Abstract
Background
Early diagnosis of chronic pancreatitis by mass spectrometry-based proteomics may result in therapies to retard or modify disease progression. We aimed to identify differences in posttranslational modifications (PTMs) in pancreatic fluid proteins from individuals with chronic pancreatitis (n=9) and non-pancreatitis controls (n=9).
Methods
We collected proteomic data from pancreatic fluid using mass spectrometry techniques. We performed database searches with emphasis on PTMs using ProteinPilot. We compared the frequency of specific PTMs in pancreatic fluid between cohorts and also to those identified in bile, gastroduodenal fluid, urine, and pancreatic duct and stellate cell lysates.
Results
We identified 97 PTMs in endoscopically-collected pancreatic fluid, of which 11 were identified exclusively in one cohort and 9 others were significantly different in frequency between cohorts. Comparing pancreatic fluid with other specimens revealed differences in specific PTM frequencies, indicating that the identified PTMs were not merely artifacts of sample processing.
Conclusions
We determined PTMs of proteins extracted from pancreatic fluid which differed in frequency in chronic pancreatitis patients verses controls. Such PTMs may serve as biomarker candidates of chronic pancreatitis upon validation with larger cohorts. The analysis of the PTM profile of pancreatic fluid proteins offers an alternative method to standard protein-based biomarker discovery.
Keywords: pancreas, pancreas juice, pancreatic function test, biomarkers, chronic pancreatitis
1. Introduction
Chronic pancreatitis (CP) is a disease manifested by severe inflammation, progressive fibrosis, intense pain, and the eventual loss of exocrine and endocrine pancreatic functions. During the past decade, diseases of the exocrine pancreas have resulted in 277,000 hospitalizations and 475,000 ambulatory care visits per year [1]. Clinical diagnosis of chronic pancreatitis is based currently on identifying advanced functional, morphological, and histological features. The non-histological “surrogate” gold standard - pancreas function testing [2] - can only diagnose moderate to late stage chronic pancreatitis with irreversible tissue damage and fibrosis are [3]. These techniques can diagnose advanced disease; however, identifying chronic pancreatitis prior to irreversible organ dysfunction would revolutionize treatment and potentially lead to therapies designed to retard or modify disease progression before the pancreas is irreversibly damaged.
Early changes in the pancreas may be detected before development of chronic pancreatitis by secretin-stimulated endoscopic pancreatic function testing (ePFT) [4–6] coupled with mass spectrometry analysis and subsequent bioinformatics [7]. Pancreatic fluid is a proximal body fluid which bathes the pancreas and contains locally secreted biomolecules that are likely to include specific markers of disease. Identifying protein biomarkers of chronic pancreatitis complement established diagnostic methods, and can uncover molecular pathways regulating clinical manifestations (i.e. signs, symptoms, and complications) [8].
In addition to identifying peptides, mass spectrometry can also determine post-translational modifications (PTMs) of peptides as a result of known mass shifts [9–11]. These modifications change the chemical nature of the amino acid and/or alter the overall structure of the intact protein. PTMs increase the functional diversity of the proteome by the covalent addition of biochemical functional groups (i.e., phosphate, carbohydrates, and acetate) to proteins [12]. As such, PTMs increase the complexity of the proteome by regulating activity, localization, and interaction with other cellular moieties. Such variations affect protein function and have a considerable impact on the biological pathways of disease. Hundreds of PTMs from mass spectrometry data can be identified using algorithms such as Paragon in ProteinPilot [13]; however PTM-based biomarkers of chronic pancreatitis have not yet been investigated. We will search a mass spectrometry data set [14] using ProteinPilot to identify differences in PTM frequencies of pancreatic fluid proteins from individuals with chronic pancreatitis (CP; n=9) and non-pancreatitis controls (NP; n=9).
The aim of this study is three-fold, 1) to catalogue the PTMs in secretin-stimulated, ePFT-collected pancreatic fluid, 2) to evaluate PTMs as biomarkers for chronic pancreatitis, and 3) to compare PTMs in pancreatic fluid to other body fluids (gastroduodenal fluid, bile, urine) and lysates from pancreatic stellate and duct cells. Our results revealed specific PTMs as exclusive to or with statistically different frequencies when comparing samples from chronic pancreatitis and control cohorts. In addition, PTM profiles differed among the various samples tested, presenting a unique set of pancreatic fluid PTMs. In this study, we have attempted to build a framework, which upon further validation in larger cohorts, will significantly enhance the development of methods for early detection of pancreatic disease.
2. Materials and Methods
Study Population
This protocol was approved by the Institutional Review Board at Brigham and Women's Hospital (BWH) (IRB # 2007-P-002480/1). The study population was comprised of adult patients referred to the Center for Pancreatic Disease at BWH, for evaluation of abdominal pain via secretin-stimulated ePFT [7]. Pancreatic fluid from a total of 18 individuals, 9 with chronic pancreatitis and 9 controls, was collected. Patient characteristics are listed in Table 1.
Table 1.
Patient characteristics
| NP1 | NP2 | NP3 | NP4 | NP5 | NP6 | NP7 | NP8 | NP9 | CP1 | CP2 | CP3 | CP4 | CP5 | CP6 | CP7 | CP8 | CP9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | male | male | male | female | female | female | male | female | male | male | female | male | female | male | male | female | female | female |
| Age (years) | 55 | 53 | 48 | 30 | 32 | 30 | 23 | 55 | 56 | 42 | 44 | 41 | 49 | 58 | 68 | 64 | 61 | 53 |
| Race | white | white | white | white | white | white | white | white | hispanic | white | white | hispanic | white | white | white | white | white | white |
| Smoker | − | + | + | − | − | − | − | − | − | − | + | + | + | + | + | + | − | + |
| Alcohol | + | − | + | − | − | − | − | − | − | − | − | + | + | − | + | + | − | + |
| TIGAR-O | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | G | G | T | T | I | T | T | I | T |
| Symptoms | pain | pain | pain, weight loss |
pain, diarrhea |
pain | pain | pain | diarrhea | pain | pain, re- current pancreatitis |
pain, re- current pancreatitis |
pain | pain | pain | pain | none | weight loss, pain |
pain |
|
CT Scan Findings |
none | none | none | none | none | none | none | none | none | calcifi- cation |
calcifi- cation dilated main duct |
calcifi- cation |
n/a | calcifi- cation |
calcifi- cation dilated main duct, atrophy |
dilated duct, calcifi- cation atrophy |
calcif- cation, dilated duct, atrophy |
calcifi- cation cyst |
| CT Grade | normal | n/a | normal | n/a | normal | n/a | normal | normal | normal | definite | definite | definite | n/a | definite | definite | definite | definite | definite |
| EUS Score | 1 | 4 | 2 | n/a | 4 | 2 | 3 | 1 | n/a | 5 | 5 | n/a | 7 | n/a | 5 | n/a | 8 | 6 |
| MRI grade | n/a | 0 | n/a | 0 | 0 | 0 | 0 | 0 | 0 | IV | IV | IV | III | IV | IV | IV | IV | IV |
|
CP based on imaging |
− | − | − | − | − | − | − | − | − | + | + | + | + | + | + | + | + | + |
|
Peak [HCO3−] |
80 | 84 | 101 | 115 | 81 | 90 | 84 | 92 | 114 | 60 | 26 | 22 | 41 | 22 | 39 | 37 | 54 | 38 |
|
Secretory dysfunction |
− | − | − | − | − | − | − | − | − | + | + | + | + | + | + | + | + | + |
TIGAR-O: T, Toxic-metabolic; I, Idiopathic; G, Genetic; A, Autoimmune; R, Recurrent and severe acute pancreatitis; O, Obstructive. EUS scores: 0–2 is normal, 3–4 is equivocal, >5 is definite CP. MRI/MRCP Grade (Cambridge classification: normal=0, equivocal=I, mild=II, moderate=III, severe=IV). Secretory dysfunction: yes (+) <80, no (−) ≥80. n/a, not available.
