Abstract
Objective
To describe serum 25(OH)D changes after Roux-en-Y gastric bypass (RYGB) and to determine if fat mass (FM) loss and vitamin D intake are associated with changes in serum levels.
Design/Methods
We investigated the relationship between serum 25(OH)D and 1) FM, 2) weight, 3) % excess weight loss (EWL), and 4) BMI, after controlling for potential confounders using a mixed effects linear model in 20 women before and up to 1-year post-RYGB. Subcutaneous (SAT) and visceral adipose tissue (VAT) vitamin D concentrations at time of RYGB were also evaluated.
Results
Weight and FM decreased 1-year after surgery by 45±1kg and 37±1kg, respectively while 25(OH)D increased by 10±2 ng/mL. Weight, FM, BMI, and %EWL changes were associated with 25(OH)D change. VAT had on average 21% more vitamin D per gram than SAT and concentrations were highly correlated.
Conclusions
Although weight loss may lead to increased serum 25(OH)D after RYGB, low levels remain a concern in some patients 1-year post-surgery. Additional research is needed to clarify the relationship between adipose storage of vitamin D and serum 25(OH)D in obesity, and how that relationship might change after surgery. This could lead to improved clinical management of vitamin D in this ever-growing clinical population.
INTRODUCTION
The worldwide obesity epidemic is associated with numerous conditions of public health concern, including low vitamin D levels [1]. Serum 25 hydroxyvitamin D (25(OH)D) levels < 30ng/mL and < 20ng/mL were previously used to define vitamin D insufficiency and deficiency, respectively [2], but recently the Institute of Medicine (IOM) redefined deficiency to < 12ng/mL based upon bone health indicators [3]. The IOM also redefined serum 25(OH)D concentrations from 12–19ng/mL as inadequate; 20–29ng/mL is the old definition. In contrast, the Endocrine Society Guidelines [4] have recently advised that the lower end of the acceptable range should be 30ng/mL based on the study by Priemel et al [5], which demonstrated asymptomatic osteomalacia between serum 25(OH)D levels of 20–30ng/mL. For the purposes of consistency we will adhere to the new IOM guidelines.
It is well-documented that obese individuals have relatively low vitamin D levels [6, 7]. Moreover, BMI [8], body weight [8], and fat mass (FM) [8, 9] are inversely related to serum 25(OH)D levels. The potential health ramifications of this observation are many, given the association between low vitamin D status and risk for metabolic syndrome [10], hypertension [11], and some cancers [11]. Given that obese individuals are at risk for these comorbidities, it is necessary to better understand the causes for and potential solutions to the problem of low vitamin D status in this population.
High obesity prevalence has resulted in growing numbers of bariatric surgeries worldwide [12]. The Roux-en-Y gastric bypass (RYGB) is the most common bariatric surgery and is considered the most effective for long-term weight loss [13]. A recent meta-analysis [14] reported that the average weight loss post-RYGB is approximately 45kg. A substantial amount of this weight loss is FM, with studies reporting losses of 6.9kg [15] to 35.9kg [16] one [15] to 24 [16] months post-RYGB, making this a unique population in which to study vitamin D changes, considering that it is a fat soluble vitamin. However, prospective data on the effect of FM loss on vitamin D levels post-RYGB are lacking. The majority of studies have only evaluated vitamin D in relation to weight status pre-operatively [17] or postoperatively in light of BMI and weight (but not body composition) changes [18–22]. More comprehensive evaluations of FM loss in relation to change in circulating 25(OH)D concentration are needed. Another limitation of many prospective studies is that quantification of vitamin D intake is not always included [18–20, 22] or the methods are incompletely described [17].
Our objective was to describe post-RYGB serum 25(OH)D changes and to determine if FM loss and vitamin D intake are associated with these changes up to one year after surgery. An additional objective was to measure vitamin D concentrations in two adipose tissue depots, VAT and SAT, at the time of RYGB. We hypothesized the following: 1) after RYGB, serum 25(OH)D levels would increase; 2) anthropometric changes (decrease in FM, body weight, and BMI, and percent excess weight loss (%EWL)) would be associated with increased serum 25(OH)D levels; and 3) VAT and SAT would both contain comparable concentrations of vitamin D.
METHODS AND PROCEDURES
Twenty-nine women undergoing the laparoscopic RYGB procedure were recruited from the University of Minnesota’s Weight Loss Surgery Center. This study included five visits: 30–70 days pre-RYGB (Baseline); two weeks post-RYGB (Week 2); six weeks post-RYGB (Week 6); six months post-RYGB (Week 24); and one year post-RYGB (Week 52). The study visit schedule allowed for the evaluation of early (e.g. Weeks 2 and 6) and late (e.g. Weeks 24 and 52) post-operative outcomes. Exclusion criteria included: use of corticosteroids, testosterone, or anabolic agents; pacemaker; liver, renal, or heart failure; pulmonary hypertension; untreated thyroid disease; cancer; Type 1 or uncontrolled Type 2 diabetes mellitus (defined by HbA1c > 7%); pregnancy; and/or previous weight loss surgery. The study protocol was approved by the Institutional Review Board and the General Clinical Research Center (GCRC) at the University of Minnesota. Subjects provided written, informed consent before participating.
Serum 25(OH)D Assay
A fasting blood sample was collected at each visit and serum was frozen at −70° C until assay using the Diasorin Liaison total 25(OH)D assay method (Diasorin, Stillwater, MN). This method uses a two-step competitive chemiluminescence (CLIA) assay with a unique antibody to 25OH D2 and D3, allowing for determination of total serum 25(OH)D. The method detects a sample concentration range of 4–150ng/mL with analytical sensitivity < 1ng/mL. Samples were assayed in duplicate, with mean value recorded.
