Abstract
Objective
To examine the incidence of single or multiple organ failure postburn and its resultant clinical outcomes during acute hospitalization.
Summary Background Data
Patient outcomes are inherently dependent on intact organ function; however, burn injury affects the structure and function of almost every organ, but especially lung, liver, kidney and heart. Therefore, single-organ failure and/or multiorgan failure (MOF) are thought to contribute significantly to postburn morbidity and mortality but to date no large trial examining the effects of MOF on postburn outcomes exists.
Methods
Incidence of MOF was monitored in 821 pediatric burn patients during acute hospitalization. Patients were divided into groups based on the incidence of single organ specific failure, MOF, and non-MOF. The DENVER2 score was used to assess organ specific scores for lung, liver, kidney and heart. The patient’s demographics, injury characteristics, and outcome parameters were recorded.
Results
Respiratory failure has the highest incidence in the early phase of postburn injury, and decreases starting 5 days postburn. Cardiac failure was noted to have the highest incidence throughout hospital stay. Incidence of hepatic failure increases with the length of hospital stay and is associated with a high mortality during the late phase of the acute hospital stay. Renal failure has an unexpectedly low incidence but is associated with a high mortality during the first three weeks postburn injury. Three or more organ failure is associated with very high mortality.
Conclusion
This is the first large study in burn patients to determine the incidence of organ specific failure and outcome. The results of this study confirmed the expected chronologic incidence of organ-specific failure and yield the long-term mortality of liver and renal failure. (NCT00673309)
Keywords: burn, organ failure, inflammation, hypermetabolism, morbidity, mortality
INTRODUCTION
Intact organ function is essential for positive outcome of burn patients; however, burn injury affects the structure and function of almost every organ.1 Therefore, organ failure (whether single or multiple) is a significant contributor to postburn morbidity and mortality. The hyper-metabolic and inflammatory postburn response is associated with vast catabolism, protein and amino acid degradation, insulin resistance and hyperglycemia, as well as lipolysis, all of which can contribute to organ failure, especially the liver.1–3 Inhalation injury impairs the respiratory function by damaging the alveolar epithelium; however, is not the exclusive cause of respiratory failure.4–6 Renal failure can result from trauma, and inappropriate fluid resuscitation.7–9 Cardiac failure postburn can be a result of cardio-myocyte apoptosis, dilative cardiomyopathy due to over-resuscitation, or cardio-toxic agents.1, 10–12 As previously emphasized, intact organ function is essential to recovery from severe thermal injury. Therefore, a discrepancy between demand and function exist that impairs postburn morbidity and mortality.
Besides the challenge of treating organ failure, at times, it can be very difficult to detect or monitor single- or multiple-organ failure (MOF). Several attempts have been made in the past to validate established scoring systems such as the DENVER2 criteria in the burn patient population.13 This patient population is at a high risk of developing organ failure and would benefit from accurate assessment of organ functions. The DENVER2 score monitors the cardiac, respiratory, renal, and liver function of the body.14 Unlike other scoring systems, it can be applied to patients of any age and weight, as the quantified parameters are consistent in all patient populations. The validity and predictive value of the assessment of organ function with other scoring systems has been controversial especially in the burn patient population. Due to the physiologic changes in the body and treatment requirements such as high-fluid resuscitation, the accuracy of the scoring assessment has been questioned by several studies. However, others and we15 hypothesize that the DENVER2 score represents an adequate method for screening and monitoring organ failure.
Despite the need to monitor and detect organ failure in burns, to date, there are only few studies looking at the incidence of single or MOF. Therefore, the first aim of this study was to determine the incidence of organ failure and to identify the critical time points for organ specific disorders following severe burn injury during acute hospitalization. Secondly, we analyzed the incidence of organ failure and correlated organ failure with the outcome of each organ failure or the combination of multiple organs.
PATIENTS AND METHODS
Eight-hundred twenty-one pediatric patients with burns over 30% total burn surface area (TBSA) admitted to our burn center were included in the study. Organ function or MOF was and is one of the main outcomes of our studies. Therefore, this study is not a retrospective analysis; it is a prospective ongoing study with prospective analysis.
In a first assignment, patients were grouped according the incidence of MOF in non-MOF and MOF groups using the DENVER2 criteria as described below. To determine the effects of specific organ failure, patients were assigned to groups according the occurrence of specific organ failures utilizing the same score system. Organ failure was determined in patients having a score according the DENVER2 definitions greater than two for each organ.
On admission, patients were resuscitated according to the Galveston formula with 5000 cc/m2 TBSA burned+2000 cc/m2 TBSA lactated Ringer’s solution given in increments over the first 24 hours. Within 48 hours of admission, all patients underwent total burn wound excision and the wounds were covered with autograft. Any remaining open areas were covered with homograft. This was repeated until all open wound areas were covered with autologous skin.
All patients underwent the same nutritional treatment according to a standardized protocol as previously published.1, 16, 17 The nutritional route of choice in our patient population was enteral nutrition via a naso-duodenal or naso-gastric tube. Parenteral nutrition was only given in rare instances if the patient could not tolerate enteral feeds.
