Abstract
The manner in which the heterotrimeric G protein complexes Gβ1γ2 and Gαiβ1γ2 interact with membranes is likely related to their biological function. We combined complementary measurements from sum frequency generation (SFG) vibrational and attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy to determine the possible membrane orientations of Gβ1γ2 and the Gαiβ1γ2 heterotrimer more precisely than could be achieved using SFG alone. The most likely orientations of Gβ1γ2 and the Gαiβ1γ2 heterotrimer were both determined to fall within a similar narrow range of twist and tilt angles, suggesting that Gβ1γ2 may bind to Gαi without a significant change in orientation. This “basal” orientation seems to depend primarily on the geranylgeranylated C-terminus of Gγ2 along with basic residues at the N-terminus of Gαi, and suggests that activated G protein-coupled receptors (GPCRs) must reorient G protein heterotrimers at lipid bilayers to catalyze nucleotide exchange. The innovative methodologies developed in this paper can be widely applied to study the membrane orientation of other proteins in situ.
Keywords: Sum frequency generation (SFG) vibrational spectroscopy, attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy, membrane protein orientation determination, heterotrimeric G protein, supported lipid bilayer, α-helix
Introduction
The orientation of peptides and proteins at interfaces plays a critical role in many research areas and applications such as biocompatibility, marine antifouling coatings, biosensors and biochips, membrane protein functions, and antimicrobial activity and selectivity.1–8 However, the orientation of peptides and proteins at solid/liquid interfaces is difficult to analyze, particularly in situ and with molecular level detail. In recent years, we have demonstrated that sum frequency generation (SFG) vibrational spectroscopy can be used to determine the interfacial orientation of simple peptides that adopt various secondary structures (such as α-helices, 310-helices, and anti-parallel β-sheets).9–20 We have also shown that SFG can be used to study the orientation of α-helical domains of interfacial proteins.21–22 However, for proteins with more complicated structures, a single measurement yields a broad range of likely orientations. We hypothesized that complementary measurements obtained from attenuated total reflectance - Fourier transform infrared (ATR-FTIR) spectroscopy10,23–24 can be combined with SFG data to obtain a more precise and detailed picture of how a molecule orients at an interface. In ATR-FTIR, a total internal reflection scheme is used to produce reasonable surface sensitivity (on the order of hundreds of nanometers to microns) based on the penetration depth of the evanescent wave into the sample. ATR-FTIR has been used to study the orientation of a wide variety of α-helical25–27 and β-sheet28–29 peptides, but because ATR-FTIR by itself only produces a limited number of measurements, studies of larger proteins30–34 have typically relied on the assumption that all secondary structural elements are roughly aligned in the protein (e.g. proteins with a β-barrel structure). However, the fold of most proteins does not follow this assumption. In this work, we demonstrate that a more precise orientation for proteins with more complex folds such as the Gαiβ1γ2 heterotrimer and Gβ1γ2 subunit, can be achieved by a combination of SFG and ATR-FTIR measurements.
Heterotrimeric G proteins (Gαβγ) are comprised of three subunits (Gα, Gβ, and Gγ), with Gβ and Gγ forming a constitutive heterodimer (Gβγ).35 When Gα is bound to GDP, it forms an inactive complex with Gβγ that serves as the substrate for activated G protein-coupled receptors (GPCRs), which catalyze the release of GDP and the binding of GTP to Gα. Upon activation of the GPCR the Gα GTP and Gβγ subunits are released and can independently interact with and regulate additional proteins that propagate signals within the cell.36 The Gβγ subunit is essential for coupling the heterotrimeric G protein to activated GPCRs, although it does not appear to make direct interactions with the receptor.37 Gβγ facilitates membrane localization of the Gαβγ heterotrimer via C-terminal prenylation of the Gγ subunit, but it may also allosterically promote nucleotide exchange or help dictate a particular orientation of the heterotrimer that is more optimal for engaging GPCRs. Free Gβγ subunits also play a major role in recruiting G protein-coupled receptor kinase 2 (GRK2) to the cell membrane.38–40 In previous work, sum frequency generation (SFG) studies were used to determine possible orientations of GRK2-Gβ1γ2 and Gβ1γ2, and we demonstrated that Gβ1γ2 changes its orientation with respect to the membrane upon binding to GRK2.41 However, the limited number of direct experimental measurements hindered attempts to narrow the molecular orientation to ranges of twist and tilt angles (defined in Figure 1) smaller than 20–30°. Herein we used a combination of SFG and ATR-FTIR to determine the orientation of Gβ1γ2 and the Gαiβ1γ2 heterotrimer. By combining orientation information from multiple spectroscopic measurements of several related proteins with common binding partners, we show it is possible to more accurately determine membrane orientations, and ascertain whether the formation of higher order complexes induces changes in orientation that could have biological consequences.
Figure 1.

The Gαiβ1γ2 heterotrimer and definition of twist (ψ), tilt (θ) and azimuthal (φ) angles which rotate the protein from the molecular (x′, y′, z′) to the macroscopic (X, Y, Z) coordinate system. Gβ1γ2 is shown in yellow, and Gαi is shown in cyan. An approximate membrane plane (defined to be consistent with previous studies of GRK2-Gβ1γ2)41, is shown as a grey rectangle, and lies parallel to the X-Y plane. The Gαiβ1γ2 heterotrimer is depicted in the reference orientation (ψ=0°, θ=0°, φ=0°) used as a starting point for data analysis. In our calculations, the molecule is rotated using an Euler rotation scheme according to three angles: first twist (ψ) then tilt (θ), and finally azimuthal (φ).
