Abstract
Study Design
An in vitro study to investigate the anti-inflammatory effects of fullerol on mouse dorsal root ganglia (DRG) under TNF-α induction.
Objective
To evaluate the potential of a free radical scavenger, fullerol nanoparticles, to prevent DRG tissue and neuron inflammatory responses under TNF-α induction in vitro.
Summary of Background Data
Low back pain is one of the most common reasons for clinician visits in western societies. Symptomatic intervertebral disc (IVD) degeneration is strongly implicated as a cause of low back pain as it results in DRG inflammation. Increased production of reactive oxygen species (ROS) is associated with DRG inflammation.
Methods
With or without fullerol treatment, DRG tissue and DRG neurons isolated from wild type C3H/HeNCrl mice were cultured under TNF-α induction. The amount of intracellular ROS was measured with H2DCFDA fluorescence staining. Cellular apoptosis was detected via terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. The expression of inflammatory as well as anti-oxidative enzyme genes in neurons was analyzed by real-time PCR. In addition, inflammatory cytokine expression in DRG tissue was determined by immunofluorescence staining and enzyme-linked immunosorbent assay (ELISA).
Results
Fluorescence staining results indicated that TNF-α markedly increased the production of intracellular ROS and the number of apoptotic cells. Under fullerol treatment cellular apoptosis was reduced along with concomitant suppression of ROS. The expression of inflammatory cytokines IL-1 β, IL-6, COX-2, and PGE2, was also inhibited by fullerol in a dose-dependent manner. Furthermore, fullerol-treated cells exhibited up-regulation of anti-oxidative enzyme genes SOD2 and catalase.
Conclusion
The results obtained from this study clearly suggest that fullerol treatment suppresses the inflammatory responses of DRG and neurons, as well as cellular apoptosis by decreasing the level of ROS and potentially enhancing anti-oxidative enzyme gene expression. Therefore, fullerol has potential to serve as a novel therapeutic agent for low back pain treatment.
Keywords: low back pain, intervertebral disc degeneration, dorsal root ganglia, neuroinflammation, reactive oxygen species, fullerol, free radical scavenging, TNF-α, inflammatory cytokine, anti-oxidative enzyme.
INTRODUCTION
Low back pain is one of the most common pathophysiological conditions for clinical visits, with a lifetime prevalence of 60%–90%.1, 2 Ingrowth of nociceptive neural fiber into deeper parts of the degenerative intervertebral disc (IVD) is considered as one of the most widely accepted pathomechanisms related to chronic discogenic pain.3 In normal disc, the outer layers of the annulus fibrosus (AF) are innervated by sensory nerve endings from the dorsal root ganglia (DRG).4 As the disc degeneration proceeds, disc inflammation may promote axonal growth of afferent fibers innervating the disc by secreting proinflammatory mediators, such as tumor necrosis factor (TNF) and interleukin 6 (IL-6).5 The pain signal could be triggered as the neurons of the DRG transmit the inflammatory signal through the spinal cord to the pain centers of the brain.6 Thus, relieving the inflammatory tension of the DRG would be of great significance to treat low back pain caused by IVD degeneration.
Clinically, epidural injection of steroids, such as corticosteroids which decrease inflammation through inhibition of prostaglandins, has been well accepted practice and a rational approach to treat low back pain. However, some absolute contraindications for this strategy exist: infection, history of severe allergic reaction to any of the injected materials and local malignancy.7 In addition, steroids have no tissue specificity and affect not only inflammation, but also development, homeostasis, metabolism and cognition.8 Administration of inhibitors for inflammatory mediators has also been investigated to treat low back pain. The cytokine TNF-α is a mediator of chronic pain produced by the onset of inflammation or sensory nerve damage. The levels of TNF-α are shown to be elevated in animal models of inflammatory response or neuropathic pain around the spinal cord and at the site of injury.9 Several clinical trials showed that application of the TNF-α inhibitor etancercept onto the spinal nerve produced pain relief.10–12 However, it was noted that etancercept has serious potential toxicity and widespread adoption of this method should await further studies.10 Therefore, discovering alternative novel agents to treat low back pain is of utmost importance.
Reactive oxygen species (ROS), at a moderate level, are recognized to be physically involved in cell signaling and required for biochemical energetics of life. However, when produced in excess, ROS can be associated with several pathological conditions including cellular inflammatory responses.13, 14 Under potentially pathological conditions, a cellular oxidative burst, or respiratory burst occurs resulting in synthesis of an array of soluble factors, such as ROS, TNF-α, and IL-1 α/β, which further induce neuronal dysfunction and degeneration.15 Therefore, attenuation of oxidative tension by quenching ROS may be a novel way to rescue DRG inflammation caused by IVD degeneration, towards low back pain treatment.
Recently, fullerene (C60) and its derivatives have drawn a great deal of attention in biomedical research fields due to their anti-oxidative features. Fullerene is considered to function as a “free radical sponge” and is shown to quench varieties of free radicals more efficiently than conventional antioxidants.16 The anti-oxidative properties of fullerene are attributed to a highly delocalized π double bond system forming the carbon cage.17 Gharbi et al18 demonstrated that fullerene has strong protective effects on rodent liver against free radical damage due to its antioxidative features.
In the current study, we investigated the anti-inflammatory effects of fullerol, a polyhydroxylated derivative of fullerene, which possesses good biocompatibility and excellent efficiency in eliminating reactive free radicals.19 It was hypothesized that fullerol nanoparticles protect DRG tissue and neurons from TNF-α-induced inflammatory responses by decreasing oxidative tension as well as other pathways. Our results demonstrated the anti-inflammatory effects of fullerol on TNF-α-induced mouse DRG tissue and neurons. This study is of clinical significance for relieving low back pain caused by IVD degeneration.
