Abstract
OBJECTIVE
To evaluate the efficacy and safety of prophylactic misoprostol use at cesarean delivery for reducing intraoperative and postoperative hemorrhage.
STUDY DESIGN
Systematic review and meta-analysis of randomized controlled trials.
RESULTS
Seventeen studies (3174 women) were included of which 7 evaluated misoprostol versus oxytocin and 8 evaluated misoprostol plus oxytocin versus oxytocin. Overall, there were no significant differences in intraoperative and postoperative hemorrhage between sublingual or oral misoprostol and oxytocin. Rectal misoprostol, compared with oxytocin, was associated with a significant reduction in intraoperative and postoperative hemorrhage. The combined use of sublingual misoprostol and oxytocin, compared with the use of oxytocin alone, was associated with a significant reduction in the mean decrease in hematocrit (mean difference, −2.1%; 95% confidence interval [CI], −3.4 to −0.8) and use of additional uterotonic agents (relative risk, 0.33; 95% CI, 0.18-0.62). Compared with oxytocin alone, buccal misoprostol plus oxytocin reduced the use of additional uterotonic agents; rectal misoprostol plus oxytocin decreased intraoperative and postoperative blood loss, mean fall in hematocrit, and use of additional uterotonic agents; and intrauterine misoprostol plus oxytocin reduced the mean fall in hemoglobin and hematocrit. Women receiving misoprostol, alone or combined with oxytocin, had a higher risk of shivering and pyrexia.
CONCLUSION
Misoprostol combined with oxytocin appears to be more effective than oxytocin alone in reducing intraoperative and postoperative hemorrhage during caesarean section. There were no significant differences in intraoperative and postoperative hemorrhage when misoprostol was compared to oxytocin. However, these findings were based on a few trials with methodological limitations.
Keywords: Blood loss, oxytocin, pregnancy, postpartum hemorrhage, uterotonics
INTRODUCTION
Cesarean delivery is the most common major surgical procedure performed on women worldwide and its rates continue to rise steadily in both developed and developing countries.1-8 In 2007, the global cesarean delivery rate was estimated to be 15%.9 Postpartum hemorrhage is a major contributor to maternal mortality, mainly in developing countries.10 Recent studies from developed countries report an increase in the rate of postpartum hemorrhage, which has been attributed (at least in part) to a rise in the rate of cesarean delivery.11-14 Large population- and hospital-based cohort studies have attributed this to uterine atony after vaginal or cesarean deliveries.11,13,14 Cesarean delivery, often performed because of “dystocia”, may predispose a patient to uterine atony. This has been traditionally attributed to either myometrial fatigue or impaired contractility at the site of the uterine incision.
Postpartum hemorrhage following a cesarean delivery has been defined as blood loss over 1000 ml15, based on a study from the early 1960s.16 Recent studies have estimated that the prevalence of postpartum hemorrhage after cesarean delivery ranges from 0.6% to 6.4% (median, 3%), although the frequency depends on the criteria used to define this condition and the method used to calculate blood loss.17-26
The efficacy of routine administration of uterotonic agents, mainly oxytocin, to reduce the frequency of postpartum hemorrhage after vaginal birth is well-established.27 It has been assumed that the benefits of injectable uterotonic agents observed for vaginal births also apply to cesarean deliveries; yet, this has not been rigorously demonstrated. An updated guideline of the Royal College of Obstetricians and Gynecologists on cesarean delivery recommends a slow intravenous bolus dose of 5 IU of oxytocin after delivery of the neonate to ensure adequate uterine contractility, minimize delay in the delivery of the placenta, reduce intraoperative blood loss and prevent postpartum hemorrhage.28 In contrast, in the United States, the practice is to use an oxytocin infusion instead of a bolus dose.29 Regardless of the mode of administration, oxytocin use in the setting of cesarean delivery may result in maternal adverse effects, such as hypotension and tachycardia.30
Misoprostol, a prostaglandin E1 analogue with strong uterotonic properties, has been suggested as an alternative to injectable uterotonic agents for preventing postpartum hemorrhage following vaginal or cesarean deliveries. A recent Cochrane review found that oral misoprostol was associated with a higher risk of severe postpartum hemorrhage and use of additional uterotonics after vaginal birth when compared to conventional uterotonic agents.31 However, oral or sublingual misoprostol was found to be more effective than placebo in reducing severe postpartum hemorrhage and blood transfusion after vaginal birth. The use of misoprostol during cesarean delivery to prevent hemorrhage attributable to uterine atony has received less attention and its effectiveness has not been systematically evaluated.
We conducted a systematic review and meta-analysis of all available randomized controlled trials to assess the efficacy and safety of prophylactic misoprostol use at cesarean delivery for reducing intraoperative and postoperative hemorrhage.
MATERIALS AND METHODS
The study was conducted using a prospectively prepared protocol, and is reported using the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines for meta-analysis of randomized controlled trials.32
Data sources and searches
We searched MEDLINE, EMBASE, CINAHL, and LILACS (all from inception to February 20, 2013), the Cochrane Central Register of Controlled Trials (http://www.mrw.interscience.wiley.com/cochrane/cochrane_clcentral_articles_fs.html) (1960 to February 20, 2013), ISI Web of Science (http://www.isiknowledge.com) (1960 to February 20, 2013), Research Registers of ongoing trials (www.clinicaltrials.gov, www.controlled-trials.com, www.centerwatch.com, www.anzctr.org.au, http://www.nihr.ac.uk, and www.umin.ac.jp/ctr), and Google scholar using a combination of keywords and text words related to misoprostol, cesarean delivery and hemorrhage. Congress proceedings of international society meetings of maternal-fetal and reproductive medicine and international meetings on postpartum hemorrhage or cesarean delivery, reference lists of identified studies, textbooks, previously published systematic reviews, and review articles were also searched. Experts in the field were contacted to identify further studies. No language restriction was applied.
Study selection
We included randomized controlled trials in which misoprostol (alone or in combination) was used to reduce perioperative hemorrhage in women undergoing cesarean delivery compared with either another uterotonic agent or placebo/no uterotonic agent. Studies were included irrespective of women’s risk status for postpartum hemorrhage, dose, and route of administration. Trials were excluded if they were quasi-randomized or if they evaluated only the effect of misoprostol on intestinal motility after cesarean delivery. Published abstracts alone were excluded if additional information on methodological issues and results could not be obtained.
Two investigators (AC-A and AN) independently reviewed all potentially relevant articles for eligibility. Disagreements regarding trial eligibility were resolved by consensus.
Outcome measures
The prespecified primary outcome measures were the mean intraoperative and postoperative blood loss, and the mean decrease in hemoglobin and hematocrit (difference between preoperative and postoperative levels). In addition, we also chose the use of additional uterotonic agents as a primary outcome because obstetricians are likely to intervene (when uterine atony does not respond to therapy) by employing an additional agent(s). Secondary outcome measures included blood loss >500 and >1000 ml, blood transfusion, mean postoperative hemoglobin and hematocrit, shivering, pyrexia (≥38 °C), nausea, vomiting, diarrhea, abdominal pain, headache, any side effect, neonatal outcomes, and costs.
Assessment of risk of bias
We assessed the risk of bias using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions.33 Seven domains related to risk of bias were assessed in each trial since there is evidence that these issues are associated with biased estimates of treatment effect: 1) random sequence generation; 2) allocation concealment; 3) blinding of participants and personnel; 4) blinding of outcome assessment; 5) incomplete outcome data; 6) selective reporting; and 7) other bias. The assessments were classified as “low risk” of bias, “high risk” of bias or “unclear risk” of bias. In addition, we evaluated the technique of assessment of blood loss used in each study and classified it as objective, subjective, unmeasured, or unreported. The risk of bias in each trial included was assessed individually by two reviewers (AC-A and AN). Any differences of opinion regarding assessment of risk of bias were resolved by discussion.
Data extraction
Two reviewers (AC-A and AN) independently extracted data from each eligible study using a standardized data abstraction form. There was no blinding of authorship. From each article, reviewers extracted data on participants (inclusion and exclusion criteria, number of women randomized, baseline characteristics, and country and date of recruitment), study characteristics (randomization procedure, concealment allocation method, blinding of clinicians, women and outcome assessors, completeness of outcome data for each outcome, including attrition and exclusions from the analysis, intention to treat analysis, anesthesia used, and method of assessment of blood loss used), details of interventions (aim, loading and maintenance dose, route, duration of treatment, and use of alternative uterotonic agents), and outcomes (definitions used, number of outcome events/total number, and mean ± standard deviation for each outcome). In an attempt to obtain additional data, we contacted seven authors by e-mail of whom two responded. Disagreements in extracted data were resolved by discussion among reviewers.
Statistical analysis
All statistical analyses were performed according to the guidelines of the Cochrane Collaboration.34 We analyzed outcomes on an intention to treat basis. If this was not clear from the original article we then carried out a re-analysis when possible. If we found no evidence of a substantial difference in study populations, interventions, or outcome measurements, we performed a meta-analysis. For dichotomous data, we calculated the summary relative risk (RR) with 95% confidence interval (CI). For continuous data, we used the mean difference (MD) if outcomes were measured in the same way among trials, or standardized mean difference if the same outcome was measured in a variety of ways, with 95% CI. Analyses were stratified by route of misoprostol administration, irrespective of dose used, as follows: sublingual, oral, buccal, rectal, and intrauterine. Further subgroup analyses were planned to assess primary outcomes according to risk status for intraoperative/postoperative hemorrhage, gestational age at cesarean delivery, type of anesthesia, whether the cesarean delivery was unplanned, method of assessment of blood loss, and dose of misoprostol but they were not undertaken due to the small number of trials included in each comparison.
