Abstract
Breast cancer survivors are at increased risk for cognitive dysfunction, which reduces quality of life. Neuroimaging studies provide critical insights regarding the mechanisms underlying these cognitive deficits as well as potential biologic targets for interventions. We measured several metabolite concentrations using 1H magnetic resonance spectroscopy as well as cognitive performance in 19 female breast cancer survivors and 17 age-matched female controls. Women with breast cancer were all treated with chemotherapy. Results indicated significantly increased choline (Cho) and myo-inositol (mI) with correspondingly decreased N-acetylaspartate (NAA)/Cho and NAA/mI ratios in the breast cancer group compared to controls. The breast cancer group reported reduced executive function and memory, and subjective memory ability was correlated with mI and Cho levels in both groups. These findings provide preliminary evidence of an altered metabolic profile that increases our understanding of neurobiologic status post-breast cancer and chemotherapy.
Keywords: MR Spectroscopy, Breast Cancer, Cognition, Prefrontal Cortex, Chemotherapy
Introduction
According to the National Cancer Institute, breast cancer is diagnosed in 1 out of every 8 women during the lifetime (NCI 2012). Although survival rates have steadily increased over the past decades, many of these women experience long-term effects of the disease and its treatments. Cognitive impairments are among the most common quality of life complaints, affecting up to 75 % of patients (Janelsins et al. 2011). These impairments reduce quality of life, hinder workplace performance and make it more difficult to follow treatment regimens (Stilley et al. 2010).
Cognitive studies implicate the prefrontal cortex as a region of significant vulnerability to the effects of breast cancer and/or its treatments (Wefel et al. 2011). Neuroimaging studies by our laboratory and others’ have consistently demonstrated abnormal prefrontal cortex function following chemotherapy (Silverman et al. 2007; Kesler et al. 2009a; McDonald et al. 2010; de Ruiter et al. 2011b; Kesler et al. 2011; Bruno et al. 2012; McDonald et al. 2012a; McDonald et al. 2012b). These studies demonstrate that prefrontal cortex is the most commonly affected region in breast cancer irrespective of imaging modality (e.g. VBM, fMRI, PET) and even when whole brain analyses are conducted. Additionally, prefrontal cortex-mediated cognitive domains, including executive function, attention and memory, are the most common areas of deficit following breast cancer chemotherapy (Jansen et al. 2005; Vardy 2009; Janelsins et al. 2011; Wefel et al. 2011; Wefel and Schagen 2012). The prefrontal cortex shows significant susceptibility to injury, stress and disease (Arnsten 2011), as well as to normal aging (Lemaitre et al. 2010).
Prefrontal cortex abnormalities have been associated with subjective difficulties in everyday, real-world executive behaviors (Kesler et al. 2011; McDonald et al. 2012b). These findings suggest that prefrontal status may be a sensitive biomarker for patients’ cognitive complaints. Importantly, executive-prefrontal deficit is the best predictor of medication adherence in breast cancer patients (Stilley et al. 2010) and thus may be indirectly associated with health status.
1H-magnetic resonance spectroscopy (1H-MRS) has been shown to be highly sensitive to metabolic abnormalities underlying cognitive deficits (Wang et al. 2011). MRS is a neuroimaging technique that provides in vivo measurement of various neurometabolites that are markers of brain parenchymal integrity and function. To date the number of 1H-MRS studies in breast cancer patients is limited. One previous study was conducted in 8 patients with breast cancer focusing on the parieto-occipital region (Brown et al. 1998). A more recent study involving a larger sample measured metabolite levels in the left centrum semiovale of breast cancer survivors (de Ruiter et al. 2011a). We extended upon these findings by measuring several metabolite concentrations in the prefrontal cortex of women treated for breast cancer and age-matched healthy female controls. The metabolites examined included N-acetylaspartate (NAA), myo-inositol (mI), choline containing compounds (Cho), glutamine + glutamate (Glx) and creatine containing compounds (Cr). We hypothesized that the breast cancer group would show altered metabolite levels in the prefrontal cortex corresponding to reduced cognitive function.
We included NAA, Cr and NAA/Cr as these have been examined in other brain regions among women with breast cancer, as noted above. NAA is considered a marker of neuronal health, viability and/or number. However, NAA can also reflect reversible dysfunction of neuronal tissue (Maddock and Buonocore 2012). Previous studies indicate that gray matter volume, particularly in prefrontal regions, is initially reduced following breast cancer chemotherapy with recovery in certain regions over time (McDonald et al. 2010; McDonald et al. 2012b). NAA levels may provide further insight into gray matter atrophy and recovery following breast cancer. Cr is critically involved in energy metabolism and homeostasis (Maddock and Buonocore 2012), which may have implications for chemotherapy-related cognitive fatigue (Schifitto et al. 2011). mI is associated with microglial activity (Maddock and Buonocore 2012) which may be relevant for a proinflammatory-cytokine model of chemotherapy-related neurotoxicity (Tangpong et al. 2006; Seruga et al. 2008).
Cho is a marker of neuronal density and/or rate of membrane turnover (Maddock and Buonocore 2012). mI and Cho and particularly NAA/mI and NAA/Cho have been shown to be highly sensitive to cognitive decline (Bozgeyik et al. 2008). Glx arises from both neurons and glial cells, providing insight regarding the function of glutamatergic systems in the brain (Maddock and Buonocore 2012). Glutamate is a major neurotransmitter involved in learning and memory processes and has been shown to be reduced in individuals with cognitive impairment (Francis 2003). There are other metabolites that could be examined as well. Considering our sample size, we emphasized those that have the most robust spectra peaks in the context of our 1H-MRS pulse sequence in combination with particular relevance for breast cancer-related cognitive dysfunction.