MATERIALS AND METHODS
Isolation and Culture of DRG Tissue and Neurons
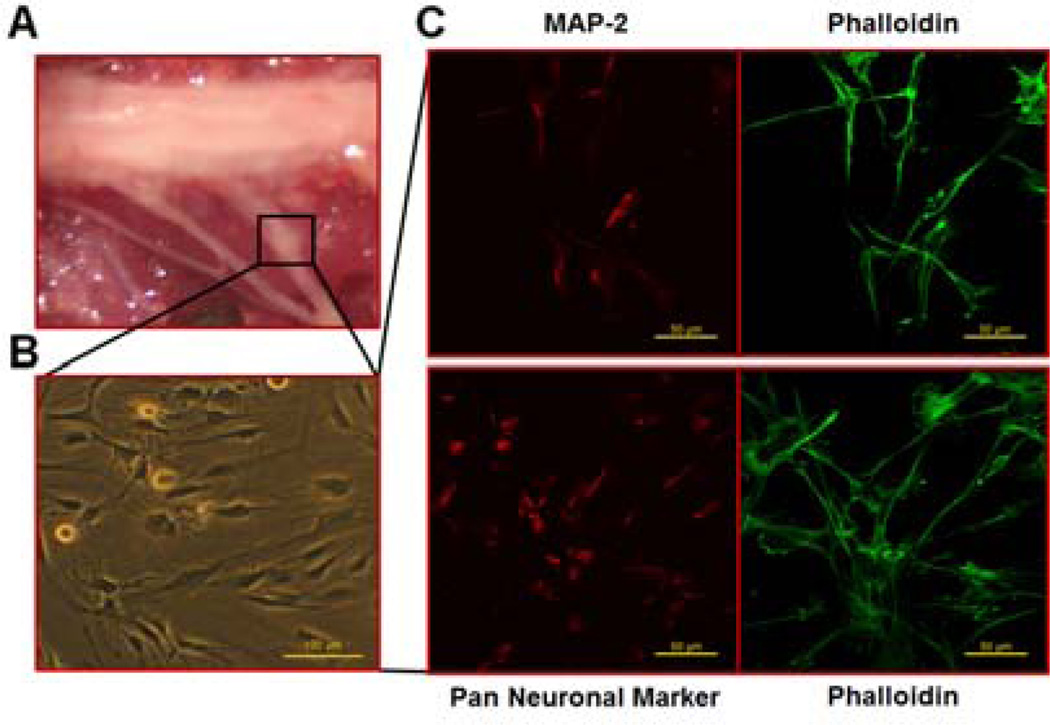
Animal protocols were approved by the Institutional Animal Care & Use Committee at University of Virginia. Twenty male C3H/HeNCrl wild-type mice of one month old were purchased from Charles River Laboratories (Wilmington, MA). Mice were sacrificed with CO2 asphyxiation followed by cervical dislocation. The bilateral DRG were immediately collected from the spinal column (Fig. 1A), and cultured in growth medium (GM, F-12 medium supplemented with 10% fetal bovine serum (FBS, Life Technologies Corporation, Grand Island, NY), 100 U/mL penicillin, 100 µg/mL streptomycin and 10 ng/ml nerve growth factors (NGF, BD Biosciences, San Jose, CA)).
Fig. 1.
Isolation of mouse DRG and characterization of neurons. The DRG from C3H/HeNCrl mice were dissected from the spinal column (A) and neurons were harvested and cultured on coverslips (B). Bar scale=100 µm. Immunofluorescence staining (C) was performed to detect MAP-2 (red) and Pan Neuronal Marker (red). Cells were counter-stained with Alexa Fluor 488-conjugated phalloidin (green). Bar scale=50 µm.
To isolate neurons, the DRG were incubated in Hank’s Balanced Salt Solution (HBSS, free of Ca2+ and Mg2+, Life Technologies Corporation) containing 40 U/mL papain (Worthington Biochemical Corporation, Lakewood, NJ) at 37°C for 10 min, followed by incubation with HBSS containing 0.2% collagenase (SERVA Electrophoresis GmbH, Heidelberg, Germany) at 37°C for 20 min, and then mechanically dissociated with plastic pipettes. Growth medium was added to stop digestion and the cells were centrifuged at 1×103 rpm for 5 min. The cell pellet was re-suspended with GM and seeded onto poly-D-lysine/laminin-coated coverslips (BD Biosciences) in 24-well culture plates at a density of 1×104 cells/cm2. Culture medium was changed every 3 days.
The isolated cells were first performed with immunofluorescence staining to detect their expression of neuronal markers. Cell cultures and DRG were both treated with different doses of fullerol (Materials and Electrochemical Research Corporation, Tucson, AZ). Intracellular ROS detection, Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) assay to appraise cellular apoptosis, as well as real-time PCR to detect inflammatory marker expression, were performed on cell cultures. Accordingly, immunofluorescence staining and Enzyme-linked Immunosorbent Assay (ELISA) were carried out on DRG tissue to detect inflammatory marker expression.
Immunofluorescence Staining
After 7 days, the primary cell cultures were fixed with 4% paraformaldehyde for 15 min, washed with PBS twice and then permeabilized with PBS containing 0.25% Triton X-100 for 10 min. After blocked with blocking buffer (3% BSA and 5% goat serum in PBS) for 30 min, polyclonal rabbit anti-mouse microtubule-associated protein 2 (MAP-2, 1:500, Santa Cruz Biotechnology, Santa Cruz, CA) or Pan Neuronal Marker (1:500, EMD Millipore, Billerica, MA) primary antibodies were applied at 4°C overnight, and the signals were visualized with a secondary antibody of goat anti-rabbit IgG conjugated with Alexa Fluor 594 (Life Technologies Corporation). Cellular F-actin was stained with Alexa Fluor 488–conjugated phalloidin (Life Technologies Corporation). The images were taken under an Olympus Fluoview 300 confocal microscope.
For immunofluorescence staining, the DRG were fixed with ice-cold 4% paraformaldehyde for 30 min and then placed in 5% sucrose-PBS for 1 h and in 15% sucrose-PBS for 1 h. Specimens were embedded in CRYO-OCT Compound (Andwin Scientific, Tryon, NC) and frozen at −80°C. The 7-µm sections were washed with PBS twice and then permeabilized with PBS containing 0.25% Triton X-100 for 10 min. After blocked with blocking buffer (3% BSA and 5% goat serum in PBS) for 30 min, polyclonal rabbit anti-mouse IL-1 β (1:50, Santa Cruz Biotechnology) or IL-6 (1:500, Abcam, Cambridge, MA) primary antibodies were applied at 4°C overnight. The signals were visualized with Alexa Fluor 488-conjugated goat anti-rabbit IgG (Life Technologies Corporation). Nuclei were counter-stained with TO-PRO®-3 Iodide (1:5000, Life Technologies Corporation). Immunofluorescence images were taken under an Olympus BX51 fluorescence microscope.
Intracellular ROS Detection
The primary neurons were seeded onto poly-D-lysine/laminin-coated coverslips and cultured in 24-well plates with GM. After 7 days, the medium was changed to GM, GM+10 ng/mL TNF-α (Cell Signaling Technology, Danvers, MA) with 0, 0.1, 1, or 10 µM fullerol. Three days later, the cells were incubated with serum free medium containing 10 µM H2DCFDA (Life Technologies Corporation) for 30 min. Cells were then washed twice with PBS and the green fluorescence was observed under an Olympus BX51 fluorescence microscope.
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
The primary neurons were treated as for ROS detection. Three days later, the cellular apoptosis was assessed using TUNEL assay kit (Roche Applied Science, Indianapolis, IN) as previously described.20 The TUNEL-positive (apoptotic) nuclei were stained as green fluorescence. Nuclei were counter-stained with propidium iodide (PI, 50 µg/mL, Life Technologies Corporation) as red fluorescence.