Heterogeneity of the results among studies was tested with the quantity I2, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance.35 A value of 0% indicates no observed heterogeneity, whereas I2 values of 50% or more indicate a substantial level of heterogeneity. A fixed effects model was used if substantial statistical heterogeneity was not detected. If there was substantial statistical heterogeneity, a random effects model was used to pool data across studies if causes of heterogeneity could not be determined and the average treatment effect was considered clinically meaningful. One of the most important sources of bias in the conduct of clinical trials evaluating the efficacy of misoprostol to reduce hemorrhage at cesarean delivery is the lack of blinding which is likely to influence the measurement of intraoperative and postoperative blood loss and use of additional uterotonic agents. We performed a pre-defined sensitivity analysis to explore the impact of study quality on the effect size for the primary outcomes by including only trials with adequate concealment allocation and double masked.
The number needed to treat (NNT) for benefit or harm with 95% CI was calculated for the outcomes for which there was a statistically significant reduction or increase in risk difference based on control event rates in the included trials.36 We planned to assess publication and related biases37 but this was not performed because of paucity of trials in each comparison. Analyses were performed with the Review Manager (RevMan) version 5.1.7 (The Nordic Cochrane Centre, Denmark).
RESULTS
Study selection, details, and quality
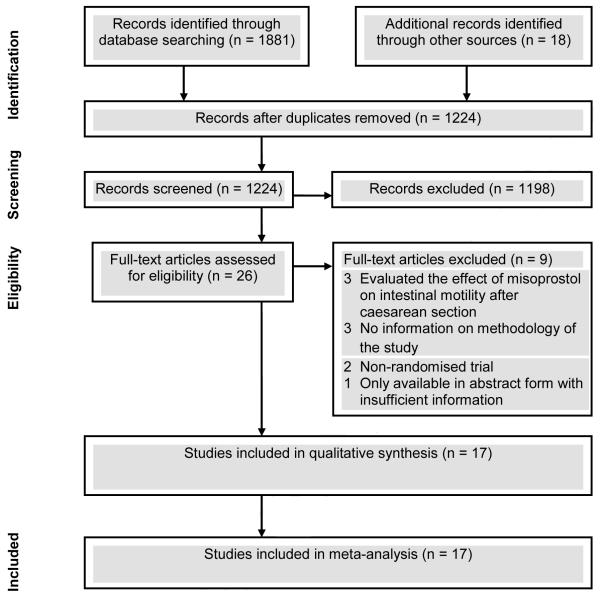
The searches yielded 1899 citations, of which 26 were considered to be potentially eligible (Figure 1). Nine studies were excluded. Three of these studies evaluated only the effect of misoprostol on intestinal motility after cesarean delivery, three provided very little information on the methodology, two were non-randomized controlled trials, and the remainder was available only in abstract form with insufficient information on methods and results. The list of studies excluded is available from the authors upon request. Seventeen studies,38-54 including 3174 women, fulfilled inclusion criteria of which seven evaluated misoprostol versus oxytocin (n=700),39,40,42,45,47,49,54 one evaluated misoprostol versus ergometrine (n=374),51 eight evaluated misoprostol plus oxytocin versus oxytocin (n=1918),41,43,44,46,48,50,52,53 and one 3-arm trial evaluated misoprostol versus oxytocin versus misoprostol plus oxytocin (n=182).38
Figure 1.
Study selection process
The main characteristics of studies included in the review are shown in Table 1. Thirteen trials were conducted in developing countries (three in India, two in Egypt, and one each in China, Iran, Thailand, Pakistan, Nigeria, Tunisia, Mexico, and Ecuador), two in the United Kingdom, and one each in the United States and Switzerland. Seven studies included women undergoing elective cesarean delivery and ten included women undergoing both elective and emergency cesarean delivery. Regional anesthesia was used in 15 studies and general anesthesia in two studies. Twelve studies excluded women with any risk factors associated with an increased risk of intraoperative or postoperative hemorrhage, three excluded women with some risk factors, and four did not report on exclusion criteria. The sample size ranged from 40 to 400 (median, 174). Of the 17 trials included in the review, eight evaluated misoprostol using the sublingual route, four using the oral route, three using the rectal route, and one each used buccal and intrauterine routes in doses of 200 g (two studies), 400 g (nine studies), 500 g (one study), 600 g (one study), and 800 g (four studies). The most common dose used in studies evaluating the sublingual route was 400 g, and 800 g in studies that evaluated the rectal and intrauterine routes. Misoprostol was used after delivery of the neonate in 12 studies and before delivery of the neonate in five studies (orally38 or rectally47 at the time of peritoneal incision, rectally just before onset of cesarean section51 or after urinary catheter placement,52 and sublingually after tracheal intubation53). It should be noted that in seven studies that compared misoprostol with placebo, all women received an intravenous infusion of oxytocin in doses ranging from 5 IU to 20 IU. Overall, misoprostol was compared with oxytocin in 16 trials and with ergometrine in one trial. Nine trials used oxytocin 20 IU and six 10 IU intravenously. The remaining study used oxytocin 20 IU intramyometrially. The main primary outcome measures were intraoperative blood loss (12 studies), difference between preoperative and postoperative hemoglobin and/or hematocrit levels (10 studies), postoperative blood loss (six studies), use of additional uterotonic agents (five studies), and drug-related adverse effects (two studies).
TABLE 1.
Characteristics of studies included in the systematic review
| First author, year | Location | Inclusion/exclusion criteria | Sample size |
Interventions | Primary outcome |
|---|---|---|---|---|---|
| Zhao,38 1998 | China | •Inclusion: women undergoing elective cesarean section under regional anesthesia •Exclusion: unreported |
182 | (1) Misoprostol 600 μg orally at the time of peritoneal incision (n=60) (2) Oxytocin 20 IU injected into uterine muscle followed by 20 IU intravenous bolus after delivery of the baby (n=58) (3) Misoprostol 600 μg orally plus oxytocin 20 IU injected into uterine muscle after delivery of the neonate (n=64) |
Postoperative blood loss |
| Acharya,39 2001 | United Kingdom |
•Inclusion: women undergoing elective cesarean section under regional anesthesia •Exclusion: unreported |
60 | (1) Misoprostol 400 μg orally after cord clamping (n=30) (2) Oxytocin 10 IU intravenous after cord clamping (n=30) |
Intraoperative blood loss and difference between preoperative and postoperative hemoglobin and hematocrit |
| Lokugamage,40 2001 | United Kingdom |
•Inclusion: women undergoing elective or emergency cesarean section under spinal or epidural anesthesia •Exclusion: ≥2 previous cesarean sections or a history of previous uterine rupture |
40 | (1) Misoprostol 500 μg orally plus placebo intravenous bolus after cord clamping (n=20) (2) Oxytocin 10 IU intravenous bolus plus oral placebo tablets after cord clamping (n=20) |
Intraoperative blood loss, difference between preoperative and postoperative hemoglobin, and use of additional uterotonic agents |
| Hamm,41 2005 | United States |
•Inclusion: women undergoing elective or emergency cesarean section under regional anesthesia •Exclusion: unreported |
352 | (1) Misoprostol 200 μg placed in the buccal space after cord clamping (n=173) (2) Placebo tablet placed in the buccal space after cord clamping (n=179) •All women received oxytocin 20 IU intravenous infusion over a period of 8 hours after cord clamping |
Need for additional uterotonic agents |
| Vimala,42 2006 | India | •Inclusion: women with singleton term pregnancy undergoing elective or emergency cesarean section under spinal anesthesia •Exclusion: women with any risk factors associated with an increased risk of postpartum hemorrhage (anemia, multiple gestation, antepartum hemorrhage, polyhydramnios, prolonged labor, ≥2 previous cesarean sections and/or a history of previous uterine rupture, and current or previous history of heart or liver disease, renal disorders or known coagulopathy |
100 | (1) Misoprostol 400 μg sublingually after delivery of the baby (n=50) (2) Oxytocin 20 IU intravenous infusion over a period of 6 hours after delivery of the baby (n=50) |
Intraoperative blood loss, difference between preoperative and postoperative hemoglobin, and need for additional uterotonic agents |
| Lapaire,43 2006 | Switzerland | •Inclusion: women undergoing indicated or elective cesarean section under spinal anesthesia •Exclusion: women undergoing emergency cesarean delivery within 30 min of admission, fetal distress, fetal malformations, preeclampsia, HELLP syndrome, hypersensitivity to prostaglandins, coagulopathy, severe systemic disorders, an ASA class III or greater, severe asthma, prior myomectomy, and fever |
56 | (1) Misoprostol 800 μg orally plus intravenous infusion of normal saline over a period of 8 hours after cord clamping (n=28) (2) Oxytocin 20 IU intravenous infusion over a period of 8 hours plus oral placebo tablet after cord clamping (n=28) •All women received oxytocin 5 IU intravenous bolus after cord clamping |
Intraoperative and postoperative blood loss, and drug-related adverse effects |
| Quiroga Diaz,44 2009 | Mexico | •Inclusion: women with singleton or multiple pregnancy undergoing elective cesarean section under regional anesthesia •Exclusion: women with placenta previa, blood dyscrasias, enlarged myomatous uterus, and obstetric hemorrhage secondary to uterine laceration |
