Abstract
Hormone therapy is the mainstay of adjuvant treatment for hormone receptor positive (HR-positive) nonmetastatic breast cancer. We evaluated adjuvant hormone therapy (AHT) initiation among Medicaid-insured women aged 21–64 years with stage I–III HR-positive breast cancer. We used multivariable logistic regression to identify independent predictors of AHT initiation. Within 1 year of diagnosis, 68% (1049/1538) initiated AHT; by 18 months, 80% (1168/1461) initiated AHT. In multivariable analysis, women less likely to initiate AHT had more comorbidity (≥2 vs none: adjusted odds ratio (AOR) = 0.55; 95% CI = 0.32 to 0.97), more advanced disease (stage III vs I: AOR = 0.27; 95% CI = 0.18 to 0.39), and no radiation after breast conserving surgery (AOR = 0.15; 95% CI = 0.10 to 0.22). Race, age, and history of mental health disorders were not independently associated with initiation of AHT. Among initiators of AHT, 58% (604/1049) were adherent to treatment for the year after initiation. Despite comprehensive prescription coverage, only 39% (604/1538) received optimal AHT including prompt initiation and adherence for the year after treatment. Partnerships between Medicaid programs and cancer registries may help identify at-risk women and facilitate the implementation of quality improvement strategies.
Each year in the United States, more than 100 000 women are diagnosed with potentially curable, hormone receptor positive (HR-positive) breast cancer (1). Adjuvant hormone therapy (AHT) reduces the recurrence risk by 40% (2). National guidelines recommend 5 years of AHT (3,4) starting within a year of diagnosis for nearly all women with nonmetastatic disease (5). Nonwhite race (6–9), low socioeconomic status (7,10–14), disability (15,16), comorbidity (17), and young age (18–21) are associated with worse breast cancer outcomes. These worse outcomes could be attributable to lower odds of receiving effective therapies, such as AHT. Because these risk factors predominate among Medicaid-insured women, evaluation of AHT initiation in this population is a priority.
Our goal was to measure AHT initiation by nonelderly Medicaid-insured women with nonmetastatic breast cancer. We used data from a linkage between the New York State Cancer Registry (NYSCR), the New York State (NYS) Medicaid program, Medicare, and a state-wide hospital discharge and ambulatory surgery records system (Statewide Planning and Research Cooperative System, SPARCS) (22). These sources provided cancer diagnosis, stage, and initial treatment, as well as healthcare utilization, including prescription use (23,24). The cohort included women aged 21–64 years diagnosed during 2004–2006 with primary incident stage I–III HR-positive breast cancer; all were continuously enrolled in Medicaid from the month prior through 1 year after diagnosis or until death.
AHT initiation was ascertained on the basis of one or more pharmacy claims for tamoxifen or an aromatase inhibitor (AI, including exemestane, letrozole, and anastrozole) as denoted by drug codes (25). AHT is covered by NYS Medicaid at a cost of $0–3 per prescription. Multivariable logistic regression identified independent predictors of AHT initiation, and consequently results are reported in odds ratios. To determine each woman’s medication possession ratio, defined as prescription supply/time (days), we evaluated pharmacy claims for AHT for the year following initiation (or until censored by disenrollment). A ratio of 80% or more was considered adherent, which is standard in claims-based studies (20,21,26–29). We used two-sided P less than .05 to determine statistical significance. The research was approved by the Institutional Review Boards at the Dana-Farber Cancer Institute and the NYS Department of Health.
Of 1538 women, 68% (1049/1538) initiated AHT within the year after diagnosis (Figure 1). Because 98% (1509/1538) of these women filled one or more prescriptions during this period, it is implausible that women are unaware of or unable to access their benefits. Among AHT initiators, 58% (604/1049) were adherent with therapy over the following year. On multivariable analysis, higher comorbidity was associated with lower odds of AHT initiation (comorbidity index 1 vs 0: adjusted odds ratio [AOR] = 0.65, 95% CI = 0.42 to 1.01; ≥2 vs 0: AOR = 0.55, 95% CI = 0.32 to 0.97).