Vitamin D2 and D3 Assay of Intraoperative Adipose and Pre-RYGB Serum
For the purposes of describing serum and adipose depot vitamin D concentrations, an additional baseline serum sample was analyzed for vitamin D2 and D3 by NMS Laboratories (Willow Grove, PA) using LC-MS-MS. During the RYGB procedure, abdominal subcutaneous (SAT) and visceral (VAT) compartment adipose tissue samples were collected by the surgeon (<10 grams in total). Briefly, a subcutaneous adipose sample was extracted through a linear incision in the abdominal wall using scissors and electrocautery. A visceral adipose sample was taken from the greater omentum coming off the transverse colon using a harmonic scalpel. Samples were quick frozen in liquid nitrogen and then stored at −70° until assay.
Adipose vitamin D2 and vitamin D3 concentrations were analyzed using a high-performance liquid chromatography (HPLC) method developed at Heartland Assays, LLC for food [23] and modified for the measurement of vitamin D2 and D3 in adipose tissue. The adipose samples were first saponified in methanolic KOH. The sample was spiked with an internal standard [3H]-vitamin D3, (30 Curies/mmole) to estimate recovery losses. After 60 mins at 60° C the saponified material was extracted 3 times with 1 volume of hexane/ethyl acetate (85/15). The extract was washed with water, collected and dried under vacuum. The dried extract was re-suspended in 1.0ml of hexane/methylene chloride 90/10 v/v), applied to a hand packed solid-phase extraction cartridge, eluted with methylene chloride/2-propanol (99.8/0.2) and dried. The residue was re-suspended in hexane/methylene chloride/alcohols (85/15/0.2), and applied to Agilent HPLC ZORBAX SIL column, and the vitamin D fraction was collected, and dried. The residue was re-suspended in hexane/alcohols (99.5/0.5), and again applied to an HPLC ZORBAX NH2 column, and the vitamin D fraction was collected and dried for final purification. The final purification for vitamin D2 and vitamin D3 by HPLC was achieved using a Vydac ODS column. Vitamin D2 and vitamin D3 were quantified by comparison of the UV peak areas with known standards and corrected for recovery. Vitamin D2 is baseline resolved from Vitamin D3. Method validation consisted of addition of 60ng of both vitamin D2 and vitamin D3 plus [3H}-vitamin D3 to 10 fat samples. Average recovery of the vitamin D2 and Vitamin D3 was 98.5±5%.
Dietary Intake Assessment
Information regarding dietary and supplemental vitamin D intake was obtained at each visit through the use of diet records. During the week prior to each testing visit, subjects recorded their food, beverage, and vitamin/mineral supplement intake on two assigned weekdays and one weekend day. Recording errors were minimized by providing the subjects with detailed verbal and written instructions on how to record their intake, including estimation of portion size using food models, measuring cups and spoons for illustration purposes. Diet records were reviewed at each testing visit and subjects were queried for missing details regarding condiments and cooking methods. Data were analyzed using the Food Processor software (SQL edition, version 10.4 ESHA Research, Salem, OR). To estimate daily vitamin D intake during the week prior to the visit, an average of the recorded intake was calculated.
Anthropometric Measurements
Height was measured to the nearest 0.1cm by a wall-mounted stadiometer (model S100; AYRTON Corporation, Prior Lake, MN) at Baseline only. At all testing visits, weight was measured to the nearest 0.1kg on a digital scale (model 5002, SCALE-TRONIX, White Plains, NY). Body mass index (BMI) was calculated as body weight/height2 (kg/m2). Percent excess weight loss (%EWL) was calculated using the following equation:
Total Body Water Measurement
Total body water (TBW) was determined by deuterium dilution, as previously described [24]. Briefly, a fasted Baseline urine sample was collected prior to initiation of the dilution protocol at 7AM of Day 2. A weighed dose of deuterium oxide (99.9 atom %; Isotec, Miamisburg, OH) providing 0.1g/kg body weight was IV pushed over a 5-minute period. The syringe containing the deuterium oxide was weighed before and after administration in order to determine the precise amount that was administered to the subject. Hourly urine samples were collected, as well as a final urine sample at 4-hour post-infusion. All urine samples were tightly sealed, packed on dry ice, and stored at −70 °C until analysis. Baseline, and 3- and 4-hour post-dose urine samples were shipped to Michael Jensen’s laboratory at the Mayo Clinic (Rochester, MN) for analysis. TBW was calculated from the average deuterium enrichment of the 3- and 4-hour urine samples and then reduced by 4% to correct for exchange with the non-aqueous compartment [25].
Fat Mass Determination
FM was determined using body weight and fat-free mass (FFM) (calculated using TBW measurements). FM was estimated by:
where 0.73 is the standard hydration coefficient of FFM [25].
Season
The date of the testing visit was used to classify the serum 25(OH)D sample as either Fall/Winter (October – March) or Spring/Summer (April – September), in accordance with Ernst et al [26].
Surgical Technique
All individuals underwent the laparoscopic RYGB procedure following standard protocol at the University of Minnesota Weight Loss Surgery Center. A small gastric pouch (approximately 15mL) was created and the duodenum and proximal portion of the jejunum were bypassed to create a Roux-limb. In most cases, the length of the Roux-limb was 150cm, and the length of the pancreaticobiliary limb was 100cm, according to the University of Minnesota Weight Loss Surgery Center protocol.
Statistical Analysis
In unadjusted models, we evaluated the relationship between serum 25(OH)D and FM, body weight, BMI, and %EWL using Spearman’s correlation. We then investigated the relationship between serum 25(OH)D and FM, body weight, BMI, and %EWL while controlling for dietary/supplemental vitamin D intake, season, and Baseline age. We estimated changes over time using a mixed effects linear model with a random effect for subject to model the correlation of repeated measurements within each subject. Logarithmic transformations were performed on dietary/supplemental vitamin D intake and back-transformed to the original scale for analyses in Table 1. A P value < 0.05 was considered significant. SAS software (version 9.2, SAS Institute, Inc., Cary, NC) was utilized for statistical analyses.
Table 1.
