Abstract
Fear extinction is a reduction in conditioned fear following repeated exposure to the feared cue in the absence of any aversive event. Extinguished fear often reappears after extinction through spontaneous recovery. Animal studies suggest that spontaneous recovery can be abolished if extinction occurs within minutes of acquisition. However, a limited number of human extinction studies have shown that short interval extinction does not prevent the return of fear. For this reason, we performed an in-depth parametric analysis of human fear extinction using fear-potentiated startle. Using separate single-cue and differential conditioning paradigms, participants were fear conditioned and then underwent extinction either 10 min (Immediate) or 72 hr (Delayed) later. Testing for spontaneous recovery occurred 96 hr after acquisition. In the single cue paradigm, the Immediate and Delayed groups exhibited differences in context, but not fear, conditioning. With differential conditioning, there were no differences in context conditioning and the Immediate group displayed less spontaneous recovery. Thus, the results remain inconclusive regarding spontaneous recovery and the timing of extinction and are discussed in terms of performing translational studies of fear in humans.
Keywords: fear conditioning, fear extinction in humans, spontaneous recovery, startle
Anxiety disorders such as specific phobia and posttraumatic stress disorder (PTSD) can be conceptualized as the result of fear conditioning in affected patients (Bouton, Mineka, & Barlow, 2001). Ambient neutral stimuli that are present when an individual is exposed to a traumatic event (or unconditioned stimulus; US) can become conditioned stimuli (CSs) that evoke conditioned fear responses. Exposure therapy, or the repeated presentation of a fear-evoking CS in the absence of aversive consequences, is currently the most effective treatment for anxiety disorders (Foa, 2000). Extinction is the reduction in the magnitude of a conditioned response that occurs after repeated presentation of the previously reinforced CS in the absence of the US.
Extinction occurs in a great variety of organisms and response systems and in both appetitive and aversive Pavlovian conditioning paradigms. The mechanism of extinction has been the subject of some debate historically, although contemporary theories generally attribute the phenomenon to a new “inhibitory” learning process that leaves previous learning intact, rather than an erasure or “unlearning” of the association between the CS and the US (Bouton & Bolles, 1985; Davis, 2000; Davis, Falls, & Gewirtz, 2000; Falls & Davis, 1995; Myers & Davis, 2002; Rescorla, 2001). The idea that extinction is a form of new learning is supported by the observation that extinguished conditioned responses return with the passage of time (spontaneous recovery; Pavlov, 1927), a change in context (renewal; Bouton & Bolles, 1979), and after unsignaled presentations of the US (reinstatement; Bouton & Bolles, 1979; Pavlov, 1927; Rescorla & Heth, 1975).
Recent animal studies, however, suggest that extinction of conditioned fear may be mediated by different mechanisms depending on the interval at which extinction training is initiated after fear acquisition. Myers, Ressler, and Davis (2006) reported that extinction of fear-potentiated startle is resistant to reinstatement, renewal, and spontaneous recovery when extinction training is initiated 10 min after acquisition, whereas all three recovery effects are observed when extinction training is initiated 72 hr after acquisition (Myers et al., 2006). More recently, Maren and Chang (2006) also reported resistance to spontaneous recovery after immediate (15 min) extinction of conditioned freezing, provided that conditioned fear expression during extinction training is not so high as to prevent extinction from occurring (Maren & Chang, 2006). Quirk (2002) observed spontaneous recovery of conditioned freezing in rats in which fear was extinguished 1 hr after fear conditioning, suggesting that the critical time period for disrupting the return of conditioned fear in this species is between 10 min and 1 hr (Quirk, 2002). Taken together, these observations suggest that, in rats, immediate and delayed fear extinction proceed by different neurobiological mechanisms and that extinction initiated within minutes of conditioned fear acquisition may actually erase fear memory, potentially by interfering with ongoing fear memory consolidation.