Patient demographics (age, date of burn and admission, sex, burn size, and depth of burn) and concomitant injuries such as inhalation injury, sepsis, morbidity, and mortality were recorded. Sepsis was defined as previously published.1, 16, 17
Organ failure was assessed using the DENVER2 definitions (Supplemental Tables S1, S2) prospectively during the entire acute hospital stay. Organ-specific functions were assessed continuously during the hospitalization. MOF was set as a total score >3 out of 12 maximum points for two or more organs for a minimum of two consecutive days. The worst daily score was used to assess the organ failure. Severe organ failure for a single organ was set at >2 points out of three maximum points of the daily average of the assessed organ DENVER2 score (Supplemental Tables S1, S2). We further determined time between operations as a measure for wound healing/re-epithelization.
Proteins and cytokines
Blood and/or urine was collected from burn patients at admission, pre-operatively, and 5 days postoperatively for 4 weeks for serum hormone, protein, cytokine and urine hormone analysis. Blood was drawn in a serum-separator collection tube and centrifuged for 10 minutes at 1320 rpm. The serum was removed and stored at −70°C until assayed.
Serum hormones and acute phase proteins were determined using HPLC, nephelometry (BNII, Plasma Protein Analyzer Dade Behring, MD), and ELISA techniques. The Bio-Plex Human Cytokine 17-Plex panel was used with the Bio-Plex Suspension Array System (Bio-Rad, Hercules, CA) to profile expression of seventeen inflammatory mediators as previously published.1
Patient data was collected and recorded prospectively using the clinical information system Emtek by physicians, nurses and supportive staff. Data was processed and analyzed with Microsoft Access®, Excel® Microsoft Corporation Inc. (Redmond, WA, USA).
Ethics and statistics
The study was reviewed and approved by the Institutional Ethics Review Board of the University of Texas Medical Branch, Galveston, Texas. Prior to the study, each subject, parent or child’s legal guardian had to sign a written informed consent form. Statistical methods such as Student’s t-test, Chi-square test, logistic regression and Kaplan-Meier Survival Analysis (log-Rank) were used where appropriate. Data are expressed as means±SD or SEM, where appropriate. Statistical significance was accepted at p<0.05. Participating patients were part of a study registered at www.clinicaltrials.gov #NCT00673309.
RESULTS
Demographics and clinical outcomes of non-MOF vs. MOF patients
A total of 821 burn patients were included in the study. MOF occurred in 157 burn patients while 664 burn patients did not develop MOF during the hospital stay. Both patient populations were similar in demographics and did not show significant differences in gender, ethnicity, and age (Table 1). Burn size was significantly greater in patients developing MOF when compared to patients with no MOF (non-MOF: 51±16 % TBSA, MOF: 69±18%, p<0.00001); in addition, full thickness burn was greater in MOF patients compared to non- MOF patients (non-MOF: 34±24%, MOF: 58±27%, p<0.00001). Furthermore, the incidence of inhalation injury was significantly higher in MOF patients compared to non-MOF (non- MOF: 192 (29%), MOF: 89 (57%), p<0.00001). Time injury to admission to the burn center did not differ among the groups (non-MOF: 3.6±4.3, MOF 3.3±4.0, p=0.4).
Table 1.
Demographic characteristics at hospital admission.
| no MOF | MOF | p value | |
|---|---|---|---|
| n | 664 | 157 | |
| Gender | |||
| Male (n) | 436 | 95 | 0.26185 |
| Female (n) | 228 | 62 | |
| Ethnicity | |||
| African American (n) | 53 | 8 | 0.14147 |
| Caucasian (n) | 111 | 21 | |
| Hispanic (n) | 487 | 123 | |
| Other | 13 | 5 | |
| Age at admit (years) | 7.3 ± 5.2 | 8.0 ± 5.8 | |
| Inhalation Injury n (%) | 192 (29) | 89 (57) | <0.00001 |
| Type of burn | |||
| Flame n (%) | 444 (67) | 124 (79.0) | 0.016 |
| Scald n (%) | 166 (25) | 25 (16) | |
| Other n (%) | 54 (8) | 8 (5) | |
| TBSA burn % | 51 ± 16 | 69 ± 18 | <0.00001 |
| TBSA third % | 34 ± 24 | 58 ± 27 | <0.00001 |
| Burn to admit (days) | 3.6 ± 4.3 | 3.3 ± 4.0 | 0.38695 |
Patients are stratified according the incidence of MOF. Patients with MOF had a larger burn size and a higher incidence of inhalation injury.
Overall, patients that developed MOF had significantly worse outcomes compared to burn patients that did not develop MOF (Table 2). Confirming our stratification, we found that burn patients developing MOF had significantly greater maximum DENVER2 scores when compared to non-MOF patients (non-MOF: 2.8±1.1, MOF 6.2±1.7, p<0.00001). MOF patients needed significantly more surgeries (non-MOF: 3.3±2.5, MOF: 6.3±4.6, p<0.00001), however time between surgeries did not differ (non-MOF: 4.8±1.7, MOF: 4.9±2.3 days, p=0.3). The length of hospital stay (LOS) was longer in the MOF group (non-MOF: 22.9±18.2, MOF: 44.1±39.3 days, p<0.00001) which was confirmed when LOS was normalized to TBSA burn size (non-MOF: 0.4±0.3, MOF: 0.6±0.5 days/%TBSA burn, p<0.00001). MOF was associated with a significant increased incidence of major infections (non-MOF: 2.1±2.3, MOF 3.3±2.7, p<0.00001) and Sepsis (non-MOF: 30 (5%), MOF 49 (31%), p<0.00001). All these worse outcomes are associated with a significantly higher mortality (non-MOF: 15 (2%), MOF 65 (41%), p<0.00001).