Subject Characteristics
| Pre-RYGB (n=20) | Week 2 (n=17) | Week 6 (n=20) | Week 26 (n=20) | Week 52 (n=20)§ | |
|---|---|---|---|---|---|
| Age (years) | 48 ± 2 | ||||
| Body Weight (kg) | 132 ± 4 | 125 ± 4*** | 118 ± 4*** | 98 ± 4*** | 88 ± 4*** |
| Body Weight Change (kg) | −7 ± 1*** | −14 ± 1*** | −35 ± 1*** | −45 ± 1*** | |
| BMI (kg/m2) | 48 ± 1 | 45 ± 1*** | 43 ± 1*** | 35 ± 1*** | 32 ± 1*** |
| Fat Mass (kg) | 69 ± 3 | 67 ± 3 | 61 ± 3*** | 42 ± 3*** | 32 ± 3*** |
| Fat Mass Change (kg) | −2 ± 2 | −8 ± 1*** | −28 ± 1*** | −37 ± 1*** | |
| Serum 25(OH)D (ng/mL) | 19 ± 2 | 22 ± 2 | 25 ± 2** | 24 ± 2* | 29 ± 2*** |
| Serum 25(OH)D Change (ng/mL) | 3 ± 2 | 6 ± 2** | 5 ± 2* | 10 ± 2*** | |
| Dietary/Supplemental Vitamin D intake (IU) (Geometric mean (95% confidence interval)) | 129 (71–235) | 352 (180–687)* | 602 (325–1114)*** | 558 (306–1018)*** | 417 (229–760)** |
: Significantly different from Pre-RYGB at p<0.05
: Significantly different from Pre-RYGB at p<0.01
Significantly different from Pre-RYGB at p<0.001
For 25OH-D, n=19
Abbreviations: RYGB: Roux-en-Y gastric bypass; 25(OH)D: 25 hydroxyvitamin D
Note: Unless otherwise noted, data are Mean ± SEM
RESULTS
Twenty of twenty-nine women completed at least four study visits. Baseline BMI and FM values were significantly greater in those who completed the study (n=20) compared to those who did not (n=9). Three subjects did not attend the Week 2 visit secondary to not feeling well after surgery and serum 25(OH)D analysis was not available for one subject at the Week 52 visit due to insufficient sample collection.
Subjects for this analysis resided primarily in the Minneapolis/Saint Paul metropolitan area, which has a latitude of 44° 59′ North. All participants were Caucasian with a mean age of 48 years. Body weight, BMI, FM, serum 25(OH)D, and dietary/supplemental vitamin D intake at Baseline and subsequent study visits are presented in Table 1. One year after surgery, body weight and FM decreased by 45±1kg and 37±1kg, respectively (P<0.001) while serum 25(OH)D increased by 10±2 ng/mL (P<0.001) (Mean ± SEM) (Table 1). At Baseline, serum 25(OH)D levels were all below 30ng/mL with 10 subjects < 20ng/mL and by Week 52, six subjects had levels greater than 30ng/mL. Additionally, two subjects at Baseline and one at Week 52 met the new serum 25(OH)D deficiency criteria of < 12ng/mL.
Seasonality and Serum 25(OH)D
Serum 25(OH)D levels and change in serum 25(OH)D levels were analyzed for differences between seasons (Fall/Winter and Spring/Summer) at all testing visits. We were unable to detect a seasonal effect for either the absolute value or the change in serum 25(OH)D (P>0.05) (data not shown).
Vitamin D2 and Vitamin D3 in SAT, VAT, and Pre-RYGB Serum
Vitamin D2 and vitamin D3 concentrations in intraoperative SAT and VAT and pre-RYGB serum are presented in Table 2. SAT and VAT vitamin D analyses were performed on 13 of the 29 subjects who completed the Baseline visit. While vitamin D3 was present in all adipose and serum samples, vitamin D2 was not always detected. VAT vitamin D3 and VAT total vitamin D (D2 and D3) concentrations were greater than that of SAT, by 19 and 21%, respectively (P<0.01). In addition, SAT and VAT total vitamin D were highly correlated (R2 = 0.99, P<0.0001).
Table 2.
Baseline Average Vitamin D2 and Vitamin D3 in SAT, VAT, and Serum and Serum 25(OH)D (n=13)
| Location of Sample | Vitamin D2 | Vitamin D3 | Total Vitamin D | 25(OH)D |
|---|---|---|---|---|
| SAT (ng/g) | 10 ± 8 | 29 ± 9 | 38 ± 11 | |
| VAT (ng/g) | 13 ± 10 | 36 ± 10** | 48 ± 14** | |
| Serum (ng/mL) | 0.7 ± 0.5 | 33 ± 4 | 34 ± 4 | 21 ± 2 |
: Significantly different than SAT (P<0.01); Mean ± SEM
Abbreviation: 25(OH)D: 25 hydroxyvitamin D; SAT: Subcutaneous Adipose Tissue; VAT: Visceral Adipose Tissue
Change in Serum 25OH Vitamin D Levels
In unadjusted correlations, change in serum 25(OH)D was positively correlated with change in body weight at Week 26 (data not shown). We then adjusted for potential influencing factors on serum 25OH vitamin D change, including vitamin D intake, age, and visit. Overall, a 10kg loss of FM and body weight was associated with a 2.8±1.3ng/mL (mean±SEM; P=0.04) and 3.9±1.4ng/mL (P=0.008) increase in serum 25(OH)D, respectively, controlling for aforementioned confounders (Table 3). Similarly, a one-unit reduction in BMI and a 10% change in EWL was associated with a 0.84±0.4ng/mL (mean±SEM; P=0.04) and 2.1±0.8ng/mL (P=0.01) increase in serum 25(OH)D, respectively, controlling for the aforementioned confounders. Increased age was also significantly associated with decreased serum 25(OH)D in all four models (data not shown).
Table 3.