Perhaps consistent with this idea, Cain, Godsil, Jami, and Barad (2005) reported that immediate extinction is not affected by the L-type voltage-gated calcium channel (L-VGCC) inhibitor nifedipine, whereas delayed extinction is impaired (Cain et al., 2005). Mao, Hsiao, and Gean (2006) found that fear extinction initiated 1 hr after fear acquisition reversed a fear conditioning-induced change in a particular glutamate receptor (the GluR1 subunit of the AMPA receptor) within the amygdala, whereas this reversal did not occur when extinction was initiated 24 hr after acquisition (Mao et al., 2006). In a very recent report, Kim et al. (2007) found that intra-amygdala injection of an mGluR1 antagonist impairs fear extinction 48 hr after, but not 2 hr after, acquisition; this suggests that amygdala mGluR1 receptors are necessary for the expression of conditioned fear extinction at a time point after the consolidation of learned fear (Kim et al., 2007). Lin et al. (Lin, Lee, & Gean, 2003; Lin, Yeh, et al., 2003) have hypothesized that fear extinction may correspond to synaptic depotentiation, a reversal of long-term potentiation that returns synapses to baseline synaptic efficacy, within the basolateral amygdala (Lin et al., 2003, Lin, Lee, Huang, Wang, & Gean, 2005; Lin, Yeh, et al., 2003).
To date, the “unlearning” hypothesis of extinction, which was suggested by Myers et al. (2006), has not been supported by human studies of conditioned fear extinction. However, the majority of previous studies of the return of fear after extinction have used skin conductance response (LaBar & Phelps, 2005; Milad, Orr, Pitman, & Rauch, 2005; Vansteenwegen et al., 2005), reaction time task performance (Hermans et al., 2005), and verbal ratings of fear and US expectancy (Neumann, Lipp, & Cory, 2007; Vansteenwegen et al., 2005). To the best of our knowledge, only two human studies have shown a return of fear after extinction using fear-potentiated startle measures. Fear-potentiated startle is the relative increase in the magnitude of the acoustic startle reflex when elicited in the presence of a previously neutral cue (e.g., colored light) that was repeatedly paired with an aversive US (e.g., an airblast to the throat or shock). Fear-potentiated startle is a well-characterized, objective measure of fear that is ideal for conducting translational studies of fear and anxiety (Davis, 1986). The first study of the return of fear-potentiated startle in humans was our previous report (Norrholm et al., 2006), in which we observed significant reinstatement when unsignaled USs were presented immediately after extinction training. Extinction training occurred 24 hr after acquisition in the latter study. The second study of the return of fear-potentiated startle in humans was conducted by Alvarez, Johnson, & Grillon (2007), using a virtual environment to present different experimental contexts. In an attempt to replicate the rodent findings of Myers et al. (2006), Alvarez and colleagues (2007) found significant renewal using fear-potentiated startle, skin conductance, and fear ratings when extinction occurred immediately after acquisition.
The rodent studies on which our translational extinction studies are based typically use a single visual cue that is repeatedly paired with an aversive footshock. Recent human studies, using either skin conductance or acoustic startle measures, have used a differential conditioning procedure in which one cue is paired to an aversive stimulus (CS+), whereas a second cue is not reinforced (CS−; Alvarez et al., 2007). The aim of the present study was to perform an in-depth parametric analysis of human conditioned fear extinction, in which participants were presented with (a) a single CS to which they were fear conditioned and for which fear was extinguished or (b) a differential conditioning paradigm (A+/B−) similar to that used in our previous work (Norrholm et al., 2006). Participant fear was extinguished 10 min or 72 hr after acquisition; these time points were based on a protocol similar to that used by Myers et al. (2006).
Method
Subjects and Materials
Ninety-two subjects (47 women, 45 men) aged 18 to 54 years (M = 27.9 ± 0.9 years) participated in this study after signing an informed consent form approved by the Emory University Institutional Review Board and the Atlanta Veterans Affairs Medical Center (VAMC) Research and Development Committee. All subjects were screened for visual impairment using an eye chart and for auditory impairment using a pure threshold audiometer (Grason-Stadler, Model GS1710). To be included, subjects were required to have corrected 20/20 vision without color blindness and to be able to detect tones at 30 dB [A] SPL at frequencies ranging from 250 to 4000 Hz. In addition, subjects were screened and excluded for current or past psychiatric illness, including alcohol or drug dependence, through interview and urine toxicology. Enrolled subjects were randomly assigned to the single-cue or differential conditioning experiments, as well as the immediate and delayed subgroups within each set of experiments.