Methods
Participants
This study included 19 right-handed women with a history of primary breast cancer (stages I–III) and 17 right-handed healthy female controls ages 40–75. Groups were matched for age, education, IQ and minority status although there were slightly (p=0.09) more women who were post-menopausal in the breast cancer group (Table 1). All women in the breast cancer group were treated with chemotherapy (Adriamycin/Cytoxan=5, Adriamycin/Cytoxan/Taxol or Taxotere = 1, Cytoxan/Methotrexate/5-Fluororacil = 2, Cytoxan/Taxol or Taxotere = 10, Cytoxan/Taxol or Taxotere/5-Fluororacil=1) and were, on average, 5.3±4. 2 years off-therapy (range 1–13 years). Eight women had a history of tamoxifen treatment and 14 also received radiation (7 had both radiation and tamoxifen). Participants were excluded for neurologic, psychiatric or medical conditions known to affect cognitive function (e.g. learning disability, brain injury, mood disorder) as well as MRI contraindications. Participants who were currently taking psychoactive medications (e.g. antidepressants, sedatives, anticonvulsants) were also excluded due to the possible effects on the 1H-MRS signal. Participants in both groups were recruited via the Army of Women (http://www.armyofwomen.org) as well as website and listserv advertisements. This study was approved by the Stanford University Institutional Review Board and informed consent was obtained from all participants.
Table 1.
Demographic Data. Data are shown as mean (standard deviation) unless otherwise indicated
| BC N=19 | Controls N=17 | Statistic | p | d | |
|---|---|---|---|---|---|
| Age (years) | 55.1 (8.4) | 55.9 (8.6) | t=0.265 | 0.79 | 0.09 |
| Education (years) | 17.0 (3.9) | 16.0 (3.4) | t=1.57 | 0.13 | 0.27 |
| Minority | N=3 (16 %) | N=2 (13 %) | X2=0.00 | 1.00 | 0.00 |
| Post-Menopausal | N=14 (74 %) | N=7 (44 %) | X2=3.24 | 0.09 | 0.77 |
| Disease Stage 1, 2, 3 | 15.8 %, 26.3 %, 7.9 % |
d effect size
Magnetic resonance imaging
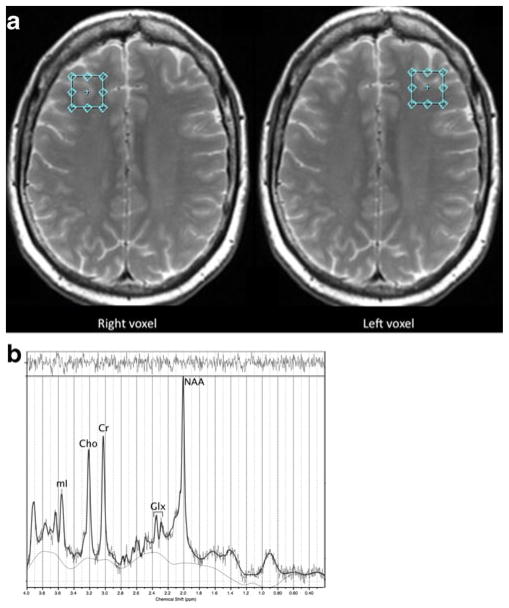
MR scans were acquired on a General Electric 3.0 Tesla Signa scanner, software version LX 20.1, with an 8-channel head coil (GE Medical Systems, Milwaukee, WI). A T2-weighted axial anatomical series involved a fast spin echo accelerated pulse sequence (TR=4000 msec, TE=17 msec, TE2=85 msec, flip angle=80°, slice thickness=4 mm, interleaved with T1 slices, # of averages=32, field of view= 240 mm). A 2 cm isotropic MRS voxel (TR=2000 msec, TE=29 msec, TE2=85 msec) of approximately half gray and half white matter was prescribed in the prefrontal cortex on the first slice superior to the lateral ventricles, in the lateral most regions just anterior of the lateral ventricles. Because the slice thickness was 4 mm, the voxel was placed anywhere from 0 mm to 4 mm above the lateral ventricles as far lateral as possible while remaining in the cerebrum and visually maintaining approximately equal parts gray and white matter (see Fig. 1). This voxel prescription has been well established in previous studies by our group and others (Chang et al. 2003; Chang et al. 2009; Kesler et al. 2009b) and was designed based on known anatomical landmarks in consultation with MRS imaging experts at the Stanford Lucas Center. MRS was acquired for the left and right hemispheres separately. CHESS was used to attain water suppression.
Fig. 1.
Panel A shows examples of the magnetic resonance spectroscopy (MRS) voxel prescription within the left and right prefrontal cortex (voxels are prescribed in radiologic convention). Panel B provides an example MRS spectrum from a participant in the breast cancer group
MRS analysis
Processing of the MRS spectra occurred via the fully automated LC Model (Provencher 2001). Eddy current correction and water scaling were used. Spectra were considered usable by visual inspection and/or if LC Model indicated %SD, a measure of resolution and noise level, less than 20 % (see Fig. 1). One spectrum from the control group was excluded. Metabolite levels from left and right hemispheres were averaged given that we had no hypotheses regarding lateralization based on previous literature and to reduce the number of statistical comparisons in this small, preliminary sample. In 5 cases, only data from a single hemisphere (4 left, 1 right) was available, which was used in place of averaging both hemispheres.
Masks were created in MarsBar (http://marsbar.sourceforge.net) such that the mask center corresponded to the coordinates of the participant’s individual MRS voxel prescription. The T2 images were segmented using FSL (http://www.fmrib.ox.ac.uk/fsl/), tissue volumes within the masks were extracted for each participant using REX (http://web.mit.edu/swg/software.htm) and the mean percentage of each tissue type in each voxel was calculated. Between-group analysis of these mean (left + right) ratios revealed no significant differences (Table 1).
Neuropsychological assessment
All participants completed tests of cognitive function including Matrix Reasoning and Information subtests of the Wechsler Adult Intelligence Scale 4th Edition (WAIS-IV) (Wechsler 2008) used to derive an estimate of global intelligence (IQ), Delis-Kaplan Executive Function System (DKEFS) Letter Fluency subtest (Homack et al. 2005), Neuropsychological Assessment Battery (NAB) Categories test (Stern and White 2005), Hopkins Verbal Learning Test-Revised (HVLT-R) (Benedict et al. 1998) and Wisconsin Card Sorting Test (WCST) (Heaton 2004). We used the Perseverative Errors outcome of the WCST given our previous research showing that this score discriminates between breast cancer survivors and healthy controls (Kesler et al. 2011).