Table 2.
Clinical outcomes and hospital course.
| no MOF | MOF | p value | |
|---|---|---|---|
| n | 664 | 157 | |
| Max DENVER2 | 2.8 ± 1.1 | 6.2 ± 1.7 | <0.00001 |
| OR n | 3.3 ± 2.5 | 6.3 ± 4.6 | <0.00001 |
| Time between OR (days) | 4.8 ± 1.7 | 4.9 ± 2.3 | 0.3 |
| LOS ICU (days) | 23 ± 18 | 44 ± 39 | <0.00001 |
| LOS/TBSA (days/%) | 0.4 ± 0.3 | 0.6 ± 0.5 | <0.00001 |
| MOF n (%) | 0 (0) | 157 (100) | <0.00001 |
| Sepsis n (%) | 30 (5) | 49 (31) | <0.00001 |
| Major infections n | 2.1 ± 2.3 | 3.3 ± 2.7 | <0.00001 |
| Mortality n (%) | 15 (2) | 65 (41) | <0.00001 |
All major clinical outcome parameters showed impaired in the MOF group. The indicator for wound healing (time between the operations) did not significantly differ between groups.
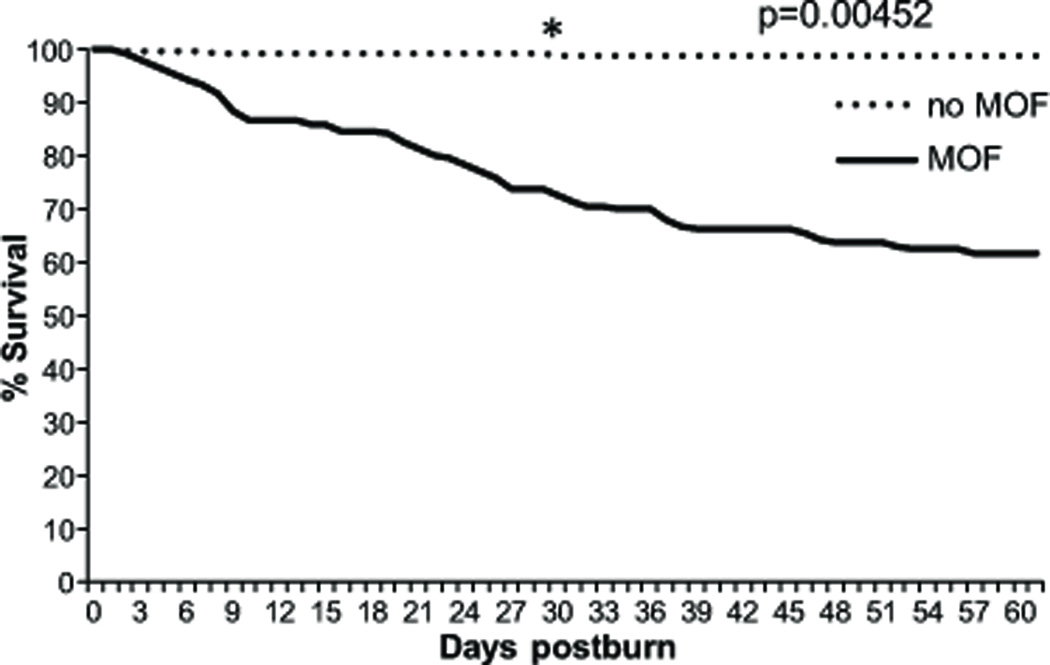
Kaplan-Meier survival analysis showed that the mortality rate had no peak but was rather constant during the first 60 days after burn injury (Figure 1).
Figure 1. Survival curve for the first 60 days after injury.
The mortality rate in patients without MOF was significantly higher during the acute hospitalization. Mortality after day 30 plateaued in the non-MOF group, whereas patients with MOF showed a constant mortality rate till day 60.
Biochemical markers
In order to obtain further insights, we conducted some biochemical analyses. Both patient populations - non-MOF and MOF - showed elevated systemic glucose levels (Figure 2). In patients with MOF glucose levels were significantly more elevated along the hospital course and reached similar levels around day 50 postburn. Inflammatory markers followed the same pattern (Figure 3). IL-6 and CRP had a significantly higher expression over the first 60 days. MCP-1 and TNF-α assimilated beginning day 41.
Figure 2.
Daily average glucose levels, an indicator for stress induced insulin resistance and metabolic dysfunction is associated with the incidence of MOF. Significantly higher systemic glucose levels can be seen in patients with incidence of MOF.
Figure 3.
Biochemical markers for inflammation such as IL-6, MCP-1, TNF-α, and CRP compared between the MOF and non-MOF group.