Model of Change in Serum 25(OH)D Levels from Baseline
| Effect | Change in Serum 25(OH)D Estimate (ng/mL) | Standard Error | Pr > |t| |
|---|---|---|---|
| Fat Mass Change from Baseline (10 kg) | 2.8 | 1.3 | 0.04 |
| Weight Change from Baseline (10 kg) | 3.9 | 1.4 | 0.008 |
| BMI Change from Baseline (1 kg/m2 unit) | 0.84 | 0.4 | 0.04 |
| Percent EWL from Baseline (10%) | 2.1 | 0.8 | 0.01 |
Abbreviation: 25(OH)D: 25 hydroxyvitamin D; EWL: excess weight loss
Note: Models adjusted for age, vitamin D intake, and visit
DISCUSSION
In this study we evaluated serum 25(OH)D status before and after RYGB and investigated how FM and vitamin D intake are associated with this change. In addition, we described the vitamin D content of VAT and SAT at the time of RYGB. We found that low serum 25(OH)D levels are pervasive before RYGB with the mean level < 20ng/mL. The number of subjects with serum 25(OH)D levels < 20ng/mL decreased within one year after RYGB and the mean level significantly increased from pre- to post-RYGB, with the one year group average of 29ng/ml falling above the new definition of adequate (> 20ng/mL). Changes in anthropometrics were positively associated with the change in serum 25(OH)D.
Serum 25(OH)D Status Before and After RYGB
Low circulating 25(OH)D levels were apparent before surgery and this is in line with data reported by other investigators in patients before RYGB [1, 18, 19, 22]. Although a significant concern, the low circulating 25(OH)D concentration observed before bariatric surgery is not surprising considering that this is a widespread phenomenon in individuals in this weight category [6, 7]. In fact, in a cross-sectional study by Goldner et al [1], a higher prevalence of low 25(OH)D levels was found in pre-RYGB women compared to non-obese controls (61% vs 12%, P<0.0001). Vitamin and mineral supplements, including vitamin D are often prescribed following bariatric surgery, however our prevalence data and that of others indicate that routine supplementation of vitamin D should occur pre-operatively as well.
On average, subjects in our study experienced a modest increase in serum 25(OH)D concentrations after RYGB. This is in contrast to a recent study with similar methodology and subject number [17], in which a significant change was not observed at 1 year after RYGB. Of note, the study by Pramyothin et al employed a more aggressive supplementation protocol with vitamin D2. It is somewhat surprising that we observed a significant increase in serum 25(OH)D concentrations despite lower vitamin D intake throughout the study, which otherwise had a similar overall design. Potential reasons for this discrepancy include differences in surgical technique and baseline subject characteristics, and compliance to and form of the vitamin D supplementation regimen (e.g. D2 vs D3). Subjects in the study by Pramyothin et al had higher average baseline vitamin D status (23ng/mL vs 19ng/mL) and reported vitamin D intake. Moreover, a majority of the subjects in our study were taking vitamin D3 supplements (compared to vitamin D2 in the Pramyothin study), which is known to be more effective at raising serum 25(OH)D levels. It should also be noted that, in the Pramyothin study, the dietary intake data were evaluated by self-report, and few additional protocol details were provided. This could have contributed error. In contrast, we obtained vitamin D intake data by employing a strict protocol utilizing three-day dietary records that involved intensive subject training and review by dietitian-supervised staff.
Although we reported a significant overall increase in serum 25(OH)D concentration, not all subjects reached adequacy. In our study, about 85% of the subjects had serum 25(OH)D concentrations in the newly defined adequate range (≥ 20ng/mL) at Week 52. Improved, yet persistently low 25(OH)D levels have been reported by others at one year or more after RYGB [16, 19, 27, 28]. A significant reduction in weight and FM occurred by one year after RYGB, and consequently we expected to see improved serum 25(OH)D levels in all subjects. However, despite significant reduction in adiposity, subjects in our study were still considered obese and for some individuals, perhaps not enough weight was lost to substantially impact the serum 25(OH)D concentration. We evaluated the BMI, weight loss, and FM loss data for the three subjects who did not achieve adequate serum 25(OH)D levels at Week 52 (< 20ng/mL). All three were obese, but their weight and FM loss data was variable (range: 30–66kg, 29–58kg, respectively). Carlin et al, who reported low serum 25(OH)D levels (< 20ng/mL) post-RYGB, also had subjects that were classified as obese 12 months after RYGB [22]. These observations support a role for more aggressive supplementation to facilitate optimization of serum 25(OH)D into the adequate range. Carlin et al [22] prospectively evaluated vitamin D supplementation (unspecified if it was D2 or D3) and BMI status in 60 vitamin D deficient women before and after RYGB. Subjects were randomized to receive either 1) 50,000IU vitamin D weekly and 800IU vitamin D daily or 2) 800IU vitamin D daily. BMI decreased in both groups after surgery (statistical significance not reported). Those in the high vitamin D supplement group, despite remaining obese at one-year post-RYGB, had serum 25(OH)D levels that were considered adequate by the new definition (mean = 38ng/mL) and were 60% greater than the low supplement group (P<0.001) (mean = 15ng/mL). Subjects in our study were advised by the Weight Loss Surgery Center to take a daily multi-vitamin after surgery (vitamin D content 400–800IU), which is similar to that consumed by the low supplement group in the Carlin et al study. Our finding of serum 25(OH)D levels < 20ng/mL in a small number of subjects (n=3) one year after surgery would support the position that the level of vitamin D intake observed in our subjects is too low. In addition, our calculations suggest that FM vitamin D stores would not be able to support an 800IU daily requirement in most subjects by six months post-RYGB. These findings further highlight the importance of vitamin D supplementation after RYGB and suggest that the frequently recommended daily dosage of 400–800IU might not be sufficient for this patient population. Carlin et al reported that the weekly dosage of 50,000IU was safe, although additional research is needed to establish optimal dosing in this population. In addition, we cannot rule out the possibility that UVB exposure was likely not enough to promote adequate serum 25(OH)D levels, considering that production of vitamin D is only possible for a maximum of seven months per year in Minnesota [29] and subjects were likely not exposing substantial areas of skin after surgery. Until more is known about the risk-benefit of sun exposure and vitamin D health we remain cautious about UVB exposure recommendations, but believe modest and safe exposure is essential for vitamin D health, especially in those with low vitamin D dietary/supplemental intake.
