Abstract
Deep insights into the structural, molecular and functional phenotypes underlying addiction have been made possible through in vivo neuroimaging techniques implemented in non-human and human primates. In addition to providing evidence that many of the neural alterations detected in stimulant-dependent individuals can emerge solely through experience with drugs, these studies have identified potential biological phenotypes that influence addiction liability. Here, we review recent advances that have been made in understanding the pathophysiology of stimulant addiction using neuroimaging techniques in non-human primates. Evidence indicates that dysfunction of the dopamine system can be both a cause and consequence of stimulant use and that this bi-directional relationship may be mediated by the ability of individuals to exert inhibitory control over behaviors. Further, recent data has demonstrated an involvement of the serotonin system in addiction-related behaviors and neurobiology, suggesting that the relationship between dopamine and serotonin systems may be altered in addiction. This approach aids in the development of novel targets that can be used in the treatment of addiction.
Introduction
Over the past 30 years, an increasingly long and detailed list of structural, functional and molecular phenotypes that differentiate the brains of people suffering from various forms of drug addiction from those of control subjects has been revealed by systematic neuroimaging studies (1–7). These changes include alterations in gray matter density and white matter integrity, altered patterns of localized activation or functional coupling between brain regions and apparent alterations in neurotransmitter receptor function; as a result, it is now clear that clinically-significant addictions are linked to measurable and reliable patterns of neural dysfunction. Moreover, these biological phenotypes can sometimes be correlated with total years of drug use, severity of addiction, degree of cognitive/behavioral dysfunction or other clinically-meaningful indices. Nevertheless, much remains unknown. For the most part, the origins and determining influences of these neurobiological deficits are not fully understood. Specifically, the degree to which they are susceptibility factors (pre-dating the onset of drug abuse) or are consequences of the addiction process or its treatment are mostly unknown, though recent studies of human addicts and their relatives are beginning to tease apart this question (8). More importantly, the mechanistic relationships between individual observed biological markers and clinically-relevant behavior or symptoms are poorly understood. Finally, it is mostly unknown which - if any - of these biological features of addiction normalize with successful abstinence. Animal models have aided in delineating these otherwise difficult-to-study phenomena and relationships.
The vast majority of basic neuroscience research on addiction that uses animal models has involved rats and mice. Rodent models have some disadvantages when it comes to exposing the relationships described above. Firstly, they are often not suitable for high resolution molecular, structural or functional neuroimaging, despite the translational potential of these approaches (allowing for the bridging of human and animal research). Secondly, though study of the biological determinants of addiction has increasingly focused on the role for frontal cortical regions, rodents have structurally and functionally underdeveloped frontal regions (9). Thirdly, addiction research has identified both genetic and biological markers of individual risk for addiction, but the total inter-individual variability in rodents is, by design, very low (due to the breeding strategies that led to the strains available today). And finally, the molecular features of brain regions conserved between rodents and non-human and human primates (e.g., the striatum) may themselves be different, meaning that systems-level drug responses may be different, as well (10). Non-human primate studies often involve necessarily few subjects, are inherently low throughput and are relatively costly, but the translational juxtaposition of non-human primate studies, bridging evidence obtained in rodent models of addiction with those obtained in humans and vice versa, provides their greatest scientific advantage. For these reasons, non-human primate model systems afford substantial advantages in the search for the biological causes of addiction.
Here, we review recent progress that has utilized neuroimaging modalities in non-human primate model systems to generate answers to some of the questions indicated above. From identifying the neural systems and molecules engaged during the initial drug experience to the neuroadaptations associated with long-term drug use, these studies have pinpointed crucial mechanisms for further study. In addition, this work is increasingly suggesting how individual differences affect acute and chronic drug responses in a manner that illuminates our understanding of susceptibility and resilience.
Initial Drug Responses and the Early Progression with Repeat Use
One of the most significant conceptual and practical advantages of animal models is the ability to prospectively identify the neural mechanisms that are affected during the initiation of drug intake/experience, as well as the associated progressive changes that happen during the transition to early, repeat use. Because of ethical reasons, very few studies in human subjects have examined these effects, often focusing on assessments of drug-induced neural responses in individuals that have developed problematic, habitual or clinically-impairing patterns of drug intake. Therefore, animal models, particularly ones that involve a dimension of neuroimaging (to allow back translation to the limited human studies), are crucial.
