Abstract
The highly-effective and selective isoxazoline insecticide A1443 is known to potently displace [3H]ethynylbicycloorthobenzoate ([3H]EBOB) binding to house fly head membranes with an IC50 of 0.2 nM in a manner characteristic of GABA-gated chloride channel antagonists. To further define its mode of action, we prepared phenyl-labeled [3H]A1443 as described with a specific activity of 14 Ci/mmol. This new radioligand with an apparent IC50 of about 0.4 nM is poorly displaced by most insecticides acting at the [3H]EBOB site. Interestingly, the isoxazoline binding site is directly coupled to the avermectin GABA/glutamate chloride channels activator site. These findings revive interest in the insect GABA/glutamate receptor as an insecticide target.
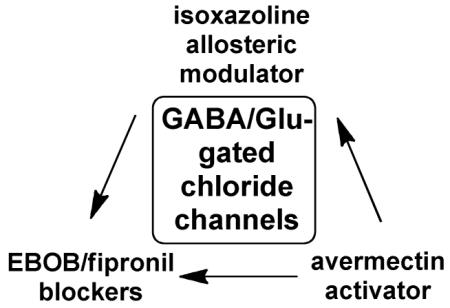
There is an urgent need for novel insecticides acting in new ways to avoid target site resistance, the principal cause for loss of pesticide effectiveness.1 Major current insecticides act as antagonists of the GABA-gated chloride channel (fipronil and endosulfan) or activators of chloride flux (avermectin (AVE)).2 Some of the newest candidate insecticides are the isoxazolines which are of great current interest as a new chemotype3-12 possibly acting at a novel target in the chloride channel more sensitive in insects than mammals.9,13 These reported advantages are based on electrophysiology studies with cloned and expressed receptors and a single binding assay with [3H]ethynylbicycloorthobenzoate ([3H]EBOB) for noncompetitive antagonists.13,14 It is important to more thoroughly consider these deductions by direct target site binding assays with the isoxazoline itself.
The present goal was to radiolabel the most potent of the currently reported isoxazoline insecticides. Synthesis of a triazole isoxazoline candidate radioligand was previously reported (Figure 1)7 without biological results to establish its utility. Isoxazoline A1443 was the compound of choice for the current study primarily because of its potency and potential action at a novel target.13,15 A1443 was prepared following a modification of the patented procedure8,12 (Figure 2). Cleavage of the N-(t-butylcarbamoyl) (N-Boc) protecting group of dipeptide 1 using 4 N HCl in dioxane (rt, 30 min), followed by condensation of the resulting amine salt with benzoic acid 2 (R=H) under standard peptide coupling techniques (EDCI, DMAP, rt, 16 h), or by its prior conversion to an acyl chloride (SOCl2, catalytic N,N-dimethylformamide, 80 °C, 1.5 h), smoothly produced A1443. The spectral data of this material was consistent with that reported in the literature.
Figure 1.
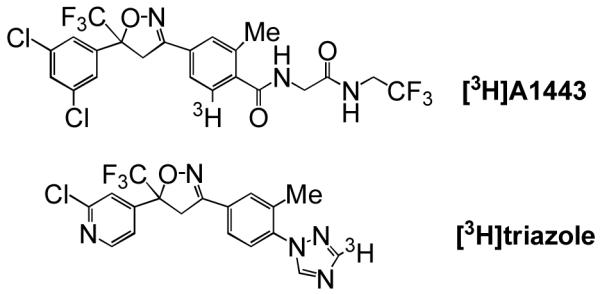
Two isoxazoline insecticide candidate radioligands. [3H]A1443 synthesis and binding are first reported here. [3H]Triazole synthesis was reported by Rauh et al.7 without biological validation.
Figure 2.