Acoustic Startle Procedure
The acoustic startle response (eyeblink component) was measured through electromyographic (EMG) recordings of the right orbicularis oculi muscle. Two 5-mm Ag/AgCl electrodes filled with electrolyte gel were placed approximately 1 cm below the pupil and 1 cm below the lateral canthus. A third ground electrode was placed behind the right ear over the mastoid. All impedances were less than 6 kΩ. EMG activity was amplified and digitized with a computerized EMG startle response monitoring system (SR-LAB, San Diego Instruments). The EMG signal was rectified and filtered at a low cutoff of 30 Hz and a high cutoff of 1 kHz. The system recorded 250 1-ms readings beginning at the onset of the startle stimulus. Subjects were seated in a sound-attenuated chamber and asked to look at a set of four lights mounted on the wall approximately 5 feet (1.524 m) in front of them. All acoustic stimuli were delivered binaurally through headphones (TDH-39-P, Maico).
General Method
The present methodology allowed us to assess baseline acoustic startle as well as the acquisition, within-session extinction, and spontaneous recovery of fear-potentiated startle. The startle probe (noise burst) was a 108-dB [A], 40-ms burst of broadband noise with a near instantaneous rise time. The aversive stimulus (US) in these studies was a 250-ms airblast with an intensity of 140 psi directed to the larynx similar to that used in previously published methods (Jovanovic et al., 2005; Norrholm et al., 2006). The airblasts were emitted by a compressed air tank connected to polyethylene tubing and controlled by a solenoid switch. The CSs were colored lights with color assignment counterbalanced across subjects.
For reinforced trials (A+ in both sets of experiments), a colored light was illuminated for a total of 4,995 ms. A startle probe (40 ms) was administered 4,000 ms after onset of the light. The airblast US (250 ms duration) was then presented 500 ms after the startle probe. The light terminated 205 ms after offset of the airblast. For nonreinforced trials (A− in the single-cue experiment or B− in the A+/B− experiment), the light was illuminated for a total of 4,245 ms. Again, a startle probe (40 ms) was administered 4,000 ms after onset of the light. The light terminated 205 ms after the startle probe. Startle probes were delivered on every trial as with previous human fear-potentiated startle studies (Ameli, Ip, & Grillon, 2001; Grillon, Baas, Lissek, Smith, & Milstein, 2004; Grillon, Dierker, & Merikangas, 1998; Jovanovic et al., 2005, Jovanovic et al., 2006; Norrholm et al., 2006).
For both sets of experiments (single cue and differential conditioning, A+/B−), subjects participated in three separate sessions: CS habituation and acquisition (Session 1), extinction training (Session 2), and the extinction test (Session 3). Each startle session began with a 1-min acclimation period consisting of 70-dB broad-band noise, which continued throughout the session as background noise. Initial startle activity was reduced with 3 presentations of the 108-dB 40-ms startle probe without the CS, referred to as noise alone (NA) trials.
Subjects were randomly assigned to one of four experimental conditions: (a) single cue, immediate (10-min) extinction; (b) single cue, delayed (72-hr) extinction; (c) differential conditioning (A+/B−), immediate extinction, or (d) differential conditioning (A+/B−), delayed extinction. During the time period between acquisition and extinction training, subjects in the immediate group were taken out of the sound booth and placed in a quiet room adjacent to the startle testing room. EMG electrodes remained in place in between sessions.
A response keypad (SuperLab, Cedrus Corp.) was used in the startle sessions in coordination with the EMG startle response monitoring system (SR-LAB, San Diego Instruments) to collect trial-by-trial ratings of US expectancy. For each presentation of the CS, subjects indicated on the response keypad whether the light would be reinforced or nonreinforced, and this response was recorded for each light presentation. Subjects pressed a button marked “+” if they expected the CS to be followed by the US (danger), a button marked “+” if they did not expect the CS to be followed by the US (safety), and a button marked “0” if they did not know what to expect (uncertain). For the purposes of data analysis, subject responses of “+” were scored as 1, responses of “0” were scored as 0, and responses of “−” were scored as −1.