For decades, it has been known that drugs of abuse produce alterations in forebrain dopamine release (11), though this is only one of a potentially large set of neurochemical effects they can produce. Invasive microdialysis studies in rodents demonstrated this effect directly (12), and subsequent PET studies suggested the same in humans (13). It was studies in non-human primates that involved a blending of microdialysis and PET imaging that allowed for the direct linkage of these datasets (14–16).
These neurochemical effects play out at a global level, however, with quite striking changes in brain-wide networks resulting from initial exposure to drugs of abuse. The effects of drugs of abuse on the central nervous system are extensive, influencing measures of global brain function and neurotransmitter-release dynamics. Experimenter-delivered or self-administered cocaine increases in vivo measures of cerebral blood flow throughout the brain (17), with the greatest increases occurring in the prefrontal cortex (17, 18). Because of the prospective nature of animal studies, it is known that drug-induced alterations in glucose utilization evolve as experience with the drug progresses (19, 20). Recently, fMRI-BOLD has been used to interrogate the cerebral response of non-human primates with a limited history of cocaine experience; cocaine administration (experimenter-delivered or self-administered) produces changes in basal ganglia BOLD responses that are opposite, in sign, to those measured in rodents but that are conceptually predicted by the cerebral metabolic rate studies conducted in humans and monkeys (21). Because of potential differences in the molecular and cellular features of brain regions (e.g., different ratios of D1 vs. D2 receptors being activated in response to drug-induced dopamine release), functional responses in rodents and primates may well be meaningfully different, revealing the importance of phylogenetically-higher model systems.
Although neuroimaging has identified many structural, functional and molecular alterations detected in stimulant-dependent individuals that have been attributed to the chronic and persistent use of drugs, there is evidence that even relatively short-term exposure to drugs can also produce robust neurochemical abnormalities similar to those detected following chronic exposure. For example, a single day of methamphetamine exposure or a single week of cocaine self-administration can measurably alter dopamine transporter and D2-like receptor availability (22, 23), respectively, in a manner similar to the differences detected in stimulant dependent individuals (24, 25). The drug-induced adaptations to the dopamine system may emerge during the initial stages of drug use, potentially influencing the initial phases of escalation of drug use (Figure 1). These rapid molecular changes likely then contribute directly to the alterations in brain function observed as a function of duration of experience with the drug (26); low prefrontal glucose utilization in human stimulant abusers is correlated with striatal D2-like receptor availability (27), and data gathered in non-human primates suggests that this is a causal relationship (from dopamine receptor activity to global measures of brain function) (28).
Figure 1.
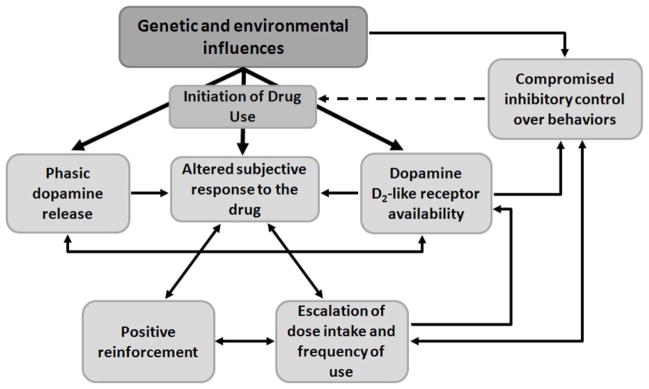
A theoretical model, based upon the available evidence, illustrating how genetic and environmental influences may impact inhibitory control of behaviors and thereby influence addiction-related processes. Compromised inhibitory control, driven by genetic and environmental influences, may impact drug use by either influencing the likelihood of initiating drug use (by potentially altering the neural mechanisms underlying the subjective response to the drug) or influence the escalation of drug use following initiation. These same genetic and/or environmental factors may also govern the bio-behavioral response of individuals to acute drug exposure, altering the subjective response to the drug and influencing the trajectory of these individuals to develop the compulsive patterns of drug-seeking and taking that are characteristic of addiction.
Identifying neuronal markers that may act as determinants for addiction
It is clear from behavioral epidemiology studies that not all individuals are at equal liability to develop a regular pattern of drug abuse, presumably because the neural responses to the drug, described in the preceding section, are systematically variable in a way that mediates the risk for developing addiction. The magnitude of the initial subjective response to the drug, itself, has been argued to be a major predictor of subsequent liability (29–31), meaning that the mechanisms that mediate initial drug response may be of interest in the search for the molecular basis of individual differences in addiction liability (Figure 1).