Figure 1.
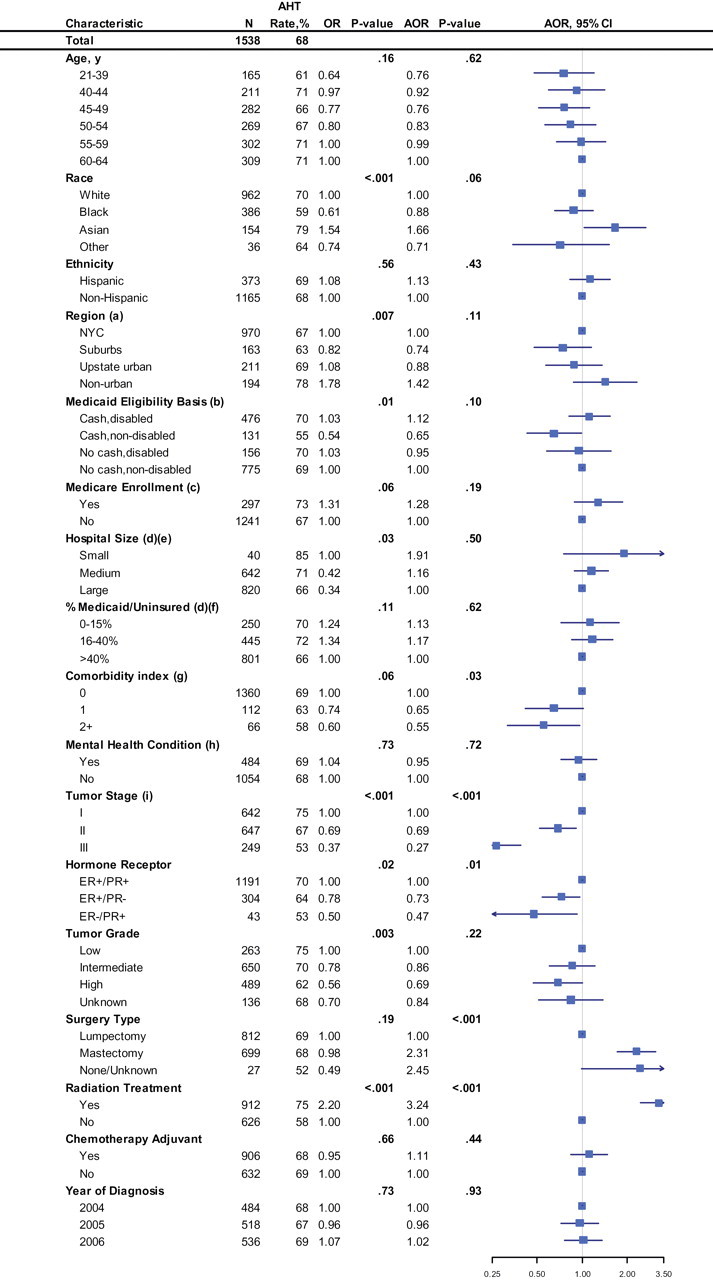
Initiation of hormone therapy rates, unadjusted and adjusted odds ratios during the year following diagnosis of women aged 21–64 years with nonmetastatic HR+ breast cancer in New York Medicaid. (a) Region was defined as NYC, the surrounding NYC suburbs, upstate urban (counties with cities of 100 000–300 000 residents), and nonurban (counties with towns <100 000). (b) Medicaid eligibility is based on New York state government criteria. (c) Concurrently enrolled Medicare patients include those with federal disability and those who turn 65 during the follow-up period. (d) Hospital affiliation is defined as the hospital of first hospital claim in linked SPARCS files for each patient after her breast cancer diagnosis. Hospital affiliation was unassigned for 36 women (2% of the cohort). These women were dropped from the multivariable analysis. (e) Hospital size was defined by the number of hospital beds: small, <100; medium, 100–400; large, >400. (f) Hospitals were categorized by percentage of total discharges in 2006 that were not covered by insurance or were covered by Medicaid insurance to characterize hospitals based on the population they serve. (g) Comorbidity index was calculated based on inpatient and outpatient Medicaid claims during the year before breast cancer diagnosis using the Charlson–Deyo–Klabunde comorbidity index. (h) Mental health condition was identified based on Medicaid claims during the year before breast cancer diagnosis (ICD9 codes 291–2, 295–8, 300–1, 303–9, 311–2, and 648.3). (i) Stage is based on American Joint Committee on Cancer, 6th edition. Two-sided P values were calculated for the group using the Wald χ2 test. The squares and lines extending outward represent point estimates and 95% confidence intervals of adjusted odds ratios, respectively. AOR = adjusted odds ratio; AHT = adjuvant hormone therapy; NYC = New York City; ER+ = estrogen receptor–positive; ER− = estrogen receptor–negative; PR+ = progesterone receptor–positive; PR− = progesterone receptor–negative; SPARCS = Statewide Planning and Research Cooperative System.