Single-Cue (A+) Method
The first session consisted of a CS habituation phase comprising four presentations of the nonreinforced CS (referred to as A−) and four NA presentations followed by an acquisition phase consisting of three blocks, with 8 trials in each block—four reinforced presentations of the CS (A+) and four NA presentations—for a total of 24 trials. The intertrial interval was randomized between 9 s and 22 s. The order of NA and A presentations was randomized. Subjects were given explicit verbal instructions before the CS habituation/acquisition session. The instructions were as follows:
During this experiment, you will hear some sudden tones and noises in addition to seeing colored lights turn on. You will also experience brief blasts of air. Your task will be to predict the occurrence of the airblasts. We are most interested in your expectation during presentation of the lights. For this reason, please press the button marked “+” if you think a light will be followed by air or press the button marked “+” if you think the light will not be followed by air. If you do not know, press the “0” sign. You should press a button for each light that turns on. You will have 3 s from the time the light turns on to press the button. If you miss a light, please move on to the next one. If at any time you want to stop the experiment, just knock on the window and we will stop the session immediately.
The “sudden tones and noises” described in the instructions refer to the 108-dB noise probes and the 70-dB background noise that was presented between startle probes.
Extinction training consisted of six blocks with 8 trials in each block (4 trials each of A− and NA), for a total of 48 trials. The order of NA and A presentations was randomized. None of the trials during extinction training was reinforced with an airblast. Again, the intertrial interval was randomized between 9 s and 22 s. Subjects were given explicit verbal instructions before the extinction training session. These instructions were as follows:
We will now proceed with the next session. Again you will hear some sudden tones and noises in addition to seeing colored lights turn on. Again, we are interested in your expectation of airblast during presentation of the lights. Try to use the knowledge you acquired in the previous session. Please press the button marked “+” if you think a light will be followed by air, or press the button marked “−” if you think the light will not be followed by air. If you do not know, press the “0” sign. You should press a button for each light that turns on. You will have 3 s from the time the light turns on to press the button. If you miss a light, please move on to the next one. If at any time you want to stop the experiment, just knock on the window and we will stop the session immediately.
The extinction test consisted of one block with eight trials (four trials each of A− and NA). The order of NA and A presentations was randomized. None of the trials during the extinction test was reinforced with an airblast. Again, the intertrial interval was randomized between 9 s and 22 s. Subjects were given explicit verbal instructions before the extinction test. These instructions were identical to those administered before extinction training.
Differential Conditioning (A+/B−) Method
The first session consisted of a CS habituation phase comprising four nonreinforced presentations of each CS (Lights A and B) and four NA presentations, followed by an acquisition phase consisting of three blocks with 12 trials in each block (4 reinforced A+ trials, 4 nonreinforced B− trials, and four NA presentations), for a total of 36 trials. The order of NA, A, and B presentations was randomized. The intertrial interval was randomized between 9 s and 22 s. Subjects were given the same explicit verbal instructions before the CS habituation/acquisition session as previously described for the single-cue experiments.
In the differential conditioning experiments, extinction training consisted of six blocks with 12 trials in each block (4 trials each of A−, B−, and NA) for a total of 72 trials. The order of NA, A, and B presentations was randomized. None of the trials during extinction training was reinforced with an airblast. Again, the intertrial interval was randomized between 9 s and 22 s. Subjects were given the same explicit verbal instructions before the extinction training session as previously described for the single-cue experiments.
The extinction test consisted of one block with 12 trials (4 trials each of A−, B−, and NA). The order of NA, A, and B presentations was randomized. None of the trials during the extinction test was reinforced with an airblast. Again, the intertrial interval was randomized between 9 s and 22 s. Subjects were given explicit verbal instructions before the extinction test, and these instructions were identical to those administered before extinction training.