Patients grouped according to specific organ failure demographics and outcome parameters
The next step was to analyze morbidity and mortality in relation to each single organ failure. We found that 586 patients had no evidence of severe organ failure according to the definition of the DENVER2 scoring system. Respiratory failure occurred in 230 patients, followed by cardiac (n=77), hepatic (n=23), and renal (n=16) failure. Demographics, injury characteristics, and clinical outcomes of each patient group were compared to patients with no organ failure and shown in Tables 3 and 4. For clarification, some patients had two or more organ failure, but were listed for each organ failure hence the sum of the patients is not 821. All groups were similar in gender distribution (p=0.9) as well as ethnicity distribution. Patients with renal failure were significantly older compared to patients without any organ dysfunction (no Organ: 7.1±5.3 years, Kidney: 15.3±2.4 years, p=0.0002). TBSA and third-degree burn size were significantly larger in all organ failure groups compared to the no organ failure group (p<0.00001), combined with a higher incidence of flame burn (p<0.00001). The largest burn size was found in the renal failure group. Interestingly, Inhalation injury did not significantly differ between groups (p=0.1).
Table 3.
Patient characteristics at admission stratified according the incidence of individual organ failure.
| All | no Organ | Heart | Liver | Lung | Kidney | p value | |
|---|---|---|---|---|---|---|---|
| n | 821 | 586 | 77 | 23 | 230 | 16 | |
| Gender | |||||||
| male n | 531 | 389 | 44 | 15 | 140 | 12 | 0.91324 |
| female n | 290 | 197 | 33 | 8 | 90 | 4 | |
| Ethnicity | * | * | |||||
| AA | 61 | 45 | 5 | 1 | 16 | 2 | 0.05 |
| C | 132 | 98 | 12 | 2 | 34 | 3 | |
| Hispanic | 610 | 430 | 56 | 18 | 176 | 11 | |
| Other | 18 | 13 | 4 | 2 | 4 | 0 | |
| Age at admission (yrs) | 7.3 ± 5.3 | 7.1 ± 5.2 | 8.3 ± 6.0 | 11.3 ± 5.9* | 7.6 ± 5.6 | 15.3 ± 2.4* | 0.00021 |
| Inhalation Injury n (%) | 281 (34) | 160 (27) | 44 (57) | 14 (61) | 118 (51) | 8 (50) | 0.09 |
| Type of burn | * | * | * | * | |||
| Flame n (%) | 568 (69.2) | 388 (66.2) | 60 (77.9) | 20 (87.0) | 176 (76.5) | 16 (100.0) | <0.00001 |
| Scald n (%) | 191 (23.3) | 151 (25.8) | 11 (14.3) | 0 (0.0) | 39 (17.0) | 0 (0.0) | |
| Other n (%) | 62 (7.6) | 47 (8.0) | 6 (7.8) | 3 (13.0) | 15 (6.5) | 0 (0.0) | |
| Burn size | |||||||
| TBSA burn % | 54.8 ± 17.6 | 50.7 ± 15.5 | 68.0 ± 18.4* | 72.8 ± 16.6* | 64.6 ± 18.7* | 80.3 ± 16.5* | <0.00001 |
| TBSA third % | 38.5 ± 26.5 | 32.4 ± 23.4 | 54.9 ± 29.4* | 59.1 ± 28.5* | 52.7 ± 27.8* | 72.4 ± 27.7* | <0.00001 |
indicate significance between the annotated group and the group no organ failure group. Please see Table 5 for patients with two or more organ failure.
Table 4.
Clinical outcomes stratified according to specific organ failure.
| All | no Organ | Heart | Liver | Lung | Kidney | p value | |
|---|---|---|---|---|---|---|---|
| n | 821 | 586 | 77 | 23 | 230 | 16 | |
| OR n | 3.9 ± 3.3 | 3.2 ± 2.5 | 5.2 ± 4.6* | 7.5 ± 4.3* | 5.6 ± 4.2* | 6.1 ± 3.1* | <0.00001 |
| LOS ICU | 26.6 ± 23.2 | 22.2 ± 18.2 | 41.5 ± 31.8* | 55.4 ± 28.0* | 41.9 ± 31.7* | 74.5 ± 81.3* | 0.04088 |
| LOS/TBSA | 0.5 ± 0.3 | 0.4 ± 0.3 | 0.7 ± 0.6* | 0.9 ± 0.3* | 0.7 ± 0.4* | 1.0 ± 1.1 | 0.0238 |
| Max DENVER2 | 3.5 ± 1.8 | 2.6 ± 0.9 | 7.2 ± 1.6* | 8.4 ± 1.6* | 5.7 ± 1.7* | 8.3 ± 1.6* | <0.00001 |
| MOF n (%) | 157 (19.1) | 3 (0.5) | 66 (85.7)* | 22 (95.7)* | 152 (66.1)* | 16 (100.0)* | <0.00001 |
| Sepsis n (%) | 79 (9.6) | 29 (4.9) | 29 (37.7)* | 17 (73.9)* | 50 (21.7)* | 12 (75.0)* | <0.00001 |
| Major infections n | 2.4 ± 2.4 | 2.2 ± 2.3 | 3.6 ± 3.0* | 4.2 ± 3.5* | 2.9 ± 2.6* | 4.3 ± 3.6* | 0.00665 |
| Mortality n (%) | 80 (10) | 11 (2) | 46 (60)* | 18 (78)* | 68 (30)* | 14 (88)* | <0.00001 |
Stars indicate significance between the annotated group and the group no organ failure group. Please see Table 5 for patients with two or more organ failure.