The degree to which biological features of addiction, particularly low dopamine D2-like receptor availability, may be a pre-existing marker of vulnerability for the disorder has been explored in non-human primate models. One of the first studies to directly test this hypothesis measured the availability of dopamine D2-like receptors in rhesus macaques using PET in order to test how individual variation in this dopaminergic marker predicted future cocaine self-administration behaviors; baseline levels of D2-like receptor availability negatively correlated with the rate at which monkeys self-administrated cocaine (23), a finding that has been subsequently confirmed in rodents (32, 33). The relationship between D2-like receptor availability and addiction liability may be, in part, mediated by the ability to exert control over behaviors, as naturally-occurring variation in D2-like receptor availability co-varies with measures of inhibitory control in drug-naïve monkeys (34). In other words, subjects with low dopamine D2 receptor availability/function are at greater risk for addictions in part because of poor control and/or reduced inhibition that leads to a greater propensity for initial substance use and a transition to regular drug abuse (Figure 1).
Specific genetic influences that determine these individual differences in monkeys have not been identified, but research has endorsed the notion that environmental factors, including psychosocial ones, are of influence. In male macaques, the acquisition of a dominant social rank after transition from individual to social housing predicted an increase in D2-like receptors and a relative resistance to cocaine reinforcement (35); this relationship supports the observation that high D2-like receptor availability, whether genetically or environmentally determined, reduces sensitivity to stimulant reinforcement. In female monkeys, on the other hand, the transition from individual to social housing – and the resulting acquisition of a relational social rank – influences both D2-like receptor availability and cocaine reinforcement, with monkeys acquiring a relatively dominant position exhibiting an increase in both D2-like receptors and sensitivity for cocaine reinforcement (36), an effect opposite to that observed in males. Therefore, environmental factors that impact the D2-like receptor can also modulate drug reinforcement, albeit in ways that may be mediated by other factors, such as sex.
Identifying neuronal markers that may reflect the progression of addiction
The drug seeking and taking behaviors that are characteristic of addiction most likely emerge over extensive periods of drug use that produce neural alterations above and beyond the acute drug effects. Because the neural impact of drugs can change over the course of use, and these changes may themselves be governed by undetected individual differences, longitudinal measurements, collected before and after drug use, have provided compelling insight into the neural effects of chronic drug exposure.
Emerging studies in non-human primates have revealed that a certain proportion of the molecular alterations detected in substance-dependent individuals are a consequence of drug exposure. For example, cocaine-induced changes in glucose utilization appear to change as experience with cocaine increases. Initially, acute cocaine administration produces an increase in glucose utilization that is localized to the prefrontal cortex; however, as experience with cocaine progresses (across a 120 day period), acute cocaine administration increases glucose utilization into surrounding cortical regions, as well as regions of the striatum (37). These neural adaptations in glucose utilization may have a functional impact on processes that depend upon the prefrontal cortex, as has been recently documented in cocaine-experienced monkeys (38).
In terms of dopamine D2-like receptors, chronic exposure to stimulants has been reported to reduce D2-like receptor availability, providing evidence that low D2-like receptor availability detected in stimulant dependent individuals may in part be a consequence of the drug (25, 27, 39). D2-like receptor availability measured before, during and after monkeys were allowed to self-administer cocaine for one year decreased by 15–20% within the initial one week of cocaine experience and remained attenuated throughout the duration of the study (23). Similar drug-induced changes to the D2-like receptor have been detected in monkeys exposed to methamphetamine, and consistent with the notion that the D2-like receptor systems plays a crucial role in positive-feedback sensitivity (34), the degree of change in D2-like receptor availability associated directly with drug-induced changes in cognitive performance (40). Thus, the ability to prospectively and longitudinally track molecular markers within the same subject has provided mechanistic insights into the neural consequences of drugs and their functional consequences.
Identifying the persistence of molecular features of addiction after abstinence
One of the most problematic features of addiction is that many individuals relapse back to drug use despite maintaining abstinence for extensive periods of time, effects that must depend upon persistence of biological adaptations caused by the drugs (Wang 2012). In humans, there is a small amount of evidence that neural recovery occurs over protracted periods of abstinence, perhaps because such a large proportion of subjects relapse within the first year of abstinence. Further, although individuals may remain abstinent from a particular drug, use of other drugs (i.e. alcohol or nicotine) may persist, potentially hindering the ability of neural systems to “fully” recover. These limitations can be controlled in animal models and, thus, much of our understanding into the persistence of drug-induced changes is based on animal models.