Data Analysis
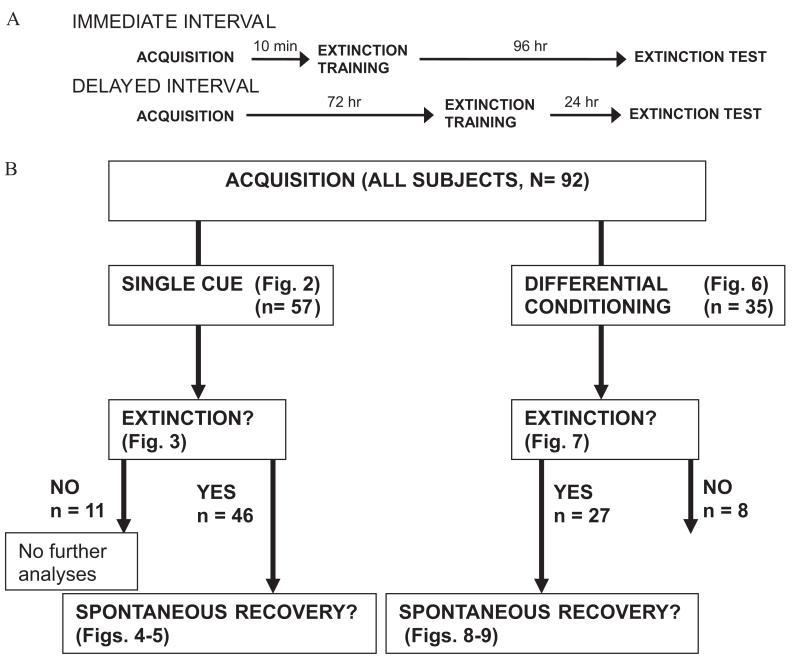
Figure 1B summarizes the distribution of subjects across experimental conditions, as well as the procedure used for assessing acquisition, within-session extinction (to rule out nonextinguishers), and spontaneous recovery (assessed in extinguishers only). Three dependent variables were used in the experiments: startle magnitude to assess startle potentiation during acquisition, difference score to assess extinction, and US expectancy ratings. Startle magnitude for each trial was calculated with the EMG startle recording software. Digital signals were full-wave rectified and smoothed by an averaging routine that calculated a rolling average of 10 data points. As previously described, the 108-dB 40-ms startle probe was presented on every trial as in our previous studies (Jovanovic et al., 2005; Jovanovic et al., 2006; Norrholm et al., 2006). The repeated presentation of the startle probe can lead to significant habituation and, as such, we calculated difference scores using an overall mean for NA in each startle session (e.g., CS habituation, acquisition, extinction training, and extinction test/reinstatement). Thus, we calculated the difference score using the following formula:
Figure 1.
(Panel A) Timing of experimental procedures. For the single-cue and differential conditioning experiments, all subjects within each paradigm received the same experimental procedures during the acquisition phase. In addition, extinction training occurred either 10 min or 72 hr after acquisition in each paradigm. Last, the time period between acquisition and the extinction test was 96 hr for both the single-cue and differential conditioning experiments. (Panel B) Flowchart illustrating the steps in data analysis and the division of subjects according to paradigm assignment (single cue vs. differential conditioning), degree of within-session extinction (identification of extinguishers), and presence of spontaneous recovery at the time of the extinction test (identification of sustained extinguishers).
To assess potentiation of startle to the CSs (both single cue and differential conditioning) and discrimination between the CS+ and CS− (differential conditioning) during CS habituation and acquisition, we used a repeated measures analysis of variance (ANOVA) with trial type (A, B, or NA) and block as within-subjects variables and startle magnitude as a dependent variable. To assess the extinction of potentiated startle during extinction training, we examined the within-session decrement in mean difference score per block after presentation of the previously reinforced CS. A one-way repeated measures ANOVA was used for this analysis, with block as a within-subjects variable and difference score to Light A as a dependent variable. To assess discrimination between the CS+ and CS− during extinction training (differential conditioning experiment), we used a repeated measures ANOVA with trial type (A or B) and block as within-subjects variables and difference score as a dependent variable. For the extinction tests, we compared the mean difference scores to the previously extinguished CS at Block 6 of extinction training and at the extinction test block.