Notably, race, age, and history of mental health disorders were not independently associated with initiation of AHT. Despite the large percentage of black women (386/1538 = 25%), there was no association between black race and AHT initiation. Overall, race was marginally associated with initiation (P = .06), a result stemming from higher initiation rates among Asians. Mental health conditions (including affective disorders/major depression, psychosis, substance abuse, anxiety, and personality disorders) were common (484/1538 = 31%) but were not associated with AHT initiation.
Breast conserving surgery and lack of radiation therapy were independently associated with lower odds of AHT initiation. Because there was an interaction between surgery type, radiation, and AHT initiation, we included this in multivariable analysis and found that women who did not have radiation following breast conserving surgery were much less likely to initiate AHT than those who did (AOR = 0.15, 95% CI = 0.10 to 0.22).
AHT initiation decreased with more advanced stage (stage I reference; stage II AOR = 0.69, 95% CI = 0.52 to 0.92; and stage III AOR = 0.27, 95% CI = 0.18 to 0.39). When we extended the time horizon to 18 months (n = 1461), AHT initiation increased from 68% to 80% (Figure 2). We found higher rates of chemotherapy use for women who initiated AHT after 12 months compared to those who initiated AHT within 12 months (Fisher’s exact test, OR = 6.25, P < .001), suggesting that receipt of chemotherapy may have contributed to the delayed AHT initiation observed for patients with more advanced stage disease. Both the lower AHT use for women not receiving standard local therapy and evidence of delay suggest a breakdown in coordination of care for these women.
Figure 2.
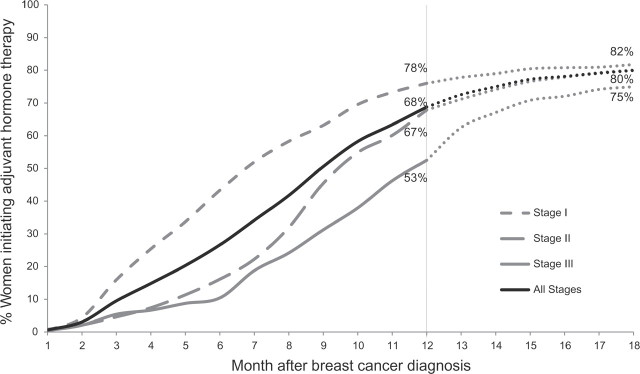
Stage-specific cumulative rates of adjuvant hormone therapy initiation by time after breast cancer diagnosis. Primary analysis of adjuvant hormone therapy initiation by 12 months is represented with solid and dashed lines (n = 1538). Analysis of adjuvant hormone therapy by 18 month is represented with dotted lines (n = 1461).
Our main study finding, a 68% AHT initiation rate in women younger than age 65 years, is most comparable to a study by Kimmick et al. (21). They studied an older cohort of Medicaid beneficiaries in North Carolina and reported an initiation rate of 70% (21) Other population-based studies have found higher AHT initiation rates (80%–85%), but these cohorts tended to be more affluent (6,30). Medicaid-insured women have lower breast cancer survival than privately insured women (31–34). Our findings suggest that one plausible cause of observed inferior survival for Medicaid-insured women with breast cancer might be underuse of AHT. That black and white women had similar rates of AHT initiation substantiates other studies demonstrating that racial disparities in breast cancer treatment and outcomes are partially mediated by poverty for which black race is often a proxy (11,35).