200 | (1) Misoprostol 800 μg intrauterine after placental extraction (n=100) (2) Placebo intrauterine after placental extraction (n=100) •All women received oxytocin 20 IU intravenous bolus over 15 min followed by an intravenous infusion at 40 mIU/min after delivery of the baby |
Difference between preoperative and postoperative hemoglobin and hematocrit |
| Eftekhari,45 2009 | Iran | •Inclusion: women with term pregnancy (37-40 weeks) undergoing elective cesarean section under general anesthesia •Exclusion: women with multiple gestation, prolonged labor (>12 hours), ≥2 previous cesarean sections, history of uterine rupture, hemoglobin <8 g/dl, coagulopathy, and history of heart, renal or liver disorders |
100 | (1) Misoprostol 400 μg sublingually after delivery of the baby (n=50) (2) Oxytocin 20 IU intravenous infusion after delivery of the baby (n=50) |
Intraoperative blood loss and difference between preoperative and postoperative hemoglobin |
| Fekih,46 2009 | Tunisia | •Inclusion: women undergoing elective or emergency cesarean section under regional anesthesia at gestational age >32 weeks of gestation •Exclusion: women with placenta previa, placental abruption, multiple gestation, gestational age <32 weeks, stillbirth, cesarean section under general anesthesia, anemia, haemostatic disorders, HELLP syndrome, history of postpartum hemorrhage or uterine rupture, ≥2 previous cesarean sections, prolonged labor, and maternal fever |
250 | (1) Misoprostol 200 μg sublingually plus oxytocin 20 IU (intravenous bolus of 10 IU and infusion of 10 IU over 30 min) after cord clamping (n=125). (2) Oxytocin 20 IU (intravenous bolus of 10 IU and infusion of 10 IU over 30 min) after cord clamping (n=125) |
Difference between preoperative and postoperative hematocrit |
| Chaudhuri,47 2010 | India | •Inclusion: women undergoing elective or emergency cesarean section under spinal anesthesia •Exclusion: women with multiple gestation, polyhydramnios, fetal macrosomia, antepartum hemorrhage, obstructed labor, anemia, severe preeclampsia, coagulopathy, ≥2 previous cesarean sections, known hypersensitivity to prostaglandins, cesarean sections with indications such as cord prolapse or gross fetal bradycardia, and cardiovascular, respiratory, liver or hematological diseases |
200 | (1) Misoprostol 800 μg rectally at the time of peritoneal incision plus placebo intravenous infusion (n=100) (2) Oxytocin 6 IU intravenous bolus over 30 min after delivery of the baby followed by an intravenous infusion at 40 mIU/min over a period of 8 hours plus placebo tablets rectally at the time of peritoneal incision (n=100) |
Intraoperative and postoperative blood loss, and difference between preoperative and postoperative hemoglobin |
| Chalermpolprapa,48 2010 |
Thailand | •Inclusion: women with singleton pregnancy undergoing elective or emergency cesarean section under spinal anesthesia •Exclusion: multiple pregnancy, gestational age <32 weeks, hypersensitivity to prostaglandins, coagulopathy, temperature >37.2 °C, hemoglobin <8 g/dl, antepartum hemorrhage, polyhydramnios, severe preeclampsia, and fetal distress |
120 | (1) Misoprostol 400 μg sublingually after cord clamping (n=60) (2) Placebo tablets sublingually after cord clamping (n=60) •All women received oxytocin 20 IU intravenous infusion after cord clamping |
Intraoperative blood loss |
| Owonikoko,49 2011 | Nigeria | •Inclusion: women with term singleton pregnancy undergoing elective or emergency cesarean section under spinal anesthesia •Exclusion: women with multiple gestation, placenta previa, antepartum hemorrhage, unexplained vaginal bleeding, cesarean section under general anesthesia, pre-existing medical illnesses (cardiovascular, renal or hepatic dysfunction), clotting disorders, severe preeclampsia, eclampsia, prolonged obstructed labor, and contraindications to prostaglandin administration |
100 | (1) Misoprostol 400 μg sublingually after delivery of the baby (n=50) (2) Oxytocin 20 IU intravenous infusion after delivery of the baby (n=50) |
Intraoperative and postoperative blood loss, difference between preoperative and postoperative hemoglobin, need for additional uterotonic agents, and drug-related adverse effects |
| Sood,50 2012 | India | •Inclusion: women undergoing elective or emergency cesarean section under spinal or epidural anesthesia •Exclusion: none |
174 | (1) Misoprostol 400 μg sublingually after cord clamping (n=90) (2) Placebo tablets sublingually after cord clamping (n=84) •All women received oxytocin 20 IU intravenous infusion (6 IU over 30 min followed by an intravenous infusion at 40 mIU/min over a period of 6 hours) after cord clamping |
Intraoperative blood loss, need for additional uterotonic agents, and difference between preoperative and postoperative hemoglobin |
| Ali,51 2012 | Pakistan | •Inclusion: women with parity ≤4 and hemoglobin >11 g/dl undergoing elective or emergency cesarean section •Exclusion: parity >4 |
374 | (1) Misoprostol 800 μg rectally just before onset of cesarean section (n=187) (2) Ergometrine 0.5 mg intravenous bolus at delivery of the baby (n=187) |
Intraoperative blood loss >500 ml and postoperative hemoglobin |
| Elsedeek,52 2012 | Egypt | •Inclusion: women with singleton pregnancy, ≥39 weeks of gestation, and parity ≤5 undergoing elective repeat cesarean section under spinal anesthesia •Exclusion: women with elective first cesarean section, hypertension, diabetes mellitus, abnormal sonographic findings, abnormal placenta, and abnormal coagulation profile |
400 | (1) Misoprostol 400 μg rectally after urinary catheter placement (n=200) (2) Placebo tablets rectally after urinary catheter placement (n=200) •All women received oxytocin 10 IU intravenous infusion over 30 min after cord clamping |
Intraoperative and postoperative blood loss, and difference between preoperative and postoperative hematocrit |
| El Tahan,53 2012 | Egypt | •Inclusion: women aged 18-35 years with uncomplicated singleton pregnancy of at least 36 weeks of gestation and ASA class I-II undergoing elective cesarean section under general anesthesia (isoflurane) •Exclusion: women with a history of allergy to prostaglandins, bronchial asthma, anemia, bleeding disorders, cardiac or inflammatory bowel disease, multiple pregnancy, preeclampsia, placenta previa, abruption placenta, previous postpartum hemorrhage, antepartum hemorrhage, parity ≥4, uterine fibroids, and intrauterine growth restriction or other fetal abnormalities |
366 | (1) Misoprostol 400 μg sublingually after tracheal intubation (n=179) (2) Placebo tablets sublingually after tracheal intubation (n=187) •All women received oxytocin 10 IU intravenous infusion after cord clamping |
Postoperative blood loss |
| Gavilanes Sáenz,54 2012 |
Ecuador | •Inclusion: women with uncomplicated singleton pregnancy ≥34 weeks of gestation undergoing elective or emergency cesarean section under regional anesthesia •Exclusion: women with severe anemia (≤8 mg/dl), multiple gestation, polyhydramnios, previous uterine rupture, current or previous coagulopathy, fetal death, and fever (>38.5 °C) |
100 | (1) Misoprostol 400 μg sublingually after delivery of the baby (n=50) (2) Oxytocin 10 IU intravenous bolus over 45 min after delivery of the baby followed by an intravenous infusion at 40 mIU/min (duration was not reported) (n=50) |
Intraoperative blood loss |
| pregnancy a history of allergy to prostaglandins, bronchial asthma, anemia, bleeding disorders, cardiac or inflammatory bowel disease, , preeclampsia, placenta previa, abruption placenta, previous postpartum hemorrhage, antepartum hemorrhage, parity ≥4, uterine fibroids, and intrauterine growth restriction or other fetal abnormalities |
IU, international unit; HELLP, hemolysis, elevated liver enzymes, and low platelet count; ASA, American Society of Anesthesiologists
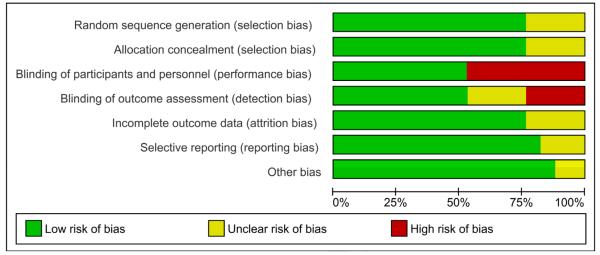
Figure 2 shows methodological quality of studies included in the systematic review. Thirteen studies had adequate generation of allocation sequence and reported adequate concealment of allocation. Nine trials were double masked, placebo controlled, 13 had an adequate handling of incomplete outcome data and 14 were free of suggestion of selective outcome reporting. Fifteen studies appeared to be free of other sources of bias. Seven trials met all seven methodological criteria and were considered to be at low risk of bias. One study met six criteria, 4 met five criteria and the remaining five met <5 criteria. Blood loss was measured using objective methods in ten studies38,42,43,45,47-49,52-54 and clinical estimation in five studies,39,40,46,50,51 unmeasured in one study44 and unreported in another.41
Figure 2.
Methodological quality of studies included in the systematic review
Misoprostol versus oxytocin (Table 2)
TABLE 2.