An alternative hypothesis for the low 25(OH)D levels observed in some subjects one-year post-RYGB is that the surgery itself is contributory. Under normal physiological conditions, dietary vitamin D is absorbed in the proximal small intestine [30]. During RYGB, the distal stomach, duodenum, and proximal jejunum are bypassed [31] resulting in micronutrient malabsorption, which could contribute to the insufficient 25(OH)D levels observed by ourselves and others [16, 19, 27, 28]. Of note, a recent report by Aarts et al [32] demonstrated that a 25% reduction in vitamin D3 absorption occurred one month after RYGB despite supplementing with a single dose of 50,000IU vitamin D3 during the postoperative period. A comparison between different bariatric surgeries (including RYGB, sleeve gastrectomy, duodenal switch) could better define the role of malabsorption in the low serum 25(OH)D levels seen after surgery. Unlike the RYGB, which is classically considered to have both malabsorptive and restrictive components and the duodenal switch which has an even more pronounced malabsorptive component, the adjustable gastric band (AGB) and sleeve gastrectomy procedures are purely restrictive weight loss surgeries, and micronutrient malabsorption would not be expected. Fish et al [28] retrospectively compared vitamin D status between RYGB and AGB subjects for two years following surgery. Compared to AGB, the prevalence of serum 25(OH)D insufficiency (at the time, defined as <30ng/mL) after RYGB was greater at 6 months (52% vs 64%), one year (41% vs 63%), and two years (50% vs 73%) after surgery (statistical significance not reported for any time point). Similarly, Gehrer et al [20] compared vitamin D status between RYGB and sleeve gastrectomy subjects and found higher incidence of low levels (serum 25(OH)D < 20ng/ml) after RYGB (RYGB: 52%, sleeve gastrectomy: 32%, P=0.02). Conversely, DiGiorgi et al [19] did not find a difference between post-RYGB and post-AGB groups in serum 25(OH)D levels at 3, 6, 12, or 24 months after surgery and Coupaye et al [18] found significantly higher serum 25(OH)D levels one year after RYGB compared to AGB. High loss to follow-up [19] and small sample size [18] might explain the lack of consistent findings in these two studies. In sum, much is known about absorption of vitamin D in normal physiology [30], however the details on absorption after RYGB have yet to be fully elucidated. Consequently, well-designed vitamin D absorption studies comparing different bariatric procedures, while controlling for UVB exposure and dietary vitamin D intake and accounting for the potential contribution from adipose tissue, are needed to enhance our understanding of issues affecting vitamin D status and optimal dosing in the RYGB patient population.
Adipose Tissue Vitamin D3 Content Before RYGB
We were able to quantify vitamin D3, in both SAT and VAT; as might be expected, most subjects did not have detectable concentrations of vitamin D2. Limited data exist on SAT and VAT vitamin D concentrations. Similar to our findings, vitamin D3 has been detected in SAT [6, 17] and VAT [17] in patients undergoing RYGB. We found a correlation between vitamin D concentrations in VAT and SAT. Additionally, in the small number of subjects for whom we had adipose samples, the vitamin D3 concentration in VAT was greater by 19% than that observed in SAT for all subjects. It is tempting to conclude from these results that vitamin D3 may be preferentially stored in VAT compared with SAT. This is an important area for further research. Because we were interested to know, at a maximum, how much vitamin D might theoretically be stored in total whole body FM, we took each of the 13 subject’s VAT vitamin D concentration and multiplied it by their total FM. In this way, we estimated the average total FM vitamin D content to be 115,353±33,628IU (mean±SEM; range: 7,120–373,043IU). If one assumes the daily consumption rate of 4,000IU/day, as posited by Heaney et al [33], needed to maintain normal serum 25(OH)D, that amount would represent on average a 29 day vitamin D supply (and based on the range of data for our subjects, it could be as low as 2 days and as high as 93 days). This provides evidence against the sequestration hypothesis.
We also attempted to evaluate the relationship between serum 25(OH)D and adipose vitamin D concentrations. Although we did not find a statistically significant relationship between serum and adipose concentrations, it is interesting to note that, among the 7 subjects for whom we had both serum 25(OH)D and adipose vitamin D concentrations, the one with the highest serum 25(OH)D also had the highest SAT and VAT vitamin D concentrations. On the other hand, the subject with the lowest serum 25(OH)D (which was 23 ng/mL, an adequate concentration) did not have the lowest adipose vitamin D concentrations in this small subsample. Larger scale studies are needed to clarify and confirm the difference in SAT and VAT vitamin D concentrations, and to further elucidate the relationship between adipose and serum vitamin D compartments.
Vitamin D Intake Before and After RYGB
In this study, we found improved vitamin D intake after RYGB. It was apparent that the subjects in our study attempted to adhere to the supplement regimen, as at the Week 52 visit, 87% of their vitamin D intake was from supplements (vs. 64% at Baseline). The importance of vitamin D supplementation in this patient population is highlighted by a component of the surgery that promotes food restriction. A small gastric pouch is created by the surgeon, which promotes early satiety [31, 34] and thereby decreases intake [34]. This alteration of the gastrointestinal tract leads to drastic reduction in food intake, from 2300 [35] to 2900 [36] kcal per day before surgery to an average intake of 1200–1400 kcal per day at least 12 months post-RYGB [35, 37]. Generally, dietary intake of vitamin D is minimal considering that few foods are naturally rich in the vitamin (e.g. oily fish) or are fortified (e.g. milk); however in a population that is already restricting their food intake, supplementation is especially key for optimal vitamin D intake. We found that subjects in our study compensated for the reduced ability to consume vitamin D containing foods by taking supplements. Nonetheless, there was a broad range of vitamin D intake in our subjects, therefore caution is warranted when interpreting these results.