Our study’s primary limitation is that we could not distinguish between women who did not receive and those who received but did not fill an AHT prescription. Second, our analysis did not capture women without insurance or with intermittent Medicaid coverage who are presumed at even greater risk for underuse.
In summary, among nonelderly women with HR-positive breast cancer insured through a Medicaid program that provided universal coverage for AHT, 20% never initiated AHT, 12% started therapy after an excessive delay, and 29% were not adherent to therapy. Therefore, only 39% received optimal therapy. Given the substantial survival benefit associated with AHT, interventions to eliminate these deficits should be prioritized. In light of the large proportion of women insured by Medicaid and the persistence of inferior outcomes for poor and nonwhite women, it is strategic to focus quality improvement efforts on the Medicaid-insured.
This benchmarking study was made possible by partnership between clinical experts, NYS Medicaid, and cancer registry leadership. From a practical perspective, if state health departments were able to expedite linkage of their tumor registries and Medicaid data, they could identify beneficiaries who fail to receive recommended care. Once identified, outreach programs complementary to traditional navigator models could contact these individuals and/or their care providers to foster compliance with high-impact interventions, such as AHT.
Footnotes
Funding
This work was supported by Cooperative Agreement S3888 from the Association of Schools of Public Health/Centers for Disease Control and Prevention (to MJS), Susan G. Komen for the Cure Career Catalyst in Disparities Award (to MJH), and National Cancer Institute (grant no. R01-CA131847 to DS).
Notes
The analysis, interpretation, and writing of the manuscript rests with the study authors, who assume responsibility for its contents. Data was presented in part on June 6, 2011, at the American Society of Clinical Oncology annual meeting in Chicago, IL. The authors report no conflicts of interest.
References
- 1. Cancer Statistics. Surveillance, Epidemiology, and End Results. http://seer.cancer.gov/statistics/. Accessed March 15, 2011
- 2. Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. [DOI] [PubMed]
- 3. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer 2011, version 2.2011. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed June 8, 2011
- 4. Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28(23):3784–3796. [DOI] [PMC free article] [PubMed]
- 5. The Quality Oncology Practice Initiative Summary of Fall 2011 measures. 2011; http://qopi.asco.org/Documents/QOPI Fall2011MeasuresSummary.pdf. Accessed November 4, 2011
- 6. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol.2006;24(9):1357–1362 [DOI] [PubMed]
- 7. Freedman RA, Virgo KS, He Y, et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117(1):180–189. [DOI] [PubMed]
- 8. Hershman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of Southwest Oncology studies S8814/S8897. J Clin Oncol.2009;27(13):2157–2162 [DOI] [PMC free article] [PubMed]
- 9. Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Research and Treatment.2003;81(1):21–31 [DOI] [PubMed]
- 10. Ansell D, Whitman S, Lipton R, Cooper R. Race, income, and survival from breast cancer at two public hospitals. Cancer.1993;72(10):2974–2978 [DOI] [PubMed]
- 11. Bassett MT, Krieger N. Social class and black-white differences in breast cancer survival. American Journal of Public Health.1986;76(12):1400–1403 [DOI] [PMC free article] [PubMed]
- 12. Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, Edwards MS. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA.1998;279(22):1801–1807 [DOI] [PubMed]