Real-time PCR Assay
After 3 days of treatment as described above, total RNA from neurons was extracted using the RNeasy Mini Kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. The concentration of RNA was determined from the optical absorbance at 260 nm. Complementary DNA (cDNA) was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions.
Real-time PCR was performed with iQ™ 5 multicolor real-time PCR Detection System (Bio- Rad, Hercules, CA) as previously described.21 The expression of inflammatory genes IL-6 and cyclooxygenase-2 (COX-2), as well as anti-oxidative enzyme genes superoxide dismutase 2 (SOD2) and catalase, was evaluated. The 18S rRNA was used as an internal control to normalize the signals from genes of interest. Sequences of primers, individual annealing temperature and amplicon lengths are shown in Table 1.
Table 1.
Sequences of Primers and Real-time PCR Conditions
| Gene | Primers (F=forward; R=reverse) | Amplicon size (bp) |
Annealing temperature (°C) |
|---|---|---|---|
| IL-6 | F: 5’-TTCCATCCAGTTGCCTTCTTG-3’ | 101 | 58 |
| R: 5’-TTGGGAGTGGTATCCTCTGTGA-3’ | |||
| COX-2 | F: 5’-TGAGTACCGCAAACGCTTCTC-3’ | 151 | 58 |
| R: 5’-TGGACGAGGTTTTTCCACCAG-3’ | |||
| SOD2 | F: 5’-ATGTTACAACTCAGGTCGCTCTTC-3’ | 135 | 56 |
| R: 5’-TGATAGCCTCCAGCAACTCTCC-3’ | |||
| catalase | F: 5’-GAACGAGGAGGAGAGGAAAC-3’ | 95 | 56 |
| R: 5’-TGAAATTCTTGACCGCTTTC-3’ | |||
| 18s | F: 5’-CGGCGACGACCCATTCGAAC-3’ | 99 | 58 |
| R: 5’-GAATCGAACCCTGATTCCCCGTC-3’ |
Enzyme-linked Immunosorbent Assay (ELISA)
Freshly isolated L4 DRG were cultured in 24-well plates with GM. Two days later, the medium was changed to GM, GM+25 ng/mL TNF-α with 0, 0.1, 1, or 10 µM fullerol in which both NGF and the 10% FBS were removed to avoid background noise. Twenty-four hours later, the supernatant was collected to analyze the contents of IL-6 (eBioscience, San Diego, CA) and prostaglandin E2 (PGE2, Thermo Scientific, Rockford, IL) via ELISA according to the manufacturers’ instructions. The DRG were fixed for immunofluorescence staining as described above. It showed that the DRG were healthy after 24 h starvation.
Statistical Analysis
All experiments were performed at least in triplicate and data were presented as mean ± standard deviation (SD). Statistical analyses for quantitative assays were performed by One-Way ANOVA assuming equal variance using SPSS 11.0 software. A p-value of less than 0.05 was considered statistically significant.
RESULTS
Characterization of Neurons Isolated from DRG
Cells isolated from DRG and cultured with GM for 7 days displayed typical neuronal morphology, axons extending outward and several neurites converging on the cells (Fig. 1B). The purity of neurons was evaluated by immunofluorescence staining for both MAP-2 (Fig. 1C upper panel) and Pan Neuronal Marker (Fig. 1C lower panel). MAP-2 is a neuron-specific protein that stabilizes microtubules in the dendrites of postmitotic neurons.22 Pan Neuronal Marker was detected by a polyclonal antibody blend that Millipore has developed to react against key somatic, nuclear, dendritic, and axonal proteins distributed across the pan-neuronal architecture. It is shown that more than 80% of the cells positively expressed both neuronal markers.
Fullerol Decreased Intracellular ROS Content
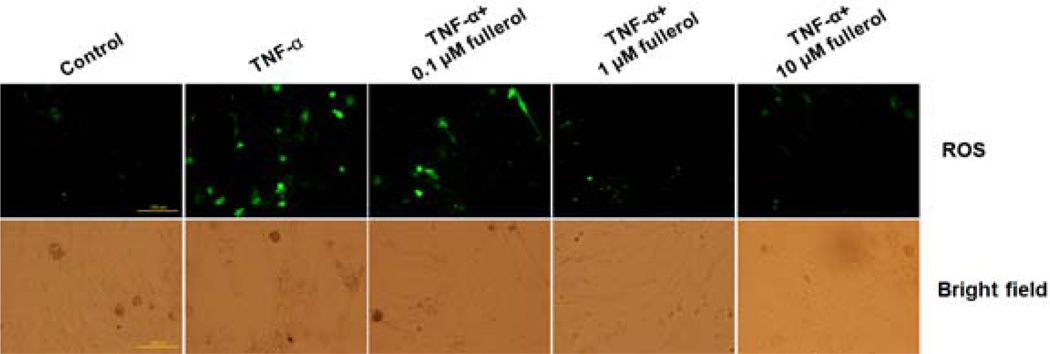
To evaluate the anti-oxidative effects of fullerol, we performed fluorescence staining using H2DCFDA as an indicator to measure intracellular ROS content. Upon cleavage of the acetate groups by intracellular oxidation, the non-fluorescent H2DCFDA would be converted to the highly fluorescent 2',7'-dichlorofluorescein (DCF). As shown in Fig. 2, the number of positively stained cells (green fluorescence) was higher in the TNF-α-treated group compared with that in the control group, and the green fluorescence signal decreased with the addition of fullerol in a dose-dependent manner with the least amount of ROS in both 1 and 10 µM fullerol-treated groups.
Fig. 2.
Fullerol suppressed intracellular ROS in a dose-dependent manner. Neurons were cultured with growth medium (GM), GM+10 ng/mL TNF-α, GM+10 ng/mL TNF-α+0.1 µM fullerol, GM+10 ng/mL TNF-α+1 µM fullerol, or GM+10 ng/mL TNF-α+10 µM fullerol. At day 3, intracellular ROS intensity was detected by fluorescence staining using H2DCFDA as probe. The corresponding bright-field pictures of cell culture were taken. Bar scale=100 µm.