Relationships Between Serum 25(OH)D and Anthropometrics
In our analyses, we found positive relationships between change in anthropometrics (FM, body weight, BMI, and %EWL) and change in serum 25(OH)D, in analyses that took into account other factors that could affect post-operative 25(OH)D concentration. The mechanisms linking increased serum 25(OH)D concentration with decreased FM/body weight are not well understood. In those with obesity-related conditions that could potentially affect vitamin D metabolism (e.g. NAFLD), weight loss may ameliorate the condition, possibly resulting in improved serum 25(OH)D concentrations. Indeed, with FM/body weight loss, the degree of obesity-related inflammation generally improves, as measured by the reduction of pro-inflammatory cytokines (e.g. IL-6) [38]. Recent research indicates that serum 25(OH)D and IL-6 are inversely related [39]. Therefore, with weight loss-mediated improvement in inflammation (e.g. decreased IL-6 levels), reduced turnover of vitamin D may occur, resulting in increased circulating levels. However, this relationship has not been extensively evaluated and additional prospective studies are needed.
In our study, both weight and FM loss were associated with a rise in serum 25(OH)D concentration, with weight loss having the strongest association. In four separate models, we assessed change in serum 25(OH)D levels after RYGB in relation to changes in anthropometrics after accounting for potential confounders (vitamin D intake, age, and study visit). Weight loss, FM loss, BMI decrease, and %EWL were significantly associated with change in serum 25(OH)D levels. Importantly, body weight had the most significant association with change in serum 25(OH)D.
In all of the adjusted models, vitamin D intake was not significantly associated with serum 25(OH)D levels. Arunabh et al also did not find strong correlations between vitamin D intake and serum 25(OH)D levels [9]. Our vitamin D intake data were highly variable at all visits, which could have affected our ability to detect a relationship between it and change in vitamin D status in our models. In addition, vitamin D intake in our subjects may not have been high enough to promote a change in serum 25(OH)D. Continued research on the optimal oral vitamin D dosage in these patients is warranted.
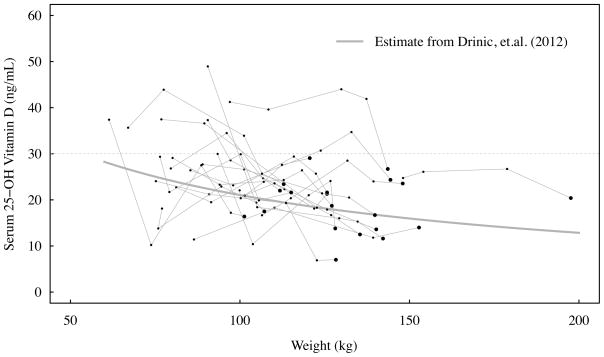
Our findings appear to be consistent with a recent study [40] that suggests an alternative to the earlier adipose sequestration hypothesis [7]. Utilizing linear and hyperbolic modeling methods, Drincic et al [40] reported that a greater volume of distribution in obese compared to normal weight individuals accounts for the lower serum 25(OH)D concentrations in obesity and thus obese individuals likely require higher doses to reach normal concentrations. Our data certainly support this more recent line of thinking. Utilizing the average VAT vitamin D concentration in 13 subjects (48 ± 14ng/g, which is 1,936 ± 541IU/kg; mean±SEM), we can extrapolate the total vitamin D content anticipated to be released using the average fat mass loss for the total sample of 37kg. This amount (71,621IU) would have released vitamin D at a rate of 1,377IU/week. This observation suggests that the release of vitamin D from adipose stores is clearly not the primary contributor to the rise of serum vitamin D following RYGB. Furthermore, by using our body weight data we can independently evaluate the hyperbolic modeling equation proposed by Drincic et al [40]. From Figure 1 in Drinic et al, one can calculate the fitted constants a = 58.01715 and b = 56.92308, for serum 25(OH)D in ng/mL and body weight in kg, respectively. Figure 1 in the present paper depicts observed serum 25(OH)D data at all visits plotted with the estimate from the Drincic et al paper. Observed mean change in serum 25(OH)D from Baseline to the Week 52 visit was similar to the predicted value (+9.9 ng/mL vs +5.5 ng/mL; P=0.090), and this suggests that the Drincic et al model adequately predicts serum 25(OH)D levels.
Figure 1.
Plot of serum 25(OH)D levels on body weight for 20 women at each study visit. Each black line represents a study participant. Each heavy dot represents the Baseline visit. The grey line represents the 25(OH)D estimate from the model developed by Drincic et al. Drinic model is: 25(OH)D = ab/(b − weight).
25(OH)D: 25 hydroxyvitamin D
Limitations of this study include a small sample comprised solely of Caucasian women, the lack of direct sun exposure data, and statistical modeling that only highlights associations. We were also not able to measure gut vitamin D absorption, and the intake levels may not reflect actual amounts of vitamin D absorbed. Although we measured vitamin D in SAT and VAT at Baseline, our study would have been stronger had we been able to perform similar analyses after RYGB. In addition, although subjects met with a registered dietitian at their pre- and postoperative visits at the Weight Loss Surgery Center and were educated about the importance of taking a vitamin D supplement, compliance was an issue, as determined from diet records. Larger prospective studies in a racially diverse population that assess FM, vitamin D intake, sun exposure, and vitamin D concentrations in adipose tissue before and after RYGB are needed.
The strengths of this study include its prospective study design with data collection before and at several time points for one year after RYGB. We also measured FM, vitamin D intake, and SAT and VAT vitamin D concentrations, which have not been commonly reported in this patient population. Our study employed methods and a research approach similar to that of Holick et al [17]. Recently Holick’s group reported on serum 25(OH)D changes after RYGB and characterized vitamin D in various adipose depots [17]. Our dietary intake data appear to be stronger based on methodology; we followed a strict protocol utilizing three-day dietary records that involved intensive subject training and review by dietitian-supervised staff. Furthermore, we have actual FM data obtained through the deuterium dilution method. We also conducted a more robust statistical analysis that included adjusted models which allowed us to account for additional potential factors that might impact serum 25(OH)D concentrations.