Results
Paradigm 1: Single Cue: Immediate Versus Delayed Extinction
CS habituation and acquisition phases: Fear-potentiated startle
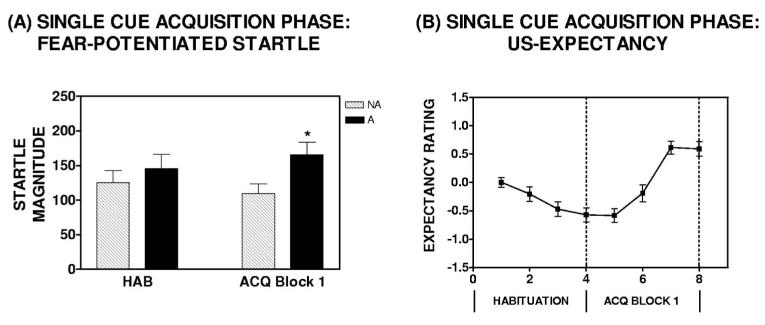
Participants were randomly assigned to the immediate group (10-min acquisition-to-extinction interval) or the delayed group (72-hr acquisition-to-extinction interval). Given that the timing manipulation was applied postacquisition, all subjects received the same experimental procedures during the CS habituation and acquisition phases. As such, there was no significant difference between the immediate and delayed groups with regard to baseline startle. There was no significant main effect of Group, F(1, 38) = 0.08, p = .77; or Block × Group interaction comparing baseline startle magnitude during the CS habituation and acquisition phases of the experiment, F(1, 38) = 0.81, p = .37. Fear-potentiated startle developed rapidly in all participants and was evident by the end of the first acquisition block; A repeated measures ANOVA revealed a significant Block × Trial Type interaction comparing startle magnitude on NA trials to that on A+ trials during the CS habituation phase and the first block of the acquisition phase, F(1, 39) = 8.1, p < .01 (see Figure 2A). There was no significant difference between the immediate and delayed groups with regard to acquisition of conditioned fear across CS habituation and acquisition phases; there was no significant Block × Trial Type × Group interaction, F(1, 38) = 1.26, p = .27.
Figure 2.
Summary of the acquisition phase in the single-cue paradigm. (Panel A) Rapid development of fear-potentiated startle. There was a significant Block × Trial Type interaction comparing startle magnitude in the absence and presence of the conditioned stimulus (CS) during the CS habituation phase and the first block of the acquisition phase, F(1, 39) = 8.1, p < .01, as indicated by an asterisk. ACQ = acquisition phase; A = light CS plus noise probe; HAB = habituation phase; NA = noise probe alone. Error bars represent standard error of the mean. (Panel B) Unconditioned stimulus (US) expectancy: Subjects in both the immediate and delayed groups displayed rapid development of fear conditioning with a significant increase in danger ratings of the CS+ (Light A) from habituation to acquisition, F(1, 34) = 207, p < .001. Expectancy ratings were scored as follows: danger = 1, uncertain = 0, and safety = −1. Error bars represent standard error of the mean.
CS habituation and acquisition phases: US expectancy ratings
Similar to startle measures, US expectancy ratings also indicated rapid development of declarative knowledge for the CS–US contingency. There was a significant effect of Trial from the last habituation trial to the last trial of acquisition phase Block 1, F(1, 34) = 44.1, p < .001 (see Figure 2B). During acquisition, subject responses on the keypad indicated successful declarative knowledge for the CS–US contingency on the basis of expectancy ratings of danger on A trials; there was a main effect of Trial, F(1, 34) = 2.07, p < .001 (see Figure 2B). There was no significant difference between subjects in the immediate and delayed groups with regard to US expectancy ratings during the CS habituation and acquisition phases; there was no significant Group × Trial interaction, F(1, 28) = 0.57, p = .46.
Extinction training (within-session extinction): Fear-potentiated startle
Subjects were randomly assigned to one of two experimental conditions for the extinction training phase: (a) a 10-min acquisition-to-extinction interval (immediate) or (b) a 72-hr acquisition-to-extinction interval (delayed). The dependent variable for these analyses was the difference score calculated according to the aforementioned formula.
Similar to our previous investigation of extinction of fearpotentiated startle in humans (Norrholm et al., 2006), significant within-session extinction was not observed in all participants. Participants who met the 50% extinction criterion (Block 1 vs. Block 6 of extinction training) were classified as extinguishers. The following analyses were conducted only on the extinguishers subset of the immediate and delayed groups (see Figure 1B).