Materials
CellStripper (25-056-CL) was purchased from Mediatech (Manassas, VA). SeeBluePlus2 Pre-Stained standard (LC5925), LDS (lithium dodecyl sulfate) sample buffer (NP0008), NuPAGE 4–12% Bis-Tris polyacrylamide gels (NP0335), SimplyBlue Coomassie stain (LC0665), and MES-SDS (2-(N-morpholino)ethanesulfonic acid-sodium dodecyl sulfate) electrophoresis buffer (NP002) were from Invitrogen (Carlsbad, CA). Sequencing-grade modified trypsin (V5111) was obtained from Promega (Madison, WI). Other reagents and solvents were from Sigma-Aldrich and Burdick & Jackson, respectively. The PaDC cell line, hTERT-HPNE (CRL-4023), was purchased from ATCC (Manassas, VA). The PaSC cell line was a kind gift from Dr. Raul Urrutia, Mayo Clinic, Minneapolis, MN.
Experimental Workflow
The general workflow for the overall analysis (Figure 1) was as follows: 1) Samples were collected; 2) proteins were extracted from the sample via chemical precipitation for body fluids and detergent extraction for cells; 3) proteins were fractionated via SDS-PAGE; 4) proteins were subjected to GeLC-MS/MS; 5) RAW files were converted to Mascot generic files (mgfs); 6) ProteinPilot was used for database searching; 7) PTMs were tabulated using the ProteinPilot Descriptive Statistics Template; and 8) the statistical significance of differentially identified PTM frequencies was determined using SAS (v. 9.2).
Figure 1. Experimental workflow.
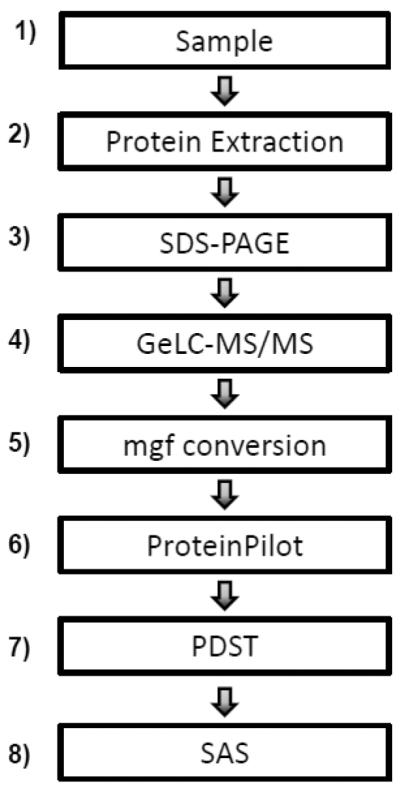
1) Samples are collected. 2) Proteins are extracted from the sample, via chemical precipitation for body fluids and detergent extraction for cells. 3) Proteins are fractionated via SDS-PAGE. 4) Proteins are subjected to GeLC-MS/MS. 5) RAW files are converted to mascot generic files (mgf). 6) Database searching is performed using ProteinPilot. 7) ProteinPilot Descriptive Statistics Template (PDST) is used to tabulate PTMs. 8) SAS is used to determine the statistical significance of differentially identified PTM frequencies.
Sample preparation
Pancreatic fluid collection (ePFT method)
The ePFT procedure was performed as described previously and the 30-minute time point following secretin stimulation was used for proteomic analysis [15].
TCA precipitation of pancreatic fluid, bile and urine
Ice-cold 100% TCA (25 μL) was added to 200 μL of each pancreatic fluid and bile sample, while 125 μL of ice-cold 100% TCA was added to 1 mL of each urine sample. The samples were vortexed for 5 seconds and incubated at 4°C for 2 hours. The samples were centrifuged at 20,000×g at 4°C for 30 minutes and the supernatant carefully aspirated. One milliliter of 100% ice-cold acetone was added to the pellets, which were briefly vortexed and incubated at −20°C for 1 hour. The samples were centrifuged again at 20,000×g at 4°C for 30 minutes, and the pellets washed twice with 100% ice-cold acetone. The final pellets were allowed to air dry at room temperature. Twenty microliters of LDS sample buffer (1× concentration) were added to each pellet.
Acetone precipitation of gastroduodenal fluid
Ice-cold 100% acetone (four sample volumes; 800 μL total) was added to 200 μL of gastroduodenal fluid, vortexed briefly, and incubated at −20°C for 3 hours. The samples were then centrifuged at 20,000×g at 4°C for 30 minutes. The supernatants were carefully aspirated and the pellets allowed to air dry at room temperature. Twenty microliters of LDS sample buffer (1× concentration) was added to each pellet.
Cell growth and harvesting of pancreatic stellate cells (PaSC) and pancreatic duct cells (PaDC)
Cell growth and propagation were similar to that previously performed using rodent PaSC [16, 17]. In brief, immortalized pancreatic duct and stellate cell lines of human origin were propagated in Dulbecco's modified Eagle's-F12 medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Upon achieving 90% confluency, the growth media was aspirated and the cells were washed 3 times with ice-cold phosphate-buffered saline (PBS). Twenty-four hours after the addition of fresh media, the cells were dislodged with non-enzymatic CellStripper, harvested by scraping following the addition of 10 mL PBS and pelleted by centrifugation at 3,000 × g for 5 min at 4°C, after which the supernatant was removed.
Cell lysis and protein extraction
One milliliter of TBSp (50 mM Tris, 150 mM NaCl, pH 7.4 supplemented with 1× Roche Complete protease inhibitors), 1% Triton X-100 and 0.5% SDS was added to each 80–90% confluent 10cm plate of cells. Cells were homogenized with 12 passages through a 27 gauge (1.25 inches long) needle and incubated on ice with gentle agitation for 1 hour. The homogenate was sedimented by ultracentrifugation at 100,000 × g for 60 minutes at 4°C. Protein concentrations were determined using the bicinchoninic acid (BCA) assay (23225, ThermoFisher Scientific). One hundred micrograms of protein were fractionated via SDS-PAGE.
SDS-PAGE analysis
LDS sample buffer, with 50mM DTT (dithiothreitol), was added to each sample to achieve 1× concentration. After incubation at 56°C for 1 hour and subsequent cooling, samples were alkylated with 1% acrylamide for 30 minutes at 23°C. Proteins (approximately 100 μg) were fractionated by SDS-PAGE at 150 volts in MES buffer for 45 minutes. Gels were rinsed in deionized water for 10 minutes, fixed in 45% methanol/45% water/10% acetic acid for 30 minutes, stained with SimplyBlue Coomassie for 1 hour, and destained overnight in deionized water.
GeLC-MS/MS analysis
In brief, each gel lane was divided into 7–10 sections and proteins in each gel section were digested with trypsin [18, 19]. Peptides extracted from each gel section were fractionated by a nanoflow reversed-phase ultra-high pressure liquid chromatography system (nanoLC, Eksigent) in-line with a linear trap quadrupole-Fourier transform ion cyclotron mass spectrometer (LTQ-FT Ultra, Thermo Scientific). The reversed-phase liquid chromatography columns (15 cm × 100 μm ID) were packed in-house (Magic C18, 5 μm, 100 Å, Michrom BioResources). Samples were analyzed with a 60-minute linear gradient (0–35% acetonitrile with 0.2% formic acid), and data were acquired in a data-dependent manner, with 6 MS/MS scans for every full scan spectrum.