We also administered domain-specific self-report measures including the Behavioral Rating Inventory of Executive Function (BRIEF) (Roth et al. 2005) and the Multifactorial Memory Questionnaire Ability Scale (MMQ) (Troyer and Rich 2002). Additionally, we administered the Clinical Assessment of Depression (CAD), a self-rating measure of depression, anxiety and fatigue (Aghakhani and Chan 2007). The neuropsychological assessment was conducted on the day of MRS acquisition. All scores were converted to T scores (mean=50 +/− 10) based on published normative data for each measure.
Statistical analyses
Metabolite levels
Group differences in mean absolute metabolite concentrations were assessed using one-way analysis of covariance (ANCOVA) in SPSS 19 (www.spss.com). Metabolites included NAA, mI, Cho, Glx and Cr. We also examined metabolite ratios including NAA/Cr, NAA/Cho and NAA/mI using ANCOVA. Analyses were controlled for mean spectra-related confounds (voxel gray matter composition, signal-to-noise and full-width half maximum) (Prescot, et al. 2011). Age, menopausal status, tamoxifen and CAD scores were also included as covariates; however, these model terms were removed from the model if insignificant at the 0.05 level to improve parsimony.
Neuropsychological status
Differences in cognitive tests and subjective measures were assessed using ANCOVA with age, menopausal status, tamoxifen, radiation and CAD scores included as covariates (removed from the model if p>0.05).
Exploratory correlational analyses
Relationships between menopausal status, age, education level, abnormal metabolite concentrations (those that differed between groups) and abnormal neuropsychological measures (scores that differed between groups) were explored within each group separately using two-tailed Pearson or Spearman correlations as appropriate. Time off-therapy, tamoxifen, radiation and disease stage were also examined in the breast cancer group.
Results
Metabolite levels
As shown in Table 2, the breast cancer group demonstrated significantly increased mI and Cho with decreased NAA/Cho and NAA/mI levels compared to controls. NAA, NAA/Cr, Cr, and Glx were not significantly different between groups. Age, menopausal status, radiation, tamoxifen and CAD scores did not contribute significantly to the metabolite ANCOVA models.
Table 2.
Metabolite Data. Data are shown as mean (standard deviation) IU unless otherwise indicated
| BC N=19 | Controls N=16 | Statistic | p | d | |
|---|---|---|---|---|---|
| NAA | 6.39 (0.82) | 6.47 (0.77) | F=0.07 | 0.80 | 0.10 |
| mI | 4.59 (0.72) | 3.88 (0.78) | F=8.71 | 0.007 | 0.95 |
| Glx | 6.76 (0.83) | 6.45 (0.85) | F=0.68 | 0.42 | 0.37 |
| Cho | 1.39 (0.23) | 1.23 (0.21) | F=4.12 | 0.05 | 0.73 |
| Cr | 5.10 (0.48) | 4.99 (0.61) | F=0.37 | 0.55 | 0.20 |
| NAA/Cr | 1.25 (1.0) | 1.30 (0.06) | F=1.73 | 0.20 | 0.07 |
| NAA/Cho | 4.63 (0.46) | 5.33 (0.88) | F=5.08 | 0.03 | 1.0 |
| NAA/mI | 1.40 (0.18) | 1.70 (0.34) | F=9.93 | 0.004 | 1.1 |
| Gray Matter % | 34.7 (9.7) | 35.2 (9.9) | F=0.02 | 0.88 | 0.05 |
| White Matter % | 65.3 (9.7) | 64.8 (9.9) | F=0.02 | 0.88 | 0.05 |
| FWHM | 0.05 (0.03) | 0.05 (0.03) | t=0.63 | 0.53 | 0.00 |
| SNR | 16.1 (4.5) | 14.7 (5.2) | t=0.83 | 0.41 | 0.29 |
d effect size, IU Institutional Units, NAA N-acetylaspartate, mI myo-inositol, Cho glycerophosphocholine + phosphocholine, Glx glutamine + glutamate, Cr creatine + phosphocreatine, FWHM full-width half maximum, SNR signal-to-noise
Neuropsychological status
As shown in Table 3, the breast cancer group demonstrated significantly elevated BRIEF and decreased MMQ scores compared to the control group indicating increased self-report of executive and memory deficits, respectively. IQ estimate (including Matrix Reasoning and Information), HVLT-R Total and Delayed Recall, Letter Fluency, Categories, WCST and CAD score were not significantly different between groups. Age was a significant covariate in the WCST and Categories models and CAD score was significant in the BRIEF model but none of the other covariates contributed to the neuropsychological ANCOVA models.
Table 3.