Materials and Methods
Protein Samples
Non-mysristoylated rat Gαi1 and myristoylated Gαi1 (myr-Gαi1) were expressed in bacteria and bovine Gβ1γ2 was expressed in High5 insect cells and the proteins were purified as previously described,42,43 and frozen in liquid nitrogen until used. The Gαiβ1γ2 heterotrimer was either formed by sequential addition of Gαi1 to Gβ1γ2 in the SFG sample cell, or by mixing them in a 1:1 ratio followed by purification of the complex on a Superdex S200 gel filtration column equilibrated with 20 mM HEPES (pH 8.0), 50 mM NaCl, and 5 mM DTT. This buffer mixture was also used as the liquid subphase for the lipid bilayer in SFG and ATR-FTIR studies.
SFG Spectroscopy
Second-order nonlinear optical spectroscopy has been widely used to study surfaces and interfaces and the relevant theoretical background has been extensively reported.44–78 The design of our SFG spectrometer is described elsewhere.45 SFG spectra from interfacial protein samples were collected at room temperature (24 °C) in a near total internal reflection geometry for the ssp and ppp polarization combinations of the sum frequency, visible, and infrared beams respectively.46 Planar supported lipid bilayers (PSLBs) were prepared on clean right-angle CaF2 prisms (Altos Photonics, Bozeman MT) using the Langmuir-Blodgett/Langmuir Schaefer method, as described previously.41 A 9:1 mixture of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) lipids was used. The lipids were purchased in a chloroform solution (Avanti Polar Lipids Inc.), and mixed to produce the desired composition. Following equilibration of the bilayer, the aqueous subphase was flushed three times with fresh buffer to remove excess lipids. Samples of the G proteins to be studied were then injected into the aqueous subphase to a final concentration of 336 nM, and allowed to diffuse to the lipid bilayer over the course of one hour, during which time the time-dependent SFG spectral intensity at 1655 cm−1 was monitored. The proteins studied are peripheral (rather than integral) membrane proteins, and thus no portion of the G proteins used in these experiments spans the lipid bilayer. Following equilibration, SFG spectra in the ppp and ssp polarization combinations were collected from the proteins associated with the lipid bilayer. More details can be found in the supporting information and previous publications.9,41
ATR-FTIR Spectroscopy
Lipid bilayers of the same composition as above were prepared on clean ZnSe substrates (Specac, UK) for ATR-FTIR experiments. Because the vibrational signal of the water O-H bending mode overlaps with the protein amide I signal, we used D2O in the buffer subphase in the ATR-FTIR experiments. Even so, strong O-H bending signal was observed from the water layer between the ATR crystal and the supported lipid bilayer prepared by the Langmuir-Blodgett/Langmuir Schaefer method. Because it is impractical to use D2O in the large Langmuir trough for the sample preparation, lipid bilayers for ATR-FTIR were prepared via the vesicle fusion method.26,27 The vesicles were prepared via extrusion through 100 nm pores (Avanti Polar Lipids) from lipid solutions reconstituted in PBS (pH 7.4) in deuterated water, after the 9:1 POPC:POPG lipid samples were dried under vacuum for 30 min to remove chloroform. The bilayer was allowed to equilibrate for one hour, after which time excess vesicle solution was removed by flushing thoroughly with fresh deuterated buffer solution. The sample was allowed to equilibrate again for an additional 30 min. Protein samples (in buffer solution with D2O as the solvent) were injected into the subphase for a target concentration of 336 nM to match the concentration used in SFG experiments, and samples were allowed to equilibrate for 2 hr prior to collection of spectra. P and s polarized spectra were collected on a Nicolet Magna IR 550 spectrometer with the ATR accessory. All spectra presented are the average of 128 scans. In order to reduce interference from water vapor present in the air, the instrument was purged with dry nitrogen prior to use, and spectra were afterwards corrected for trace amounts of water vapor using an additional background correction based on the spectrum of pure water vapor in air at 24 °C.79 The background subtraction and a baseline correction in the amide I region were performed in OMNIC 2.1, after which spectra were fit to a Gaussian lineshape using a nonlinear curve fitting algorithm in Origin 8.1. The dichroic ratio RATR was determined from the ratio of the absorbance of α-helices in the p and s polarizations of the infrared beam.27,79 Our method of calculating RATR for a protein with many separate helical segments is described in the supporting information.