Misoprostol compared with oxytocin
| Number of events/total number or Total number |
Relative risk or Mean difference (95% CI) |
|||||
|---|---|---|---|---|---|---|
| Outcome | No of trials |
Misoprostol | Oxytocin | P value |
I2 (%) |
|
| Primary outcomes | ||||||
| Mean intraoperative blood loss (ml) | ||||||
| Sublingual misoprostol | 442,45,49,54 | 200 | 200 | −55 (−115 to 5) | 0.07 | 58 |
| Oral misoprostol | 239,40 | 50 | 50 | 20 (−53 to 93) | 0.59 | 0 |
| Rectal misoprostol | 147 | 96 | 94 | −90 (−147 to −32) | 0.002 | NA |
| Mean postoperative blood loss (ml) | ||||||
| Sublingual misoprostol | 149 | 50 | 50 | −23 (−32 to −14) | <0.00001 | NA |
| Rectal misoprostol | 147 | 96 | 94 | −40 (−76 to −4) | 0.03 | NA |
| Mean fall in hemoglobin (g/dl) | ||||||
| Sublingual misoprostol | 242,45 | 100 | 100 | 0.1 (−0.2 to 0.3) | 0.61 | 0 |
| Oral misoprostol | 239,40 | 50 | 50 | 0.1 (−0.2 to 0.4) | 0.54 | 0 |
| Rectal misoprostol | 147 | 96 | 94 | −0.2 (−0.4 to 0.0) | 0.07 | NA |
| Mean fall in hematocrit (%) | ||||||
| Oral misoprostol | 139 | 30 | 30 | 0.6 (−0.7 to 1.9) | 0.38 | NA |
| Use of additional uterotonic agents | ||||||
| Sublingual misoprostol | 442,45,49,54 | 57/200 | 67/200 | 0.85 (0.64 to 1.14) | 0.27 | 33 |
| Oral misoprostol | 239,40 | 8/50 | 4/50 | 1.87 (0.21 to 16.53) | 0.57 | 63 |
| Rectal misoprostol | 147 | 11/96 | 14/94 | 0.77 (0.37 to 1.61) | 0.49 | NA |
| Secondary outcomes | ||||||
| Blood loss >500 ml | ||||||
| Sublingual misoprostol | 142 | 47/50 | 46/50 | 1.02 (0.92 to 1.14) | 0.70 | NA |
| Oral misoprostol | 140 | 17/20 | 17/20 | 1.00 (0.77 to 1.30) | 1.00 | NA |
| Rectal misoprostol | 147 | 38/96 | 51/94 | 0.73 (0.54 to 0.99) | 0.05 | NA |
| Blood loss >1000 ml | ||||||
| Sublingual misoprostol | 242,54 | 17/100 | 22/100 | 0.77 (0.44 to 1.36) | 0.37 | 0 |
| Oral misoprostol | 239,40 | 4/50 | 4/50 | 1.00 (0.27 to 3.67) | 1.00 | 0 |
| Rectal misoprostol | 147 | 1/96 | 6/94 | 0.16 (0.02 to 1.33) | 0.09 | NA |
| Blood transfusion | ||||||
| Sublingual misoprostol | 149 | 1/50 | 0/50 | 3.00 (0.13 to 71.92) | 0.50 | NA |
| Oral misoprostol | 239,40 | 2/50 | 1/50 | 1.67 (0.23 to 12.18) | 0.61 | 0 |
| Rectal misoprostol | 147 | 0/96 | 3/94 | 0.14 (0.01 to 2.67) | 0.19 | NA |
| Postoperative hemoglobin (g/dl) | ||||||
| Sublingual misoprostol | 242,45 | 100 | 100 | −0.4 (−0.9 to 0.0) | 0.06 | 66 |
| Rectal misoprostol | 147 | 96 | 94 | 0.4 (0.1 to 0.8) | 0.02 | NA |
| Shivering | ||||||
| Sublingual misoprostol | 342,49,54 | 73/150 | 4/150 | 18.25 (6.82 to 48.8) | <0.00001 | 20 |
| Oral misoprostol | 239,40 | 15/50 | 10/50 | 1.50 (0.82 to 2.75) | 0.19 | 0 |
| Rectal misoprostol | 147 | 8/96 | 1/94 | 7.83 (1.00 to 61.42) | 0.05 | NA |
| Pyrexia | ||||||
| Sublingual misoprostol | 142 | 8/50 | 2/50 | 4.00 (0.89 to 17.91) | 0.07 | NA |
| Oral misoprostol | 239,40 | 0/50 | 0/50 | Not estimable | NA | NA |
| Rectal misoprostol | 147 | 2/96 | 4/94 | 0.49 (0.09 to 2.61) | 0.40 | NA |
| Nausea | ||||||
| Sublingual misoprostol | 249,54 | 2/100 | 9/100 | 0.26 (0.07 to 1.02) | 0.05 | 0 |
| Vomiting | ||||||
| Sublingual misoprostol | 342,49,54 | 9/150 | 9/150 | 1.00 (0.43 to 2.34) | 1.00 | 0 |
| Oral misoprostol | 139 | 2/30 | 3/30 | 0.67 (0.12 to 3.71) | 0.64 | NA |
| Rectal misoprostol | 147 | 2/96 | 3/94 | 0.65 (0.11 to 3.82) | 0.64 | NA |
| Headache | ||||||
| Sublingual misoprostol | 342,49,54 | 7/150 | 12/150 | 0.60 (0.25 to 1.41) | 0.24 | 0 |
| Any side effect | ||||||
| Sublingual misoprostol | 245,49 | 53/100 | 29/100 | 1.82 (1.07 to 3.08) | 0.03 | 52 |
CI, confidence interval; NA, not applicable
Sublingual misoprostol versus oxytocin
Four trials at moderate/high risk of bias, with a total of 400 women, compared sublingual misoprostol to oxytocin.42,45,49,54 There was a trend towards a lower mean intraoperative blood loss among women that received sublingual misoprostol (MD, 55 ml) although it was not statistically significant (95% CI, −115 to 5; P=0.07; I2=58%). One study showed a significant reduction in the mean postoperative blood loss associated with the use of sublingual misoprostol (MD, −23 ml; 95% CI, −32 to −14; P<0.00001).49 The rates of both shivering and any side effect were higher among women allocated to sublingual misoprostol than among women allocated to oxytocin (49% vs 3%; RR, 18.3; 95% CI, 6.8 to 48.8; I2=20%; NNT for harm 2, 95% CI 1 to 6, and 53% vs 29%; RR, 1.82; 95% CI, 1.07 to 3.08; I2=52%; NNT for harm 4, 95% CI 2 to 49, respectively). No statistically significant differences were found between sublingual misoprostol and oxytocin for other outcomes. One study reported that the mean cost of uterotonic agents was significantly lower in the misoprostol group than in the oxytocin group (2.0 ± 0.8 vs 5.1 ± 0.9 US dollars; MD, −3.1; 95% CI, −3.4 to −2.9; P<0.00001).47 Sensitivity analysis could not be performed because none of the three trials was double masked.
Oral misoprostol versus oxytocin
Two trials with a moderate risk of bias,39,40 including 100 women, compared oral misoprostol with oxytocin. There were no significant differences between oral misoprostol and oxytocin for any of the outcomes evaluated.
Rectal misoprostol versus oxytocin
One trial at low risk of bias (n=200 women),47 compared rectal misoprostol with oxytocin. Women that used rectal misoprostol, compared with those that received oxytocin, had a statistically significant reduction in mean intraoperative and postoperative blood loss (MD, −90 ml; 95% CI, −147 to −32; P=0.002, and MD, −40 ml; 95% CI, −76 to −4; P=0.03, respectively), and blood loss >500 ml (RR, 0.73; 95% CI, 0.54 to 0.99), and a significant increase in the mean postoperative hemoglobin (MD, 0.4 g/dL; 95% CI, 0.1 to 0.8; P=0.02). Rectal misoprostol was associated with a marginally significant increase in the risk of shivering. No significant differences were seen in other outcomes.
Misoprostol plus oxytocin versus oxytocin alone (Table 3)
TABLE 3.