Fullerol Rescued TNF-α-Induced Cellular Apoptosis
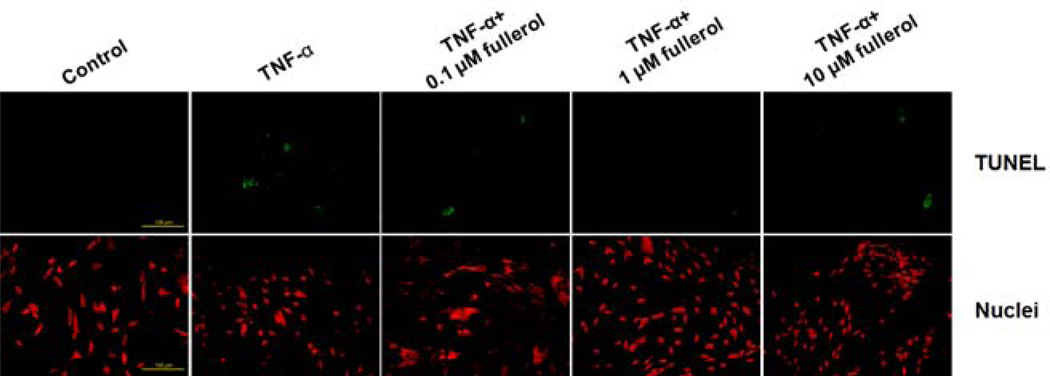
Cellular apoptosis was measured by detection of cleavage of genomic DNA during apoptosis via TUNEL assay (Fig. 3). After 3 days of culture, no detectable apoptosis was seen in control group. In contrast, TNF-α significantly increased the number of apoptotic cells (green fluorescence) when compared with the control group. Cells treated with 1 µM fullerol showed marked effect with few apoptotic cells. There was no clear protective effect in either 0.1 or 10 µM fullerol treatment group. This suggested that fullerol at 1 µM concentration has the best effects and little toxicity.
Fig. 3.
Fullerol prevented cellular apoptosis in a dose-dependent manner. Neurons were cultured as for ROS assay. At day 3, cellular apoptosis was evaluated via TUNEL assay. Apoptotic cells were stained green. Nuclei were counter-stained with propidium iodide (PI, red). Bar scale=100 µm.
Fullerol Attenuated Inflammatory Responses of DRG and Neurons
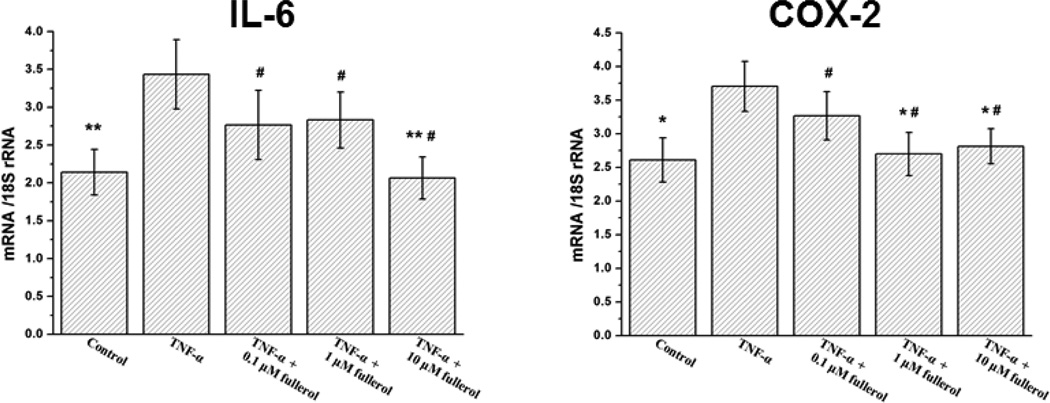
As shown in Fig. 4, fullerol suppressed cellular mRNA expression of IL-6 and COX-2 in a similar manner. TNF-α significantly increased IL-6 (p<0.01) and COX-2 (p<0.05) expression compared with respective control group, while 10 µM fullerol reduced this elevation by 40% and 24%, respectively, and both of IL-6 and COX-2 expression went down to basal control levels (p>0.05).
Fig. 4.
Fullerol suppressed neuron inflammatory gene expression. Neurons were cultured as for ROS assay. At day 3, total RNA was extracted and inflammatory gene expression of IL-6 and COX-2 was evaluated by real-time PCR. * p<0.05, ** p<0.01 compared with TNF-α-only group. # p>0.05 compared with basal control group.
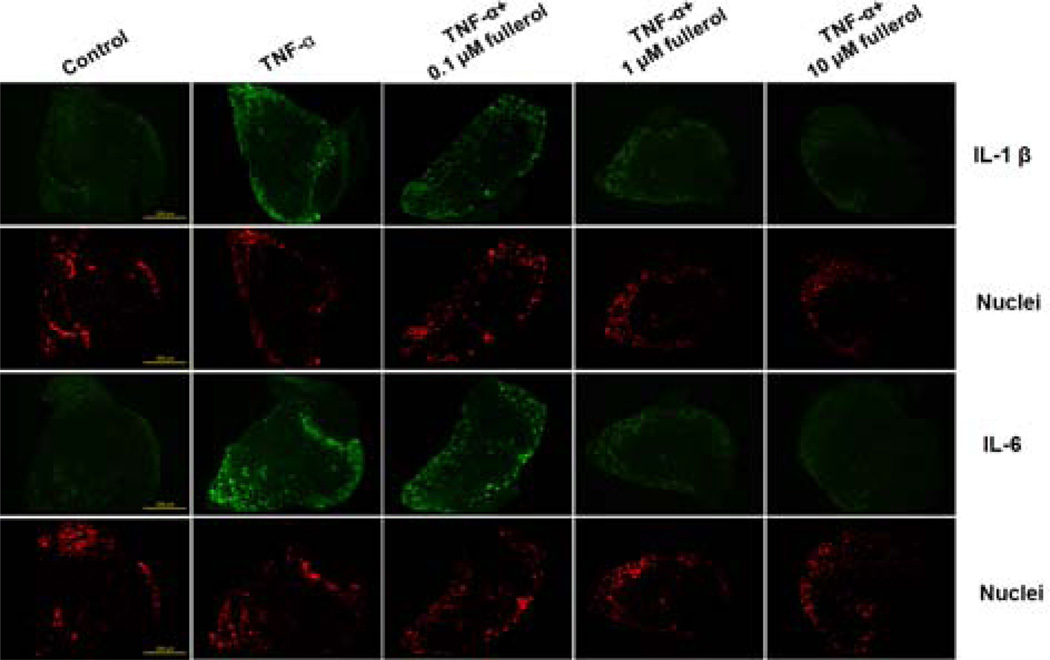
The mRNA expression results were further confirmed by in situ detection of IL-1 β and IL-6 in DRG tissue. By immunofluorescence staining, as shown in Fig. 5, TNF-α treatment markedly increased both IL-1 β and IL-6 expression compared with controls and, the green fluorescence intensity was decreased by fullerol treatment in a dose-dependent fashion. The expression of both IL-1 β and IL-6 in either 1 or 10 µM fullerol-treated group was reduced close to control groups.
Fig. 5.
Fullerol suppressed IL-1 β and IL-6 expression in DRG tissue. Mouse L4 DRG were cultured with GM, GM+25 ng/mL TNF-α, GM+25 ng/mL TNF-α+0.1 µM fullerol, GM+25 ng/mL TNF-α+1 µM fullerol, or GM+25 ng/mL TNF-α+10 µM fullerol, in which NGF and the 10% FBS were removed. Twenty-four hours later, cryostat sections of the DRG samples were got and immunofluorescence staining was performed to detect IL-1 β and IL-6 expression (green). Nuclei were counter-stained with TO-PRO®-3 Iodide (red). Bar scale=200 µm.