The results of this study suggest a need for randomized clinical trials to not only define optimal treatment strategies in this patient population, but to also understand how normalization of serum 25(OH)D levels could be beneficial for outcomes related to traditional and nontraditional roles for vitamin D in human health. As the obese population grows and more individuals seek bariatric surgery, these research questions will be of considerable importance to investigate. In summary, this line of research has particular relevance to clinicians in obesity surgery because it further underscores the importance of comprehensive nutrition management and education with regard to vitamin D.
From a clinical perspective our data suggest that weight and FM loss may promote increased serum 25(OH)D concentration post-operatively, and release of vitamin D from adipose is not likely to be the primary contributing factor. We based this conclusion on actual SAT and VAT adipose vitamin D concentrations and total body FM data. We further utilized our FM data to independently evaluate the hyperbolic model, proposed by Drincic et al [40] to describe the relationship between body size and serum 25(OH)D concentrations in obese individuals. Based on our analyses, we conclude that the volume of distribution appears to be an important contributing factor to serum 25(OH)D concentrations in obese individuals.
Acknowledgments
We thank the study participants for their commitment to this study and the team at the Weight Loss Surgery Center and the GCRC at the University of Minnesota for ongoing support of this study. We thank Dr. Michael Jensen, Charles Ford, and Jaime Gransee at the Mayo Clinic for assistance with the deuterium analyses, and Dr. Barbara McCloskey at Thomas Jefferson University for assistance with the 25(OH)D assays. We also thank Dr. Dale Schoeller for input on the TBW method.
SOURCE OF FINANCIAL OR MATERIAL SUPPORT: Funding for this study was provided by the Rhoads Research Foundation of the American Society for Parenteral and Enteral Nutrition (to CP Earthman); Center for Translational Science Activities Grant ULI-RR24150 and National Institutes of Health (NIH) Grant DK50456 (to MD Jensen); NIH Grant M01-RR00400, which supported the GCRC at the University of Minnesota; the Minnesota Agricultural Experiment Station (to CP Earthman); and Dairy Management, Inc., administered by the Dairy Research Institute (to CP Earthman).
Footnotes
Ideal body weight was determined from the Metropolitan Life Weight Tables. The middle weight of the range for a medium framed woman was used for all IBW calculations.
DISCLOSURES
None
Contributor Information
Lauren M Beckman, University of Minnesota - Department of Food Science and Nutrition, 1334 Eckles Avenue, Saint Paul, MN 55108.
Carrie P Earthman, University of Minnesota - Department of Food Science and Nutrition, 1334 Eckles Avenue, Saint Paul, MN 55108.
William Thomas, University of Minnesota - Division of Biostatistics, School of Public Health, 420 Delaware Street SE, Minneapolis, MN 55455.
Charlene W Compher, University of Pennsylvania - School of Nursing, 418 Curie Boulevard, Philadelphia, PA 19104.
Juan Muniz, University of Pennsylvania - School of Nursing, 418 Curie Boulevard, Philadelphia, PA 19104.
Ronald L. Horst, Heartland Assays, LLC, 2711 S. Loop Drive, Suite 4400 Building 4, Ames, IA 50010
Sayeed Ikramuddin, University of Minnesota – Department of Surgery, 516 Delaware Street SE, Minneapolis, MN 55454.
Todd A. Kellogg, University of Minnesota – Department of Surgery, 516 Delaware Street SE, Minneapolis, MN 55454
Shalamar D Sibley, University of Minnesota - Department of Medicine, 420 Delaware Street SE, Minneapolis, MN 55455.
References
- 1.Goldner WS, Stoner JA, Thompson J, Taylor K, Larson L, Erickson J, McBride C. Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controls. Obes Surg. 2008;18:145–150. doi: 10.1007/s11695-007-9315-8. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D: a D-Lightful health perspective. Nutr Rev. 2008;66:S182–94. doi: 10.1111/j.1753-4887.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 3.Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Dietary Reference Intakes for Calcium Vitamin D. Washington DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 4.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 5.Priemel M, von Domarus C, Klatte T, Kessler S, Schlie JMS, Proksch N, Pastor F, Netter C, Streichert T, Püschel K, Amling M. Bone mineralization defects and vitamin D deficiency: Histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25:305. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 6.Blum M, Dolnikowski G, Seyoum E, Harris SS, Booth SL, Peterson J, Saltzman E, Dawson-Hughes B. Vitamin D(3) in fat tissue. Endocrine. 2008;33:90–94. doi: 10.1007/s12020-008-9051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 8.Kremer R, Campbell PP, Reinhardt T, Gilsanz V. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab. 2009;94:67–73. doi: 10.1210/jc.2008-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88:157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 10.Kayaniyil S, Vieth R, Harris SB, Retnakaran R, Knight JA, Gerstein HC, Perkins BA, Zinman B, Hanley AJ. Association of 25(OH)D and PTH with metabolic syndrome and its traditional and nontraditional components. J Clin Endocrinol Metab. 2011;96:168–175. doi: 10.1210/jc.2010-1439. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff-Ferrari H. Health effects of vitamin D. Dermatol Ther. 2010;23:23–30. doi: 10.1111/j.1529-8019.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- 12.Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg. 2009;19:1605–1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 13.Smith BR, Schauer P, Nguyen NT. Surgical approaches to the treatment of obesity: bariatric surgery. Endocrinol Metab Clin North Am. 2008;37:943–964. doi: 10.1016/j.ecl.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 15.Lima MM, Pareja JC, Alegre SM, Geloneze SR, Kahn SE, Astiarraga BD, Chaim EA, Geloneze B. Acute effect of roux-en-y gastric bypass on whole-body insulin sensitivity: a study with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3871–3875. doi: 10.1210/jc.2010-0085. [DOI] [PubMed] [Google Scholar]