Bioinformatics and Data Analysis
Mascot generic files (“mgf”) were generated using MSconvert software (18). All data generated from the gel sections were searched against the UniProt database (downloaded November 11, 2011) using the Paragon Algorithm [13] integrated into the ProteinPilot search engine (v. 3; ABSciex). Search parameters were set as follows: sample type, identification; Cys alkylation, acrylamide; Instrument, Orbitrap/FT (1–3 ppm), LTQ (MS/MS); special factors, gel-based ID; ID focus, Biological Modifications; database, international protein index (IPI) human (v.3.69); detection protein threshold, 99.0%; and search effort, thorough ID. We defined an identified protein ≥99% confidence as determined by the Paragon Algorithm. Modifications searched for in ProteinPilot (including abbreviations, names, and monoisotopic masses) are listed in Supplemental Table 1. In addition, we used the ProteinPilot™ Descriptive Statistics Template (v. 3.001; ABSciex) to extract and quantify the frequency of post-translational modifications identified in relation to the potential modification sites. The frequency of post-transitional modifications was determined by dividing the total number of peptide-spectra matches identified and having that particular modification by the number of peptide-spectra matches having the consensus sequence for (i.e., potential of having) that particular modification.
Statistical Analysis
The results were analyzed by Wilcoxon rank-sum non-parametric testing using SAS 9.2 (Cary, NC). P-value < 0.05 was considered significant. Box plots were constructed using a Microsoft Excel add-in (Vertex42, LLC).
3. Results
PTMs were identified in secretin-stimulated ePFT-collected pancreatic fluid
Of the over 400 PTMs searched though ProteinPilot, a total of 97 different PTMs were identified in the 18 pancreatic fluid samples. Supplemental Table 2 lists all PTMs and the frequency of identification per 1,000 potential modification sites in each of the 18 samples. Supplemental File 1 includes peptides identified, along with corresponding peptides and modifications.
Several PTMs were identified exclusively in one cohort
Of the 97 different PTMs identified in pancreatic fluid, 11 were identified exclusively in the control cohort (Table 2) and 9 were exclusive to the chronic pancreatitis cohort (Table 3), as illustrated in Figure 2. However, these exclusive PTMs all occurred at a frequency of less than 1 per 1,000 potential modification sites. In addition, most of these PTMs appeared in fewer than 3 of the 9 samples in each cohort. For example, Hex(T) appeared in 3 of the 9 chronic pancreatitis samples and none of the control samples, while Carboxyethyl(K) appeared in 4 of the 9 chronic pancreatitis samples and none of the control samples.
Table 2.
PTMs identified exclusively in the NP cohort.
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency of modification per 1,000 potential modification sites | ||||||||||
|
| ||||||||||
| # NP | NP1 | NP2 | NP3 | NP4 | NP5 | NP6 | NP7 | NP8 | NP9 | |
| Allysine(K) | 1 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cation:K(C-term) | 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 0.00 |
| Cation:Na(C-term) | 2 | 0.00 | 0.06 | 0.00 | 0.26 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| GlyGly(T) | 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 | 0.08 | 0.00 | 0.00 |
| Hex(N) | 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 |
| Hydroxyallysine(K) | 1 | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Methyl(T) | 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 | 0.00 | 0.00 |
| Methylpyrroline(K) | 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 |
| Quinone(W) | 1 | 0.00 | 0.16 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Quinone(Y) | 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 |
| Oxoalanine(S) | 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 |
NP, no pancreatitis (controls); #NP, number of NP samples in which a specific PTM has been identified.
Table 3.
PTMs identified exclusively in the CP cohort.
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency of modification per 1,000 potential modification sites | ||||||||||
|
| ||||||||||
| #CP | CP1 | CP2 | CP3 | CP4 | CP5 | CP6 | CP7 | CP8 | CP9 | |
| Acetyl(K) | 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | 0.11 |
| Carbamyl(R) | 1 | 0.00 | 0.00 | 0.23 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Carboxy(K) | 2 | 0.14 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Carboxyethyl(K) | 4 | 0.14 | 0.00 | 0.00 | 0.12 | 0.00 | 0.09 | 0.13 | 0.00 | 0.00 |
| Dichloro(Y) | 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 |
| Hex(S) | 2 | 0.07 | 0.00 | 0.08 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Hex(T) | 3 | 0.11 | 0.00 | 0.11 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Nicotinyl(K) | 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | 0.00 | 0.00 |
| Trioxidation(Y) | 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 | 0.00 | 0.00 | 0.00 |
CP, no pancreatitis (controls); #CP, number of CP samples in which a specific PTM has been identified.
Figure 2.
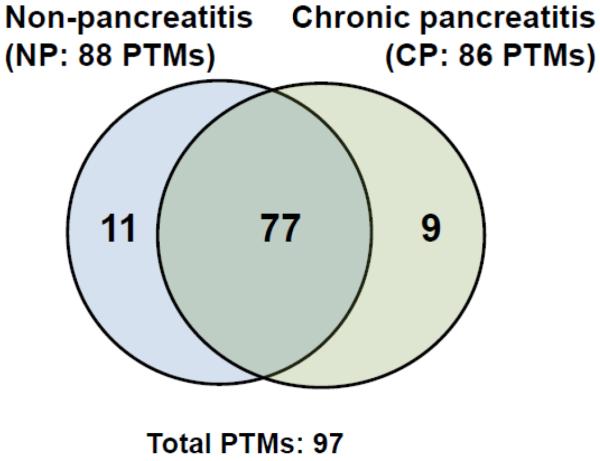
Venn diagram comparing PTMs identified in non-pancreatitis (NP) and chronic pancreatitis (CP) pancreatic fluid samples.
Significant differences in protein PTMs were identified when comparing pancreatic fluid from chronic pancreatitis (CP) patients and non-pancreatitis controls (NP)
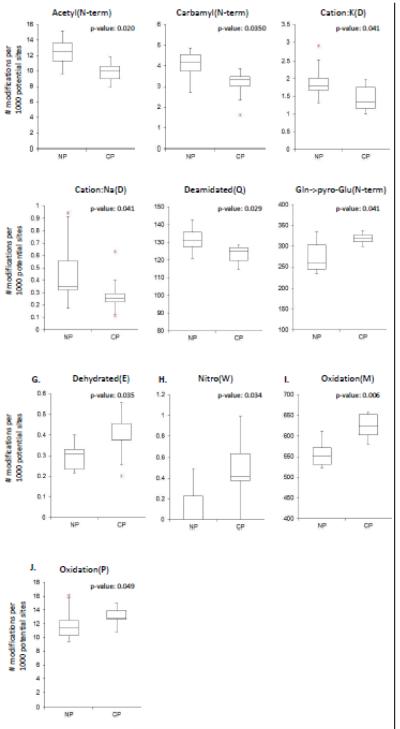
Wilcoxon rank sum tests revealed that 10 PTMs had significantly different frequencies when comparing the two cohorts (Table 4 in bold typeface). The PTMs identified in higher frequency in the control cohort were: Acetyl(N-term), Carbamyl(N-term), Cation:K(D), Cation:Na(D), Deamidated(Q), and pyro-Glu(N-term). PTMs identified in higher frequency in the chronic pancreatitis cohort included: Dehydrated(E), Nitro(W), Oxidation(M), and Oxidation(P). Box plots of PTMs with significantly higher frequency in the control cohort are shown in Figure 3 A to F, and those in the chronic pancreatitis cohort are shown in Figure 3 G to J.
Table 4.