Neuropsychological Data. Data are shown as mean (standard deviation) T-scores unless otherwise indicated
| BC N=19 | Controls N=17 | Statistic | p | d | |
|---|---|---|---|---|---|
| Estimated IQ | 56.7 (8.7) | 59.3 (6.7) | F=1.29 | 0.26 | 0.34 |
| WAIS-IV Matrix Reasoning | 57.3 (8.7) | 57.7 (9.0) | F=0.01 | 0.93 | 0.11 |
| WAIS-IV Information | 56.7 (8.0) | 60.3 (9.7) | F=0.98 | 0.33 | 0.41 |
| HVLT-R Total Recall | 52.6 (11.2) | 56.3 (8.8) | F=0.85 | 0.37 | 0.37 |
| HVLT-R Delayed Recall | 50.7 (9.6) | 53.3 (7.6) | F=0.63 | 0.43 | 0.30 |
| DKEFS Letter Fluency | 57.0 (12.7) | 63.0 (9.3) | F=2.38 | 0.13 | 0.54 |
| NAB Categories | 51.6 (9.7) | 55.6 (9.2) | F=1.37 | 0.25 | 0.42 |
| WCST Perseverative Errors | 47.0 (9.1) | 50.9 (10.1) | F=1.49 | 0.23 | 0.41 |
| BRIEF | 59.1 (10) | 53.1 (8.2) | F=4.10 | 0.05 | 0.66 |
| MMQ | 40.8 (11.2) | 58.2 (7.5) | F=25.3 | < 0.0001 | 1.82 |
| CAD Total Score | 48.8 (8.2) | 48.0 (7.2) | F=0.07 | 0.80 | 0.10 |
d effect size, IQ Intelligence Quotient, WAIS-IV Wechsler Adult Intelligence Scale – Fourth Edition, HVLT-R Hopkins Verbal Learning Test-Revised, DKEFS Delis-Kaplan Executive Function System, NAB Neuropsychological Assessment Battery, WCST Wisconsin Card Sorting Test, BRIEF Behavioral Rating Inventory of Executive Function Global Executive Composite, MMQ Multifactorial Memory Questionnaire Ability Scale, CAD Clinical Assessment of Depression
Exploratory correlational analyses
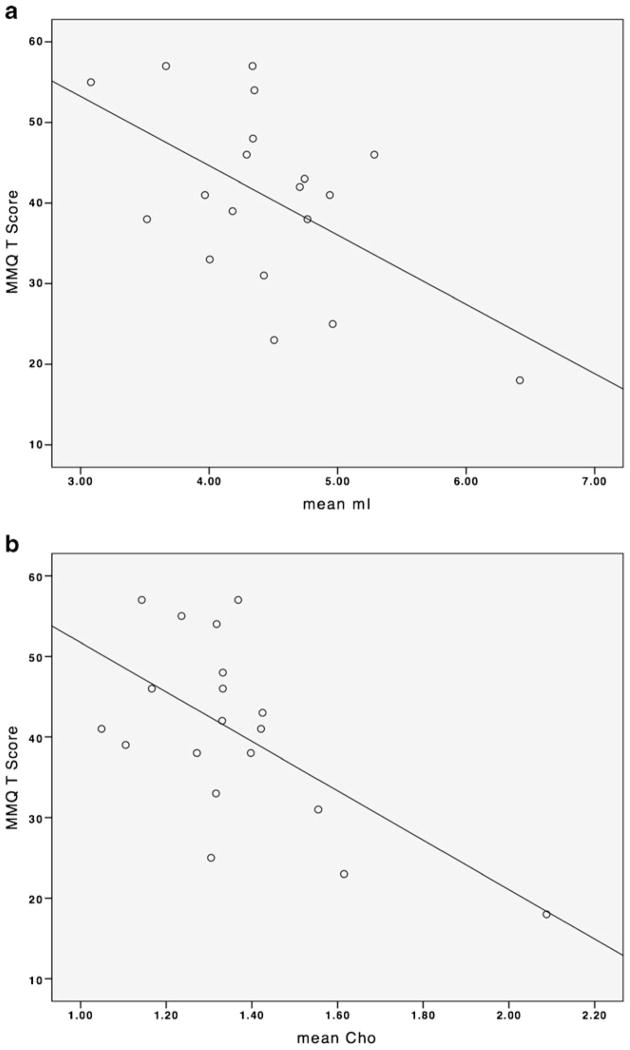
As shown in Fig. 2, in the breast cancer group, there was a significant correlation between MMQ score and Cho (r=−0.62, p=0.005) as well as mI (r=−0.55, p=0.02). There were no significant correlations in the control group. There were no significant correlations between Cho, mI, NAA/Cho, NAA/mI and the BRIEF or between NAA/Cho, NAA/mI and MMQ in either group. There was a significant positive correlation between education level and NAA/mI (r=0.61, p=0.006) in the breast cancer group only. There were no significant correlations between education and NAA/Cho, mI, Cho, MMQ or BRIEF in either group. There were no significant correlations between MMQ, BRIEF and education level in either group. There were no significant correlations between Cho, mI, NAA/Cho or NAA/mI and age, menopausal status or CAD in either group. There were no significant correlations between Cho, mI, NAA/Cho or NAA/mI and disease stage, time off-therapy, radiation or tamoxifen in the breast cancer group. There were no correlations between MMQ or BRIEF and disease stage, time off-therapy, radiation or tamoxifen in the breast cancer group.
Fig. 2.
Correlations between prefrontal metabolite levels and self-rated memory function. Panel A shows increased mI associated with decreased MMQ score in the breast cancer group. Panel B illustrates increased Cho associated with decreased MMQ score in the breast cancer group. There were no significant correlations in the control group
Discussion
The present findings demonstrated increased mI and Cho with correspondingly decreased NAA/mI and NAA/Cho in a group of chemotherapy-treated breast cancer survivors who were, on average, 5 years off-therapy. These results were observed in comparison with a sample of healthy matched female controls. A previous MRS study in breast cancer showed a decrease in NAA/Cr from chemotherapy induction to 3 months post-therapy and a decrease in Cho/Cr at 9 months post-therapy (Brown et al. 1998). Another, larger study showed decreased NAA/Cr in the left centrum semiovale at >9 years post-treatment (de Ruiter et al. 2011a). Our study confirms abnormalities in neural metabolite levels following breast cancer treatment. Additionally, we expanded upon the previous results by including additional metabolites and a focus on the prefrontal cortex.