All major clinical outcome parameters for each organ failure are shown in Table 4. It is interesting to note that the majority of patients detected via DENVER2 scoring had pulmonary failure, followed by heart, liver, and lastly kidney. This is most likely due to the scoring system that only identifies severe organ damage and hence mild episodes of renal failure or liver failure are not detected. Patients with liver failure required the most surgeries, while patients with kidney failure or liver failure had the longest LOS even when normalized for burn size. Patients with kidney or liver failure had the highest DENVER2 scores followed by cardiac and lung indicating that these patients were the sickest patients, which is reflected in the sepsis and major infection incidence.
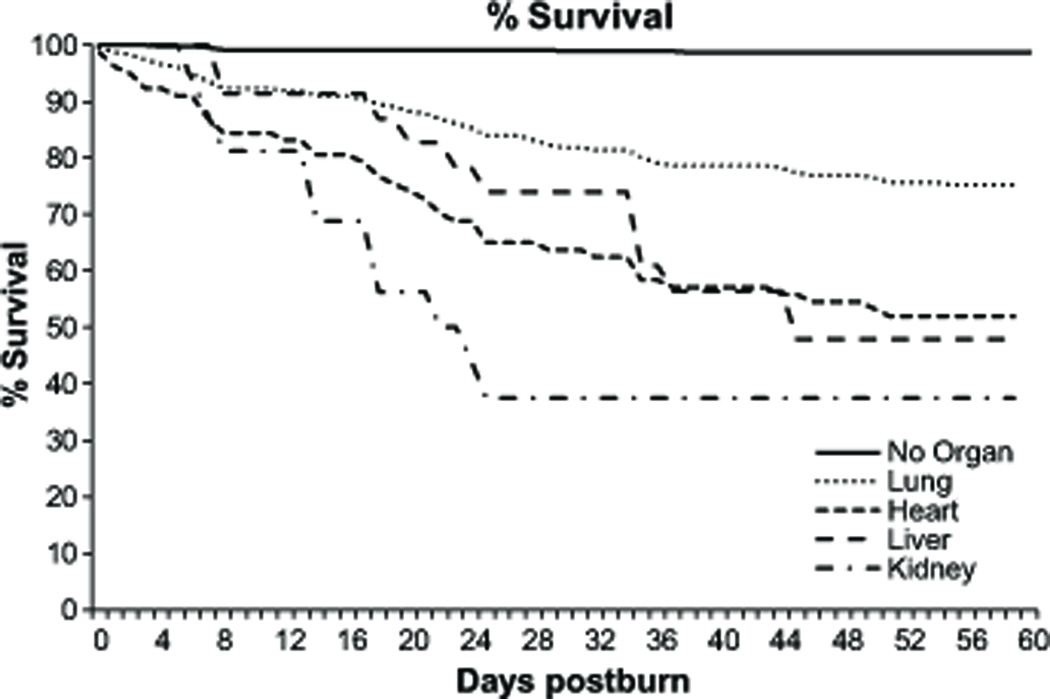
Sixty-day mortality was the highest in patients with severe kidney failure, followed by liver failure. The best outcome was in burn patients who suffered from pulmonary failure (Figure 4).
Figure 4.
Survival curve of patients stratified according specific organ failure.
Co-incidence of organ failure
Causal relationships of the co-incidence of individual organ failures are shown in Table 5A. A significant relationship was found in patients with liver failure and consecutive incidence of heart and liver failure. Also patients with cardiac failure had a significant interrelation with the incidence of liver failure. The primary incidence of respiratory and renal failure did not show a statistically significant co-incidence with other organ dysfunction. The highest incidence of a single-organ failure was noted in patients with respiratory failure. All other groups had a relatively higher incidence of two or more additional organs failing, whereas renal failure did not occur as a single organ failure in the patient population.
Table 5.
Coincidence and correlation between organ failures.
| (A) | ||||
|---|---|---|---|---|
| Heart | Lung | Kidney | Liver | |
| Heart (77) | NN | 73 | 10 | 16* |
| Lung (230) | 73 | NN | 16 | 22 |
| Kidney (16) | 10 | 16 | NN | 6 |
| Liver (23) | 16* | 22 | 6* | NN |
| (B) | ||||
|---|---|---|---|---|
| 1 Organ | 2 Organs | 3 Organs | 4 Organs | |
| Heart (77) | 4 | 51 | 18 | 4 |
| Lung (230) | 147 | 59 | 20 | 4 |
| Kidney (16) | 0 | 4 | 8 | 4 |
| Liver (23) | 1 | 4 | 14 | 4 |
(A) Displays the coincidence of the single organ failures. Logistic regression revealed a statistically significant relationship between liver failure accompanied by heart and renal failure. (B) Depicts the incidence of single and combined organ failures in the patient population.