Statistical analysis of PTMs identified in both cohorts.
|
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Modification | p-value | #NP | #CP | Median NP | Median CP | Modification | p-value | #NP | #CP | Median NP | Median CP |
|
|
|
||||||||||
| Acetyl(N-term) | 0.020 | 9 | 9 | 12.49 | 10.09 | Formyl(N-term) | 0.345 | 9 | 9 | 1.68 | 1.42 |
| Protein Terminal Acetyl(N-term) | 0.058 | 7 | 9 | 5.64 | 29.93 | Gln->pyro-Glu(N-term) | 0.041 | 9 | 9 | 258.24 | 320.24 |
| Amidated(C-term) | 0.395 | 5 | 3 | 0.06 | 0.00 | Glu->pyro-Glu(N-term) | 0.862 | 9 | 9 | 27.78 | 31.25 |
| Amino(Y) | 0.660 | 2 | 3 | 0.00 | 0.00 | GlyGly(K) | 0.929 | 7 | 5 | 0.10 | 0.12 |
| Ammonia-loss(N) | 0.069 | 9 | 9 | 0.92 | 0.43 | GlyGly(S) | 0.470 | 5 | 5 | 0.04 | 0.06 |
| Arg-add(N-term) | 1.000 | 3 | 3 | 0.00 | 0.00 | Hex(K) | 0.146 | 4 | 6 | 0.00 | 0.11 |
| Arg-loss(C-term) | 0.443 | 6 | 4 | 0.21 | 0.00 | Hex(Y) | 1.000 | 1 | 1 | 0.00 | 0.00 |
| Carbamyl(K) | 0.861 | 9 | 5 | 0.12 | 0.14 | Lys-add(N-term) | 0.723 | 7 | 5 | 0.14 | 0.07 |
| Carbamyl(N-term) | 0.035 | 9 | 9 | 4.20 | 3.34 | Lys-loss(C-term) | 0.290 | 5 | 6 | 0.11 | 0.14 |
| Carboxy(D) | 0.594 | 2 | 1 | 0.00 | 0.00 | Met->Hcy(M) | 1.000 | 1 | 1 | 0.00 | 0.00 |
| Carboxy(E) | 0.384 | 4 | 2 | 0.00 | 0.00 | Methyl(E) | 0.130 | 9 | 9 | 1.41 | 1.11 |
| Cation:K(D) | 0.041 | 9 | 9 | 1.79 | 1.34 | Methyl(H) | 0.445 | 3 | 2 | 0.00 | 0.00 |
| Cation:K(E) | 0.728 | 9 | 9 | 1.29 | 1.60 | Methyl(I) | 0.739 | 4 | 4 | 0.00 | 0.00 |
| Cation:Na(D) | 0.041 | 9 | 9 | 0.35 | 0.25 | Methyl(K) | 0.303 | 7 | 8 | 0.11 | 0.18 |
| Cation:Na(E) | 0.176 | 9 | 9 | 0.79 | 0.75 | Methyl(L) | 0.224 | 5 | 3 | 0.05 | 0.00 |
| Deamidated(N) | 0.069 | 9 | 9 | 214.09 | 204.22 | Methyl(Q) | 0.952 | 2 | 2 | 0.00 | 0.00 |
| Deamidated(Q) | 0.029 | 9 | 9 | 131.38 | 125.10 | Methyl(R) | 0.408 | 1 | 3 | 0.00 | 0.00 |
| Deamidated(R) | 0.489 | 9 | 9 | 35.81 | 34.63 | Nitro(W) | 0.034 | 3 | 7 | 0.00 | 0.41 |
| Dehydrated(D) | 0.603 | 9 | 7 | 0.21 | 0.11 | Nitro(Y) | 0.664 | 9 | 8 | 0.22 | 0.24 |
| Dehydrated(E) | 0.035 | 9 | 9 | 0.31 | 0.38 | Oxidation(D) | 0.489 | 9 | 9 | 1.79 | 1.80 |
| Dehydrated(S) | 0.232 | 8 | 7 | 0.14 | 0.06 | Oxidation(F) | 0.545 | 9 | 9 | 0.83 | 0.58 |
| Dehydrated(T) | 0.203 | 9 | 8 | 0.21 | 0.11 | Oxidation(H) | 0.152 | 9 | 9 | 17.28 | 19.76 |
| Dehydrated(Y) | 1.000 | 1 | 1 | 0.00 | 0.00 | Oxidation(K) | 0.233 | 9 | 9 | 0.81 | 1.08 |
| Delta:H(2)C(2)(H) | 0.664 | 9 | 9 | 10.21 | 9.40 | Oxidation(M) | 0.006 | 9 | 9 | 551.35 | 623.48 |
| Delta:H(2)C(2)(K) | 0.304 | 9 | 9 | 0.83 | 0.84 | Oxidation(N) | 0.728 | 9 | 9 | 1.75 | 1.82 |
| Delta:H(2)C(2)(N-term) | 0.081 | 9 | 9 | 10.74 | 8.64 | Oxidation(P) | 0.049 | 9 | 9 | 11.41 | 12.84 |
| Delta:H(4)C(2)(H) | 1.000 | 2 | 2 | 0.00 | 0.00 | Oxidation(R) | 0.931 | 9 | 9 | 1.57 | 1.43 |
| Delta:H(4)C(2)(K) | 0.506 | 2 | 1 | 0.00 | 0.00 | Oxidation(W) | 0.176 | 9 | 9 | 29.46 | 27.05 |
| Delta:H(4)C(2)(N-term) | 1.000 | 9 | 8 | 0.30 | 0.28 | Oxidation(Y) | 0.120 | 9 | 9 | 5.03 | 4.53 |
| Deoxy(D) | 0.506 | 2 | 1 | 0.00 | 0.00 | Phospho(S) | 0.408 | 3 | 1 | 0.00 | 0.00 |
| Dethiomethyl(M) | 0.703 | 5 | 3 | 0.34 | 0.00 | pyro-Glu(P) | 0.095 | 9 | 9 | 0.35 | 0.22 |
| Dioxidation(F) | 0.716 | 5 | 5 | 0.10 | 0.09 | Propionamide(C) | 1.000 | 9 | 9 | 1000.00 | 1000.00 |
| Dioxidation(K) | 0.664 | 7 | 8 | 0.21 | 0.12 | Propionamide(K) | 0.489 | 9 | 9 | 1.03 | 0.59 |
| Dioxidation(M) | 0.304 | 9 | 9 | 13.11 | 17.11 | Propionamide(N-term) | 1.000 | 9 | 9 | 2.06 | 2.09 |
| Dioxidation(P) | 0.788 | 6 | 5 | 0.11 | 0.11 | PyruvicAcidIminyl(K) | 0.506 | 2 | 1 | 0.00 | 0.00 |
| Dioxidation(R) | 0.693 | 4 | 0.00 | 0.00 | Trioxidation(W) | 0.058 | 7 | 3 | 0.61 | 0.00 | |
| Dioxidation(W) | 0.203 | 9 | 9 | 22.09 | 18.55 | Hydroxykynurenin(W) | 0.081 | 9 | 8 | 1.43 | 0.64 |
| Dioxidation(Y) | 0.103 | 8 | 8 | 0.22 | 0.71 | Kynurenin(W) | 0.069 | 9 | 9 | 5.85 | 4.48 |
| Formyl(K) | 0.198 | 4 | 1 | 0.00 | 0.00 | ||||||
NP, no pancreatitis (controls); #NP, number of NP samples in which a specific PTM has been identified; CP, no pancreatitis (controls); #CP, number of CP samples in which a specific PTM has been identified; Median, median frequency of PTM (per 1,000 potential cites); Bold = statistically significant p-value<0.005.
Figure 3. Box and whisker plots of PTMs detected in statistically different frequencies in non-pancreatitis (NP) and chronic pancreatitis (CP) pancreatic fluid samples.