Results and Discussion
Orientation of Gβ1γ2
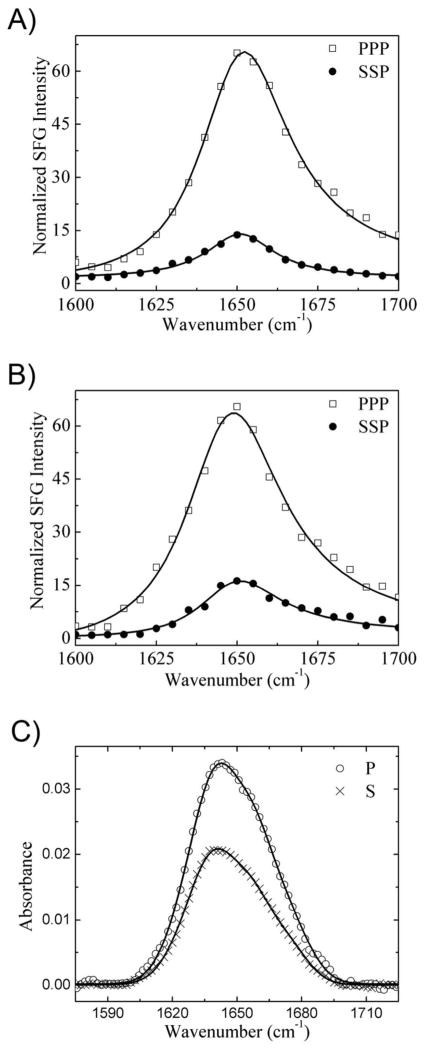
Gγ2 is lipid modified with a C-terminal hydrophobic geranylgeranyl group that localizes Gβ1γ2 to membranes. Although Gβ1γ2 has been studied previously using SFG,41,44 the limited number of resulting measurements meant that it was only possible to determine either the tilt angle (by assuming a constant twist angle)44 or very broad ranges of possible twist and tilt angles.41 Although Gβ1γ2 contains regions of non-helical secondary structures (such as β-sheets), SFG amide I signals from Gβ1γ2 were found to be dominated by contributions from α-helices, possibly due to the high symmetry of the β-propeller. In our previous work, by fitting SFG spectra in the ppp and ssp polarization combinations and correcting for differences in the Fresnel coefficients for the two polarizations, it was found that the measured ratio for the α-helical peak at 1652 cm−1 was 2.01 for Gβ1γ2.41 Unlike SFG, which only detects signals where inversion symmetry is broken, ATR-FTIR signals are generated by all secondary structures. Therefore the ATR-FTIR spectra contained vibrational peaks at 1635 cm−1, 1643 cm−1, 1657 cm− 1, and 1671 cm−1, for β-sheet, random coil/disordered structure, α-helix, and β-turn, respectively (see supporting information),27,79 resulting in a broader overall lineshape than in SFG (Figure 2). The fitted dichroic ratio RATR for the α-helical peak was 1.70.
Figure 2.
Experimental ATR-FTIR spectra of 336 nM membrane-bound Gβ1γ2 on 9:1 POPC:POPG lipid bilayer for the p and s polarizations. The circles and crosses are experimental data. The solid lines are the fitting results.
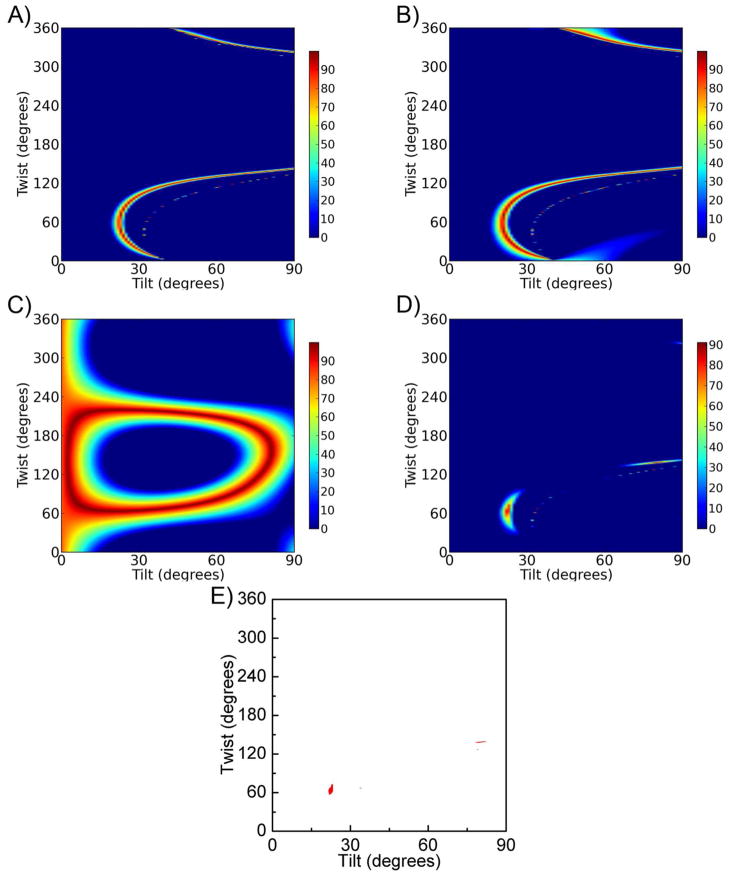
We then used the crystal structure of Gβ1γ2 from the structure of the GRK2-Gβ1γ2 complex (PDB entry 1OMW)43 to predict the expected amide I net molecular responses from helical portions of the protein (see supporting information), for comparison with the measured parameters (from SFG measurements) and the dichroic ratio RATR (from ATR-FTIR spectroscopy). The structure of Gβ does not change significantly when in complex with any of the targets that has been characterized thus far (GRK2, Gα, phosducin, SIGK peptide). We chose the 1OMW structure of Gβ1γ2 (GRK2-bound) simply because its extended N-terminal helices were not involved in crystal contacts as they are in other crystal forms, and thus this structure is more likely to represent the conformation of Gβ in solution. SFG observable quantities were calculated for all unique orientations of the heterodimer relative to a reference orientation (shown in Figure 1). As shown in Figure 3A, many possible twist and tilt angle combinations produce the experimentally determined ratio of 2.0±0.2, indicating once again that the SFG measurement alone cannot determine a single unique orientation of Gβ1γ2. Figure 3B shows orientations that would generate an ATR-FTIR dichroic ratio of 1.7±0.2. Again, many combinations of twist and tilt angle are possible, but they are distinct from matches for the SFG measurement (Figure 3A). Thus, combining the available orientations determined by the ATR-FTIR dichroic ratio RATR with constraints from the SFG ratio yields a much narrower range of possible orientations of Gβ1γ2 (Figure 3C and 3D), which fall in a narrow range of moderate twist angles (50–80°) and low tilt angles (15–35°). Additional arcs of matches exist for Gβ1γ2 at high tilt angles ~75°, but such positions would place the geranylgeranyl group quite far from the bilayer and are therefore physically unlikely. The combination of SFG and ATR-FTIR measurements points to a single favored orientation range for Gβ1γ2 on the lipid bilayer in situ. All orientations in this range correspond to closely related configurations where the C-terminus of Gγ2 would be in close proximity to the membrane, consistent with the geranylgeranyl group being the dominant membrane binding determinant of Gβ1γ2.