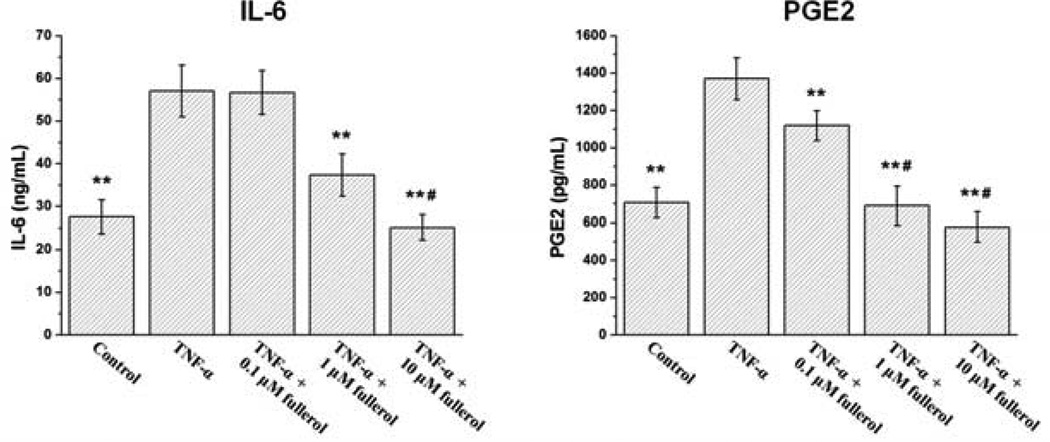
The ELISA results further confirmed the above observation quantitatively. As shown in Fig. 6, TNF-α significantly increased IL-6 expression compared with control group (p<0.01), and this promotion was inhibited by fullerol at 1 and 10 µM (p<0.01). There was also a similar dose-dependent suppressive effect of fullerol on PGE2 secretion. Notably, 0.1 µM fullerol was strong enough to significantly suppress PGE2 secretion (p<0.01) induced by TNF-α treatment. Fullerol at 1 µM concentration was enough to reduce PGE2 secretion to basal level (p>0.05). For consistency, only L4 DRG were employed for ELISA in this study.
Fig. 6.
Detection of fullerol suppressive effects on IL-6 and PGE2 expression in DRG via ELISA. Mouse L4 DRG were cultured as for immunofluorescence staining. Twenty-four hours later, the supernatant was collected to detect IL-6 and PGE2 contents via ELISA. ** p<0.01 compared with TNF-α-only group. # p>0.05 compared with basal control group.
Fullerol up-regulated the expression of SOD2 and Catalase
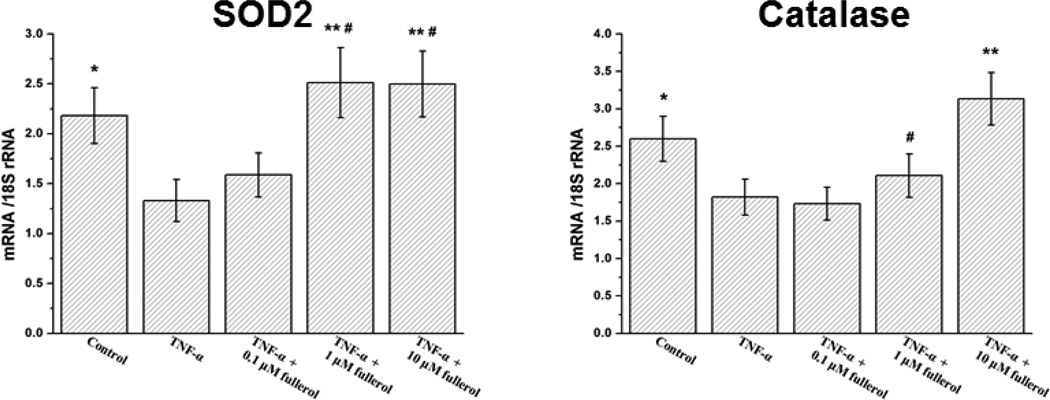
As it has been demonstrated the potent antioxidants could protect cells by promoting cellular anti-oxidative enzyme expression, 23, 24 we asked whether fullerol, as an antioxidant, would suppress neuron inflammatory responses in the same way. As shown in Fig. 7, the treatment of TNF-α dramatically decreased the expression of SOD2 and catalase mRNA expression compared with the control groups (p<0.05), while fullerol at 1 µM concentration completely rescued their expression from TNF-α assault by increasing it close to the basal levels (p>0.05). When treated with 10 µM fullerol, the expression of catalase was actually increased by 20% compared with control group (p<0.05). These results suggested that up-regulating SOD2 and catalase may contribute to the suppression of inflammation by fullerol at least partially in this model.
Fig. 7.
Fullerol enhanced neuron anti-oxidative enzyme genes SOD2 and catalase expression. Neurons were cultured as for ROS assay. At day 3, total RNA was extracted and anti-oxidative enzyme gene expression of SOD2 and catalase was evaluated by real-time PCR. * p<0.05, ** p<0.01 compared with TNF-α-only group. # p>0.05 compared with basal control group.
DISCUSSION
Most individuals, admitted to hospital with low back pain, have a long history of unsuccessful non-operative therapy.25 A therapeutic strategy without aggressive surgery may offer an attractive alternative to treat low back pain. Clinically, epidural injection of steroids and administration of inhibitors for inflammatory mediators have absolute contraindications.7, 10 To overcome these obstacles, we envisioned the possibility of fullerol injection as a novel therapeutic strategy against low back pain. The promising results obtained from this study clearly indicate that fullerol treatment has potential to treat low back pain and the strategy deserves further animal investigations aimed for clinical applications.
Fullerene is a third carbon allotrope composed of 60 carbon atoms arranged in a spherical structure. Since its first detection in 1985,26 fullerene has been explored as “free radical sponge” in many biomedical applications.27–30 As fullerene is highly hydrophobic, extensive studies were focused on the development of water-soluble fullerene derivatives to overcome this obstacle and improve its chances to be an effective antioxidant for therapeutic use.31–33 Fullerol, a polyhydroxylated derivative of fullerene, has been explored to protect endothelial cells against NO-induced damage,34 prevent doxorubicin-induced acute cardiotoxicity,35 and attenuate neutrophilic lung inflammation induced by quartz.36 With this background we performed an in vitro study to demonstrate that fullerol, a potent anti-oxidative agent, suppressed TNF-α-induced inflammatory responses of DRG tissue and neurons.