P-values ≤ 0.05 were considered strongly significant using the Wilcoxon rank-sum non-parametric test in SAS 9.2 (Cary, NC).
PTMs were identified in relatively different frequencies in pancreatic fluid, bile, gastroduodenal fluid, urine, PaSC, and PaDC
In addition to comparing differences in the PTM frequencies in pancreatic fluid, we used an analogous strategy (as that depicted in Figure 1) to compare the PTM profiles of pancreatic fluid to closely related proximal body fluids (i.e., bile and gastroduodenal fluid), a systemic fluid (urine), and cell lysates of pancreatic stellate (PaSC) and duct (PaDC) cells. Supplemental Table 2 lists the 109 PTMs that were identified among the six fluid types investigated. Included are the 97 PTMs identified in pancreatic fluid, and an additional 12 not identified in pancreatic fluid.
We first examined the 10 PTMs that had significantly higher frequencies between the chronic pancreatitis and control pancreatic fluid cohorts, to determine if these PTMs were unique to pancreatic fluid, or were artifacts of sample preparation (Table 5 and Supplementary Table 2). Several PTMs were observed in pancreatic fluid at higher frequencies than in the other sample types. Of particular interest, gastrointestinal fluids (pancreatic fluid, gastroduodenal fluid, and bile) had relatively high frequencies of glutamine deamination, Deamidated(Q): 7–12 modifications per 100 potential sites. Furthermore, although bile and pancreatic fluid were prepared identically, the frequencies of PTMs in the various fluids differed. For example, the frequency of Acetyl(N-term) in bile was 3-fold that in pancreatic fluid, while Oxidation(M) was twice as frequent in pancreatic fluid. In addition, Nitrosylation of tryptophan, Nitro(W) was identified only in pancreatic fluid, though at only 2.9 modifications per 10,000 potential sites.
Table 5.
Comparison of modifications across all sample types. The following ten modifications were identified with statistically different frequencies in the non-pancreatitis verses chronic pancreatitis pancreatic fluid samples.
|
|
||||||
|---|---|---|---|---|---|---|
| Frequency of modification per 1,000 potential modification sites | ||||||
|
| ||||||
| Modification | PF | Bile | Urine | GDF | PaSC | PaDC |
| Acetyl(N-term) | 11.13 | 16.02 | 24.80 | 8.53 | 1.97 | 41.01 |
| Carbamyl(N-term) | 3.57 | 3.61 | 0.70 | 3.01 | 0.66 | 1.43 |
| Cation:K(D) | 1.67 | 1.07 | 0.11 | 0.00 | 0.74 | 0.18 |
| Cation:Na(D) | 0.37 | 0.10 | 0.11 | 0.84 | 0.25 | 0.00 |
| Deamidated(Q) | 127.29 | 101.69 | 23.89 | 66.24 | 17.60 | 14.50 |
| Dehydrated(E) | 0.35 | 0.00 | 0.06 | 1.94 | 0.00 | 0.00 |
| Gln->pyro-Glu(N-term) | 297.88 | 410.20 | 454.55 | 451.61 | 298.25 | 363.82 |
| Nitro(W) | 0.29 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Oxidation(M) | 589.72 | 264.02 | 391.73 | 870.37 | 714.29 | 844.93 |
| Oxidation(P) | 12.35 | 6.39 | 20.34 | 4.84 | 19.42 | 13.38 |
PF, pancreatic fluid; GDF, gastroduodenal fluid; PaSC, pancreatic stellate cells; PaDC, pancreatic duct cells.
We then focused our analysis on the PTMs identified in the highest average frequencies over the 18 pancreatic fluid samples to investigate what, if any, sample-specific modifications should be added to database search parameters. Using a frequency threshold of at least 1 PTM per 1,000 potential sites, we identified 28 PTMs. Many of these PTMs showed little variation across sample types; however, some did show striking differences and may merit future investigation. In particular, Deamidated(N) was significantly more frequent in pancreatic fluid. The frequency of Protein Terminal Acetylation at the N-terminus was 15–30 fold greater in pancreatic fluid compared to cell lysates. In addition, the frequency of the pyro-Glu(N-term) modification in pancreatic fluid is approximately a 6-fold greater when compared to cell lysates. Comparisons between body fluids and cell lysates also identified notable frequency differences. For example, Carbamyl(N-term) was approximately of equal frequency (~3/1000) in pancreatic fluid, bile and gastroduodenal fluid, while the PaDC and PaSC lysates have only half that frequency. Similarly, in urine, gastroduodenal fluid, and pancreatic fluid, Oxidation(H) and Oxidation(W) appear at approximately equal frequencies (14/1000 and 30/1000, respectively), while, the cell lysates contain approximately twice the number of modifications. These data suggest that sample-specific modifications may be important additions to database search parameters.
Discussion
Post-translational modifications (PTMs) of proteins are typically associated with alterations in protein function. Moreover, PTMs may also serve to differentiate two sets of samples, regardless if the modification occurs naturally or is a procedural artifact resulting from sample preparation provided that samples are treated identically. As such, using mass spectrometry-based proteomic techniques, we have identified in secretin-stimulated, ePFT-collected pancreatic fluid, PTMs which are exclusive to either the chronic pancreatitis or control pancreatic fluid cohorts. The data suggest that such a PTM-based comparative evaluation, as we have outlined herein, may be applied and developed further as a complimentary method to protein-based biomarker investigation.
We identify several PTMs which are exclusive to either chronic pancreatitis or control cohorts, as well as other PTMs with significantly different frequencies. The PTMs identified in a lower frequency in the chronic pancreatitis cohort include: Cation:Na(D), Cation:K(D), Acetyl(N-term), Carbamyl(N-term), pyro-Glu(N-term), and Deamidated(Q). The Cation:Na(D) and Cation:K(D) modifications are adducts - of sodium and potassium, respectively - to aspartate. Generally, these modifications are considered artifacts of sample preparation and therefore should affect all samples equally. However, the significantly higher frequency of these modifications in the control cohort compared to the chronic pancreatitis cohort suggests another mechanism rather than simply being an artifact.
The function of acetylation at the N-terminus (Acetyl(N-term)) is largely unknown, but it may act as a degradation signal targeted by ubiquitin ligase [20]. Acetyl(N-term) may be a major determinant in protein sorting. For example, cytoplasmic proteins are Acetyl(N-term) modified, whereas proteins bound for the rough endoplasmic reticulum are unmodified [21]. Additionally, acetylated somatostatin analogs display prolonged and enhanced biological activities in suppressing serum growth hormones and gastrin in pancreatic tumors [22]. At the N-terminus, carbamylation can also occur. Carbamyl(N-term) is an irreversible process resulting from the nonenzymatic reaction between isocyanic acid and specific free functional groups. N-terminus carbamylations of proteins have been shown to be potential disease biomarkers; for example, the rate of carbamylation is used to monitor atherosclerosis [23]. Carbamylation also reduces the insulin-releasing activity of beta-cell-tropin, a potent insulin-releasing and lipogenic peptide hormone [24, 25].
Deamidation, the result of the removal of an amide functional group, is a common protein PTM manifested by the conversion of glutamine to glutamic acid. Deamidation(Q), may play a role in neurodegenerative diseases [26], but has not yet been investigated in the pancreas. It is also possible that deamination is the result of incorrect peak peaking [27]. The incorrect peak-picking is exacerbated when there is a difference in the size distribution of the peptides, as larger peptides produce more complex isotope distributions leading to this instrument-related artifact. However, this process is random and irrespective of the sample, particularly as the samples in this study were collected, processed and analyzed in parallel. Also, N-terminal glutamine residue can spontaneously form pyroglutamate (pyro-Glu(N-term)) particularly under acid conditions [28], but to our knowledge, its role in pancreatic disease has not been investigated.