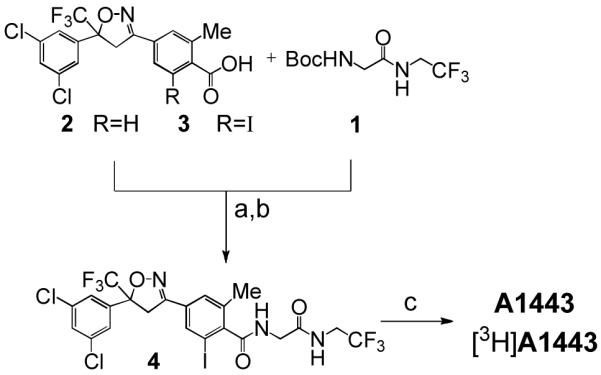
Synthesis of A1443 and [3H]A1443. (a) 4 N HCl/dioxane. (b) N,N-diisopropylethylamine (DIEA), 1-(3-dimethylaminopropyl) -3-ethylcarbodiimide (EDCI), 4-dimethylaminopyridine (DMAP). (c) 10% Pd/C, H2 or 3H2, EtOH.
Direct radioactive labeling of A1443 was investigated using organoiridium catalysis. 16,17 However, with deuterium gas (D2) as a model, no significant amounts of labeled compound were observed under the standard conditions employed ([Ir(COD)(Py)PCy3]PF6 or [(COD)IrCl]2/PPh3, or [(COD)IrCl]2/diphenylphosphinoethane, CH2Cl2, D2), returning mostly starting material. Additionally, direct bromination of A1443 was attempted using a variety of conditions, from which Pd(OAc)2/N-bromosuccinimide (NBS) appeared as a viable reagent combination. 18 Unfortunately, this approach suffered from low and irreproducible yields.
Consequently, the synthesis of [3H]A1443 was ultimately achieved as shown in Figure 2. In this manner, halogenation of benzoic acid 2 (R=H) was investigated. Initial conditions employing Pd(OAc)2 and NBS in AcOH at 100 °C produced the desired bromobenzoic acid, albeit in low yields and as a mixture of isomeric products. A more consistent process for halogenation of benzoic acid 2 (R=H), consisted of its conversion to the iodobenzoic acid (3, R=I), following a reported procedure [Pd(OAc)2, I2, PhI(OAc)2, N,N-dimethylformamide, 100 °C, 1 h]6 as a single regioisomer. Preparation of [3H]A1443 then proceeded via coupling of the amine salt resulting from N-Boc cleavage of dipeptide 1, followed by carbodiimide-mediated condensation with iodocarboxylic acid 3. To our delight, the resulting peptide, iodo-A1443 (4), underwent clean reductive deiodination with 10% Pd/C and H2 in EtOH at rt for 2 h to give the desired product, A1443. A similar procedure employing 3H2 yielded [3H]A1443 (14 Ci/mmol by mass spectrometry) (>99% radiochemical purity based on HPLC) (see the Supporting Information). [3H]A1443 was stored at 1 mCi/mL in EtOH at −20 °C.
In the binding site investigations house flies were the insect of choice because of the extensive data on their GABA receptor and sensitivity to a variety of GABAergic agents.13,14,19-23 The radioligand binding studies here used head membranes14 rather than expressed receptors of a particular type13 to better represent the native form. Specific binding was considered to be the difference between the disintegrations per minute (dpm) total labeling with 0.1 nM [3H]A1443 alone and nonspecific labeling with 0.1 nM [3H]A1443 plus 30 nM unlabeled A1443, i.e., about 75X the specific binding IC50 value. The total binding with 120 μg protein24 was typically 1,200 dpm and the specific binding 200-400 dpm or 17-33%. The specific binding dpm was increased at higher radioligand levels but the elevated nonspecific binding dpm interfered with the assay precision. Although this was a relatively low level of specific binding, it was achieved by three optimized procedural steps involving the use of membranes treated with bovine serum albumin (BSA)25 and 3-[(3-cholamidopropyldimethylammonio]-1-propanesulfonate (CHAPS)26 for the binding protein and buffer containing 2% (v/v) ethanol27 for washing the membrane after filtration.