In studies of human drug dependent individuals, differences in global brain function, compared to healthy control subjects, appear to normalize after brief periods of abstinence (41). Consistent with this data, cocaine-induced changes in glucose utilization that occur over the course of drug experience is significantly attenuated after 4 weeks of withdrawal (37). However, dopaminergic dysfunction appears to persist for a longer period of time. Decreases in D2-like receptor availability that occur in response to chronic exposure to stimulants are still present at seven weeks after the last drug administration (40) and in some monkeys, one year after drug experience (23). Thus, the ability to recover from the drug-induced adaptations may vary across neural systems, which may in turn contribute to different aspects of relapse.
Despite the persistence of dopaminergic alterations, some recovery can occur after periods of abstinence. In humans and in monkeys, DAT availability increases after several weeks of abstinence (24, 40), with similar increases being detected in the D2-like receptor systems of monkeys following abstinence from cocaine (23) and methamphetamine (40). However, as indicated by the non-human primate studies, these levels are not restored to that of baseline, indicating that protracted periods of abstinence, perhaps greater than one year for some individuals, is required for a complete neural recovery (23).
Of interest is the high amount of heterogeneity in the rate at which recovery of these dopaminergic markers occurs between individuals, irrespective of the amount of drug taken. For example, reductions in D2-like receptor availability following cocaine exposure return, in some monkeys, to levels comparable to that of baseline, while, in others, remain decreased one year after the last cocaine experience (23). The rate at which dopamine systems recovery may be mediated by genetic or environmental factors, which, if identified, could be utilized in relapse prevention therapies, though it is important to note that pre-existing factors that set up the foundation for drug abuse susceptibility cannot be expected to dissipate with duration of abstinence.
Identifying the mechanistic basis of treatments for stimulant dependence
As discussed above, dopaminergic dysfunction is a phenotype common to many forms of stimulant dependence and, as such, pharmacological strategies aimed at enhancing dopamine signaling (i.e. D2-like receptor agonists) have been proposed as potential treatments for cocaine and methamphetamine dependence. However, because of dopamine’s involvement in all aspects of reward and reinforcement, not just drug-mediated reward and basic motor processes, the side effects of these drugs often results in low treatment compliance. There is growing interest in use of anti-depressants as a treatment for stimulant dependence (42, 43), not only because of the limited side effects of these drugs, but also because many of these drugs, despite primarily targeting the serotonin systems, indirectly modify the dopamine system (44).
In a recent study, neuroimaging measures of the serotonin system were combined with microdialysis and behavioral assessments in monkeys in order to determine the effects of chronic treatment with fluoxetine, a selective serotonin reuptake inhibitor, on cocaine-related behaviors and neurobiology. Chronic treatment with fluoxetine did not directly impact cocaine self-administration, but did prevent the ability of cocaine to reinstate drug-seeking behaviors following extinction. It also attenuated the magnitude of dopamine release caused by cocaine within the striatum and increased the availability of the serotonin 2A receptors in the frontal cortex (45). Thus, enhancements in serotonin signaling may indirectly influence the dopamine system, potentially influencing addiction-related behaviors. In line with this hypothesis, recent evidence has indicated that dopamine levels in the putamen interact with serotonin in the orbitofrontal cortex to influence inhibitory control processes (46). Specifically, the interaction between dopamine and serotonin these discrete brain regions follows that of a hyperbolic function with inhibitory control processes being poorest in monkeys with low levels of orbitofrontal serotonin and putamen dopamine. Thus, chronic stimulant use may create an imbalance between cortical and subcortical neuromodulation that influences addiction-related behaviors, and restoration of the dopamine-serotonin balance may be potential treatment for stimulant dependence (47). Indeed, in vivo measures with PET have indicated that monkeys with a history of cocaine use have greater serotonin transporter availability in striatal and cortical brain regions compared to drug-naive monkeys (48, 49) which may, as discussed above, directly impact the dopaminergic signaling. Simultaneous interrogation of the both dopamine and serotonin systems across stages of drug use are needed and may provide crucial insight into how these neurotransmitter systems influence one and other and addiction-related behaviors.