- 16.Lin E, Armstrong-Moore D, Liang Z, Sweeney JF, Torres WE, Ziegler TR, Tangpricha V, Gletsu-Miller N. Contribution of Adipose Tissue to Plasma 25-Hydroxyvitamin D Concentrations During Weight Loss Following Gastric Bypass Surgery. Obesity (Silver Spring) 2010;19:588–594. doi: 10.1038/oby.2010.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pramyothin P, Biancuzzo RM, Lu Z, Hess DT, Apovian CM, Holick MF. Vitamin D in Adipose Tissue and Serum 25-Hydroxyvitamin D After Roux-en-Y Gastric Bypass. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.170. [DOI] [PubMed] [Google Scholar]
- 18.Coupaye M, Puchaux K, Bogard C, Msika S, Jouet P, Clerici C, Larger E, Ledoux S. Nutritional consequences of adjustable gastric banding and gastric bypass: a 1-year prospective study. Obes Surg. 2009;19:56–65. doi: 10.1007/s11695-008-9571-2. [DOI] [PubMed] [Google Scholar]
- 19.DiGiorgi M, Daud A, Inabnet WB, Schrope B, Urban-Skuro M, Restuccia N, Bessler M. Markers of bone and calcium metabolism following gastric bypass and laparoscopic adjustable gastric banding. Obes Surg. 2008;18:1144–1148. doi: 10.1007/s11695-007-9408-4. [DOI] [PubMed] [Google Scholar]
- 20.Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)-a prospective study. Obes Surg. 2010;20:447–453. doi: 10.1007/s11695-009-0068-4. [DOI] [PubMed] [Google Scholar]
- 21.Riedt CS, Brolin RE, Sherrell RM, Field MP, Shapses SA. True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2006;14:1940–1948. doi: 10.1038/oby.2006.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlin AM, Rao DS, Yager KM, Parikh NJ, Kapke A. Treatment of vitamin D depletion after Roux-en-Y gastric bypass: a randomized prospective clinical trial. Surg Obes Relat Dis. 2009;5:444–449. doi: 10.1016/j.soard.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Phillips KM, Byrdwell WC, Exler J, Harnly J, Holden JM, Holick MF, Hollis BW, Horst RL, Lemar LE, Patterson KY, Tarrago-Trani MT, Wolf WR. Development and validation of control materials for the measurement of vitamin D3 in selected U.S. foods. J Food Comp Anal. 2008;21:527–534. [Google Scholar]
- 24.Levitt DG, Beckman LM, Mager JR, Valentine BJ, Sibley SD, Beckman TR, Kellogg TA, Ikramuddin S, Earthman CP. Comparison of DXA and water measurements of body fat following gastric bypass surgery and a physiological model of body water, fat, and muscle composition. J Appl Physiol. 2010 doi: 10.1152/japplphysiol.00278.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoeller DA. Hydrometry. In: Roche AF, Heymsfield SB, Lohman TG, editors. Human Body Composition. Champaign, IL: Human Kinetics; 1996. pp. 25–43. [Google Scholar]
- 26.Ernst B, Thurnheer M, Schmid SM, Wilms B, Schultes B. Seasonal variation in the deficiency of 25-hydroxyvitamin D(3) in mildly to extremely obese subjects. Obes Surg. 2009;19:180–183. doi: 10.1007/s11695-008-9636-2. [DOI] [PubMed] [Google Scholar]
- 27.Dalcanale L, Oliveira CP, Faintuch J, Nogueira MA, Rondo P, Lima VM, Mendonca S, Pajecki D, Mancini M, Carrilho FJ. Long-term nutritional outcome after gastric bypass. Obesity Surgery. 2010;20:181–187. doi: 10.1007/s11695-009-9916-5. [DOI] [PubMed] [Google Scholar]
- 28.Fish E, Beverstein G, Olson D, Reinhardt S, Garren M, Gould J. Vitamin D status of morbidly obese bariatric surgery patients. J Surg Res. 2010;164:198–202. doi: 10.1016/j.jss.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 29.Plotnikoff GA. Vitamin D--the steroid hormone prescription for every patient. Minn Med. 2003;86:43–45. [PubMed] [Google Scholar]
- 30.Hines C., Jr Vitamins. Absorption and malabsorption. Arch Intern Med. 1978;138:619–621. doi: 10.1001/archinte.138.4.619. [DOI] [PubMed] [Google Scholar]
- 31.Buchwald H Consensus Conference Panel. Consensus conference statement bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Surg Obes Relat Dis. 2005;1:371–381. doi: 10.1016/j.soard.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Aarts E, van Groningen L, Horst R, Telting D, van Sorge A, Janssen I, de Boer H. Vitamin D absorption: consequences of gastric bypass surgery. Eur J Endocrinol. 2011;164:827–832. doi: 10.1530/EJE-10-1126. [DOI] [PubMed] [Google Scholar]
- 33.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux M. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 34.Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology. 2007;132:2253–2271. doi: 10.1053/j.gastro.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 35.Bobbioni-Harsch E, Huber O, Morel P, Chassot G, Lehmann T, Volery M, Chliamovitch E, Muggler C, Golay A. Factors influencing energy intake and body weight loss after gastric bypass. Eur J Clin Nutr. 2002;56:551–556. doi: 10.1038/sj.ejcn.1601357. [DOI] [PubMed] [Google Scholar]
- 36.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 37.Beckman LM, Beckman TR, Sibley SD, Thomas W, Ikramuddin S, Kellogg TA, Ghatei MA, Bloom SR, le Roux CW, Earthman CP. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass surgery. J Parenter Enteral Nutr. 2011;35:169–180. doi: 10.1177/0148607110381403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H, Kaser S, Kaser A, Tilg H. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59:1259–1264. doi: 10.1136/gut.2010.214577. [DOI] [PubMed] [Google Scholar]
- 39.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–69. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drincic A, Armas LAG, Van Diest E, Heaney R. Volumetric Dilution, Rather Than Sequestration Best Explains the Low Vitamin D Status of Obesity. Obesity. 2012 doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]