Standard conditions for the [3H]A1443/house fly head membrane binding site assay were as follows. Heads (1 g) collected14 from house fly adults (Benzon Research Inc., Carlisle, PA)25 were homogenized in 10 mL of ice-cold Buffer A (250 mM sucrose, 10 mM Tris-HCl, pH 7.5) containing 0.8% (w/v) BSA then the homogenate was filtered through four layers of nylon mesh filter screen (64 μ). After centrifugation at 1,000g for 30 min, the supernatant was collected and centrifuged at 25,000g for 1 h. The pellet was resuspended at 5 mg protein/mL in solubilization Buffer B [0.8% (w/v) BSA, 2 mg CHAPS /mg protein, 303 mM sucrose, 10 μM phenylmethanesulfonyl fluoride and 20 mM Tris-maleate, pH 7.0), 26 shaken overnight at 4 °C and centrifuged at 25,000 g for 1 h. The final membrane pellet was resuspended in Buffer C (300 mM NaCl and 10 mM phosphate-buffered pH 7.5 saline) and used for the binding assay after protein determination.
The binding assay consisted of sequential addition to incubation tubes (13 × 100 mm) of candidate inhibitor (0, 0.1, 0.3, 1, 3, 10, 30, 100, 300, 1,000 nM final concentration) in dimethylsulfoxide (5 μL), [3H]A1443 (final concentration 0.1 nM unless indicated otherwise) in ice-cold Buffer C (10 μL) and finally the membrane preparation (120 μg protein in 985 μL Buffer C). After incubation for 70 min at 22 °C, the samples were filtered followed by two 5-mL rinse washes with ice-cold Buffer C containing 2% (v/v) ethanol. Radioactivity on the filters was counted as before. 25 Each experiment was repeated 3-5 times and the results reported are the means with standard errors.
Several features of the insect isoxazoline specific binding site are evident from the preliminary studies reported here. One or more sites are highly sensitive to A1443 with an overall IC50 of about 0.4 nM possibly consisting of Kd sub-nM and low-nM components (Figure 3). The noncompetitive antagonist fipronil is less potent by several orders of magnitude (Figure 3). Other insecticidal noncompetitive antagonists also have IC50 values much greater than 1,000 nM including α-endosulfan, 12-ketoendrin, heptachlor epoxide, lindane and 3,3-bis-trifluoromethyl-bicyclo[2.2.1]heptane-2,2-dicarbonitrile (BIDN).
Figure 3.
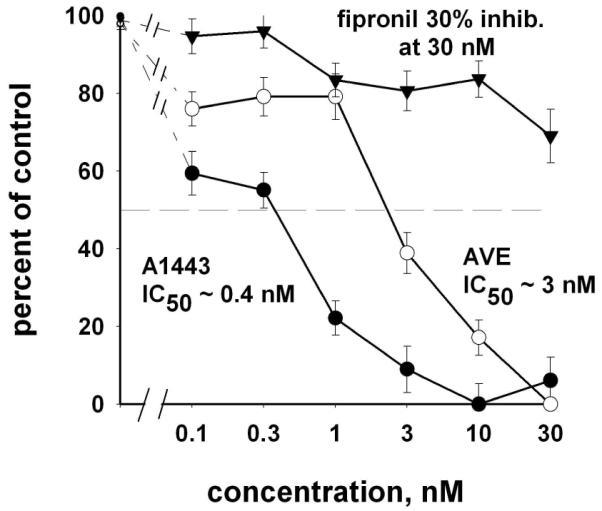
Effects of A1443, avermectin B1 and fipronil on specific binding of [3H]A1443 to the isoxazoline target in house fly head membranes.