First, we verified the anti-oxidative effects of fullerol on isolated DRG neurons. Using different doses of fullerol, we showed that fullerol markedly decreased TNF-α-induced intracellular ROS content at 1 and 10 µM concentrations. It has been revealed excessive ROS could cause mitochondrial depolarization associated with the opening of mitochondrial permeability transition pore, following which the release of small pro-apoptotic molecules would lead to activation of caspase cascades and apoptosis.17 In part, fullerol may inhibit cellular apoptosis due to its free radical scavenging potential. Accordingly, results from our study suggest that TNF-α-induced neuron apoptosis was rescued by 1 µM fullerol treatment. However, 10 µM fullerol treatment, which was most effective in suppressing ROS production, did not offer similar protective effect. Therefore, apart from decreasing ROS content, fullerol may play other roles in suppressing neuron apoptosis through separate mechanisms which need further investigation to elucidate. In addition, high concentration of fullerol may have some toxicity on the cells.
The cytokine TNF-α acts through two transmembrane receptors that upon activation release pro-inflammatory signals in target cells.37 Interaction with TNF-α can lead to early NF-κB–mediated transcription of IL-1 and IL-6, which mediate inflammatory hyperalgesia. The 3-day TNF-α treatment on DRG neurons significantly induced the gene expression of IL-6 which is a hallmark of inflammatory response.38 COX-2 is an inducible enzyme and another inflammatory marker, which produces prostaglandins that cause inflammation and pain. COX-2 expression is abundant in activated macrophages and other cells at sites of inflammation. TNF-α-treated DRG neurons up-regulated COX-2 gene expression, which was attenuated by 1 and 10 µM fullerol to the basal level. These results clearly demonstrate that fullerol suppressed the expression of the two key inflammatory genes IL-6 and COX-2 in neurons.
In addition, as revealed by immunofluorescence staining, TNF-α suppressed expression of both IL-1 β and IL-6 in DRG tissue. IL-1 β is a member of the IL-1 cytokine family, which associated with the other proinflammatory cytokines TNF-α and IL-6 plays key roles in inflammatory response.39 The induction of COX-2 by IL-1 β in the central nervous system contributes to inflammatory pain hypersensitivity. Our ELISA results revealed that fullerol treatment suppressed both IL-6 and PGE2 expression in TNF-α-induced DRG tissue. PGE2 is one of the metabolic products of cyclooxygenase and is responsible for enhanced secretion of neuropeptide release which subsequently increases excitability in sensory neurons. Interestingly, we not only found the suppressive effects of fullerol on COX-2 expression in DRG neurons, but also found the inhibition of PGE2 secretion, a product of COX-2, in DRG tissue. Taken together, we conclude that fullerol attenuated inflammatory responses of DRG under TNF-α induction.
Even though it is still controversial, TNF-α may affect different target cells by suppressing or promoting cellular anti-oxidative enzyme expression.40–42 In this study, we demonstrated that TNF-α treatment significantly inhibited neuron mRNA expression of SOD2 and catalase. Intracellular superoxide could be converted to H2O2 by SOD2, and then be catalyzed to H2O and O2 by anti-oxidative enzymes including catalase.43, 44 Therefore, SOD2 associated with catalase plays significant roles in effective augmentation of cellular anti-oxidative defense mechanisms. As has been shown that decreased concentrations of intracellular SOD and catalase led to an increase in the production of reactive free radicals,42 we hypothesize that the decreased expression of both SOD2 and catalase and further increased ROS production may be one of pathways for TNF-α-induced inflammation.
Our results showed that TNF-α dramatically decreased both SOD2 and catalase gene expression, while this effect was reversed by fullerol treatment. Superoxide anions have been demonstrated to play proinflammatory roles in many diseases, causing lipid peroxidation and oxidation, DNA damage, peroxynitrite ion formation, and recruitment of neutrophils to sites of inflammation.45 Elimination of superoxide anions by SOD and its isoenzymes can, therefore, be considered to be anti-inflammatory. MnTBAP, a mimetic of SOD suppressed inflammation responses in a lung pleurisy rat model.46 Montano et al.47 showed that the peripheral blood mononuclear cells with an Ala16Val-SOD2 polymorphism which has a decreased efficiency of SOD2 transport into targeted mitochondria, produced higher levels of proinflammatory cytokines such as IL-1, IL-6, and TNF-α. Pani et al.48 stated that even small amounts of SOD are crucial for cell resistance to inflammatory stimuli. All these results suggested that SOD plays an important role in suppression of inflammation. Similarly, it was reported that the rat liver with adjuvant-induced arthritis presented a pronounced oxidative stress which was the consequence of the strongly diminished catalase activities.49 Administration of catalase attenuated the effects of proinflammatory cytokines (IL-1β, TNF-α, and IFN-γ) in human alveolar epithelial cell line A549.50 Microencapsulated compounds of antioxidant enzyme catalase inhibited hydrogen peroxide production, nitrate synthesis and TNF release in cell culture models using endothelial cells.51 Therefore, catalase also plays an important role in suppression of inflammation. Thus, we propose that there may be two possible pathways through which fullerol nanoparticles prevented cellular inflammatory responses. The first one is that fullerol rescued cells from inflammation by directly eliminating over-produced ROS that have a potential role in causing cellular and tissue damage.52 The second one is that fullerol treatment promoted cellular anti-oxidative enzyme expression through proper signaling pathways. The understanding of the details of the underlying mechanisms warrants further investigation.
In conclusion, this is the first reported observation showing that fullerol, a potent anti-oxidative agent, suppressed TNF-α-induced ROS and inflammatory cytokine production in DRG tissue and neurons in vitro. Further in vivo animal studies are needed to demonstrate the therapeutic effects of fullerol treatment and validate this treatment strategy.
Key Points.
Fullerol nanoparticles efficiently scavenged increased ROS induced by TNF-α, and retarded neuron apoptosis.
Fullerol nanoparticles suppressed DRG and neuron inflammation in a dose-dependent manner presumably by enhancing anti-oxidative enzyme gene expression.
Fullerol injection may serve as a novel therapeutic strategy for low back pain treatment.
Acknowledgments
The manuscript submitted does not contain information about medical device(s)/drug(s). AO Foundation Focus grant funds and NIH R21 grant funds were received in support of this work.
Footnotes
No relevant financial activities outside the submitted work.