Severity of organ dysfunction over time
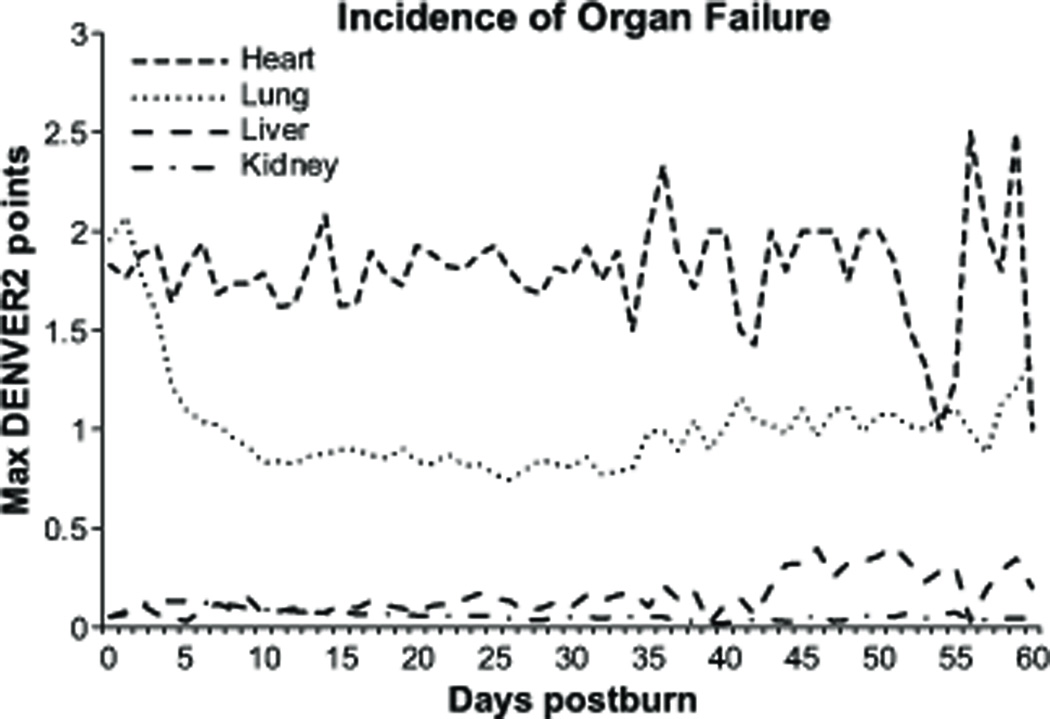
Figure 5 shows the maximum DENVER2 points for each organ during the first 60 days of all patients. Cardiac failure had a high variability; lung failure trended to normalize after the initial 10 days and increased again along with the hospitalization. Hepatic function worsened in patients with a prolonged hospital stay. Renal function was in most patients not impaired as recorded by the DENVER2 score in accordance with the low incidence of severe renal failure in the pediatric patient population.
Figure 5.
Average of DENVER2 points over time for the first 60 days after burn injury of all patients. Respiratory failure tends to decrease after within the first 10 days after injury whereas liver failure has an increasing trend over the first 60 days. Renal and cardiac dysfunction remain at relatively stable along the hospital course.
DISCUSSION
Severe burn injury is associated with profound hypermetabolic and catabolic responses. Protein catabolism leads not only to a failure of the skin barrier but also to impaired organ functions.1 Impaired organ function can result in organ failure or even in MOF, which is one of the leading causes of death in the thermally injured patient.18 Despite the common occurrence of organ failure in this patient population, there’s incomplete understanding of single/MOF. The underlying causes leading to organ failure are not clear but have been associated infections and sepsis, open burn wounds, inadequate oxygen delivery and extraction, protein degradation, insulin resistance and hyperglycemia, lipolysis, and impaired gastrointestinal function with increased bacterial translocation. It thus appears that single/MOF in the thermally injured patient is very complex, involving a multi-factorial cascade of postburn pathophysiologic sequelae.1, 12, 19
Aims of this study were to determine the incidence of single-organ failure, MOF, the time course for organ failure, and the outcomes. We found that 19% of our 821 patients had MOF as captured by the DENVER2 score. To our surprise the highest incidence of organ failure was pulmonary followed by cardiac, liver, and kidney. The worst outcome was in patients with profound kidney or liver failure. The best outcome was in burn patients with pulmonary failure. We also found that burn patients with 3 or more organ failure have an extremely poor prognosis. There were no survivors in the group with three organ failures. The poorest outcome again is associated with kidney and liver and an additional organ failure. These data clearly indicate that single/MOF is important contributors to mortality. The results of our study now necessitate investigations to determine which patients are at high risk to develop organ failure to individualize patient treatment. We believe that early detection of patients at high risk for MOF is important and would result in improved outcomes, as interventions would be implemented early in course of treatment. To our knowledge, there are several studies that are currently investigating the effect of biomarkers on the early detection of MOF.
Manifest and severe renal failure was associated with a high mortality despite recent studies showing that renal failure is usually associated with a good outcome.9,20 We found that renal failure in association with liver failure resulted in very poor outcomes. The reasons for these results are not entirely clear; however, possible explanation is that DENVER2 may only detect severe non-reversible renal failure and the milder cases of renal dysfunction/failure cannot be determined using this score. This surely leads to the question whether DENVER2 is a valid and applicable score. The discussion remains about the best score to use in burn patients. Our choice of the use of the DENVER2 score was based on its application in various multicenter trials indicating applicability and validity in burn patients.