Cho is associated with cell membrane metabolism and myelination (Bozgeyik et al. 2008) and is a precursor of acetylcholine, which is essential for memory function (Tayebati et al. 2011). NAA/Cho is used as an indicator of neuronal density to axonal density (Bozgeyik et al. 2008). Cholinergic pathways are known to subserve several cognitive processes affected by breast cancer chemotherapy including executive function, attention and memory (Kesler et al. 2009b; Newman et al. 2012; Wefel and Schagen 2012). It has been suggested that elevated Cho reflects membrane catabolism that increases free choline to counteract acetylcholine deficiency at the expense of membrane maintenance (Tayebati et al. 2011). Breast cancer and chemotherapy may increase the necessity for such compensatory mechanisms.
mI is involved in glial function and is a precursor of inositol triphosphate and phosphatidylinositol. These molecules are important second messengers in signal transduction cascades, affecting neuronal excitability and neurotransmitter release (Gamper and Shapiro 2007). Increased glial cell activation and mI accumulation occur in the context of elevated cytokine levels (Yorek et al. 1998; Schneider et al. 2012). Cytokine-mediated neuroinflammation and consequent oxidative and nitrosative damage are believed to play a critical role in neurotoxic brain injury following chemotherapy (Tangpong et al. 2006; Tangpong et al. 2007; Joshi et al. 2010). Accordingly, elevated cytokine levels are associated with cognitive dysfunction and neurobiologic alterations following breast cancer chemotherapy (Kesler et al. 2013), including abnormal frontal cortex function (Ganz et al. 2013). Our findings therefore provide further evidence supporting proinflammatory cytokine dysregulation as a potential mechanism underlying cognitive dysfunction in breast cancer.
Increased Cho and mI as well as decreased NAA/Cho and NAA/mI have been associated with aging (Bozgeyik et al. 2008). Decreased NAA/Cho, and especially NAA/mI, have also been shown to be highly predictive of Alzheimer’s dementia (Wang et al. 2011). Previous studies suggest an accelerated aging process following breast cancer chemotherapy (Ahles et al. 2010; Kesler et al. 2011; Koppelmans et al. 2011; Kesler et al. 2013). We have also previously reported results of an observational study in which we identified a subset of women with breast cancer who demonstrated progressive decline in cognitive function after chemotherapy (Wefel et al. 2010). However, there were no significant correlations between age and brain metabolites in the present study. Further study is required to determine the interactions between chemotherapy, aging and neurometabolite levels.
mI has been reported to be lower in women with breast cancer taking tamoxifen and healthy women receiving hormone replacement therapy, when compared to healthy women not receiving hormone replacement therapy (Ernst et al. 2002). Since approximately half the women with breast cancer in our study were currently or had previously received tamoxifen, our elevated mI results are even more compelling as one would expect a reduction of mI in this group. Post-hoc analysis indicated no significant difference in mI between women who received tamoxifen and those who had not (p=0.97, d=0.01). However, there was more variation in mI among the tamoxifen group (SD=1.1) compared to the no tamoxifen group (SD=0.34). The breast cancer group also had more post-menopausal women than the control group. This is expected given that chemotherapy frequently causes early menopause (Vehmanen et al. 2006). Menopausal status and tamoxifen were controlled for in the analyses, neither was a significant covariate in any of the models, nor were they correlated with metabolite levels or cognitive function. However, further research regarding the interaction between breast cancer, chemotherapy, estrogen deficiency and brain metabolism is warranted.
Unlike previous studies, we did not observe a significant decrease in NAA or NAA/Cr. As noted above, we focused on the prefrontal cortex whereas previous studies examined metabolite levels in parietal-occipital (Brown et al. 1998) and left centrum semiovale regions (de Ruiter et al. 2011a). More importantly, our MRS voxel included equal parts of gray and white matter while previous studies measured metabolite levels in white matter only. Therefore, differences in results could reflect regional and/or tissue-specific metabolite alterations. The Brown et al. study included mI but measured metabolites only acutely (Brown et al. 1998). Similar to our study, de Ruiter and colleagues investigated long-term breast cancer survivors but did not include mI (de Ruiter et al. 2011a). Therefore, the discrepancy in findings could also reflect acute versus persistent effects and further study is required to evaluate this possibility.
The present study suggests that increased Cho and mI are associated with decreased self-reported memory ability in breast cancer survivors. Prefrontal cortex is known to play a critical role in memory and learning (Leh et al. 2010) and we previously demonstrated abnormalities of the prefrontal cortex associated with verbal memory function in breast cancer survivors (Kesler et al. 2009a). Studies from our laboratory as well as others have also previously demonstrated correlations between prefrontal cortex abnormalities and elevated BRIEF scores in breast cancer patients and survivors (Kesler et al. 2011; McDonald et al. 2012b). However, we did not observe a correlation between BRIEF scores and metabolite levels in the present study despite BRIEF scores being significantly elevated in the breast cancer group. It should be noted, however, that, while the MMQ score was impaired on average in the breast cancer group, the mean BRIEF score was not clinically significant (T score of 65 or higher is considered significant (Roth et al. 2005)). The lack of correlation between altered prefrontal metabolites and BRIEF score may reflect sample characteristics, insufficient metabolite abnormalities to result in behavioral differences, or it may suggest that the specific executive behaviors measured by the BRIEF correspond better with other measures of prefrontal cortex physiology (i.e. gray matter volume, BOLD activation).
The prefrontal cortex is known to be commonly affected by a range of conditions and may have unique neurochemical properties that increase its vulnerability to injury and disease (Diamond 2011). However, it is unknown if the metabolic changes noted in the present breast cancer group are region specific or represent a more global deficit. Prefrontal cortex abnormality often points to a distributed injury (Elliott 2003) and accordingly, recent studies indicate widespread reductions in white matter integrity (de Ruiter et al. 2011a; Deprez et al. 2011; Deprez et al. 2012) and brain network organization (Bruno et al. 2012; Hosseini et al. 2012) following breast cancer treatment. Future studies of brain metabolism in breast cancer should include multi-voxel acquisitions when possible to help determine if there are regional differences in brain metabolism following breast cancer chemotherapy.
We did not find any between group differences on objective cognitive tests. However, longitudinal analysis of cognitive function tends to yield better results given that a change in individual ability level may be significant despite the group average being “normal” or comparable to controls. Additionally, a decline in cognitive function may occur in only a subset of subjects and is thus masked by the overall group mean (Wefel et al. 2004; Ouimet et al. 2009; Wefel et al. 2010). We have observed significant between group differences in previous, larger studies using these same cognitive measures (Kesler et al. 2011; Bruno et al. 2012; Kesler et al. 2013). Therefore, the present null results may also reflect limited statistical power.