Of particular interest was the interaction of the channel activator avermectin B1 with the [3H]A1443 binding site giving an IC50 of about 3 nM (Figure 3). The AVE analogs ivermectin and emamectin were also very potent inhibitors of [3H]A1443 binding. A recent electrophysiological study13 showed that A1443 at nanomolar levels blocks both GABA- and glutamate (Glu)- induced chloride currents in Xenopus oocytes expressing house fly MdGBCl and MdGluCl channels. AVE is a positive allosteric modulator for several members of the Cys-loop receptor family of ligand-gated ion channels including Glu-gated chloride channels (GluClRs) and the inhibitory GABA type A and glycine receptors (GABAARs and GlyRs). 28-31 We observed earlier the high sensitivity of [3H]EBOB binding to AVE and its analogs.20,27 AVE acts at or near a proposed anesthetic binding site in a water-filled cavity. [3H]A1443 obviously has a distinct and unique binding site among the chloride channel modulators. The primary target for isoxazoline insecticidal action is probably in the insect GABA/Glu receptors.
The isoxazolines rejuvenate the GABA/Glu receptors as an insecticide target of interest. The isoxazoline binding site appears to be different than the targets of earlier chemotypes to which resistance has been selected. The likelihood of selecting for isoxazoline resistance is unclear but for now the new subsite is a fresh start and the GABA/Glu receptors are once again favored as an insecticide target, particularly since the isoxazoline site appears to be more important in insects than in mammals.13
Supplementary Material
ACKNOWLEDGMENTS
Bob Fazio of Vitrax Company made important suggestions on radiosynthesis. C. Z. thanks Professor Lihong Qiu of China Agricultural University for academic counsel and our laboratory colleagues Suzhen Qi, Stephen Lantz, Tami Swenson for helpful advice.
Funding R. S. and P. G-R. are grateful to the National Institutes of Health (1RO1GM086374-02S1) for funding the research related to the synthesis of [3H]A1443. C.Z. was supported in part by State Scholarship Fund No. 2011635139 provided by the China Scholarship Council.
ABBREVIATIONS
- BSA
bovine serum albumin
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- DIEA
N,N-diisopropylethylamine
- EDCI
1-(3-dimethylaminopropyl)-3 ethylcarbodiimide
- DMAP
4-dimethylaminopyridine
- dpm
disintegrations per minute
- Glu
glutamate
- NBS
N-bromosuccinimide
Footnotes
Supporting Information General methods, experimental procedures, 1H and 13C NMR spectra and spectral data and HRMS-ESI data for A1443 and compounds 1-4. HPLC conditions for analysis of radiochemical purity.
Notes The authors declare no competing financial interest.
REFERENCES
- (1).Nauen R, Elbert A, Mccaffery A, Slater R, Sparks TC. In: Modern Crop Protection Compounds. 2nd ed Krämer W, Schirmer U, Jeschke P, Witschel M, editors. Vol. 1-3. Wiley-VCH Verlag; Weinheim, Germany: 2012. pp. 935–956. [Google Scholar]
- (2).Casida JE, Durkin KA. Annu. Rev. Entomol. 2013;58:99–117. doi: 10.1146/annurev-ento-120811-153645. [DOI] [PubMed] [Google Scholar]
- (3).Ikeda S, Maeda K, Furukawa H, Takii S, Mita T, Kikudui T, Mizukoshi T, Yaosara M. PCT International Application WO 085216. 2005.