Conclusions
The use of combined behavioral, neuroimaging and psychopharmacological manipulations in non-human primate models has already enabled significant advances towards understanding how specific molecules (e.g., dopamine and dopamine D2-like receptors, serotonin) mediate the susceptibility for addiction, as well as the short- and long-term neuroadaptations caused by drug experience. These systems allow us to explore these effects in the context of a primate frontal lobe, a brain region substantially elaborated across mammalian evolution. And finally, it makes it possible for animal-based research to directly address a fundamental issue: namely, that not all individuals are alike and that the molecular basis of relapsing addiction is not the same in all subjects.
As neuroimaging advances our understanding of brain dysfunction in human addicts, it will be increasingly necessary to use that modality in non-human primates to bridge that information to molecular and cellular results in rodents. In that sense, the work described here is only a beginning. As we move ever closer to the development of rational therapeutics for addiction, neuroimaging in primates may also help us to understand how those medicines achieve their clinical efficacy, thereby playing an irreplaceable role in the scientific effort to stem the impact of drug addiction.
Highlights.
The translational utility of neuroimaging has provided insight into the pathophysiology of addiction
We review neuroimaging research of stimulant addiction conducted in non-human primates
Dysfunction of the dopamine system may be a cause as well as a consequence of addiction
This biobehavioral relationship is potentially mediated by inhibitory control processes
Future directions of addiction neuroimaging studies are discussed
Acknowledgments
This article is dedicated to the memory of Jacob P. Waletzky and recognizes the unwavering commitment of his family to supporting addiction science research.
This work was supported by Public Health Service grants F31-DA028812, T32-DA024635 and R01-DA031852.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.London ED. Studying addiction in the age of neuroimaging. Marian W. Fischman Lecture given at the 2008 meeting of CPDD. Drug Alcohol Depend. 2009;100(1–2):182–5. doi: 10.1016/j.drugalcdep.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Parvaz MA, Alia-Klein N, Woicik PA, Volkow ND, Goldstein RZ. Neuroimaging for drug addiction and related behaviors. Rev Neurosci. 2011;22(6):609–24. doi: 10.1515/RNS.2011.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex. 2000;10(3):334–42. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- 5.McClernon FJ, Gilbert DG. Human functional neuroimaging in nicotine and tobacco research: basics, background, and beyond. Nicotine Tob Res. 2004;6(6):941–59. doi: 10.1080/14622200412331337394. [DOI] [PubMed] [Google Scholar]
- 6.Daglish MR, Nutt DJ. Brain imaging studies in human addicts. Eur Neuropsychopharmacol. 2003;13(6):453–8. doi: 10.1016/j.euroneuro.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Lindsey KP, Gatley SJ, Volkow ND. Neuroimaging in drug abuse. Curr Psychiatry Rep. 2003;5(5):355–61. doi: 10.1007/s11920-003-0068-3. [DOI] [PubMed] [Google Scholar]
- 8.Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335(6068):601–4. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- 9.Preuss TM. Do Rats Have Prefrontal Cortex? The Rose-Woolsey-Akert Program Reconsidered. J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14(1):21–7. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- 11.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 12.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez D, Narendran R. Imaging neurotransmitter release by drugs of abuse. Curr Top Behav Neurosci. 2010;3:219–45. doi: 10.1007/7854_2009_34. [DOI] [PubMed] [Google Scholar]
- 14.Schiffer WK, Gerasimov MR, Bermel RA, Brodie JD, Dewey SL. Stereoselective inhibition of dopaminergic activity by gamma vinyl-GABA following a nicotine or cocaine challenge: a PET/microdialysis study. Life Sci. 2000;66(13):PL169–73. doi: 10.1016/s0024-3205(00)00432-x. [DOI] [PubMed] [Google Scholar]
- 15.Laruelle M, Iyer RN, al-Tikriti MS, Zea-Ponce Y, Malison R, Zoghbi SS, Baldwin RM, Kung HF, Charney DS, Hoffer PB, Innis RB, Bradberry CW. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse. 1997;25(1):1–14. doi: 10.1002/(SICI)1098-2396(199701)25:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 16.Kimmel HL, Nye JA, Voll R, Mun J, Stehouwer J, Goodman MM, Votaw JR, Carroll FI, Howell LL. Simultaneous measurement of extracellular dopamine and dopamine transporter occupancy by cocaine analogs in squirrel monkeys. Synapse. 2012;66(6):501–8. doi: 10.1002/syn.21536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howell LL, Hoffman JM, Votaw JR, Landrum AM, Wilcox KM, Lindsey KP. Cocaine-induced brain activation determined by positron emission tomography neuroimaging in conscious rhesus monkeys. Psychopharmacology (Berl) 2002;159(2):154–60. doi: 10.1007/s002130100911. [DOI] [PubMed] [Google Scholar]