Misoprostol plus oxytocin compared with oxytocin
| Number of events/total number or Total number |
||||||
|---|---|---|---|---|---|---|
| Outcome | No of trials |
Misoprostol plus oxytocin |
Oxytocin | Relative risk or Mean difference (95% CI) |
P value |
I2 (%) |
| Primary outcomes | ||||||
| Mean intraoperative blood loss (ml) | ||||||
| Sublingual misoprostol | 446,48,50,53 | 454 | 456 | −139 (−300 to 21) | 0.09 | 99 |
| Oral misoprostol | 143 | 28 | 25 | −29 (−159 to 101) | 0.66 | NA |
| Buccal misoprostol | 141 | 173 | 179 | 24 (−16 to 64) | 0.24 | NA |
| Rectal misoprostol | 152 | 200 | 200 | −191 (−252 to −130) | <0.00001 | NA |
| Mean postoperative blood loss (ml) | ||||||
| Sublingual misoprostol | 153 | 179 | 187 | −265 (−282 to −248) | <0.00001 | NA |
| Oral misoprostol | 143 | 28 | 25 | 28 (−30 to 86) | 0.34 | NA |
| Rectal misoprostol | 152 | 200 | 200 | −139 (−166 to −112) | <0.00001 | NA |
| Mean fall in hemoglobin (g/dl) | ||||||
| Sublingual misoprostol | 346,48,50 | 275 | 269 | −0.2 (−0.5 to 0.1) | 0.13 | 66 |
| Intrauterine misoprostol | 144 | 100 | 100 | −0.6 (−0.9 to −0.3) | 0.0002 | NA |
| Mean fall in hematocrit (%) | ||||||
| Sublingual misoprostol | 346,48,53 | 364 | 372 | −2.1 (−3.4 to −0.8) | 0.0001 | 91 |
| Buccal misoprostol | 141 | 173 | 179 | −0.2 (−0.5 to 0.1) | 0.11 | NA |
| Rectal misoprostol | 152 | 200 | 200 | −3.5 (−4.2 to −2.9) | <0.00001 | NA |
| Intrauterine misoprostol | 144 | 100 | 100 | −1.8 (−2.8 to −0.7) | 0.001 | NA |
| Use of additional uterotonic agents | ||||||
| Sublingual misoprostol | 348,50,53 | 35/329 | 102/331 | 0.33 (0.18 to 0.62) | 0.0005 | 61 |
| Oral misoprostol | 143 | 0/28 | 0/25 | Not estimable | NA | NA |
| Buccal misoprostol | 141 | 45/173 | 76/179 | 0.61 (0.45 to 0.83) | 0.002 | NA |
| Rectal misoprostol | 152 | 14/200 | 36/200 | 0.39 (0.22 to 0.70) | 0.002 | NA |
| Intrauterine misoprostol | 144 | 3/100 | 6/100 | 0.50 (0.13 to 1.94) | 0.32 | NA |
| Secondary outcomes | ||||||
| Blood loss >500 ml | ||||||
| Sublingual misoprostol | 150 | 73/90 | 77/84 | 0.88 (0.79 to 1.00) | 0.05 | NA |
| Blood loss >1000 ml | ||||||
| Sublingual misoprostol | 346,48,50 | 25/275 | 29/269 | 0.85 (0.52 to 1.39) | 0.53 | 0 |
| Buccal misoprostol | 141 | 24/173 | 22/179 | 1.13(0.66 to 1.94) | 0.66 | NA |
| Blood transfusion | ||||||
| Sublingual misoprostol | 346,50,53 | 3/394 | 17/396 | 0.24 (0.02 to 2.60) | 0.24 | 65 |
| Oral misoprostol | 143 | 0/28 | 0/25 | Not estimable | NA | NA |
| Buccal misoprostol | 141 | 3/173 | 3/179 | 1.03 (0.21 to 5.06) | 0.97 | NA |
| Rectal misoprostol | 152 | 0/200 | 0/200 | Not estimable | NA | NA |
| Intrauterine misoprostol | 144 | 0/100 | 0/100 | Not estimable | NA | NA |
| Postoperative hemoglobin (g/dl) | ||||||
| Sublingual misoprostol | 248,50 | 150 | 144 | 0.1 (−0.4 to 0.6) | 0.68 | 64 |
| Oral misoprostol | 143 | 28 | 25 | −0.5 (−1.3 to 0.3) | 0.20 | NA |
| Intrauterine misoprostol | 144 | 100 | 100 | 0.6 (0.3 to 0.9) | 0.0007 | NA |
| Postoperative hematocrit (%) | ||||||
| Sublingual misoprostol | 248,53 | 239 | 247 | 1.4 (−2.2 to 5.0) | 0.44 | 95 |
| Intrauterine misoprostol | 144 | 100 | 100 | 1.6 (0.6 to 2.6) | 0.002 | NA |
| Shivering | ||||||
| Sublingual misoprostol | 446,48,50,53 | 87/454 | 43/456 | 2.01 (1.50 to 2.70) | <0.00001 | 34 |
| Oral misoprostol | 143 | 10/28 | 2/25 | 4.46 (1.08 to 18.45) | 0.04 | NA |
| Pyrexia | ||||||
| Sublingual misoprostol | 446,48,50,53 | 44/454 | 17/456 | 2.58 (1.50 to 4.45) | 0.0006 | 8 |
| Rectal misoprostol | 152 | 11/200 | 13/200 | 0.85 (0.39 to 1.84) | 0.67 | NA |
| Intrauterine misoprostol | 144 | 8/100 | 4/100 | 2.00 (0.62 to 6.43) | 0.24 | NA |
| Nausea | ||||||
| Sublingual misoprostol | 346,50,53 | 52/394 | 26/396 | 1.90 (0.87 to 4.17) | 0.11 | 64 |
| Oral misoprostol | 143 | 0/28 | 1/25 | 0.30 (0.01 to 7.02) | 0.45 | NA |
| Vomiting | ||||||
| Sublingual misoprostol | 246,50 | 20/215 | 13/209 | 1.51 (0.78 to 2.95) | 0.22 | 0 |
| Intrauterine misoprostol | 144 | 3/100 | 3/100 | 1.00 (0.21 to 4.84) | 1.00 | NA |
| Diarrhea | ||||||
| Sublingual misoprostol | 153 | 2/179 | 0/187 | 5.22 (0.25 to 108.0) | 0.28 | NA |
| Intrauterine misoprostol | 144 | 0/100 | 0/100 | Not estimable | NA | NA |
| Abdominal pain | ||||||
| Sublingual misoprostol | 153 | 24/179 | 13/187 | 1.93 (1.01 to 3.67) | 0.04 | NA |
| Intrauterine misoprostol | 144 | 7/100 | 8/100 | 0.88 (0.33 to 2.32) | 0.79 | NA |
| Headache | ||||||
| Sublingual misoprostol | 146 | 4/125 | 2/125 | 2.00 (0.37 to 10.72) | 0.42 | NA |
| Oral misoprostol | 143 | 0/28 | 1/25 | 0.30 (0.01 to 7.02) | 0.45 | NA |
| Any side effect | ||||||
| Sublingual misoprostol | 146 | 48/125 | 19/125 | 2.53 (1.58 to 4.04) | 0.0001 | NA |
| Apgar score at 1 min | ||||||
| Rectal misoprostol | 152 | 200 | 200 | −0.3 (−0.6 to 0.0) | 0.08 | NA |
| Apgar score at 5 min | ||||||
| Rectal misoprostol | 152 | 200 | 200 | 0.2 (0.1-0.3) | 0.005 | NA |
| NICU admission | ||||||
| Rectal misoprostol | 152 | 6/200 | 9/200 | 0.66 (0.23-1.88) | 0.43 | NA |
CI, confidence interval; NA, not applicable; NICU, neonatal intensive care unit
Sublingual misoprostol plus oxytocin versus oxytocin alone
Four trials, which included 910 women, evaluated this comparison.46,48,50,53 Three of these studies were double masked and had a low risk of bias.48,50,53 The combined use of sublingual misoprostol and oxytocin, compared with the use of oxytocin alone, was associated with a significant reduction in the mean decrease in hematocrit (MD, −2.1%; 95% CI, −3.4 to −0.8; P=0.001; three trials, 736 women; I2=91%) and use of additional uterotonic agents (11% vs 31%; RR, 0.33; 95% CI, 0.18 to 0.62; three trials, 660 women; I2=61%; NNT for benefit 5, 95% CI 4 to 9). In addition, there was a trend towards a decrease in both the mean intraoperative blood loss and the mean decrease in hemoglobin with the use of sublingual misoprostol plus oxytocin. One study reported a significant reduction in mean postoperative blood loss (MD, −265 ml; 95% CI, −282 to −248; P<0.00001).53
The use of sublingual misoprostol combined with oxytocin was associated with a statistically significant increase in the rates of shivering and pyrexia (19% vs 9%; RR, 2.01; 95% CI, 1.50 to 2.70; I2=34%; NNT for harm 10, 95% CI 6 to 21, and 10% vs 4%; RR, 2.58; 95% CI, 1.50 to 4.45; I2=8%; NNT for harm 17, 95% CI 8 to 54, respectively). Moreover, one study reported a significant increase in the rate of abdominal pain,53 and another showed an increase in the rate of any side effect.46 One trial in which sublingual misoprostol was administered before delivery of the baby in women undergoing cesarean delivery under general anesthesia reported that Apgar scores at 1 and 5 min and neonatal cardiovascular status did not differ significantly between the study groups.53 There were no significant differences between the groups in the risk of other outcomes.
After the sensitivity analysis limited to trials with adequate concealment allocation and double masking, the effect of the combined use of sublingual misoprostol and oxytocin on reduction in the use of additional uterotonic agents did not change (RR, 0.33; 95% CI, 0.18 to 0.62) whereas the reduction in the mean decrease in hematocrit turned non-significant (MD, −1.5%; 95% CI, −3.5 to 0.5; P=0.14). However, the reduction in the mean decrease in hemoglobin became statistically significant (MD, −0.1 g/dl; 95% CI, −0.2 to −0.1; P=0.001).
Oral misoprostol plus oxytocin versus oxytocin alone
This comparison included one trial (56 women) at low risk for bias.43 Shivering was significantly more common among women allocated to oral misoprostol plus oxytocin than among women allocated to oxytocin alone (36% vs 8%; RR, 4.46; 95% CI, 1.08 to 18.5). No significant differences were observed between the groups for other outcome measures.
Buccal misoprostol plus oxytocin versus oxytocin alone
One trial (352 women) at low risk for bias41 compared buccal misoprostol plus oxytocin with oxytocin alone. Women in the buccal misoprostol plus oxytocin group were less likely to require an additional uterotonic agent than those in the oxytocin alone group (26% vs 42%; RR, 0.61; 95% CI, 0.45 to 0.83; NNT for benefit 6, 95% CI, 4 to 14). There were no significant differences between the groups in other outcome measures.
Rectal misoprostol plus oxytocin versus oxytocin alone
One study at low risk for bias52 involving 400 women evaluated this comparison. The combined use of rectal misoprostol and oxytocin, compared with the use of oxytocin alone, was associated with a significant reduction in the mean intraoperative and postoperative blood loss (MD, −191 ml; 95% CI, −252 to −130; P<0.00001, and MD, −139 ml; 95% CI, −166 to −112; P<0.00001, respectively), mean decrease in hematocrit (MD, −3.5%; 95% CI, −4.2 to −2.9; P<0.00001), and use of additional uterotonic agents (7% vs 18%; RR, 0.39; 95% CI, 0.22-0.70; NNT for benefit 9, 95% CI 7 to 19), and a significant increase in mean Apgar score at 5 min (MD, 0.2; 95% CI, 0.1 to 0.3; P=0.005). There were no differences between rectal misoprostol plus oxytocin and oxytocin alone groups for other outcomes, including mean Apgar score at 1 min and admission to the neonatal intensive care unit.