REFERENCES
- 1.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354(9178):581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 2.Deyo RA, Weinstein JN. Low back pain. The New England journal of medicine. 2001;344(5):363–370. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- 3.Lee MKB, Lim EJ, Back SK, et al. Complete Freund's adjuvant-induced intervertebral discitis as an animal model for discogenic low back pain. Anesth Analg. 2009;109(4):1287–1296. doi: 10.1213/ane.0b013e3181b31f39. [DOI] [PubMed] [Google Scholar]
- 4.Moon HJ, Kim JH, Lee HS, et al. Annulus fibrosus cells interact with neuron-like cells to modulate production of growth factors and cytokines in symptomatic disc degeneration. Spine (Phila Pa 1976) 2012;37(1):2–9. doi: 10.1097/BRS.0b013e31820cd2d8. [DOI] [PubMed] [Google Scholar]
- 5.Inoue G, Ohtori S, Aoki Y, et al. Exposure of the nucleus pulposus to the outside of the anulus fibrosus induces nerve injury and regeneration of the afferent fibers innervating the lumbar intervertebral discs in rats. Spine (Phila Pa 1976) 2006;31(13):1433–1438. doi: 10.1097/01.brs.0000219946.25103.db. [DOI] [PubMed] [Google Scholar]
- 6.Hadjipavlou AG, Simmons JW, Yang JP, et al. Torsional injury resulting in disc degeneration in the rabbit: II. Associative changes in dorsal root ganglion and spinal cord neurotransmitter production. Journal of spinal disorders. 1998;11(4):318–321. [PubMed] [Google Scholar]
- 7.Colonno DV, Harrast MA, Herring SA. Overview of spinal interventions. Clinics in sports medicine. 2012;31(3):409–422. doi: 10.1016/j.csm.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Biddie SC, Conway-Campbell BL, Lightman SL. Dynamic regulation of glucocorticoid signalling in health and disease. Rheumatology (Oxford) 2012;51(3):403–412. doi: 10.1093/rheumatology/ker215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehrenbacher JCBT, Nicol GD, Vasko MR. Tumor necrosis factor alpha and interleukin-1beta stimulate the expression of cyclooxygenase II but do not alter prostaglandin E2 receptor mRNA levels in cultured dorsal root ganglia cells. Pain. 2005;113(1–2):113–122. doi: 10.1016/j.pain.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Tobinick EL, Britschgi-Davoodifar S. Perispinal TNF-alpha inhibition for discogenic pain. Swiss medical weekly. 2003;133(11–12):170–177. doi: 10.4414/smw.2003.10163. [DOI] [PubMed] [Google Scholar]
- 11.Tobinick E, Davoodifar S. Efficacy of etanercept delivered by perispinal administration for chronic back and/or neck disc-related pain: a study of clinical observations in 143 patients. Current medical research and opinion. 2004;20(7):1075–1085. doi: 10.1185/030079903125004286. [DOI] [PubMed] [Google Scholar]
- 12.Ohtori S, Miyagi M, Eguchi Y, et al. Epidural administration of spinal nerves with the tumor necrosis factor-alpha inhibitor, etanercept, compared with dexamethasone for treatment of sciatica in patients with lumbar spinal stenosis: a prospective randomized study. Spine (Phila Pa 1976) 2012;37(6):439–444. doi: 10.1097/BRS.0b013e318238af83. [DOI] [PubMed] [Google Scholar]
- 13.Hatanaka E, Dermargos A, Armelin HA, et al. Serum amyloid A induces reactive oxygen species (ROS) production and proliferation of fibroblast. Clin Exp Immunol. 2011;163(3):362–367. doi: 10.1111/j.1365-2249.2010.04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naha PC, Davoren M, Lyng FM, et al. Reactive oxygen species (ROS) induced cytokine production and cytotoxicity of PAMAM dendrimers in J774A.1 cells. Toxicol Appl Pharmacol. 2010;246(1–2):91–99. doi: 10.1016/j.taap.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Tweedie D, Frankola KA, Luo W, et al. Thalidomide Analogues Suppress Lipopolysaccharide-Induced Synthesis of TNF-alpha and Nitrite, an Intermediate of Nitric Oxide, in a Cellular Model of Inflammation. The open biochemistry journal. 2011;5:37–44. doi: 10.2174/1874091X01105010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krusic PJ, Wasserman E, Keizer PN, et al. Radical reactions of c60. Science. 1991;254(5035):1183–1185. doi: 10.1126/science.254.5035.1183. [DOI] [PubMed] [Google Scholar]
- 17.Markovic Z, Trajkovic V. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60) Biomaterials. 2008;29(26):3561–3573. doi: 10.1016/j.biomaterials.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Gharbi N, Pressac M, Hadchouel M, et al. [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano letters. 2005;5(12):2578–2585. doi: 10.1021/nl051866b. [DOI] [PubMed] [Google Scholar]
- 19.Qingnuan LyX, Xiaodong Z, Ruili L, et al. Preparation of (99m)Tc-C(60)(OH)(x) and its biodistribution studies. Nucl Med Biol. 2002;29(6):707–710. doi: 10.1016/s0969-8051(02)00313-x. [DOI] [PubMed] [Google Scholar]
- 20.Feng G, Wan Y, Shen FH, et al. Nucleus pulposus explant culture model. J Orthop Res. 2009;27(6):814–819. doi: 10.1002/jor.20803. [DOI] [PubMed] [Google Scholar]
- 21.Feng G, Yang X, Shang H, et al. Multipotential differentiation of human anulus fibrosus cells: an in vitro study. J Bone Joint Surg Am. 2010;92(3):675–685. doi: 10.2106/JBJS.H.01672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soltani MH, Pichardo R, Song Z, et al. Microtubule-associated protein 2, a marker of neuronal differentiation, induces mitotic defects, inhibits growth of melanoma cells, and predicts metastatic potential of cutaneous melanoma. The American journal of pathology. 2005;166(6):1841–1850. doi: 10.1016/S0002-9440(10)62493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang KA, Lee KH, Kim SY, et al. Cytoprotective effects of KIOM-79 on streptozotocin induced cell damage by inhibiting ERK and AP-1. Biological & pharmaceutical bulletin. 2007;30(5):852–858. doi: 10.1248/bpb.30.852. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Sun P, Bao Y, et al. Vitamin E renders protection to PC12 cells against oxidative damage and apoptosis induced by single-walled carbon nanotubes. Toxicol In Vitro. 2012;26(1):32–41. doi: 10.1016/j.tiv.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Ozer AF, Oktenoglu T, Sasani M, et al. Unusual cause of acute low-back pain: sudden annulus fibrosus rupture. Orthopedic reviews. 2012;4(2):e22. doi: 10.4081/or.2012.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroto HW, Heath JR, Obrien SC, et al. C-60 - Buckminsterfullerene. Nature. 1985;318(6042):162–163. [Google Scholar]
- 27.Kato S, Aoshima H, Saitoh Y, et al. Fullerene-C60 incorporated in liposome exerts persistent hydroxyl radical-scavenging activity and cytoprotection in UVA/B-irradiated keratinocytes. Journal of nanoscience and nanotechnology. 2011;11(5):3814–3823. doi: 10.1166/jnn.2011.4172. [DOI] [PubMed] [Google Scholar]