- 18**.Howell LL, Votaw JR, Goodman MM, Lindsey KP. Cortical activation during cocaine use and extinction in rhesus monkeys. Psychopharmacology (Berl) 2010;208(2):191–9. doi: 10.1007/s00213-009-1720-3. This study involved the measurement of cerebral blood flow in the same subjects before and after noncontingent administration or self-administration of cocaine. Cocaine increased cerebral blood flow in both procedures, but the anatomical pattern of activation was different: noncontingent cocaine administration most strongly increased cerebral blood flow within the dorsolateral prefrontal cortex, while cocaine self-administration did so in the anterior cingulate cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24(14):3554–62. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Functional effects of cocaine self-administration in primate brain regions regulating cardiovascular function. Neurosci Lett. 2004;370(2–3):201–5. doi: 10.1016/j.neulet.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Mandeville JB, Choi JK, Jarraya B, Rosen BR, Jenkins BG, Vanduffel W. fMRI of cocaine self-administration in macaques reveals functional inhibition of basal ganglia. Neuropsychopharmacology. 2011;36(6):1187–98. doi: 10.1038/npp.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villemagne V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, Mathews WB, Ravert HT, Musachio J, McCann UD, Ricaurte GA. Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci. 1998;18(1):419–27. doi: 10.1523/JNEUROSCI.18-01-00419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9(8):1050–6. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- 24.Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158(3):377–82. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 25.Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29(47):14734–40. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002;22(17):7687–94. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158(12):2015–21. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 28.Black KJ, Hershey T, Koller JM, Videen TO, Mintun MA, Price JL, Perlmutter JS. A possible substrate for dopamine-related changes in mood and behavior: prefrontal and limbic effects of a D3-preferring dopamine agonist. Proc Natl Acad Sci U S A. 2002;99(26):17113–8. doi: 10.1073/pnas.012260599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151(2):184–9. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- 30.King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68(4):389–99. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Wit H, Phillips TJ. Do initial responses to drugs predict future use or abuse? Neurosci Biobehav Rev. 2012;36(6):1565–76. doi: 10.1016/j.neubiorev.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315(5816):1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michaelides M, Thanos PK, Kim R, Cho J, Ananth M, Wang GJ, Volkow ND. PET imaging predicts future body weight and cocaine preference. Neuroimage. 2012;59(2):1508–13. doi: 10.1016/j.neuroimage.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Groman SM, Lee B, London ED, Mandelkern MA, James AS, Feiler K, Rivera R, Dahlbom M, Sossi V, Vandervoort E, Jentsch JD. Dorsal Striatal D2-Like Receptor Availability Covaries with Sensitivity to Positive Reinforcement during Discrimination Learning. J Neurosci. 2011;31(20):7291–9. doi: 10.1523/JNEUROSCI.0363-11.2011. This study addressed the relationship between striatal D2-like receptor availability and performance of monkeys in a task of inhibitory control. Natural variation in D2-like receptor availability did not correlate with the ability of monkeys to acquire or remember a visual discrimination, but did with the ability of subjects to reverse a stimulus-reward association. Analysis of the behavioral data revealed that D2-like receptors were related to the sensitivity of monkeys to positive, but not negative, feedback. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5(2):169–74. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 36.Nader MA, Nader SH, Czoty PW, Riddick NV, Gage HD, Gould RW, Blaylock BL, Kaplan JR, Garg PK, Davies HM, Morton D, Garg S, Reboussin BA. Social dominance in female monkeys: dopamine receptor function and cocaine reinforcement. Biol Psychiatry. 2012;72(5):414–21. doi: 10.1016/j.biopsych.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Henry PK, Murnane KS, Votaw JR, Howell LL. Acute brain metabolic effects of cocaine in rhesus monkeys with a history of cocaine use. Brain Imaging Behav. 