- 13. Sprague BL, Trentham-Dietz A, Gangnon RE, et al. Socioeconomic status and survival after an invasive breast cancer diagnosis. Cancer. 2011;117(7):1542–1551. [DOI] [PMC free article] [PubMed]
- 14. Yu XQ. Socioeconomic disparities in breast cancer survival: relation to stage at diagnosis, treatment and race. BMC Cancer.2009;9:364. [DOI] [PMC free article] [PubMed]
- 15. Iezzoni LI, Ngo LH, Li D, Roetzheim RG, Drews RE, McCarthy EP. Early stage breast cancer treatments for younger Medicare beneficiaries with different disabilities. Health Services Research.2008;43(5p1):1752–1767. [DOI] [PMC free article] [PubMed]
- 16. McCarthy EP, Ngo LH, Roetzheim RG, et al. Disparities in breast cancer treatment and survival for women with disabilities. Annals of Internal Medicine.2006;145(9):637–645 [DOI] [PMC free article] [PubMed]
- 17. Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. Journal of the American Geriatrics Society.2004;52(1):106–111 [DOI] [PMC free article] [PubMed]
- 18. The Kaiser Family Foundation Statehealthfacts.org Datasource http://pdf.kff.org/mfs/NYUS.pdf and http://pdf.kff.org/mfs/CAUS.pdf. 2011; http://www.statehealthfacts.org/profileglance.jsp?rgn=6&rgn=34. Accessed June 8, 2011
- 19. Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol.2009;27(5):713–719 [DOI] [PubMed]
- 20. Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol.2010;28(27):4120–4128 [DOI] [PMC free article] [PubMed]
- 21. Kimmick G, Anderson R, Camacho F, Bhosle M, Hwang W, Balkrishnan R. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol.2009;27(21):3445–3451 [DOI] [PMC free article] [PubMed]
- 22. Boscoe FP, Schrag D, Chen K, Roohan PJ, Schymura MJ. Building capacity to assess cancer care in the Medicaid population in New York State. Health Services Research.2011;46(3):805–820 [DOI] [PMC free article] [PubMed]
- 23. NYS Cancer Registry and Cancer Statistics Datasource. http://www.nyhealth.gov/statistics/cancer/registry/. [Webpage]. 2010; http://www.nyhealth.gov/statistics/cancer/registry/. Accessed February 14, 2011
- 24. Medicaid. Centers for Medicare and Medicaid Services2011; http://www.cms.gov/Medicaid GenInfo/. Accessed March 14, 2011
- 25. Administration UFaD. FDA National Drug Code Directory. http://www.accessdata.fda.gov/scripts/cder/ndc/default.cfm. Accessed December, 2010
- 26. Partridge AH, Archer L, Kornblith AB, et al. Adherence and persistence with oral adjuvant chemotherapy in older women with early-stage breast cancer in CALGB 49907: adherence companion study 60104. J Clin Oncol. 2010;28(14):2418–2422. [DOI] [PMC free article] [PubMed]
- 27. Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol.2008;26(4):556–562 [DOI] [PubMed]
- 28. Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Research and Treatment. 2011;125(1):191–200. [DOI] [PubMed]
- 29. Ziller V, Kalder M, Albert US, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol.2009;20(3):431–436 [DOI] [PubMed]
- 30. Svahn TH, Niland JC, Carlson RW, et al. Predictors and temporal trends of adjuvant aromatase inhibitor use in breast cancer. J Natl Compr Canc Netw.2009;7(2):115–121 [DOI] [PubMed]
- 31. Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. The New England Journal of Medicine.1993;329(5):326–331 [DOI] [PubMed]
- 32. Bradley CJ, Gardiner J, Given CW, Roberts C. Cancer, Medicaid enrollment, and survival disparities. Cancer.2005;103(8):1712–1718 [DOI] [PubMed]
- 33. Bradley CJ, Given CW, Roberts C. Disparities in cancer diagnosis and survival. Cancer.2001;91(1):178–188 [DOI] [PubMed]
- 34. McDavid K, Tucker TC, Sloggett A, Coleman MP. Cancer survival in Kentucky and health insurance coverage. Archives of Internal Medicine.2003;163(18):2135–2144 [DOI] [PubMed]
- 35. Komenaka IK, Martinez ME, Pennington RE, Jr., et al. Race and ethnicity and breast cancer outcomes in an underinsured population. Journal of the National Cancer Institute. 2010; 102(15):1178–1187. [DOI] [PubMed]