One expects that patients with liver failure would have a very poor prognosis, which was confirmed in the present study. Several studies have demonstrated that a bilirubin level above 4 mg/dl is an indicator of poor prognosis with associated high morbidity and mortality.1, 21 The livers metabolic, inflammatory, acute phase and immune functions play a pivotal role in recovery from injury in multiple modulating pleiotropic pathways.1, 2, 21, 22 The hepatic acute phase response is characterized by an increased production of acute phase proteins with a failure to produce constitutive proteins. The shift in hepatic protein synthesis leads to physiologic alterations of transport, metabolic and immune functions.1, 2, 22 Our data shows that the liver is markedly affected postburn and that the shift of the expression of hepatic derived proteins is associated with organ failure. Animal studies have shown that burn injury causes a vast alteration in the endoplasmic reticulum (ER) and mitochondria with depletion of ER calcium stores leading to hepatocyte apoptosis and intracellular inflammation.23, 24 Novel treatments to attenuate these responses may improve liver function postburn preventing hepatomegaly and impaired protein synthesis improving postburn morbidity and consecutively mortality.
Burn injury induces dramatic cardiac stress indicated by increased cardiac output, stroke volume, oxygen consumption, and cardiac index.18, 25, 26 In a recent study by Pereira et al.,25 cardiac dysfunction was the main contributor to mortality in patients under four years of age. We have further shown that the only physiologic difference between burn sizes 20 to 40, 40 to 60, 60 to 80, and 80 to 100% TBSA was caused by cardiac dysfunction, which increased in proportion to burn size.12 Williams et al.26 have recently shown that these alterations do not only persist for a short period of time but that cardiac stress continues to be present for up to two to three years postburn. All of this data together along with our findings show that cardiac stress is present immediately postburn and persists throughout acute hospitalization and up to three years.26 Despite the early and high incidence of cardiac failure, cardiac dysfunction is associated with the best outcome; and we further propose that attenuating cardiac stress (by for example administration of beta adrenergic antagonists such as non-selective β1 and β2 blockers) will improve postburn cardiac oxygenation, reduces tachycardia, improves cardiac filling, and pressure leading to an attenuated cardiac stress response and cardiac work.
Postburn pulmonary complications are not only present after inhalation injury. Patients with severe burns have inflammatory processes causing acute respiratory distress syndrome and pneumonia, which are augmented if inhalation injury is present.4, 6, 27, 28 Our data demonstrates that lung injury has one of the best survival probabilities postburn indicating that current treatment regimens such as low-tidal volume ventilation, administration of nebulized heparin, albuterol, cortisol, epinephrine (if needed), and chest physiotherapy as well as mucolysis improve pulmonary ventilation and function. We see this as an example of how early intervention for organ failure can successfully change hospital course. We fail to do so for kidney, liver, probably pancreas, and the gastrointestinal system.
Newer studies looking at proteomic and genomic profiles can help to determine which patients are at risk of developing single/MOF (Glue Grant unpublished findings). We believe that once protein(s) or gene(s) are identified, which can direct the treating physicians in identifying the patients at risk of developing organ failure; their outcomes can be dramatically improved. These novel approaches could shorten hospital length of stay and possibly decrease postburn morbidity and mortality. To test whether some biomarkers can differentiate MOF from non-MOF, we measured glucose and several cytokines and CRP. We found that MOF had significantly higher glucose levels, serum IL-6, MCP-1, TNF, and CRP at almost all time points. These markers were not predictive, however, were significantly different during hospital course.
Conclusions
The present study is the first large-scale study demonstrating the incidence of single or MOF. The general incidence of single or MOF is around 20%. Liver and renal failure had the worst outcome, while pulmonary and cardiac have a good prognosis. A combination of three or more organ failures is always fatal with no therapeutic success. We hypothesize that it is now imperative to develop markers to predict patients at high risk for MOF to improve postburn outcomes. We, therefore, would like to emphasize that early detection of organ failure and intervention are needed to improve postburn outcomes.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the individuals who participated in this clinical trial. We also would like to thank the research staff for their assistance.
Source of Funding
This study was supported by Shriners Hospitals for Children (8660, 8760, and 9145), National Institutes of Health (R01-GM56687, T32 GM008256, and P50 GM60338), and NIDRR (H133A020102).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Authors declare no conflicts of interest.