The PTMs identified in higher frequency in the chronic pancreatitis cohort were: Dehydrated(E), Nitro(W), Oxidation(M), and Oxidation(P). Dehydrated(E), or dehydrated glutamate, is a neutral loss that often occurs in mass spectrometry experiments [29]. However, its biological relevance has not been thoroughly studied. Nitro(W), or tryptophan nitrosylation, is a PTM in which a nitrosyl group is added to a protein. Nitrosylation reactions may be relevant to metabolism and are involved in a variety of pathophysiological processes [30]. For example, neuron growth factor signaling in PC12 cells has been found to induce post-translational nitration of tryptophan, subsequently causing differentiation of PC12 cells [31]. In addition, nitrosylated tryptophan has exhibited dose-dependent vasorelaxation and platelet inhibiting activity [32]. Protein oxidation plays an important pathophysiological role, affecting protein function in normal and pathological processes, and has been shown to affect protein structures. Methionine oxidation (Oxidation(M)) can induce structural changes in myosin, including a redistribution of existing structural states of the actin-binding cleft [33]. Proline oxidation (Oxidation(P)), an irreversible oxidation reaction, is a common PTM in humans [34]. The various biological consequences of proline oxidation include alteration of protein conformation and protein-protein interactions [35]. Hitherto, the roles of the aforementioned modifications in the pancreas have not been studied comprehensively. It is also imperative to note that PTMs appearing in a low number of specimens (I.e., 1 or 2 or 18 specimens) may indicate that that particular modification is unique to that individual or a small subset and not necessarily representative of the general control group, and as such a larger number of specimens must be investigated. As such, with the data set described herein, the PTMs identified in 8 or 9 specimens in each cohort and having statistically significant differences would be the primary candidate for follow-up studies.
Efforts were made to prepare samples in parallel so as to reduce the likelihood that differences in PTM frequencies are due to methods of preparation. As such, experimental variation would be minimal, so that differences in frequency of common modifications (e.g., Cation:K, Cation:Na, Deamidated Q, pyro-Glu Q, and Oxidation M) are unlikely to be artifacts of sample preparation. Pancreatic fluid, urine, and bile were prepared in a similar manner (TCA protein extraction and GeLC-MS/MS). Gastroduodenal fluid was precipitated using acetone, rather than TCA, and as such served as a negative control for TCA-precipitation-specific artifacts. Comparisons between the different body fluid types provide evidence that PTMs identified in pancreatic fluid were not merely artifacts of sample preparation. For example, sample preparation artifacts would likely appear in all three TCA-precipitated samples. However, despite similar preparation methods, few consensus PTMs were present among bile, urine and pancreatic fluid. In addition, as seen in Figure 3, significant increases in frequency of the various PTMs were evenly distributed among the chronic pancreatitis and control pancreatic fluid cohorts.
Our data also demonstrated that sample-specific preparation protocols were not a major limitation for comparing PTMs from pancreatic fluid and other fluids or lysates. The protocols used for pancreatic fluid, bile and urine were most similar, as all three body fluids were precipitated by TCA and subjected to analysis by GeLC-MS/MS. In contrast, TCA was used to extract proteins from pancreatic fluid to inactivate pancreatic enzymes, while acetone was used to extract proteins from gastroduodenal fluid because TCA accelerated auto-digestion by enzymes, such as pepsin [36, 37]. While some of the modifications may be preparation-specific, there were no clear distinctions between pancreatic fluid, bile, and urine and gastroduodenal fluid. In the PaDC and PaSC cell lysates, the majority of extracted proteins were either intracellular or membrane proteins, rather than secreted. Consequently, the cell lysates did not require chemical precipitation, but instead proteins were extracted using lysis buffer detergents. Differences in PTMs of body fluid and lysate proteins were expected, as secreted proteins and intracellular/membrane proteins were processed differently in the cell (e.g., cleavage of signal peptides is prevalent).
We performed the protein database search using ProteinPilot, as it allows for hundreds of different modifications to be considered in a single database query. This extensive number of searched PTMs cannot be matched currently with more commonly used search engines, such as SEQUEST [38] and Mascot [39], which set limits on the number of fixed and variable modifications available to the end user. Additionally, our workflow incorporated the ProteinPilot Descriptive Statistics Template allowing automated tallying of modifications present in peptides from identified proteins and facilitates the comparison of PTMs between cohorts. This template eliminated the need for manual tallies or additional computational efforts, thereby reducing any associated errors. This methodology can be universally applied by any researcher with one of many freely available mascot generic file (mgf) converters, such as MSconvert [40], and commercial ProteinPilot software.
Future studies may investigate specific PTMs in pancreatic fluid. For example, studies correlating observed PTM frequency to the activity of enzyme(s) responsible for the modification, or to the activity of enzyme(s) being modified. Such studies could be performed in cell culture models, including, but not limited to, using RNAi technology [41, 42]. Cell culture-based investigation may reveal aberrant activity in biochemical pathways and thus insights into the cellular mechanisms of the disease, differentiating primary from secondary biomarker PTMs. As with all such studies, analyzing a large number of samples is vital for future experiments [43].
Conclusions
In summary, we have identified PTMs that appeared at different frequencies in the ePFT-collected pancreatic fluid of chronic pancreatitis patients compared to non-pancreatitis controls. Upon further validation with a larger data set, the use of the ePFT collection technique coupled with GeLC-MS/MS analysis of pancreatic fluid proteins and PTM-centric bioinformatics has significant potential for advancing exocrine pancreas research. Future investigation of differentially identified PTMs in chronic pancreatitis should incorporate a wider spectrum of disease, specifically patients with mild, moderate and severe chronic pancreatitis to ensure that identified PTM frequency differences are sensitive enough to be detected in early disease and are not solely markers of advanced disease. Such studies will also require an increase in patient numbers for both the biomarker discovery and validation phases. Finally, insight into changes in pathophysiology over time will result from longitudinal studies that investigate the progression of chronic pancreatitis in patients at different stages of disease, with the added benefit of each subject serving as their own control. The natural course of chronic pancreatitis remains unclear and such studies may provide potential prognostic biomarkers, complementing current radiologic and imaging techniques. In conclusion, we have established a workflow designed to compare global protein PTM differences which may be readily applied to related proteomic studies of diseases of the exocrine pancreas.
Supplementary Material
Significance.
The early diagnosis of chronic pancreatitis is paramount in developing strategies to modify, retard, or halt disease progression. In the present study, we compared post-transitional modifications (PTMs) of proteins extracted from pancreatic fluid of chronic pancreatitis patients verses a control cohort. With many mass spectrometry-based proteomics workflows aimed to identify and quantify proteins, data for PTMs typically comes gratis, in that such data are collected during protein sequencing and, as such, require only downstream bioinformatics processing. We identified a total of 20 PTMs which were exclusive to or significantly different between cohorts. Upon validation with larger cohorts these PTMs may serve as biomarker candidates of chronic pancreatitis. PTM profiling of pancreatic fluid proteins is complementary to standard protein-based biomarker discovery, and may be readily applied to studies of pancreatic disease.
Highlights
PTMs were identified in secretin-stimulated ePFT-collected pancreatic fluid.
Significant differences in protein PTMs were identified between cohorts.
PTMs were identified in different frequencies in specimens of different origins.
Identified PTMs may serve as biomarker candidates of chronic pancreatitis.
Table 6.