Intrauterine misoprostol plus oxytocin versus oxytocin alone
One trial (200 women) at low risk of bias44 compared intrauterine misoprostol plus oxytocin with oxytocin alone. The use of intrauterine misoprostol combined with oxytocin was associated with a significant reduction in the mean decrease in hemoglobin and hematocrit (MD, −0.6 g/dl, 95% CI, −0.9 to −0.3; P=0.0002, and MD, −1.8%; 95% CI, −2.8 to −0.7; P=0.001, respectively), and higher levels of mean postoperative hemoglobin and hematocrit (MD, 0.6 g/dl; 95% CI, 0.3 to 0.9; P=0.0007, and MD, 1.6%; 95% CI, 0.6 to 2.6; P=0.002, respectively). The rates of use of additional uterotonic agents, blood transfusion, and adverse maternal effects did not differ significantly between the groups.
Other comparisons
A three-arm trial at high risk of bias,38 involving 182 women, compared oral misoprostol versus intramyometrial oxytocin versus oral misoprostol plus intramyometrial oxytocin. Women in the oral misoprostol alone and oral misoprostol plus intramyometrial oxytocin groups had a lower mean postoperative blood loss than those in the intramyometrial oxytocin alone group (MD, −133 ml; 95% CI, −155 to −111; P<0.00001, and MD, −137 ml; 95% CI, −158 to −116; P<0.00001, respectively). There were no significant differences between oral misoprostol alone and oral misoprostol plus intramyometrial oxytocin groups in mean postoperative blood loss. Another trial at high risk of bias (374 women)51 compared rectal misoprostol with intravenous ergometrine. Women that received rectal misoprostol had a significantly lower rate of both intraoperative blood loss >500 ml and postoperative hemoglobin levels <9 g/dl than those that received intravenous ergometrine (7% vs 13%; RR, 0.52; 95% CI, 0.27 to 0.98, for both outcomes). No other outcome measures were reported in this study.
COMMENT
Principal findings
Several systematic reviews have been published recently on the use of misoprostol for prevention of postpartum hemorrhage after vaginal delivery.55-57 This is the first study that has systematically evaluated the use of misoprostol for reducing intraoperative and postoperative blood loss in women undergoing cesarean delivery. The pooled evidence in our systematic review shows that, overall, the combined use of misoprostol and oxytocin appears to be more effective than oxytocin alone in reducing intraoperative and postoperative hemorrhage at cesarean delivery. The evidence was strongest for the subgroup of trials that used sublingual and rectal misoprostol. The reduction in intraoperative and postoperative hemorrhage associated with the combined use of misoprostol and oxytocin could be explained by the initial rapid effect of oxytocin followed by the sustained effect of misoprostol on uterine contractility. In fact, after a single intravenous injection, oxytocin appears in the circulation within 15 seconds, reaches maximum concentrations in 60 seconds and has a short half-life (4-10 minutes).58 In contrast, the peak concentration after sublingual and rectal administration of misoprostol is achieved at about 30 and 40-65 minutes, respectively, with a duration of action of about 3 and 4 hours, respectively.59
In addition, we found no statistically significant differences between misoprostol and oxytocin in reducing intraoperative and postoperative hemorrhage at cesarean delivery. Nevertheless, it is noteworthy that this finding is based on a few small trials with methodological limitations, mainly lack of double masking. Trials comparing misoprostol with oxytocin should be considered “equivalence or non-inferiority trials” designed to evaluate whether misoprostol is not superior but equivalent to or not inferior to oxytocin.60 None of the trials that compared misoprostol with oxytocin were planned or had the statistical power to evaluate equivalence or non-inferiority between the two agents. Therefore, the lack of statistical significance between misoprostol and oxytocin found in our review does not imply that these drugs have the same efficacy in reducing perioperative hemorrhage at cesarean delivery. Overall, the rates of side effects, mainly shivering and pyrexia, were higher among women that received misoprostol alone or combined with oxytocin than among women that received oxytocin alone. The increased risk of side effects was more apparent in trials that used sublingual misoprostol than in trials that used other routes of misoprostol administration. There were no significant differences in Apgar score at 1 and 5 minutes and admission to the neonatal intensive care unit between misoprostol and oxytocin groups, although these outcomes were reported in only two of the five trials that used misoprostol before delivery of the neonate.
Strengths and limitations of the study
The reliability and robustness of our results are supported by the use of the most rigorous methodology for performing a systematic review and meta-analysis of randomized controlled trials, the comprehensive literature search without language restrictions and including the grey literature and conference proceedings which identified studies published in English, Spanish, French, Chinese, and Thai, the inclusion of a relatively large number of studies in the systematic review most of which were published in the last years, the strict assessment of methodological quality of included trials, the quantitative summary of the evidence, the performance of subgroup analyses according to route of administration of misoprostol and combined use of oxytocin, and the sensitivity analysis restricted to trials at low risk of bias.
Some limitations of this study should be acknowledged. First, there was substantial statistical heterogeneity in several of the meta-analyses performed; therefore, our findings should be interpreted in this context. Nevertheless, in most of the comparisons, the estimates showed the same direction of effect, which could suggest the absence of clinical heterogeneity among the studies. We used random effects models to pool data across studies to attempt to incorporate any heterogeneity and explored possible sources of heterogeneity such as study quality. In addition, we stratified analyses by route of misoprostol administration and the combined use of oxytocin. Despite this, we could not explain most of the heterogeneity, which might be due to differences in study population, doses of misoprostol and oxytocin, cesarean delivery technique, surgical experience, method of assessment of blood loss, or trial implementation. Second, some subgroup analyses were based on small numbers of patients. As a result, our analysis was limited in its power to estimate effects within these subgroups and to detect differences, if any exist, among women in predetermined subgroups. Third, several studies did not report results of important primary outcome measures included in our review, such as postoperative blood loss and decrease in hematocrit in trials comparing misoprostol and oxytocin, and postoperative blood loss and decrease in hemoglobin in trials comparing misoprostol plus oxytocin and oxytocin alone. Thus, our meta-analysis may be underpowered for such outcomes. It is possible that, if these results were reported more consistently, effect sizes might be different. Finally, like any systematic review, ours is limited by the quality of original data. Only about half of the trials included in the meta-analyses were considered to be at low risk of bias. Nevertheless, sensitivity analyses restricted to high quality trials comparing misoprostol plus oxytocin and oxytocin alone upheld most results obtained with the overall meta-analyses.
Thus far, no systematic review on the prophylactic use of misoprostol at cesarean delivery for reducing intraoperative and postoperative hemorrhage has been published. A recent Cochrane review assessed the effectiveness of prophylactic prostaglandin use as part of the routine management of the third stage of labor for the prevention of postpartum hemorrhage.31 A total of 57 trials that evaluated misoprostol were included in this review. Only seven trials included in our review were also included in the Cochrane review.40-46 Data provided by these studies were pooled with data from studies that evaluated misoprostol in women after vaginal delivery. This review found that oral or sublingual misoprostol, compared with placebo, reduced significantly the risk of severe postpartum hemorrhage and blood transfusion. Nevertheless, when compared with oxytocin, oral misoprostol was associated with a significant increase in the risk of severe postpartum hemorrhage and use of additional uterotonic agents, but with a trend toward fewer blood transfusions. Overall, misoprostol was associated with an increased risk of shivering and pyrexia compared with both placebo and oxytocin. No results were reported for women receiving misoprostol at cesarean delivery.
Carbetocin, a synthetic analogue of human oxytocin with structural modifications that increase its half-life (thereby prolonging its pharmacological effects), has been proposed for the prevention of postpartum hemorrhage following cesarean delivery. A recent Cochrane review reported that carbetocin decreased significantly the use of additional uterotonic agents in women undergoing cesarean delivery when compared to oxytocin (RR, 0.64; 95% CI, 0.51 to 0.81; four trials, 1173 women).61 There were no differences between carbetocin and oxytocin in postpartum hemorrhage >500 mL and >1000 ml, intraoperative blood loss, blood transfusion, decrease in hemoglobin, and adverse effects. Our systematic review and meta-analysis showed that the combined use of sublingual misoprostol and oxytocin was superior to oxytocin alone, because the combination significantly reduced the need for additional uterotonic agents by 67% (RR, 0.33; 95% CI, 0.18 to 0.62). Moreover, the use of combined sublingual misoprostol and oxytocin was associated with a significant reduction in the mean decrease in hematocrit and a trend towards a decrease in both the mean intraoperative blood loss and the mean decrease in hemoglobin. It should be noted that the Society of Obstetricians and Gynecologists of Canada recommends the use of carbetocin instead of oxytocin in elective cesarean delivery for the prevention of postpartum hemorrhage and to decrease the need for additional uterotonic agents.62
Implications for practice
Currently, there is insufficient evidence to establish equivalence between misoprostol and oxytocin in the control of intraoperative and postoperative hemorrhage during cesarean delivery. Moreover, misoprostol is associated with increased rates of side effects. Therefore, misoprostol by itself cannot be recommended to replace oxytocin as first-line prophylactic uterotonic agent for the control of blood loss at cesarean delivery. If future equivalence or non-inferiority trials show that misoprostol is as efficacious as oxytocin in the reduction of perioperative hemorrhage at cesarean delivery, then misoprostol could be considered as an option in settings in which use of oxytocin is not feasible.