Figure 3.
The possible orientations of Gβ1γ2 determined by A) SFG ( ), B) ATR-FTIR (dichroic ratio RATR =1.7 ± 0.2), and C) the combination of SFG and ATR-FTIR measurements. The effect of experimental errors (such as uncertainty in the Fresnel coefficients) is accounted for using a coloring scheme based on how well the calculated and experimentally measured quantities agree for each possible orientation, within specified error bars (±10%).41 If the calculated ratio does not match the experimental value within ±10%, a score of 0 is assigned. In panel C), the total score is calculated as the product of the scores for all individual criteria. A score of 100% indicates an exact match for all experimental measurements. The shaded areas indicate orientations of Gβ1γ2 that are considered to be physically reasonable, according to previously defined criteria.41 D) The possible orientation areas with a score ≥ 70% (red).
Orientation of Gαiβ1γ2
Although Gαi is N-terminally myristoylated in cells, we first chose to examine the effect of non-myristoylated Gαi on complex formation Gβγ to avoid the complication of spectroscopic signals originating from membrane bound Gαi in addition to those from a reconstituted Gαiβ1γ2 heterotrimer. Indeed, no SFG signals could be detected from non-myristoylated Gαi alone (data not shown). However, SFG amide I signal could be readily detected for the Gαiβ1γ2 heterotrimer (Figure 4A) and exhibited strong contributions from α-helices centered at 1652 cm−1. SFG spectra for a pre-formed Gαiβ1γ2 heterotrimer were identical to those obtained by adding the two subunits to the lipid bilayer subphase sequentially (first Gβ1γ2, followed by Gαi), but distinct from the spectra of Gβ1γ2 alone,41 demonstrating that the Gαiβ1γ2 heterotrimer can be formed at the membrane in situ in our experiments. It was found that the measured ratio for the α-helical peak centered at 1652 cm−1 was 2.67. Possible values of for various orientations of Gαiβ1γ2 were calculated (see supporting information) based on a modified structure of the Gαiβ1γ2 heterotrimer (PDB entry 1GP2)80 in which the structure of the Gβ1γ2 portion was taken from a structure of GRK2-Gβ1γ2 (PDB entry 1OMW)43 for the reasons described above. The calculated and experimentally determined orientation parameters were compared, yielding possible orientations of Gαiβ1γ2 (Figure 5A). Once again, the SFG results alone were insufficient to uniquely determine the overall orientation of the protein.
Figure 4.
SFG Amide I region signals from A) 336 nM Gαiβ1γ2 heterotrimer or B) myr-Gαiβ1γ2 heterotrimer interacting with a 9:1 POPC:POPG lipid bilayer. C) ATR-FTIR Amide I region signals from 336 nM Gαiβ1γ2 heterotrimer interacting with a 9:1 POPC:POPG lipid bilayer. The circles and crosses are experimental data. The solid lines are the fitting results.
Figure 5.
The possible orientations of Gαiβ1γ2 determined by A) the SFG ratio of (2.7 ± 0.3) and the possible orientations of myr-Gαiβ1γ2 determined by B) the SFG ratio of (2.5 ± 0.3). Orientations of Gαiβ1γ2 at which the calculated values best match experimentally measured values for C) the ATR-FTIR dichroic ratio RATR (1.8 ± 0.2). The product of these two measurements of Gαiβ1γ2 (panel D) further narrows the range of possible orientations. E) Same plot as D) but only showing orientation areas with a score ≥ 70% (red).
ATR-FTIR spectra were also collected for Gαiβ1γ2 (Figure 4C), and the calculated relationship between dichroic ratio RATR and heterotrimer orientation (supporting information) was compared to the experimentally measured value of RATR=1.75 based on the α-helical peak center at 1657 cm−1 (see supporting information). The broad range of possible matches for the ATR-FTIR measurement is shown in Figure 5C, and the greatly narrowed range of matches obtained by combining ATR-FTIR and SFG measurements is shown in Figures 5D and 5E. The best matches fall in a very narrow range of moderate twist angles (~65°) and low tilt angles (~23°), with some additional matches at high tilt angles (75–85°) that can be rejected as physically unlikely. Thus, the combination of SFG and ATR-FTIR spectroscopy makes it possible to determine the orientation of Gαiβ1γ2 more precisely than using either technique alone.
To ensure that the myristoyl group has no impact on the membrane orientation of Gαiβ1γ2, we also collected SFG spectra from samples of the myr-Gαiβ1γ2 heterotrimer. SFG amide I signals could be detected (Figure 4B) with strong contributions from α-helices centered at 1652 cm−1. The spectral features detected from the myr-Gαiβ1γ2 heterotrimer (Figure 4B) were very similar to those from the non-myristoylated Gαiβ1γ2 heterotrimer (Figure 4A). The measured ratio for the α-helical peak centered at 1652 cm−1 was determined to be 2.51, very close to the value of Gαiβ1γ2 heterotrimer (2.67). Making the reasonable assumption that the structure of Gαiβ1γ2 is unaffected by the myristoyl group, we were able to calculate the expected SFG observables, and found a similar range of possible orientations for myr-Gαiβ1γ2 (Figure 5B) as for Gαiβ1γ2 (Figure 5A). This suggests that the myristoyl group does not substantially impact the orientation of Gαiβ1γ2, consistent with the fact that the myristoyl group is predicted to be in close proximity to the geranylgeranyl group of Gγ2 in the complex, and both modifications occur on flexible regions of their respective proteins.