REFERENCES
- 1.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeschke MG. The hepatic response to thermal injury: is the liver important for postburn outcomes? Mol Med. 2009;15:337–351. doi: 10.2119/molmed.2009.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeschke MG, Mlcak RP, Finnerty CC, Herndon DN. Changes in liver function and size after a severe thermal injury. Shock. 2007;28:172–177. doi: 10.1097/shk.0b013e318047b9e2. [DOI] [PubMed] [Google Scholar]
- 4.Barrow RE, Przkora R, Hawkins HK, et al. Mortality related to gender, age, sepsis, and ethnicity in severely burned children. Shock. 2005;23:485–487. [PubMed] [Google Scholar]
- 5.Cox RA, Burke AS, Oliveras G, et al. Acute bronchial obstruction in sheep: histopathology and gland cytokine expression. Exp Lung Res. 2005;31:819–837. doi: 10.1080/01902140600574967. [DOI] [PubMed] [Google Scholar]
- 6.Cox RA, Mlcak RP, Chinkes DL, et al. Upper airway mucus deposition in lung tissue of burn trauma victims. Shock. 2008;29:356–361. doi: 10.1097/shk.0b013e31814541dd. [DOI] [PubMed] [Google Scholar]
- 7.Barrow RE, Jeschke MG, Herndon DN. Early fluid resuscitation improves outcomes in severely burned children. Resuscitation. 2000;45:91–96. doi: 10.1016/s0300-9572(00)00175-1. [DOI] [PubMed] [Google Scholar]
- 8.Chrysopoulo MT, Jeschke MG, Dziewulski P, et al. Acute renal dysfunction in severely burned adults. J Trauma. 1999;46:141–144. doi: 10.1097/00005373-199901000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Jeschke MG, Barrow RE, Wolf SE, Herndon DN. Mortality in burned children with acute renal failure. Arch Surg. 1998;133:752–756. doi: 10.1001/archsurg.133.7.752. [DOI] [PubMed] [Google Scholar]
- 10.Ballard-Croft C, Carlson D, Maass DL, Horton JW. Burn trauma alters calcium transporter protein expression in the heart. J Appl Physiol. 2004;97:1470–1476. doi: 10.1152/japplphysiol.01149.2003. [DOI] [PubMed] [Google Scholar]
- 11.Horton JW, White DJ, Maass D, et al. Calcium antagonists improve cardiac mechanical performance after thermal trauma. J Surg Res. 1999;87:39–50. doi: 10.1006/jsre.1999.5726. [DOI] [PubMed] [Google Scholar]
- 12.Jeschke MG, Mlcak RP, Finnerty CC, et al. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007;11:R90. doi: 10.1186/cc6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss M, Huber-Lang M, Taenzer M, et al. Different patient case mix by applying the 2003 SCCM/ESICM/ACCP/ATS/SIS sepsis definitions instead of the 1992 ACCP/SCCM sepsis definitions in surgical patients: a retrospective observational study. BMC Med Inform Decis Mak. 2009;9:25. doi: 10.1186/1472-6947-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore FA, Sauaia A, Moore EE, et al. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501–512. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Klein MB, Silver G, Gamelli RL, et al. Inflammation and the host response to injury: an overview of the multicenter study of the genomic and proteomic response to burn injury. J Burn Care Res. 2006;27:448–451. doi: 10.1097/01.BCR.0000227477.33877.E6. [DOI] [PubMed] [Google Scholar]
- 16.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 17.Hart DW, Wolf SE, Zhang XJ, et al. Efficacy of a high-carbohydrate diet in catabolic illness. Crit Care Med. 2001;29:1318–1324. doi: 10.1097/00003246-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Williams FN, Herndon DN, Hawkins HK, et al. The leading causes of death after burn injury in a single pediatric burn center. Crit Care. 2009;13:R183. doi: 10.1186/cc8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 20.Kallinen O, Maisniemi K, Bohling T, et al. Multiple organ failure as a cause of death in patients with severe burns. J Burn Care Res. 2012;33:206–211. doi: 10.1097/BCR.0b013e3182331e73. [DOI] [PubMed] [Google Scholar]
- 21.Barrow RE, Hawkins HK, Aarsland A, et al. Identification of factors contributing to hepatomegaly in severely burned children. Shock. 2005;24:523–528. doi: 10.1097/01.shk.0000187981.78901.ee. [DOI] [PubMed] [Google Scholar]
- 22.Jeschke MG, Barrow RE, Herndon DN. Extended hypermetabolic response of the liver in severely burned pediatric patients. Arch Surg. 2004;139:641–647. doi: 10.1001/archsurg.139.6.641. [DOI] [PubMed] [Google Scholar]
- 23.Jeschke MG, Finnerty CC, Herndon DN, et al. Severe injury is associated with insulin resistance, endoplasmic reticulum stress response, and unfolded protein response. Ann Surg. 2012;255:370–378. doi: 10.1097/SLA.0b013e31823e76e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeschke MG, Gauglitz GG, Song J, et al. Calcium and ER stress mediate hepatic apoptosis after burn injury. J Cell Mol Med. 2009;13:1857–1865. doi: 10.1111/j.1582-4934.2008.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira CT, Barrow RE, Sterns AM, et al. Age-dependent differences in survival after severe burns: a unicentric review of 1,674 patients and 179 autopsies over 15 years. J Am Coll Surg. 2006;202:536–548. doi: 10.1016/j.jamcollsurg.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Williams FN, Herndon DN, Suman OE, et al. Changes in cardiac physiology after severe burn injury. J Burn Care Res. 2011;32:269–274. doi: 10.1097/BCR.0b013e31820aafcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finnerty CC, Herndon DN, Jeschke MG. Inhalation injury in severely burned children does not augment the systemic inflammatory response. Crit Care. 2007;11:R22. doi: 10.1186/cc5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finnerty CC, Herndon DN, Przkora R, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–19. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.