The results of our review show that misoprostol as an adjunct to oxytocin is more effective than oxytocin alone for reducing intraoperative and postoperative blood loss during cesarean delivery. Hence, the use of misoprostol, possibly 400 μg sublingually after cord clamping, in addition to oxytocin, should be considered in women undergoing elective or emergency cesarean delivery under regional or general anesthesia. This prophylactic strategy could be especially useful in women undergoing emergency cesarean delivery or under general anesthesia, which are well-known risk factors for postpartum hemorrhage associated with this surgical procedure.17,22,23,63 Some trials have reported that misoprostol has the additional effect of increasing intestinal motility after cesarean delivery and allowing early oral feeding, which could reduce the risk of postoperative ileus and shortened hospital stay length, and allow early breastfeeding.64-66 This effect has been reported in trials that administered misoprostol rectally after surgery, but it is unclear if similar effects can be obtained if the drug is administered sublingually.
Finally, it should be noted that although 13 of 17 studies were conducted indeveloping countries, the results of this systematic review and meta-analysis are very likely applicable to industrialized ones. In fact, the median "mean intraoperative blood loss" and “mean duration of surgery” in the control group of the 4 studies conducted in developed countries was very similar to that in the control group of the 13 studies conducted in developing countries (650 versus 651 ml, and 42 versus 43 min, respectively). Moreover, the cesarean section technique used was very similar in all 17 studies included in the review.
Implications for research
Equivalence or non-inferiority randomized controlled trials are needed to compare the efficacy of misoprostol and oxytocin in reducing perioperative hemorrhage at cesarean delivery. Such trials should have sufficient statistical power to establish equivalence or non-inferiority between the two drugs, be double-masked to minimize the risk of bias in the assessment of the outcomes, and measure intraoperative and postoperative blood loss as objectively and accurately as possible.
Further research on the combined use of misoprostol and oxytocin could focus on determining the best route of administration and the optimal dose of misoprostol for reducing perioperative hemorrhage at cesarean delivery, the cost-effectiveness of this intervention, and the short- and long-term consequences of infants exposed to misoprostol in utero. Moreover, trials comparing misoprostol plus oxytocin versus carbetocin would be justified.
Condensation.
The combined use of misoprostol and oxytocin appears to be more effective than oxytocin alone in reducing intraoperative and postoperative hemorrhage during cesarean delivery.
Acknowledgments
Financial support: This research was supported, in part, by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: the authors report no conflicts of interest.
REFERENCES
- 1.Gibbons L, Belizan JM, Lauer JA, Betran AP, Merialdi M, Althabe F. Inequities in the use of cesarean section deliveries in the world. Am J Obstet Gynecol. 2012;206:331, e1–19. doi: 10.1016/j.ajog.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 2.Ananth CV, Vintzileos AM. Trends in cesarean delivery at preterm gestation and association with perinatal mortality. Am J Obstet Gynecol. 2011;204:505, e1–8. doi: 10.1016/j.ajog.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Troendle J, Reddy UM, Laughon SK, Branch DW, Burkman R, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203:326.e1–326.e10. doi: 10.1016/j.ajog.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klemetti R, Che X, Gao Y, Raven J, Wu Z, Tang S, Hemminki E. Cesarean section delivery among primiparous women in rural China: an emerging epidemic. Am J Obstet Gynecol. 2010;202:65, e1–6. doi: 10.1016/j.ajog.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 5.Getahun D, Strickland D, Lawrence JM, Fassett MJ, Koebnick C, Jacobsen SJ. Racial and ethnic disparities in the trends in primary cesarean delivery based on indications. Am J Obstet Gynecol. 2009;201:422, e1–7. doi: 10.1016/j.ajog.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 6.Brennan DJ, Robson MS, Murphy M, O’Herlihy C. Comparative analysis of international cesarean delivery rates using 10-group classification identifies significant variation in spontaneous labor. Am J Obstet Gynecol. 2009;201:308, e1–8. doi: 10.1016/j.ajog.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Tang S, Li X, Wu Z. Rising cesarean delivery rate in primiparous women in urban China: evidence from three nationwide household health surveys. Am J Obstet Gynecol. 2006;195:1527–32. doi: 10.1016/j.ajog.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 8.Bailit JL, Love TE, Mercer B. Rising cesarean rates: are patients sicker? Am J Obstet Gynecol. 2004;191:800–3. doi: 10.1016/j.ajog.2004.01.051. [DOI] [PubMed] [Google Scholar]
- 9.Betrán AP, Merialdi M, Lauer JA, Bing-Shun W, Thomas J, Van Look P, et al. Rates of caesarean section: analysis of global, regional and national estimates. Paediatr Perinat Epidemiol. 2007;21:98–113. doi: 10.1111/j.1365-3016.2007.00786.x. [DOI] [PubMed] [Google Scholar]
- 10.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 11.Joseph KS, Rouleau J, Kramer MS, Young DC, Liston RM, Baskett TF. Investigation of an increase in postpartum hemorrhage in Canada. BJOG. 2007;114:751–9. doi: 10.1111/j.1471-0528.2007.01316.x. [DOI] [PubMed] [Google Scholar]
- 12.Ford JB, Roberts CL, Simpson JM, Vaughan J, Cameron CA. Increased postpartum hemorrhage rates in Australia. Int J Gynaecol Obstet. 2007;98:237–43. doi: 10.1016/j.ijgo.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994-2006. Am J Obstet Gynecol. 2010;202:353, e1–6. doi: 10.1016/j.ajog.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110:1368–73. doi: 10.1213/ANE.0b013e3181d74898. [DOI] [PubMed] [Google Scholar]
- 15.American College of Obstetricians and Gynecologists ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 76. Postpartum hemorrhage. Obstet Gynecol. 2006;108:1039–47. doi: 10.1097/00006250-200610000-00046. [DOI] [PubMed] [Google Scholar]
- 16.Pritchard JA, Baldwin RM, Dickey JC, Wiggins KM, Reed GP, Bruce DM. Blood volume changes in pregnancy and the puerperium. II. Red blood cell loss and changes in apparent blood volume during and following vaginal delivery, cesarean section, and cesarean plus total hysterectomy. Am J Obstet Gynecol. 1962;84:1271–82. [Google Scholar]
- 17.Magann EF, Evans S, Hutchinson M, Collins R, Lanneau G, Morrison JC. Postpartum hemorrhage after cesarean delivery: an analysis of risk factors. South Med J. 2005;98:681–5. doi: 10.1097/01.SMJ.0000163309.53317.B8. [DOI] [PubMed] [Google Scholar]
- 18.Al-Zirqi I, Vangen S, Forsén L, Stray-Pedersen B. Effects of onset of labor and mode of delivery on severe postpartum hemorrhage. Am J Obstet Gynecol. 2009 Sep;201(3):273, e1–9. doi: 10.1016/j.ajog.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum hemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol. 2008;22:999–1012. doi: 10.1016/j.bpobgyn.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Kolås T, Øian P, Skjeldestad FE. Risks for peroperative excessive blood loss in cesarean delivery. Acta Obstet Gynecol Scand. 2010;89:658–63. doi: 10.3109/00016341003605727. [DOI] [PubMed] [Google Scholar]
- 21.Kramer MS, Dahhou M, Vallerand D, Liston R, Joseph KS. Risk factors for postpartum hemorrhage: can we explain the recent temporal increase? J Obstet Gynaecol Can. 2011;33:810–9. doi: 10.1016/S1701-2163(16)34984-2. [DOI] [PubMed] [Google Scholar]
- 22.Chang CC, Wang IT, Chen YH, Lin HC. Anesthetic management as a risk factor for postpartum hemorrhage after cesarean deliveries. Am J Obstet Gynecol. 2011;205:462, e17. doi: 10.1016/j.ajog.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 23.Skjeldestad FE, Oian P. Blood loss after cesarean delivery: a registry-based study in Norway, 1999-2008. Am J Obstet Gynecol. 2012;206:76, e1–7. doi: 10.1016/j.ajog.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 24.Larsson C, Saltvedt S, Wiklund I, Pahlen S, Andolf E. Estimation of blood loss after cesarean section and vaginal delivery has low validity with a tendency to exaggeration. Acta Obstet Gynecol Scand. 2006;85:1448–52. doi: 10.1080/00016340600985032. [DOI] [PubMed] [Google Scholar]
- 25.Stafford I, Dildy GA, Clark SL, Belfort MA. Visually estimated and calculated blood loss in vaginal and cesarean delivery. Am J Obstet Gynecol. 2008;199:519, e1–7. doi: 10.1016/j.ajog.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 26.Toledo P, McCarthy RJ, Burke CA, Goetz K, Wong CA, Grobman WA. The effect of live and web-based education on the accuracy of blood-loss estimation in simulated obstetric scenarios. Am J Obstet Gynecol. 2010;202:400, e1–5. doi: 10.1016/j.ajog.2009.10.881. [DOI] [PubMed] [Google Scholar]