Post-hoc analysis indicated no correlations between BRIEF and MMQ with any of the objective tests used in this study which is consistent with previous studies of self-report measures in breast cancer (Castellon et al. 2004; Quesnel et al. 2009; Vardy 2009). Self-report measures of cognitive function tend to be correlated with psychiatric distress (Vardy 2009). In the present study, CAD score was a significant covariate in the BRIEF model but not the MMQ. Although participants were excluded for diagnosed mood disorder or psychoactive medications and were matched for psychiatric symptom report, these symptoms can affect cognitive and prefrontal function (Wefel et al. 2011). In this study, there were no significant correlations between CAD scores and metabolite levels or cognitive function. However, further evaluation of the potential effects of depression on brain metabolism in breast cancer is required. Overall, the fact that certain self-report measures, particularly those that are domain specific like the MMQ, are correlated with neurobiologic status suggests that these measures may be of significant benefit in studies of cognition and cancer.
Lower education was associated with lower NAA/mI in the breast cancer group. These findings suggest that chemotherapy-treated women may be more vulnerable to the effects of lower cognitive reserve (Stern 2009) on brain physiology. Our results are consistent with previous studies demonstrating an association between lower education level and decreased performance on measures of prefrontal-mediated skills including executive functioning (Kesler et al. 2011) and processing speed (Ahles et al. 2010) in women with breast cancer. Education level, like other proxies of cognitive reserve suggests a higher level of mental activity that is neuroprotective (Stern 2009). Recent evidence suggests that cognitive training may decrease prefrontal mI in individuals with mild cognitive impairment (Boripuntakul et al. 2012). Patients with breast cancer status post chemotherapy have been found to exhibit improved cognitive function after participating in a neuroplasticity-based cognitive retraining intervention (Von Ah et al. 2012). It remains to be determined if this improvement is a result of alterations in brain physiology and/or cognitive reserve.
In addition to possible behavioral interventions, our findings may help inform future pharmacologic treatments for cognitive dysfunction following breast cancer chemotherapy. For example, treatment with donepezil, an acetylcholin-esterase inhibitor, has been shown to decrease mI levels with associated improvements in cognitive function in patients with dementia (Henigsberg et al. 2011). Mice treated with chemotherapy and donepezil demonstrated greater preservation of cognitive function compared to mice treated with chemotherapy alone (Winocur et al. 2011). When phosphatidyl inositol (a product of myo-inositol) is inhibited in rats, cytokine migration across the blood–brain barrier is blocked (Yang et al. 2009). Longitudinal studies using larger samples are required to determine if mI and/or Cho represent viable targets for potential pharmacotherapies.
Limitations of this study include small sample size, which restricts the interpretation of the results. The large time-range for time-off-therapy also limits the results as there may be differences in early and long-term survivors that were not detectable in this small sample. The present findings do not provide information regarding the differential effects of cancer versus chemotherapy treatments on metabolite levels and we did not have information regarding chemotherapy dose. Longitudinal studies are required to determine if between group differences in memory and executive functioning actually reflect changes from pre-illness status and to help determine the specific effects of disease and treatment parameters over time. Our study was restricted to prefrontal metabolite levels as we expected it a priori to be an area of significant vulnerability. Future studies with larger sample sizes are needed to expand these findings to include other brain regions, additional cognitive domains and examine the potential effects of specific chemotherapy regimens, hormonal blockade treatments, psychiatric status and other medical variables.
In conclusion, our study demonstrates preliminary evidence of increased mI and Cho concentrations as well as decreased NAA/Cho and NAA/mI ratios in women treated for breast cancer. The profile of metabolic differences is similar to that seen in normal and pathological aging. Although we did not observe associations between age and metabolite levels in this sample, our results provide further insight regarding brain injury in breast cancer survivors. Specifically, these findings suggest that breast cancer and/or chemotherapy may alter glial function, neuronal density and/or axonal density in the prefrontal cortex. Further, certain alterations in neurometabolism may be associated with increased memory complaints and lower cognitive reserve. If replicated in larger, longitudinal studies, our findings may have implications for future interventions such as providing biologic targets for pharmacotherapy.
Acknowledgments
This study was funded by National Institutes of Health (1 DP2 OD004445-01: SK).
Contributor Information
Shelli R. Kesler, Email: skesler@stanford.edu, Department of Psychiatry and Behavioral Sciences, Stanford University, 401 Quarry Rd., Stanford, CA 94305-5795, USA. Stanford Cancer Institute, Palo Alto, CA 94304, USA
Christa Watson, Department of Psychiatry and Behavioral Sciences, Stanford University, 401 Quarry Rd., Stanford, CA 94305-5795, USA. Memory and Aging Center, Department of Neurology, University of California at San Francisco, San Francisco, CA 94143, USA.
Della Koovakkattu, Department of Psychiatry and Behavioral Sciences, Stanford University, 401 Quarry Rd., Stanford, CA 94305-5795, USA.
Clement Lee, Department of Psychiatry and Behavioral Sciences, Stanford University, 401 Quarry Rd., Stanford, CA 94305-5795, USA.
Ruth O’Hara, Department of Psychiatry and Behavioral Sciences, Stanford University, 401 Quarry Rd., Stanford, CA 94305-5795, USA.