The modifications with the highest frequency comparing pancreatic fluid (PF), bile, gastroduodenal fluid (GDF), urine, pancreatic stellate cells (PaSC), and pancreatic duct cells (PaDC).
|
|
||||||
|---|---|---|---|---|---|---|
| Frequency of modification per 1,000 potential modification sites | ||||||
|
| ||||||
| Modification | PF | Bile | Urine | GDF | PaSC | PaDC |
| Acetyl(N-term) | 10.65 | 16.02 | 8.53 | 24.80 | 1.97 | 41.01 |
| Deamidated(N) | 208.86 | 170.25 | 135.72 | 101.60 | 48.53 | 62.41 |
| Deamidated(Q) | 126.98 | 101.69 | 66.24 | 23.89 | 17.60 | 14.50 |
| Deamidated(R) | 35.34 | 21.37 | 7.85 | 6.39 | 11.92 | 5.68 |
| Delta:H(2)C(2)(H) | 10.02 | 11.42 | 15.92 | 18.87 | 9.51 | 7.90 |
| Dioxidation(M) | 14.35 | 2.16 | 7.41 | 10.18 | 2.72 | 15.19 |
| Dioxidation(W) | 21.60 | 13.09 | 0.00 | 29.89 | 53.94 | 7.76 |
| Gln->pyro-Glu(N-term) | 309.07 | 410.20 | 451.61 | 454.55 | 298.25 | 363.82 |
| Glu->pyro-Glu(N-term) | 30.12 | 31.89 | 27.03 | 39.04 | 4.72 | 5.55 |
| Oxidation(H) | 18.31 | 6.48 | 19.11 | 14.07 | 5.95 | 4.65 |
| Oxidation(M) | 591.25 | 264.02 | 870.37 | 391.73 | 714.29 | 844.93 |
| Oxidation(P) | 12.54 | 6.39 | 4.84 | 20.34 | 19.42 | 13.38 |
| Oxidation(W) | 27.05 | 10.29 | 37.88 | 29.41 | 16.60 | 7.76 |
| Protein Terminal Acetyl(N-term) | 17.76 | 65.60 | 0.00 | 0.00 | 321.31 | 568.03 |
Acknowledgments
Funds were provided by the following NIH grants: 1 F32 DK085835-01A1 (JP), 1 R21 DK081703-01A2 (DC) and 5 P30 DK034854-24 (Harvard Digestive Diseases Center; DC). We would like to thank the Burrill family for their generous support through the Burrill Research Grant. We would also like to thank members of the Steen Laboratory at Children's Hospital Boston, in particular John FK Sauld and Ali Ghoulidi for their technical assistance and critical reading of the manuscript. In addition, we thank members of the Center for Pancreatic Disease at Brigham and Women's Hospital, particularly Shadeah Suleiman for her technical assistance.
Abbreviations
- ePFT
endoscopic pancreatic function test
- GeLC-MS/MS
SDS-PAGE-based protein fractionation
- LTQ-FTICR
linear trap quadrupole- Fourier transform ion cyclotron resonance mass spectrometry
- TCA
trichloroacetic acid
Footnotes
Joao A. Paulo (current address) Harvard Medical School Department of Cell Biology 240 Longwood Ave Boston, MA 02115 joao_paulo@post.harvard.edu phone: +1-401-368-2925 fax:: +1-617-264-5277
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests The authors declare no competing interests.
Author contributions JP and VK carried out the experiments and drafted the original manuscript. SB provided technical assistance. JP, HS, PB, and DC conceived of the study, and participated in its design and coordination. All authors helped to draft the manuscript and approved the final manuscript.
References
- [1].James S. Opportunities and challenges at NIDDK in digestive diseases research. Gastroenterology. 2007;132:1219–20. doi: 10.1053/j.gastro.2007.02.025. [DOI] [PubMed] [Google Scholar]
- [2].DiMagno EP, Go VL, Summerskill WH. Relations between pancreatic enzyme ouputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973;288:813–5. doi: 10.1056/NEJM197304192881603. [DOI] [PubMed] [Google Scholar]
- [3].Chowdhury R, Bhutani MS, Mishra G, Toskes PP, Forsmark CE. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas. 2005;31:63–8. doi: 10.1097/01.mpa.0000164451.69265.80. [DOI] [PubMed] [Google Scholar]
- [4].Conwell DL, Zuccaro G, Jr., Vargo JJ, Morrow JB, Obuchowski N, Dumot JA, et al. An endoscopic pancreatic function test with cholecystokinin-octapeptide for the diagnosis of chronic pancreatitis. Clin Gastroenterol Hepatol. 2003;1:189–94. doi: 10.1053/cgh.2003.50028. [DOI] [PubMed] [Google Scholar]
- [5].Conwell DL, Zuccaro G, Jr., Vargo JJ, Trolli PA, Vanlente F, Obuchowski N, et al. An endoscopic pancreatic function test with synthetic porcine secretin for the evaluation of chronic abdominal pain and suspected chronic pancreatitis. Gastrointest Endosc. 2003;57:37–40. doi: 10.1067/mge.2003.14. [DOI] [PubMed] [Google Scholar]
- [6].Wu B, Conwell DL. The endoscopic pancreatic function test. Am J Gastroenterol. 2009;104:2381–3. doi: 10.1038/ajg.2008.181. [DOI] [PubMed] [Google Scholar]
- [7].Paulo JA, Kadiyala V, Lee LS, Banks PA, Conwell DL, Steen H. Proteomic analysis (GeLC-MS/MS) of ePFT-collected pancreatic fluid in chronic pancreatitis. J Proteome Res. 2012;11:1897–912. doi: 10.1021/pr2011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Paulo JA, Lee LS, Wu B, Banks PA, Steen H, Conwell DL. Mass spectrometry-based proteomics of endoscopically collected pancreatic fluid in chronic pancreatitis research. Proteomics Clin Appl. 2011;5:109–20. doi: 10.1002/prca.201000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Parker CE, Mocanu V, Mocanu M, Dicheva N, Warren MR. Mass Spectrometry for Post-Translational Modifications. In: Alzate O, editor. Neuroproteomics. Boca Raton; FL: 2010. [PubMed] [Google Scholar]
- [10].Salzano AM, Crescenzi M. Mass spectrometry for protein identification and the study of post translational modifications. Ann Ist Super Sanita. 2005;41:443–50. [PubMed] [Google Scholar]
- [11].Schweppe RE, Haydon CE, Lewis TS, Resing KA, Ahn NG. The characterization of protein post-translational modifications by mass spectrometry. Acc Chem Res. 2003;36:453–61. doi: 10.1021/ar020143l. [DOI] [PubMed] [Google Scholar]
- [12].Walsh CT, Garneau-Tsodikova S, Gatto GJ., Jr. Protein posttranslational modifications: the chemistry of proteome diversifications. Angew Chem Int Ed Engl. 2005;44:7342–72. doi: 10.1002/anie.200501023. [DOI] [PubMed] [Google Scholar]
- [13].Shilov IV, Seymour SL, Patel AA, Loboda A, Tang WH, Keating SP, et al. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics. 2007;6:1638–55. doi: 10.1074/mcp.T600050-MCP200. [DOI] [PubMed] [Google Scholar]
- [14].Paulo JA, Kadiyala V, Lee LS, Banks PA, Conwell DL, Steen H. Proteomic Analysis (GeLC-MS/MS) of ePFT-Collected Pancreatic Fluid in Chronic Pancreatitis. Journal of proteome research. 2012 doi: 10.1021/pr2011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Paulo JA, Lee LS, Wu B, Repas K, Mortele KJ, Banks PA, et al. Identification of pancreas-specific proteins in endoscopically (endoscopic pancreatic function test) collected pancreatic fluid with liquid chromatography--tandem mass spectrometry. Pancreas. 2010;39:889–96. doi: 10.1097/MPA.0b013e3181cf16f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Paulo JA, Urrutia R, Banks PA, Conwell DL, Steen H. Proteomic analysis of a rat pancreatic stellate cell line using liquid chromatography tandem mass spectrometry (LC-MS/MS) J Proteomics. 2011;75:708–17. doi: 10.1016/j.jprot.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Paulo JA, Urrutia R, Banks PA, Conwell DL, Steen H. Proteomic analysis of an immortalized mouse pancreatic stellate cell line identifies differentially-expressed proteins in activated vs nonproliferating cell states. Journal of proteome research. 2011;10:4835–44. doi: 10.1021/pr2006318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Neubauer G, Mann M. Mapping of phosphorylation sites of gel-isolated proteins by nanoelectrospray tandem mass spectrometry: potentials and limitations. Analytical chemistry. 1999;71:235–42. doi: 10.1021/ac9804902. [DOI] [PubMed] [Google Scholar]