2010;4(3–4):212–9. doi: 10.1007/s11682-010-9100-5. This paper deals with how acute cocaine-induced alterations in glucose utilization change over the course of cocaine self-administration. In drug-naïve monkeys, acute exposure to cocaine increased glucose utilization only in the prefrontal cortex. However, as experience with cocaine increased over 60 cocaine self-administration sessions, acute cocaine-induced increases in glucose utilization expanded into other regions of the frontal cortex and the striatum. This cocaine-induced activation was significantly attenuated after four weeks of forced abstinence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Gould RW, Gage HD, Nader MA. Effects of chronic cocaine self-administration on cognition and cerebral glucose utilization in Rhesus monkeys. Biol Psychiatry. 2012;72(10):856–63. doi: 10.1016/j.biopsych.2012.05.001. By combining in vivo measures of glucose utilization with cognitive assessments before and after monkeys were allowed to self-administer cocaine, this study demonstrated that experience with cocaine impaired performance in two tasks of behavioral flexibility. In cocaine-naïve monkeys, glucose utilization increased during an extra-dimensional shift in several brain regions implicated in inhibitory control, but this pattern of activation was markedly missing in the cocaine-experienced monkeys. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14(2):169–77. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 40.Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, London ED, Jentsch JD. Dysregulation of d2-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci. 2012;32(17):5843–52. doi: 10.1523/JNEUROSCI.0029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148(5):621–6. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- 42.Oliveto A, Poling J, Mancino MJ, Williams DK, Thostenson J, Pruzinsky R, Gonsai K, Sofuoglu M, Gonzalez G, Tripathi S, Kosten TR. Sertraline delays relapse in recently abstinent cocaine-dependent patients with depressive symptoms. Addiction. 2012;107(1):131–41. doi: 10.1111/j.1360-0443.2011.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moeller FG, Schmitz JM, Steinberg JL, Green CM, Reist C, Lai LY, Swann AC, Grabowski J. Citalopram combined with behavioral therapy reduces cocaine use: a double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse. 2007;33(3):367–78. doi: 10.1080/00952990701313686. [DOI] [PubMed] [Google Scholar]
- 44.Zangen A, Overstreet DH, Yadid G. Increased catecholamine levels in specific brain regions of a rat model of depression: normalization by chronic antidepressant treatment. Brain Res. 1999;824(2):243–50. doi: 10.1016/s0006-8993(99)01214-7. [DOI] [PubMed] [Google Scholar]
- 45**.Sawyer EK, Mun J, Nye JA, Kimmel HL, Voll RJ, Stehouwer JS, Rice KC, Goodman MM, Howell LL. Neurobiological changes mediating the effects of chronic fluoxetine on cocaine use. Neuropsychopharmacology. 2012;37(8):1816–24. doi: 10.1038/npp.2012.29. This study evaluated how acute and chronic exposure to a purported pharmacological treatment for substance dependence, a selective serotonin reuptake inhibitor (fluoxetine), altered cocaine-taking behaviors and neurobiology. Fluoxetine did not directly influence cocaine self-administration, but suppressed cocaine-induced reinstatement and cocaine-induced dopamine overflow. Chronic exposure to fluoxetine increased availability of serotonin 2A receptors in the frontal cortex which remained elevated after a 6-week washout period. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Groman SM, James AS, Seu E, Crawford MA, Harpster SN, Jentsch JD. Monoamine Levels Within the Orbitofrontal Cortex and Putamen Interact to Predict Reversal Learning Performance. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320(2):757–65. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- 48.Banks ML, Czoty PW, Gage HD, Bounds MC, Garg PK, Garg S, Nader MA. Effects of cocaine and MDMA self-administration on serotonin transporter availability in monkeys. Neuropsychopharmacology. 2008;33(2):219–25. doi: 10.1038/sj.npp.1301420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49**.Gould RW, Gage HD, Banks ML, Blaylock BL, Czoty PW, Nader MA. Differential effects of cocaine and MDMA self-administration on cortical serotonin transporter availability in monkeys. Neuropharmacology. 2011;61(1–2):245–51. doi: 10.1016/j.neuropharm.2011.04.007. This dataset deals with the availability of the serotonin transporter in monkeys with a history of cocaine or 3,4-methyldioxymethamphetamine (MDMA) self administration. Cortical SERT availability in cocaine-experienced monkeys was significantly greater than that of drug-naïve monkeys, but lowest in MDMA-experienced monkeys. This study extended previous findings that drugs of abuse differentially alter the serotonin system. [DOI] [PMC free article] [PubMed] [Google Scholar]