Misty L. Mahaffey, Stanford Cancer Institute, Palo Alto, CA 94304, USA
Jeffrey S. Wefel, Department of Neuro-Oncology, University of Texas M.D. Anderson Cancer Center, Houston, TX 77030, USA
References
- Aghakhani A, Chan EK. Test Reviews: Bracken, B. A., & Howell, K. (2004). Clinical Assessment of Depression. Odessa, FL: Psychological Assessment Resources. Journal of Psychoeducational Assessment. 2007;25:416–422. [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. Journal of Clinical Oncology. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. International Journal of Developmental Neuroscience: the official Journal of the International Society for Developmental Neuroscience. 2011;29:215–223. doi: 10.1016/j.ijdevneu.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test – revised: normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Boripuntakul S, Kothan S, Methapatara P, Munkhetvit P, Sungkarat S. Short-term effects of cognitive training program for individuals with amnestic mild cognitive impairment: a pilot study. Physical & Occupational Therapy in Geriatrics. 2012;30:138–149. [Google Scholar]
- Bozgeyik Z, Burakgazi G, Sen Y, Ogur E. Age-related metabolic changes in the corpus callosum: assessment with MR spectroscopy. Diagnostic and Interventional Radiology. 2008;14:173–176. [PubMed] [Google Scholar]
- Brown MS, Stemmer SM, Simon JH, Stears JC, Jones RB, Cagnoni PJ, Sheeder JL. White matter disease induced by high-dose chemotherapy: longitudinal study with MR imaging and proton spectroscopy. AJNR American Journal of Neuroradiology. 1998;19:217–221. [PMC free article] [PubMed] [Google Scholar]
- Bruno J, Hosseini SM, Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis. 2012;48:329–338. doi: 10.1016/j.nbd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26:955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman N, Dienes K, Barnea-Goraly N, Reiss A, Ketter T. Decreased N-acetylaspartate in children with familial bipolar disorder. Biol Psychiatry. 2003;53:1059–1065. doi: 10.1016/s0006-3223(02)01744-4. [DOI] [PubMed] [Google Scholar]
- Chang K, Karchemskiy A, Kelley R, Howe M, Garrett A, Adleman N, Reiss A. Effect of divalproex on brain morphometry, chemistry, and function in youth at high-risk for bipolar disorder: a pilot study. J Child Adolesc Psychopharmacol. 2009;19:51–59. doi: 10.1089/cap.2008.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, Lavini C, Linn SC, Boven E, van Dam FS, Schagen SB. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: Converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. 2011a doi: 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FS, Nederveen AJ, Boven E, Schagen SB. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011b;32:1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Van den Stock J, Smeets A, Christiaens MR, Leemans A, Van Hecke W, Vandenberghe J, Vandenbulcke M, Sunaert S. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Human Brain Mapping. 2011;32:480–493. doi: 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez S, Amant F, Smeets A, Peeters R, Leemans A, Van Hecke W, Verhoeven JS, Christiaens MR, Vandenberghe J, Vandenbulcke M, Sunaert S. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30:274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- Diamond A. Biological and social influences on cognitive control processes dependent on prefrontal cortex. Progress in Brain Research. 2011;189:319–339. doi: 10.1016/B978-0-444-53884-0.00032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. Executive functions and their disorders. British Medical Bulletin. 2003;65:49–59. doi: 10.1093/bmb/65.1.49. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Cooray D, Salvador C, Jovicich J, Walot I, Boone K, Chlebowski R. The effects of tamoxifen and estrogen on brain metabolism in elderly women. J Natl Cancer Inst. 2002;94:592–597. doi: 10.1093/jnci/94.8.592. [DOI] [PubMed] [Google Scholar]
- Francis PT. Glutamatergic systems in Alzheimer’s disease. Int J Geriatr Psychiatry. 2003;18:S15–21. doi: 10.1002/gps.934. [DOI] [PubMed] [Google Scholar]
- Gamper N, Shapiro MS. Regulation of ion transport proteins by membrane phosphoinositides. Nature reviews. Neuroscience. 2007;8:921–934. doi: 10.1038/nrn2257. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DHS, Geist C, Breen EC, Irwin MR, Cole SW. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain, Behavior, and Immunity. 2013;30:S99–S108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin card sorting test computer version 4 -research edition (WCST:CV4) Odessa: Psychological Assessment Resources; 2004. [Google Scholar]
- Henigsberg N, Kalember P, Hrabac P, Rados M, Bajs M, Kovavic Z, Loncar M, Madzar T. 1-H MRS changes in dorsolateral prefrontal cortex after donepezil treatment in patients with mild to moderate Alzheimer’s disease. Collegium Antropologicum. 2011;35(Suppl 1):159–162. [PubMed] [Google Scholar]
- Homack S, Lee D, Riccio CA. Test review: Delis-Kaplan executive function system. J Clin Exp Neuropsychol. 2005;27:599–609. doi: 10.1080/13803390490918444. [DOI] [PubMed] [Google Scholar]
- Hosseini SM, Koovakkattu D, Kesler SR. Altered small-world properties of gray matter networks in breast cancer. BMC Neurol. 2012;12:28. doi: 10.1186/1471-2377-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Seminars in Oncology. 2011;38:431–438. doi: 10.1053/j.seminoncol.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer. 2005;104:2222–2233. doi: 10.1002/cncr.21469. [DOI] [PubMed] [Google Scholar]
- Joshi G, Aluise CD, Cole MP, Sultana R, Pierce WM, Vore M, St Clair DK, Butterfield DA. Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: implications for oxidative stress-mediated chemobrain. Neuroscience. 2010;166:796–807. doi: 10.1016/j.neuroscience.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Bennett FC, Mahaffey ML, Spiegel D. Regional brain activation during verbal declarative memory in metastatic breast cancer. Clin Cancer Res. 2009a;15:6665–6673. doi: 10.1158/1078-0432.CCR-09-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Lightbody AA, Reiss AL. Cholinergic dysfunction in fragile X syndrome and potential intervention: a preliminary 1H MRS study. Am J Med Genet A. 2009b;149A:403–407. doi: 10.1002/ajmg.a.32697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Kent JS, O’Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol. 2011;68:1447–1453. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, Dhabhar FS. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30:S109–S116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, de Ruiter MB, van der Lijn F, Boogerd W, Seynaeve C, van der Lugt A, Vrooman H, Niessen WJ, Breteler MM, Schagen SB. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1888-1. [DOI] [PubMed] [Google Scholar]
- Leh SE, Petrides M, Strafella AP. The neural circuitry of executive functions in healthy subjects and Parkinson’s disease. Neuropsychopharmacology. 2010;35:70–85. doi: 10.1038/npp.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, Mattay VS. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiology of aging. 2010 doi: 10.1016/j.neurobiolaging.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH. MR spectroscopic studies of the brain in psychiatric disorders. Current topics in behavioral neurosciences. 2012 doi: 10.1007/7854_2011_197. [DOI] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Research and Treatment. 2010;123:819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012a doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: A replication and extension study. Brain Behav Immun. 2012b doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCI. Breast Cancer Risk in American Women. 2012 Sep 24; Retrieved 01/30/2013, 2013, from http://www.cancer.gov/cancertopics/factsheet/detection/probability-breast-cancer.