- 27.Begley CM, Gyte GM, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labor. Cochrane Database Syst Rev. 2011;11:CD007412. doi: 10.1002/14651858.CD007412.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Collaborating Centre for Women’s and Children’s Health . NICE clinical guideline. RCOG Press; London: 2011. Caesarean section. [Google Scholar]
- 29.Bonanno C, Gaddipati S. Mechanisms of hemostasis at cesarean delivery. Clin Perinatol. 2008;35:531–47. doi: 10.1016/j.clp.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Dyer RA, Butwick AJ, Carvalho B. Oxytocin for labor and caesarean delivery: implications for the anaesthesiologist. Curr Opin Anaesthesiol. 2011;24:255–61. doi: 10.1097/ACO.0b013e328345331c. [DOI] [PubMed] [Google Scholar]
- 31.Tunçalp Ö , Hofmeyr GJ, Gülmezoglu AM. Prostaglandins for preventing postpartum hemorrhage. Cochrane Database Syst Rev. 2012;8:CD000494. doi: 10.1002/14651858.CD000494.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 The Cochrane Collaboration; [updated March 2011]. 2011. [Google Scholar]
- 34.Deeks JJ, Higgins JPT, Altman DG, Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 The Cochrane Collaboration; [updated March 2011]. 2011. Chapter 9: Analysing data and undertaking meta-analyses. [Google Scholar]
- 35.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–12. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analyses detected by a simple graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Li X, Peng Y. Clinical study on reduction of postpartum bleeding in cesarean section by misoprostol [in Chinese] Zhonghua Fu Chan Ke Za Zhi. 1998;33:403–5. [PubMed] [Google Scholar]
- 39.Acharya G, Al-Sammarai MT, Patel N, Al-Habib A, Kiserud T. A randomized, controlled trial comparing effect of oral misoprostol and intravenous syntoc inon on intra-operative blood loss during cesarean section. Acta Obstet Gynecol Scand. 2001;80:245–50. doi: 10.1034/j.1600-0412.2001.080003245.x. [DOI] [PubMed] [Google Scholar]
- 40.Lokugamage AU, Paine M, Bassaw-Balroop K, Sullivan KR, Refaey HE, Rodeck CH. Active management of the third stage at caesarean section: a randomized controlled trial of misoprostol versus syntocinon. Aust N Z J Obstet Gynaecol. 2001 Nov;41(4):411–4. doi: 10.1111/j.1479-828x.2001.tb01319.x. [DOI] [PubMed] [Google Scholar]
- 41.Hamm J, Russell Z, Botha T, Carlan SJ, Richichi K. Buccal misoprostol to prevent hemorrhage at cesarean delivery: a randomized study. Am J Obstet Gynecol. 2005;192:1404–6. doi: 10.1016/j.ajog.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 42.Vimala N, Mittal S, Kumar S. Sublingual misoprostol versus oxytocin infusion to reduce blood loss at cesareansection. Int J Gynaecol Obstet. 2006;92:106–10. doi: 10.1016/j.ijgo.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Lapaire O, Schneider MC, Stotz M, Surbek DV, Holzgreve W, Hoesli IM. Oral misoprostol vs. intravenous oxytocin in reducing blood loss after emergency cesarean delivery. Int J Gynaecol Obstet. 2006;95:2–7. doi: 10.1016/j.ijgo.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 44.Quiroga Díaz R, Cantú Mata R, Tello Gutiérrez HE, Puente Villalobos M, Montemayor Garza R, Martínez Mendoza A. Intrauterine misoprostol for the prevention of bleeding cesarean [in Spanish] Ginecol Obstet Mex. 2009;77:469–74. [PubMed] [Google Scholar]
- 45.Eftekhari N, Doroodian M, Lashkarizadeh R. The effect of sublingual misoprostol versus intravenous oxytocin in reducing bleeding after caesarean section. J Obstet Gynaecol. 2009;29:633–6. doi: 10.1080/01443610903061744. [DOI] [PubMed] [Google Scholar]
- 46.Fekih M, Jnifene A, Fathallah K, Ben Regaya L, Memmi A, Bouguizene S, et al. Benefit of misoprostol for prevention of postpartum hemorrhage in cesarean section: a randomized controlled trial [in French] J Gynecol Obstet Biol Reprod (Paris) 2009;38:588–93. doi: 10.1016/j.jgyn.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Chaudhuri P, Banerjee GB, Mandal A. Rectally administered misoprostol versus intravenous oxytocin infusion during cesarean delivery to reduce intraoperative and postoperative blood loss. Int J Gynaecol Obstet. 2010;109:25–9. doi: 10.1016/j.ijgo.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Chalermpolprapa MD. Efficacy of sublingual misoprostol in prevention of postpartum hemorrhage in cesarean section: a randomized double-blinded, placebo-controlled trial [in Thai] Reg 4-5 Med J. 2010;29:325–35. [Google Scholar]
- 49.Owonikoko KM, Arowojolu AO, Okunlola MA. Effect of sublingual misoprostol versus intravenous oxytocin on reducing blood loss at cesarean section in Nigeria: a randomized controlled trial. J Obstet Gynaecol Res. 2011;37:715–21. doi: 10.1111/j.1447-0756.2010.01399.x. [DOI] [PubMed] [Google Scholar]
- 50.Sood AK, Singh S. Sublingual misoprostol to reduce blood loss at cesarean delivery. J Obstet Gynecol India. 2012;62:162–7. doi: 10.1007/s13224-012-0168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ali R, Hina F. Postpartum hemorrhage: comparison of efficacy of ergometrine with misoprostol in prophylaxis in cesarean section. Prof Med J. 2012;19:86–90. [Google Scholar]
- 52.Elsedeek MS. Impact of preoperative rectal misoprostol on blood loss during and after elective cesarean delivery. Int J Gynaecol Obstet. 2012;118:149–52. doi: 10.1016/j.ijgo.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 53.El Tahan MR, Warda OM, Rashad A, Yasseen AM, Ramzy EA, Ahmady MS, et al. Effects of Preoperative Sublingual Misoprostol on Uterine Tone during Isoflurane Anesthesia for Cesarean Section. Rev Bras Anestesiol. 2012;62:625–35. doi: 10.1016/S0034-7094(12)70162-9. [DOI] [PubMed] [Google Scholar]
- 54.Gavilanes Sáenz VP, Morales Carrasco MF, Velasco Jácome SM. Postgraduate Thesis. Universidad Central del Ecuador, Quito, Ecuador; 2012. Comparison of the efficacy between misoprostol and oxytocin in the prevention of hemorrhage during cesarean section in pregnant women at ≥34 weeks’ gestation in the Gynecological and Obstetrical hospital “Isidro Ayora”, 2011-2012 [in Spanish] Retrieved from http://www.dspace.uce.edu.ec/bitstream/25000/634/1/T-UCE-0006-18.pdf. [Google Scholar]
- 55.Chu CS, Brhlikova P, Pollock AM. Rethinking WHO guidance: review of evidence for misoprostol use in the prevention of postpartum hemorrhage. R Soc Med. 2012;105:336–47. doi: 10.1258/jrsm.2012.120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oladapo OT, Fawole B, Blum J, Abalos E. Advance misoprostol distribution for preventing and treating postpartum hemorrhage. Cochrane Database Syst Rev. 2012;2:CD009336. doi: 10.1002/14651858.CD009336.pub2. [DOI] [PubMed] [Google Scholar]
- 57.Chelmow D. Postpartum hemorrhage: prevention. Clin Evid (Online) 2011;2011 [PMC free article] [PubMed] [Google Scholar]
- 58.Gibbens D, Boyd NR, Crocker S, Baumber S, Chard T. The circulating levels of oxytocin following intravenous and intramuscular administration of syntometrine. J Obstet Gynaecol Br Commonw. 1972;79:644–6. doi: 10.1111/j.1471-0528.1972.tb14215.x. [DOI] [PubMed] [Google Scholar]
- 59.Tang OS, Gemzell-Danielsson K, Ho PC. Misoprostol: pharmacokinetic profiles, effects on the uterus and side-effects. Int J Gynaecol Obstet. 2007;99(Suppl 2):S160–7. doi: 10.1016/j.ijgo.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Treadwell J, Uhl S, Tipton K, Singh S, Santaguida L, Sun X, Berkman N, Viswanathan M, Coleman C, Shamliyan T, Wang S, Ramakrishnan R, Elshaug A. Assessing equivalence and noninferiority. Agency for Healthcare Research and Quality; Rockville, MD: 2012. AHRQ Publication No. 12-EHC045-EF. [PubMed] [Google Scholar]
- 61.Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum hemorrhage. Cochrane Database Syst Rev. 2012;4:CD005457. doi: 10.1002/14651858.CD005457.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leduc D, Senikas V, Lalonde AB, Ballerman C, Biringer A, Delaney M, et al. Active management of the third stage of labor: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can. 2009;31:980–93. doi: 10.1016/S1701-2163(16)34329-8. [DOI] [PubMed] [Google Scholar]
- 63.Häger RM, Daltveit AK, Hofoss D, Nilsen ST, Kolaas T, Øian P, et al. Complications of cesarean deliveries: rates and risk factors. Am J Obstet Gynecol. 2004;190:428–34. doi: 10.1016/j.ajog.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 64.Hu BM, Liu X, Chen ZY. Effect of misoprostol on gastrointestinal peristalsis after cesarean section [in Chinese] Chin J Prim Med Pharm. 2008;15:1297–8. [Google Scholar]
- 65.Çayan F, Doruk A, Sungur MA, Dilek S. Comparison of the different dosages of rectal misoprostol on intestinal motility and pain score in high risk cesarean delivery. Turkiye Klinikleri J Med Sci. 2010;30:1154–9. [Google Scholar]
- 66.Adanikin AI, Orji EO, Fasubaa OB, Onwudiegwu U, Ijarotimi OA, Olaniyan O. The effect of post-cesarean rectal misoprostol on intestinal motility. Int J Gynaecol Obstet. 2012;119:159–62. doi: 10.1016/j.ijgo.2012.05.033. [DOI] [PubMed] [Google Scholar]