Comparison of the orientation of Gβ1γ2 alone and in complex with Gαi
Comparison of the most likely orientations for Gβ1γ2 (Figure 3C) and Gαiβ1γ2 (Figure 5D), reveals that there is substantial overlap between the two plots, suggesting that it is possible for the Gαiβ1γ2 heterotrimer to form without reorientation of Gβ1γ2. To consider the possibility of a common orientation more quantitatively, we directly combined all four available measurements (two ratios and two dichroic RATR ratios) in one plot, thereby testing the assumption that Gβ1γ2 and Gαiβ1γ2 adopt the same orientation. Figure 6A shows the orientations of both Gβ1γ2 and the Gαiβ1γ2 heterotrimer that would satisfy all of the SFG and ATR-FTIR measurements. The most likely orientation range has twist from 50–80° and low tilt angles from 20–25°, with an assigned score of 68% at ψ=67°, θ=23°. Because overall scores are the product of scores for all individual measurements, this indicates that all individual criteria would provide good quality matches between experimental and calculated parameters ( or better). Thus, it is reasonable to assume that Gβ1γ2 does not reorient upon formation of the heterotrimer. A proposed shared orientation for Gβ1γ2 and Gαiβ1γ2 is shown in Figure 7. In this pose, the orientation of Gβγ at the membrane would allow association with GDP-bound inactive Gα subunits in a manner that would not create an overlap between Gα and the membrane plane. Furthermore, the relatively static orientation of Gβ1γ2 at the membrane allows for the disassociation from a complex with Gα and rapid association with other signaling molecules, such as GRK2. These results also indicate that the amino acid sequence of Gα does not contain regions that strongly influence the membrane orientation of the Gαβγ heterotrimer, consistent with the lack of SFG signals obtained for non-myristoylated Gαi. Thus, this deduced orientation may be exhibited by all families of heterotrimeric G proteins.
Figure 6.

Assessment of whether the Gβ1γ2 subunit and Gαiβ1γ2 can adopt similar orientations at the membrane. A) Scores for orientations that satisfy all four measurements (two ratios and two dichroic RATR ratios). These scores would represent good matches for all experimentally measured parameters, and suggests that Gβ1γ2 does not greatly change its orientation when bound to Gαi. B) The same plot as A) but only showing the orientation area with a score ≥50% (red).
Figure 7.

Possible membrane orientation of A) Gβ1γ2, and B) Gαiβ1γ2 as determined from our experimental measurements (twist=67°, tilt=23°). Two views related by 90° are shown. The plane of the membrane relative to the protein is shown as a blue rectangle.
Conclusions
In this work, we combined SFG and ATR-FTIR to determine the membrane orientation of Gβ1γ2 and the Gαiβ1γ2 heterotrimer in situ. SFG and ATR-FTIR vibrational spectroscopies measure complementary independent parameters, and the combination of techniques was shown to provide a more precise orientation than could be obtained using either technique alone. The most likely orientations of Gβ1γ2 fall in a range of moderate twist angles (50–80°) and low tilt angles (15–35°), whereas the most likely orientation of the Gαiβ1γ2 heterotrimer falls in a very narrow range of moderate twist angles (~65°) and low tilt angles (~23°). According to our measurements, it is entirely possible that Gβ1γ2 can bind to Gαi without changing its orientation (twist = 67°, tilt = 23°) relative to the lipid bilayer. In this orientation (Figure 7), the parts of the protein closest to the lipid bilayer are the C-terminus of Gγ2 and basic residues at the extreme N-terminus of Gαi (which are conserved among all Gα subunits). Our analysis of the myr-Gαiβ1γ2 complex indicated that the myristoyl group does not strongly dictate the orientation of the heterotrimeric G protein complex (Gαβγ), in which the Gβ1γ2 subunits do not undergo a large conformational change in response to binding the Gαi. This could be due to close proximity of the lipid modifications in the complex (N-terminus of Gαi and C-terminus of Gβ1γ2) to each other and on flexible regions of the protein or due to the longer geranylgeranylation modification of Gβ1γ2O which likely dominates the membrane localization of the heterotrimeric G protein complex compared to the shorter myristoyl chain of Gα.
A low-resolution structure of transducin (Gαtβ1γ1) bound to PC lipid tubules was previously determined in helical reconstructions from cryo electron micrographs.81 This work suggested that the heterotrimer binds to the tubules via two strong contacts, which were modeled as the N-terminus and C-terminus of the Gαt subunit, the former of which presumably included the farnesylated C-terminus of Gγ1. Their proposed membrane orientation is significantly different than that suggested in our study (Figure 7) because it suggests that the C-terminus of transducin is involved in membrane binding, whereas we find no evidence that regions other than the extreme N-terminus of Gαi are involved. The conflicting results could reflect several experimental differences. The first could be the influence of helical crystal contacts on the orientation of the transducin-lipid tubule complex in the EM study. Furthermore, although close homologs, the proteins involved in the two studies were distinct, and Gγ1 is farnesylated instead of geranylgeranylated like Gγ2. Finally, there were differences in the composition of the lipid environment used in each study. Whereas the electron microscopy study used neutral lipids, we used a 9:1 mix of POPC and POPG lipids that more closely reflects the charge of the inner leaflet of the membrane surface. In future studies it will be interesting to examine the effect of a change in lipid composition on the orientation of heterotrimeric G proteins and their complexes.
