioxid Redox Signal. 2005;7:1704–1714. doi: 10.1089/ars.2005.7.1704. [DOI] [PubMed] [Google Scholar]
- 31.Isbrucker RA, Bausch J, Edwards JA, Wolz E. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 1: genotoxicity. Food Chem Toxicol. 2006;44:626–635. doi: 10.1016/j.fct.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Isbrucker RA, Edwards JA, Wolz E, Davidovich A, Bausch J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: dermal, acute and short-term toxicity studies. Food Chem Toxicol. 2006;44:636–650. doi: 10.1016/j.fct.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Ho CT. Polyphenolic chemistry of tea and coffee: a century of progress. J Agric Food Chem. 2009;57:8109–8114. doi: 10.1021/jf804025c. [DOI] [PubMed] [Google Scholar]
- 34.de Mejia EG, Ramirez-Mares MV, Puangpraphant S. Bioactive components of tea: cancer, inflammation and behavior. Brain Behav Immun. 2009;23:721–731. doi: 10.1016/j.bbi.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Bertram B, Bollow U, Rajaee-Behbahani N, Burkle A, Schmezer P. Induction of poly(ADP-ribosyl)ation and DNA damage in human peripheral lymphocytes after treatment with (−)-epigallocatechin-gallate. Mutat Res. 2003;534:77–84. doi: 10.1016/s1383-5718(02)00245-0. [DOI] [PubMed] [Google Scholar]
- 36.Weisburg JH, Weissman DB, Sedaghat T, Babich H. In vitro cytotoxicity of epigallocatechin gallate and tea extracts to cancerous and normal cells from the human oral cavity. Basic Clin Pharmacol Toxicol. 2004;95:191–200. doi: 10.1111/j.1742-7843.2004.pto_950407.x. [DOI] [PubMed] [Google Scholar]
- 37.Kanadzu M, Lu Y, Morimoto K. Dual function of (--)-epigallocatechin gallate (EGCG) in healthy human lymphocytes. Cancer Lett. 2006;241:250–255. doi: 10.1016/j.canlet.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 38.Lambert JD, Sang S, Yang CS. Possible controversy over dietary polyphenols: benefits vs risks. Chem Res Toxicol. 2007;20:583–585. doi: 10.1021/tx7000515. [DOI] [PubMed] [Google Scholar]
- 39.Hong J, Lu H, Meng X, Ryu JH, Hara Y, Yang CS. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62:7241–7246. [PubMed] [Google Scholar]
- 40.Ketron AC, Gordon ON, Schneider C, Osheroff N. Oxidative Metabolites of Curcumin Poison Human Type II Topoisomerases. Biochemistry. 2013;52:221–227. doi: 10.1021/bi3014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sang S, Lee MJ, Hou Z, Ho CT, Yang CS. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J Agric Food Chem. 2005;53:9478–9484. doi: 10.1021/jf0519055. [DOI] [PubMed] [Google Scholar]
- 42.Ishino N, Yanase E, Nakatsuka S. Epimerization of tea catechins under weakly acidic and alkaline conditions. Biosci Biotechnol Biochem. 2010;74:875–877. doi: 10.1271/bbb.90884. [DOI] [PubMed] [Google Scholar]
- 43.Kingma PS, Greider CA, Osheroff N. Spontaneous DNA lesions poison human topoisomerase IIα and stimulate cleavage proximal to leukemic 11q23 chromosomal breakpoints. Biochemistry. 1997;36:5934–5939. doi: 10.1021/bi970507v. [DOI] [PubMed] [Google Scholar]
- 44.Fortune JM, Osheroff N. Merbarone inhibits the catalytic activity of human topoisomerase IIα by blocking DNA cleavage. J Biol Chem. 1998;273:17643–17650. doi: 10.1074/jbc.273.28.17643. [DOI] [PubMed] [Google Scholar]
- 45.Byl JA, Fortune JM, Burden DA, Nitiss JL, Utsugi T, Yamada Y, Osheroff N. DNA topoisomerases as targets for the anticancer drug TAS-103: primary cellular target and DNA cleavage enhancement. Biochemistry. 1999;38:15573–15579. doi: 10.1021/bi991791o. [DOI] [PubMed] [Google Scholar]
- 46.Pitts SL, Jablonksy MJ, Duca M, Dauzonne D, Monneret C, Arimondo PB, Anklin C, Graves DE, Osheroff N. Contributions of the D-ring to the activity of etoposide against human topoisomerase IIα: potential interactions with DNA in the ternary enzyme-drug-DNA complex. Biochemistry. 2011;50:5058–5066. doi: 10.1021/bi200531q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ketron AC, Denny WA, Graves DE, Osheroff N. Amsacrine as a topoisomerase II poison: importance of drug-DNA interactions. Biochemistry. 2012;51:1730–1739. doi: 10.1021/bi201159b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuramochi H, Okamoto K, Nishikawa K, Nagamine S, Takahashi K. Antitumor effect of etoposide and its analogs. Gan To Kagaku Ryoho. 1985;12:2196–2201. [PubMed] [Google Scholar]
- 49.Froelich-Ammon SJ, McGuirk PR, Gootz TD, Jefson MR, Osheroff N. Novel 1-8-bridged chiral quinolones with activity against topoisomerase II: stereospecificity of the eukaryotic enzyme. Antimicrob Agents Chemother. 1993;37:646–651. doi: 10.1128/aac.37.4.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McClendon AK, Osheroff N. DNA topoisomerase II, genotoxicity, and cancer. Mutat Res. 2007;623:83–97. doi: 10.1016/j.mrfmmm.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osheroff N. Effect of antineoplastic agents on the DNA cleavage/religation reaction of eukaryotic topoisomerase II: inhibition of DNA religation by etoposide. Biochemistry. 1989;28:6157–6160. doi: 10.1021/bi00441a005. [DOI] [PubMed] [Google Scholar]
- 52.Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S, Johansson SL, Patil KD, Gross ML, Gooden JK, Ramanathan R, Cerny RL, Rogan EG. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc Natl Acad Sci USA. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kingma PS, Corbett AH, Burcham PC, Marnett LJ, Osheroff N. Abasic sites stimulate double-stranded DNA cleavage mediated by topoisomerase II: anticancer drugs mimic endogenous DNA lesions. J Biol Chem. 1995;270:21441–21444. doi: 10.1074/jbc.270.37.21441. [DOI] [PubMed] [Google Scholar]
- 54.Kingma PS, Osheroff N. The response of eukaryotic topoisomerases to DNA damage. Biochim Biophys Acta. 1998;1400:223–232. doi: 10.1016/s0167-4781(98)00138-9. [DOI] [PubMed] [Google Scholar]
- 55.Sabourin M, Osheroff N. Sensitivity of human type II topoisomerases to DNA damage: stimulation of enzyme-mediated DNA cleavage by abasic, oxidized and alkylated lesions. Nucleic Acids Res. 2000;28:1947–1954. doi: 10.1093/nar/28.9.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Velez-Cruz R, Riggins JN, Daniels JS, Cai H, Guengerich FP, Marnett LJ, Osheroff N. Exocyclic DNA lesions stimulate DNA cleavage mediated by human topoisomerase IIα in vitro and in cultured cells. Biochemistry. 2005;44:3972–3981. doi: 10.1021/bi0478289. [DOI] [PubMed] [Google Scholar]