- (4).Mita T, Kikuchi T, Mizukoshi T, Yaosaka M, Komoda M. JP Patent 308471. 2007
- (5).Lahm GP, Pahutski TF, Long JK, Smith BK, Xu M, Holyoke CW, Jr., Barry JD, Cordova D, Smith R. 238th Amer. Chem. Soc. National Meeting; Washington, D.C.. AGRO; 2009. p. 159. Abstract. [Google Scholar]
- (6).Renold P, Zambach W, Maienfisch P, Muehlebach M. WO Patent 080250. 2009
- (7).Rauh JJ, Lahm GP, Pahutski TF, Ullas GV, Filer CN. J. Labelled. Compd. Radiopharm. 2010;53:50–51. [Google Scholar]
- (8).Takeshi M, Furukawa Y, Iwasa M, Komoda M. EP Patent 2151437. 2010
- (9).Cordova D. J. Pestic. Sci. 2011;36:148. [Google Scholar]
- (10).Mita T, Furukawa Y, Iwasa M, Komoda M. US Patent 0144797. 2010
- (11).Lahm GP, Shoop WL, Xu M. US Patent 8231888. 2012
- (12).Kousaka H, Fukuya S, Moriyama Y, Yaosaka M, Mizukoshi T. EP Patent 2308857. 2011
- (13).Ozoe Y, Asahi M, Ozoe F, Nakahira K, Mita T. Biochem. Biophys. Res. Commun. 2010;391:744–749. doi: 10.1016/j.bbrc.2009.11.131. [DOI] [PubMed] [Google Scholar]
- (14).Deng Y, Palmer CJ, Casida JE. Pestic. Biochem. Physiol. 1991;41:60–65. [Google Scholar]
- (15).Woods DJ, Vaillancourt VA, Wendt JA, Meeus PF. Future Med. Chem. 2011;3:887–896. doi: 10.4155/fmc.11.39. [DOI] [PubMed] [Google Scholar]
- (16).Valsborg JS, Sørensen L, Foged C. J. Labelled Compd. Radiopharm. 2001;44:209–214. [Google Scholar]
- (17).Cross PWC, Ellames GJ, Gibson JS, Herbert JM, Kerr WJ, McNeill AH, Mathers TW. Tetrahedron. 2003;59:3349–3358. [Google Scholar]
- (18).Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Casida JE. Arch. Insect Biochem. Physiol. 1993;22:13–23. doi: 10.1002/arch.940220104. [DOI] [PubMed] [Google Scholar]
- (20).Deng Y, Clark J. Molecular Action of Insecticides on Ion Channels. Americal Chemical Society; Washington D.C.: 1995. pp. 230–250. (ACS Symposium Series). [Google Scholar]
- (21).Salgado VL, Schnatterer S, Holmes KA. In: Modern Crop Protection Compounds. 2nd ed Krämer W, Schirmer U, Jeschke P, Witschel M, editors. Vol. 1-3. Wiley-VCH Verlag; Weinheim, Germany: 2012. pp. 1283–1305. [Google Scholar]
- (22).Ozoe Y, Ishaaya I. Biorational Control of Arthropod Pests. Springer; New York: 2009. pp. 131–162. [Google Scholar]
- (23).Buckingham SD, Sattelle DB, Gilbert LI, Gill SS. Insect Pharmacology Channels, Receptors, Toxins and Enzymes. Academic Press; London, UK: 2010. pp. 29–64. [Google Scholar]
- (24).Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- (25).Isaacs AK, Qi S, Sarpong R, Casida JE. Chem. Res. Tox. 2012;25:1571–1573. doi: 10.1021/tx300326m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Seifert J, Casida JE. J. Neurochem. 1985;44:110–116. doi: 10.1111/j.1471-4159.1985.tb07119.x. [DOI] [PubMed] [Google Scholar]
- (27).Deng Y, Casida JE. Pestic. Biochem. Physiol. 1992;43:116–122. [Google Scholar]
- (28).Pitterna T. In: Modern Crop Protection Compounds. 2nd ed Krämer W, Schirmer U, Jeschke P, Witschel M, editors. Vol. 1-3. Wiley-VCH Verlag; Weinheim, Germany: 2012. pp. 1305–1326. [Google Scholar]
- (29).Dawson GR, Wafford KA, Smith A, Marshall GR, Bayley PJ, Schaeffer JM, Meinke PT, McKernan RM. J. Pharmacol. Exp. Therap. 2000;295:1051–1060. [PubMed] [Google Scholar]
- (30).Wolstenholme AJ. Invert. Neurosci. 2010;10:5–10. doi: 10.1007/s10158-010-0105-y. [DOI] [PubMed] [Google Scholar]
- (31).Lynagh T, Lynch JW. Trends Pharmacol. Sci. 2012;33:432–441. doi: 10.1016/j.tips.2012.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