The observation that Gβ1γ2 does not seem to change its orientation significantly as it engages Gαi suggests that the geranylgeranyl modification of Gγ2 is most important determinant of the orientation of Gαiβ1γ2 in membranes, at least in the context of the model phospholipid bilayer we employed. Other regions of the heterotrimer do not lie in the plane of the membrane, such as the C-terminus of Gα, which forms the primary contact with activated integral membrane GPCRs (our model system contains lipids, but not GPCRs)37. Thus, when the heterotrimer encounters an activated GPCR, it will need to change its membrane orientation as a result of additional strong interactions created between the receptor and Gα. The “basal” orientations of Gβ1γ2 and the Gαiβ1γ2 determined in this work may be of physiological relevance because they allow unhindered access to the protein-interaction surfaces of each complex (i.e. the Gα-binding face of the β-propeller of Gβ1 and the C-terminus and other regions of Gα that interact with GPCRs). For example, a previous study showed that altering the location of the prenylation site in Gγ5 by deleting 10 residues immediately N-terminal to the modified cysteine (Gγ5-Δ55-64) abrogates the ability of Gβγ5-Δ55-64 to activate the effector enzyme phospholipase Cβ.82 Although there are other possible explanations82, it is tempting to speculate that this phenotype at least in part reflects a change in the orientation of Gβγ5-Δ55–64 at the membrane that either makes it more difficult for Cβ to access its binding site on Gβγ, or constrains the resulting complex into an orientation that is not optimal for phospholipid hydrolysis.
It remains possible that the protein can change its conformation upon interacting with membrane. However, both Gβ1γ2 and GaiGβ1γ2 interact with membranes primarily through lipid-modified regions that are intrinsically disordered. Because these regions are not coupled to the core structure of the complex, it seems unlikely that their interaction with membrane will induce a large conformational change. Thus we are comfortable with the required assumption that Gβ1γ2 and Gαi do not change conformation upon protein-protein interactions and membrane binding.
We believe that the combination of SFG and ATR-FTIR will provide a foundation for future studies on larger protein complexes, and will enable studies of how each component affects the overall orientation of the complex in order to accommodate additional binding partners. With the help of other advanced approaches currently being developed (e.g. isotope labeling), we believe that in the future it will be possible to deduce an even more accurate orientation for a large protein complex using combined vibrational spectroscopic studies.
Supplementary Material
Acknowledgments
We thank Mark R. Nance, Dr. Valerie Tesmer, and Tracy Quay for technical assistance, and Bei Ding for insightful discussion. This work was supported by NIH grant GM081655 to ZC and JT, and by NIH grants HL071818 and HL086865 (to J.T.).
Footnotes
Supporting Information Available:
Details of the theory and orientation analysis methodologies relevant to SFG and ATR-FTIR, as well as additional information about spectral fitting results for ATR-FTIR. This information is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Horbett TA, Brash JL. Proteins at Interfaces II: Fundamentals and Applications (ACS Symposium) American Chemical Society; Washington, DC: 1995. [Google Scholar]
- 2.Giangaspero A, Sandri L, Tossi A. Eur J Biochem. 2001;268:5589–5600. doi: 10.1046/j.1432-1033.2001.02494.x. [DOI] [PubMed] [Google Scholar]
- 3.Morris MC, Deshayes S, Heitz F, Divita G. Biol Cell. 2008;100:201–217. doi: 10.1042/BC20070116. [DOI] [PubMed] [Google Scholar]
- 4.Tweedle MF. Acc Chem Res. 2009;42:958–968. doi: 10.1021/ar800215p. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Xu Z, Peng L, Fang X, Yin X, Xu N, Cen P. Peptides. 2006;27:931–940. doi: 10.1016/j.peptides.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Jin W. Anal Chim Acta. 2002;461:1–36. [Google Scholar]
- 7.Benkovic SJ, Hammes-Schiffer S. Science. 2003;301:1196–1202. doi: 10.1126/science.1085515. [DOI] [PubMed] [Google Scholar]
- 8.Grozea CM, Walker GC. Soft Matter. 2009;5:4088–4100. [Google Scholar]