- [19].Steen H, Kuster B, Fernandez M, Pandey A, Mann M. Detection of tyrosine phosphorylated peptides by precursor ion scanning quadrupole TOF mass spectrometry in positive ion mode. Analytical chemistry. 2001;73:1440–8. doi: 10.1021/ac001318c. [DOI] [PubMed] [Google Scholar]
- [20].Hwang CS, Shemorry A, Varshavsky A. N-terminal acetylation of cellular proteins creates specific degradation signals. Science (New York, NY. 2010;327:973–7. doi: 10.1126/science.1183147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Forte GM, Pool MR, Stirling CJ. N-terminal acetylation inhibits protein targeting to the endoplasmic reticulum. PLoS Biol. 2011;9:e1001073. doi: 10.1371/journal.pbio.1001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pinski J, Milovanovic S, Yano T, Hamaoui A, Radulovic S, Cai RZ, et al. Biological activity and receptor binding characteristics to various human tumors of acetylated somatostatin analogs. Proc Soc Exp Biol Med. 1992;200:49–56. doi: 10.3181/00379727-200-43393. [DOI] [PubMed] [Google Scholar]
- [23].Jaisson S, Pietrement C, Gillery P. Carbamylation-derived products: bioactive compounds and potential biomarkers in chronic renal failure and atherosclerosis. Clinical chemistry. 2011;57:1499–505. doi: 10.1373/clinchem.2011.163188. [DOI] [PubMed] [Google Scholar]
- [24].Dunmore SJ, Panico M, Etienne AT, Morris HR, Beloff-Chain A. The effects of structural modifications on the insulin-releasing activity of beta-cell-tropin. Biochem J. 1987;244:797–800. doi: 10.1042/bj2440797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dunmore SJ, Cawthorne MA, Hislop DC, Morton JL, Beloff-Chain A. Beta-cell tropin- and glucose-induced hypersecretion of insulin and amylin from perfused fatty rat pancreas. J Endocrinol. 1993;137:375–81. doi: 10.1677/joe.0.1370375. [DOI] [PubMed] [Google Scholar]
- [26].Hasan Q, Alluri RV, Rao P, Ahuja YR. Role of glutamine deamidation in neurodegenerative diseases associated with triplet repeat expansions: a hypothesis. J Mol Neurosci. 2006;29:29–33. [PubMed] [Google Scholar]
- [27].Hao P, Ren Y, Alpert AJ, Sze SK. Detection, evaluation and minimization of nonenzymatic deamidation in proteomic sample preparation. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.O111.009381. O111 009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Podell DN, Abraham GN. A technique for the removal of pyroglutamic acid from the amino terminus of proteins using calf liver pyroglutamate amino peptidase. Biochem Biophys Res Commun. 1978;81:176–85. doi: 10.1016/0006-291x(78)91646-7. [DOI] [PubMed] [Google Scholar]
- [29].Neta P, Pu QL, Kilpatrick L, Yang X, Stein SE. Dehydration versus deamination of N-terminal glutamine in collision-induced dissociation of protonated peptides. Journal of the American Society for Mass Spectrometry. 2007;18:27–36. doi: 10.1016/j.jasms.2006.08.016. [DOI] [PubMed] [Google Scholar]
- [30].Simon DI, Mullins ME, Jia L, Gaston B, Singel DJ, Stamler JS. Polynitrosylated proteins: characterization, bioactivity, and functional consequences. Proceedings of the National Academy of Sciences of the United States of America; 1996. pp. 4736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kawasaki H, Shigenaga A, Uda M, Baba T, Ogawa H, Takamori K, et al. Nitration of tryptophan in ribosomal proteins is a novel post-translational modification of differentiated and naive PC12 cells. Nitric Oxide. 2011;25:176–82. doi: 10.1016/j.niox.2011.05.005. [DOI] [PubMed] [Google Scholar]
- [32].Zhang YY, Xu AM, Nomen M, Walsh M, Keaney JF, Jr., Loscalzo J. Nitrosation of tryptophan residue(s) in serum albumin and model dipeptides. Biochemical characterization and bioactivity. J Biol Chem. 1996;271:14271–9. [PubMed] [Google Scholar]
- [33].Klein JC, Moen RJ, Smith EA, Titus MA, Thomas DD. Structural and functional impact of site-directed methionine oxidation in myosin. Biochemistry. 2011;50:10318–27. doi: 10.1021/bi201279u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].McKnight JA, Hird FJ. The oxidation of proline by mitochondrial preparations. Comp Biochem Physiol B. 1986;85:289–94. doi: 10.1016/0305-0491(86)90002-7. [DOI] [PubMed] [Google Scholar]
- [35].Gorres KL, Raines RT. Prolyl 4-hydroxylase. Critical reviews in biochemistry and molecular biology. 2010;45:106–24. doi: 10.3109/10409231003627991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Paulo JA, Lee LS, Wu B, Repas K, Banks PA, Conwell DL, et al. Proteomic analysis of endoscopically (endoscopic pancreatic function test) collected gastroduodenal fluid using in-gel tryptic digestion followed by LC-MS/MS. Proteomics Clin Appl. 2010;4:715–25. doi: 10.1002/prca.201000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Paulo JA, Lee LS, Wu B, Repas K, Banks PA, Conwell DL, et al. Optimized sample preparation of endoscopic collected pancreatic fluid for SDS-PAGE analysis. Electrophoresis. 2010;31:2377–87. doi: 10.1002/elps.200900762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Eng JK, Mccormack AL, Yates JR. An Approach to Correlate Tandem Mass-Spectral Data of Peptides with Amino-Acid-Sequences in a Protein Database. Journal of the American Society for Mass Spectrometry. 1994;5:976–89. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- [39].Perkins DN, Pappin DJC, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–67. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- [40].Kessner D, Chambers M, Burke R, Agus D, Mallick P. ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics. 2008;24:2534–6. doi: 10.1093/bioinformatics/btn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tuschl T. Mammalian Cell Culture and Preparation of Cells for RNAi in 24-Well Plates. Cold Spring Harbor Protocols. 2006;2006 doi: 10.1101/pdb.prot4341. pdb.prot4341. [DOI] [PubMed] [Google Scholar]
- [42].van Leeuwen F, Gottschling DE. Assays for gene silencing in yeast. Methods Enzymol. 2002;350:165–86. doi: 10.1016/s0076-6879(02)50962-9. [DOI] [PubMed] [Google Scholar]
- [43].Dakna M, Harris K, Kalousis A, Carpentier S, Kolch W, Schanstra JP, et al. Addressing the challenge of defining valid proteomic biomarkers and classifiers. BMC Bioinformatics. 2010;11:594. doi: 10.1186/1471-2105-11-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.