- Newman EL, Gupta K, Climer JR, Monaghan CK, Hasselmo ME. Cholinergic modulation of cognitive processing: insights drawn from computational models. Front Behav Neurosci. 2012;6:24. doi: 10.3389/fnbeh.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet LA, Stewart A, Collins B, Schindler D, Bielajew C. Measuring neuropsychological change following breast cancer treatment: an analysis of statistical models. J Clin Exp Neuropsychol. 2009;31:73–89. doi: 10.1080/13803390801992725. [DOI] [PubMed] [Google Scholar]
- Prescot AP, Locatelli AE, Renshaw PF, Yurgelun-Todd DA. Neurochemical alterations in adolescent chronic marijuana smokers: a proton MRS study. Neuroimage. 2011;57:69–75. doi: 10.1016/j.neuroimage.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher S. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Quesnel C, Savard J, Ivers H. Cognitive impairments associated with breast cancer treatments: results from a longitudinal study. Breast Cancer Res Treat. 2009;116:113–123. doi: 10.1007/s10549-008-0114-2. [DOI] [PubMed] [Google Scholar]
- Roth RM, Isquith PK, Gioia G. Behavioral rating inventory of executive function - adult version. Lutz: Psychological Assessment Resources; 2005. [Google Scholar]
- Schifitto G, Deng L, Yeh TM, Evans SR, Ernst T, Zhong J, Clifford D. Clinical, laboratory, and neuroimaging characteristics of fatigue in HIV-infected individuals. J Neurovirol. 2011;17:17–25. doi: 10.1007/s13365-010-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Weber-Fahr W, Schweinfurth N, Ho YJ, Sartorius A, Spanagel R, Pawlak CR. Central metabolite changes and activation of microglia after peripheral interleukin-2 challenge. Brain Behav Immun. 2012;26:277–283. doi: 10.1016/j.bbi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, Waddell K, Petersen L, Phelps ME, Ganz PA. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, White T. NAB Categories Test. Lutz: Psychological Assessment Resources; 2005. [Google Scholar]
- Stilley CS, Bender CM, Dunbar-Jacob J, Sereika S, Ryan CM. The impact of cognitive function on medication management: three studies. Health Psychology: official Journal of the Division of Health Psychology, American Psychological Association. 2010;29:50–55. doi: 10.1037/a0016940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangpong J, Cole MP, Sultana R, Joshi G, Estus S, Vore M, St Clair W, Ratanachaiyavong S, St Clair DK, Butterfield DA. Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiology of Disease. 2006;23:127–139. doi: 10.1016/j.nbd.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Tangpong J, Cole MP, Sultana R, Estus S, Vore M, St Clair W, Ratanachaiyavong S, St Clair DK, Butterfield DA. Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: insight into the mechanism of chemobrain. J Neurochem. 2007;100:191–201. doi: 10.1111/j.1471-4159.2006.04179.x. [DOI] [PubMed] [Google Scholar]
- Tayebati SK, Tomassoni D, Di Stefano A, Sozio P, Cerasa LS, Amenta F. Effect of choline-containing phospholipids on brain cholinergic transporters in the rat. Journal of the Neurological Sciences. 2011;302:49–57. doi: 10.1016/j.jns.2010.11.028. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Rich JB. Psychometric properties of a new metamemory questionnaire for older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2002;57:P19–P27. doi: 10.1093/geronb/57.1.p19. [DOI] [PubMed] [Google Scholar]
- Vardy J. Cognitive function in breast cancer survivors. Cancer Treat Res. 2009;151:387–419. doi: 10.1007/978-0-387-75115-3_24. [DOI] [PubMed] [Google Scholar]
- Vehmanen L, Elomaa I, Blomqvist C, Saarto T. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. Journal of Clinical Oncology: official Journal of the American Society of Clinical Oncology. 2006;24:675–680. doi: 10.1200/JCO.2005.02.3515. [DOI] [PubMed] [Google Scholar]
- Von Ah D, Carpenter JS, Saykin A, Monahan P, Wu J, Yu M, Rebok G, Ball K, Schneider B, Weaver M, Tallman E, Unverzagt F. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2012;135:799–809. doi: 10.1007/s10549-012-2210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Xiao S, Li X, Ding B, Ling H, Chen K, Fang Y. Using proton magnetic resonance spectroscopy to identify mild cognitive impairment. International psychogeriatrics/IPA. 2011:1–9. doi: 10.1017/S1041610211000962. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 4. San Antonio: The Psychological Corporation; 2008. [Google Scholar]
- Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12:267–275. doi: 10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Vardy J, Ahles T, Schagen SB. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. The Lancet Oncology. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- Winocur G, Binns MA, Tannock I. Donepezil reduces cognitive impairment associated with anti-cancer drugs in a mouse model. Neuropharmacology. 2011;61:1222–1228. doi: 10.1016/j.neuropharm.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Yang B, Akhter S, Chaudhuri A, Kanmogne GD. HIV-1 gp120 induces cytokine expression, leukocyte adhesion, and transmigration across the blood–brain barrier: modulatory effects of STAT1 signaling. Microvasc Res. 2009;77:212–219. doi: 10.1016/j.mvr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorek MA, Dunlap JA, Thomas MJ, Cammarata PR, Zhou C, Lowe WL., Jr Effect of TNF-alpha on SMIT mRNA levels and myo-inositol accumulation in cultured endothelial cells. Am J Physiol. 1998;274:C58–71. doi: 10.1152/ajpcell.1998.274.1.C58. [DOI] [PubMed] [Google Scholar]