- 9.Nguyen K, Le Clair S, Ye S, Chen Z. J Phys Chem B. 2009;113:12169–12180. doi: 10.1021/jp904153z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Lee S-H, Chen Z. J Phys Chem B. 2008;112:2281–2290. doi: 10.1021/jp077556u. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Chen X, Clarke ML, Chen Z. Proc Natl Acad Sci USA. 2005;102:4978–4983. doi: 10.1073/pnas.0501206102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen K, King JT, Chen Z. J Phys Chem B. 2010;114:8291–8300. doi: 10.1021/jp102343h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang P, Ramamoorthy A, Chen Z. Langmuir. 2011;27:7760–7767. doi: 10.1021/la201048t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye S, Li H, Wei F, Jasensky J, Boughton AP, Yang P, Chen Z. J Am Chem Soc. 2012;134:6237–6243. doi: 10.1021/ja2110784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang T, Li D, Lu X, Khmaladze A, Han X, Ye S, Yang P, Xu G, He N, Chen Z. J Phys Chem C. 2011;115:7613–7620. doi: 10.1021/jp200546h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thennarasu S, Huang R, Lee DK, Yang P, Maloy L, Chen Z, Ramamoorthy A. Biochemistry. 2010;49:10595–10605. doi: 10.1021/bi101394r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding B, Chen Z. J Phys Chem B. 2012;116:2545–2552. doi: 10.1021/jp209604m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye S, Nguyen K, Chen Z. J Phys Chem B. 2010;114:3334–3340. doi: 10.1021/jp911174d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han X, Soblosky L, Slutsky M, Mello CM, Chen Z. Langmuir. 2011;27:7042–7051. doi: 10.1021/la200388y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye S, Nguyen K, Boughton AP, Mello CM, Chen Z. Langmuir. 2010;26:6471–6477. doi: 10.1021/la903932w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke ML, Wang J, Chen Z. J Phys Chem B. 2005;109:22027–22035. doi: 10.1021/jp054456k. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen K, Soong R, Im S, Waskell L, Ramamoorthy A, Chen Z. J Am Chem Soc. 2010;132:15112–15115. doi: 10.1021/ja106508f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Wang J, Boughton AP, Kristalyn CB, Chen Z. J Am Chem Soc. 2007;129:1420–1427. doi: 10.1021/ja067446l. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Paszti Z, Clarke ML, Chen X, Chen Z. J Phys Chem B. 2007;111:6088–6095. doi: 10.1021/jp070383o. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen KT, Le Clair SV, Ye S, Chen Z. J Phys Chem B. 2009;113:12358–12363. doi: 10.1021/jp904154w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey S, Tamm LK. Biophys J. 1991;60:922–930. doi: 10.1016/S0006-3495(91)82126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamm LK, Tatulian SA. Quart Rev Biophys. 1997;30:365–429. doi: 10.1017/s0033583597003375. [DOI] [PubMed] [Google Scholar]
- 28.Marsh D. J Mol Biol. 2004;338:353–367. doi: 10.1016/j.jmb.2004.02.061. [DOI] [PubMed] [Google Scholar]
- 29.Marsh D. Biophys J. 1997;72:2710–2718. doi: 10.1016/S0006-3495(97)78914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Páli T, Marsh D. Biophys J. 2001;80:2789–2797. doi: 10.1016/S0006-3495(01)76246-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramakrishnan M, Qu J, Pocanschi CL, Kleinschmidt JH, Marsh D. Biochemistry. 2005;44:3515–3523. doi: 10.1021/bi047603y. [DOI] [PubMed] [Google Scholar]
- 32.Rodionova NA, Tatulian SA, Surrey T, Jaehnig F, Tamm LK. Biochemistry. 1995;34:1921–1929. doi: 10.1021/bi00006a013. [DOI] [PubMed] [Google Scholar]
- 33.Gray C, Tamm LK. Protein Science. 1997;6:1993–2006. doi: 10.1002/pro.5560060920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tatulian SA, Hinterdorfer P, Baber G, Tamm LK. EMBO J. 1995;14:5514–5523. doi: 10.1002/j.1460-2075.1995.tb00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lodowski DT, Barnhill JF, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJG. Acta Crystallogr Sec D: Biol Crystallogr. 2003;59:936–939. doi: 10.1107/s0907444903002622. [DOI] [PubMed] [Google Scholar]
- 36.Neves SR, Ram PT, Iyengaar R. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 37.Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJG. Science. 2005;310:1686–1690. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- 39.Wood JF, Wang J, Benovic JL, Ferkey DM. J Biol Chem. 2012;287:12634–12644. doi: 10.1074/jbc.M111.336818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evron T, Daigle TL, Caron MG. Trends Pharmacol Sci. 2012;33:154–164. doi: 10.1016/j.tips.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boughton AP, Yang P, Tesmer VM, Ding B, Tesmer JJ, Chen Z. Proc Natl Acad Sci USA. 2011;108:E667–E673. doi: 10.1073/pnas.1108236108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linder ME, Gilman AG. Methods Enzymol. 1991;195:202–215. doi: 10.1016/0076-6879(91)95167-i. [DOI] [PubMed] [Google Scholar]
- 43.Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJG. Science. 2003;300:1256–1262. doi: 10.1126/science.1082348. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Boughton AP, Tesmer JJG, Chen Z. J Am Chem Soc. 2007;129:12658–12659. doi: 10.1021/ja075542w. [DOI] [PubMed] [Google Scholar]
- 45.Yang P, Wu FG, Chen Z. J Phys Chem C. 2013;117:3358–3365. doi: 10.1021/jp3099522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Even MA, Chen X, Schmaier AH, Waite JH, Chen Z. J Am Chem Soc. 2003;125:9914–9915. doi: 10.1021/ja036373s. [DOI] [PubMed] [Google Scholar]