- 28.Xiao L, Aoshima H, Saitoh Y, et al. The effect of squalane-dissolved fullerene-C60 on adipogenesis-accompanied oxidative stress and macrophage activation in a preadipocyte-monocyte co-culture system. Biomaterials. 2010;31(23):5976–5985. doi: 10.1016/j.biomaterials.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 29.Yudoh K, Shishido K, Murayama H, et al. Water-soluble C60 fullerene prevents degeneration of articular cartilage in osteoarthritis via down-regulation of chondrocyte catabolic activity and inhibition of cartilage degeneration during disease development. Arthritis and rheumatism. 2007;56(10):3307–3318. doi: 10.1002/art.22917. [DOI] [PubMed] [Google Scholar]
- 30.Yudoh K, Karasawa R, Masuko K, et al. Water-soluble fullerene (C60) inhibits the osteoclast differentiation and bone destruction in arthritis. International journal of nanomedicine. 2009;4:233–239. doi: 10.2147/ijn.s7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolff DJ, Papoiu AD, Mialkowski K, et al. Inhibition of nitric oxide synthase isoforms by tris-malonyl-C(60)-fullerene adducts. Archives of biochemistry and biophysics. 2000;378(2):216–223. doi: 10.1006/abbi.2000.1843. [DOI] [PubMed] [Google Scholar]
- 32.Lin AM, Fang SF, Lin SZ, et al. Local carboxyfullerene protects cortical infarction in rat brain. Neuroscience research. 2002;43(4):317–321. doi: 10.1016/s0168-0102(02)00056-1. [DOI] [PubMed] [Google Scholar]
- 33.Tong J, Zimmerman MC, Li S, et al. Neuronal uptake and intracellular superoxide scavenging of a fullerene (C60)-poly(2-oxazoline)s nanoformulation. Biomaterials. 2011;32(14):3654–3665. doi: 10.1016/j.biomaterials.2011.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lao F, Li W, Han D, et al. Fullerene derivatives protect endothelial cells against NO-induced damage. Nanotechnology. 2009;20(22):225103. doi: 10.1088/0957-4484/20/22/225103. [DOI] [PubMed] [Google Scholar]
- 35.Torres VM, Srdjenovic B, Jacevic V, et al. Fullerenol C60(OH)24 prevents doxorubicin-induced acute cardiotoxicity in rats. Pharmacological reports : PR. 2010;62(4):707–718. doi: 10.1016/s1734-1140(10)70328-5. [DOI] [PubMed] [Google Scholar]
- 36.Roursgaard M, Poulsen SS, Kepley CL, et al. Polyhydroxylated C60 fullerene (fullerenol) attenuates neutrophilic lung inflammation in mice. Basic & clinical pharmacology & toxicology. 2008;103(4):386–388. doi: 10.1111/j.1742-7843.2008.00315.x. [DOI] [PubMed] [Google Scholar]
- 37.Barbara JA, Smith WB, Gamble JR, et al. Dissociation of TNF-alpha cytotoxic and proinflammatory activities by p55 receptor- and p75 receptor-selective TNF-alpha mutants. The EMBO journal. 1994;13(4):843–850. doi: 10.1002/j.1460-2075.1994.tb06327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu XJ, Hao JX, Andell-Jonsson S, et al. Nociceptive responses in interleukin-6-deficient mice to peripheral inflammation and peripheral nerve section. Cytokine. 1997;9(12):1028–1033. doi: 10.1006/cyto.1997.0243. [DOI] [PubMed] [Google Scholar]
- 39.Turner NAMR, Warburton P, O'Regan DJ, et al. Mechanism of TNFalpha-induced IL-1alpha, IL-1beta and IL-6 expression in human cardiac fibroblasts: effects of statins and thiazolidinediones. Cardiovasc Res. 2007;76(1):81–90. doi: 10.1016/j.cardiores.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Brown MD, Feairheller DL, Thakkar S, et al. Racial differences in tumor necrosis factor-alpha-induced endothelial microparticles and interleukin-6 production. Vascular health and risk management. 2011;7:541–550. doi: 10.2147/VHRM.S22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yen GC, Chen YC, Chang WT, et al. Effects of polyphenolic compounds on tumor necrosis factor-alpha (TNF-alpha)-induced changes of adipokines and oxidative stress in 3T3-L1 adipocytes. Journal of agricultural and food chemistry. 2011;59(2):546–551. doi: 10.1021/jf1036992. [DOI] [PubMed] [Google Scholar]
- 42.Araki S, Dobashi K, Kubo K, et al. N-acetylcysteine attenuates TNF-alpha induced changes in secretion of interleukin-6, plasminogen activator inhibitor-1 and adiponectin from 3T3-L1 adipocytes. Life sciences. 2006;79(25):2405–2412. doi: 10.1016/j.lfs.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Chen YAM, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16(7):1040–1052. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- 44.Flekac M, Skrha J, Hilgertova J, et al. Gene polymorphisms of superoxide dismutases and catalase in diabetes mellitus. BMC medical genetics. 2008;9:30. doi: 10.1186/1471-2350-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li C, Zhou HM. The role of manganese superoxide dismutase in inflammation defense. Enzyme research. 2011;2011:387176. doi: 10.4061/2011/387176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cuzzocrea S, Zingarelli B, Costantino G, et al. Beneficial effects of Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), a superoxide dismutase mimetic, in carrageenan-induced pleurisy. Free radical biology & medicine. 1999;26(1–2):25–33. doi: 10.1016/s0891-5849(98)00142-7. [DOI] [PubMed] [Google Scholar]
- 47.Montano MA, da Cruz IB, Duarte MM, et al. Inflammatory cytokines in vitro production are associated with Ala16Val superoxide dismutase gene polymorphism of peripheral blood mononuclear cells. Cytokine. 2012;60(1):30–33. doi: 10.1016/j.cyto.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 48.Pani G, Colavitti R, Bedogni B, et al. Mitochondrial superoxide dismutase: a promising target for new anticancer therapies. Current medicinal chemistry. 2004;11(10):1299–1308. doi: 10.2174/0929867043365297. [DOI] [PubMed] [Google Scholar]
- 49.Comar JF, Babeto de Sa-Nakanishi A, de Oliveira AL, et al. Oxidative state of the liver of rats with adjuvant-induced arthritis. Free radical biology & medicine. 2012 doi: 10.1016/j.freeradbiomed.2012.12.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Muroya M, Chang K, Uchida K, et al. Analysis of cytotoxicity induced by proinflammatory cytokines in the human alveolar epithelial cell line A549. Bioscience trends. 2012;6(2):70–80. [PubMed] [Google Scholar]
- 51.Oettinger CW, D'Souza MJ. Microencapsulated drug delivery: a new approach to pro-inflammatory cytokine inhibition. Journal of microencapsulation. 2012;29(5):455–462. doi: 10.3109/02652048.2012.658443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kemp K, Gray E, Mallam E, et al. Inflammatory cytokine induced regulation of superoxide dismutase 3 expression by human mesenchymal stem cells. Stem cell reviews. 2010;6(4):548–559. doi: 10.1007/s12015-010-9178-6. [DOI] [PubMed] [Google Scholar]