- 47.Shen YR. Nature. 1989;337:519–525. [Google Scholar]
- 48.Eisenthal KB. Chem Rev. 1996;96:1343–1360. doi: 10.1021/cr9502211. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Somorjai GA. J Am Chem Soc. 2003;125:3150–3158. doi: 10.1021/ja028987n. [DOI] [PubMed] [Google Scholar]
- 50.Richmond GL. Chem Rev. 2002;102:2693–2724. doi: 10.1021/cr0006876. [DOI] [PubMed] [Google Scholar]
- 51.Kim J, Cremer PS. Chem Phys Chem. 2001;2:543–546. doi: 10.1002/1439-7641(20010917)2:8/9<543::AID-CPHC543>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Gang W, Lu R, Rao Y, Wu B. Int Rev Phys Chem. 2005;24:191–256. [Google Scholar]
- 53.Bain CD. J Chem Soc, Dalton Trans. 1995;91:1281–1296. [Google Scholar]
- 54.Haupert LM, Simpson GJ. Annu Rev Phys Chem. 2009;60:345–365. doi: 10.1146/annurev.physchem.59.032607.093712. [DOI] [PubMed] [Google Scholar]
- 55.Liljeblad JFD, Bulone V, Rutland MW, Johnson CM. J Phys Chem C. 2011;115:10617–10629. [Google Scholar]
- 56.Li G, Ye S, Morita S, Nishida T, Osawa M. J Am Chem Soc. 2004;126:12198–12199. doi: 10.1021/ja046183x. [DOI] [PubMed] [Google Scholar]
- 57.Barth C, Jakubczyk D, Kubas A, Anastassacos F, Brenner-Weiss G, Fink K, Schepers U, Brase S, Koelsch P. Langmuir. 2012;28:8456–8462. doi: 10.1021/la301241s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Engelhardt K, Rumpel A, Walter J, Dombrowski J, Kulozik U, Braunschweig B, Peukert W. Langmuir. 2012;28:7780–7787. doi: 10.1021/la301368v. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, Conboy JC. J Am Chem Soc. 2004;126:8894–8895. doi: 10.1021/ja031570c. [DOI] [PubMed] [Google Scholar]
- 60.Ye S, Liu G, Li H, Chen F, Wang X. Langmuir. 2012;28:1374–1380. doi: 10.1021/la203690p. [DOI] [PubMed] [Google Scholar]
- 61.Ma G, Liu DF, Allen HC. Langmuir. 2004;20:11620–11629. doi: 10.1021/la0487343. [DOI] [PubMed] [Google Scholar]
- 62.Weidner T, Breen NF, Li K, Drohny GP, Castner DG. Proc Natl Acad Sci USA. 2010;107:13288–13293. doi: 10.1073/pnas.1003832107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weidner T, Breen NF, Drobny GP, Castner DG. J Phys Chem B. 2009;113:15423–15426. doi: 10.1021/jp908773c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weidner T, Dubey M, Breen NF, Ash J, Baio JE, Jaye C, Fischer DA, Drobny GP, Castner DG. J Am Chem Soc. 2012;134:8750–8753. doi: 10.1021/ja301711w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu L, Ma G, Yan EC. J Am Chem Soc. 2010;132:5405–5412. doi: 10.1021/ja909546b. [DOI] [PubMed] [Google Scholar]
- 66.Fu L, Liu J, Yan EC. J Am Chem Soc. 2011;122:8094–8097. doi: 10.1021/ja201575e. [DOI] [PubMed] [Google Scholar]
- 67.Chen X, Flores SC, Lim SM, Zhang YJ, Yang TL, Kherb J, Cremer PS. Langmuir. 2010;26:16447–16454. doi: 10.1021/la1015862. [DOI] [PubMed] [Google Scholar]
- 68.Campen RK, Ngo TTM, Sovago M, Ruysschaert JM, Bonn M. J Am Chem Soc. 2010;132:8037–8047. doi: 10.1021/ja100838q. [DOI] [PubMed] [Google Scholar]
- 69.Wang HF, Troxler T, Yeh AG, Dai HL. J Phys Chem C. 2007;111:8708–8715. [Google Scholar]
- 70.Jen SH, Dai HL. J Phys Chem B. 2006;110:23000–23003. doi: 10.1021/jp0644762. [DOI] [PubMed] [Google Scholar]
- 71.Ye H, Abu-Akeel A, Huang J, Katz HE, Gracias DH. J Am Chem Soc. 2006;128:6528–6529. doi: 10.1021/ja060442w. [DOI] [PubMed] [Google Scholar]
- 72.Ye H, Gu Z, Gracias DH. Langmuir. 2006;22:1863–1868. doi: 10.1021/la052030r. [DOI] [PubMed] [Google Scholar]
- 73.Ye H, Huang J, Park JR, Katz HE, Gracias DH. J Phys Chem C. 2007;111:13250–13255. [Google Scholar]
- 74.Li Q, Kuo CW, Yang Z, Chen P, Chou KC. Phys Chem Chem Phys. 2009;11:3436–3442. doi: 10.1039/b821045d. [DOI] [PubMed] [Google Scholar]
- 75.Chen P, Kung KY, Shen YR, Somorjai GA. Surf Sci. 2001;494:289–297. [Google Scholar]
- 76.Leung BO, Yang Z, Wu SSH, Chou KC. Langmuir. 2012;28:5724–5728. doi: 10.1021/la204805x. [DOI] [PubMed] [Google Scholar]
- 77.Yang Z, Li Q, Gray MR, Chou KC. Langmuir. 2010;26:16397–16400. doi: 10.1021/la1020737. [DOI] [PubMed] [Google Scholar]
- 78.Chen X, Yang T, Kataoka S, Cremer PS. J Am Chem Soc. 2007;129:12272–12279. doi: 10.1021/ja073869r. [DOI] [PubMed] [Google Scholar]
- 79.Tatulian SA, Jones LR, Reddy LG, Stokes DL, Tamm LK. Biochemistry. 1995;34:4448–4456. doi: 10.1021/bi00013a038. [DOI] [PubMed] [Google Scholar]
- 80.Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Z, Melia TJ, He F, Yuan C, Mcgough A, Schmid MF, Wensel TG. J Biol Chem. 2004;279:33937–33945. doi: 10.1074/jbc.M403404200. [DOI] [PubMed] [Google Scholar]
- 82.Akgoz M, Azpiazu I, Kalyanaraman V, Gautam N. J Biol Chem. 2002;277:19573–19578. doi: 10.1074/jbc.M201546200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.