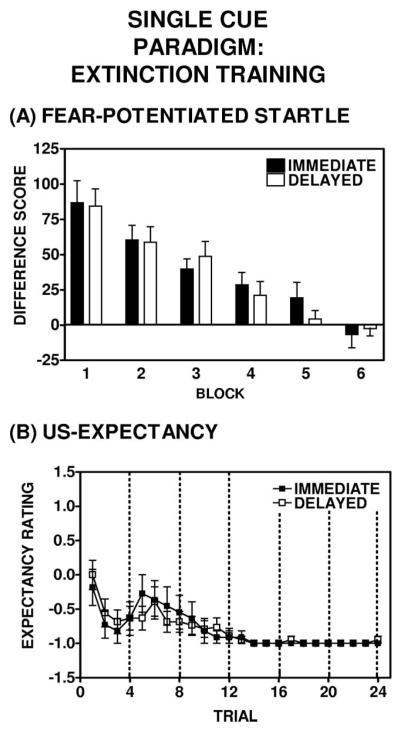
Extinguishers in the immediate (n = 12) and delayed (n = 19) groups displayed a mean within-session extinction decrement of 108 ± 12% and 119 ± 12%, respectively. A repeated measures ANOVA revealed a main effect of Block: For the immediate group, F(1, 11) = 23.6, p = .001; and for the delayed group, F(1, 18) = 52.8, p = .001 (see Figure 3A). The degree of within-session extinction did not differ between extinguishers in the immediate and delayed groups, as measured by difference score; a repeated measures ANOVA revealed that there was no main effect of Group, F(1, 29) = 0.005, p = .94 (see Figure 3A), and there was no significant difference between the immediate and delayed extinguishers with regard to potentiated startle at the end of extinction training. As found in a one-way ANOVA comparing mean difference score during extinction Block 6, F(1, 38) = 0.03, p = .87.
Figure 3.
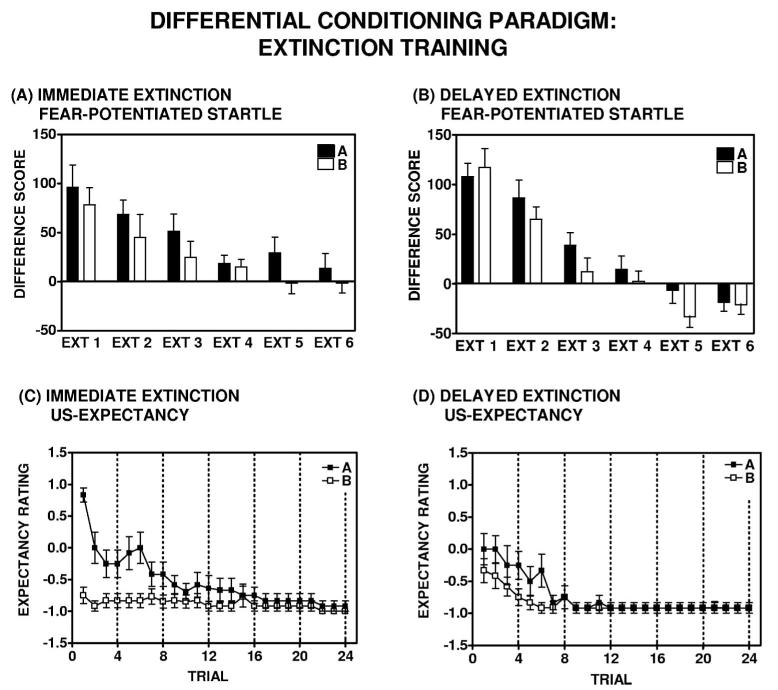
Single cue paradigm: Summary of extinction training in the extinguishers. (Panel A) Within-session extinction of fear-potentiated startle, as expressed as difference scores from baseline, in the immediate (closed bars) and delayed (open bars) extinguishers. Extinguishers were defined as those subjects that displayed at least 50% extinction of fearpotentiated startle during extinction training (Block 6 vs. Block 1). There was no difference between the immediate and delayed extinguishers with respect to the magnitude within-session extinction over the course of the session. Each block consisted of four trials. (Panel B) Subjects’ unconditioned stimulus (US) expectancy ratings from the immediate (closed squares) and delayed (open squares) extinguisher subgroups. US expectancy responses on the first four trials of extinction training (Block 1) revealed significant recall of the habituation phase that preceded acquisition during the subjects’ previous test session. Extinguishers from each time group also showed a significant within-session decrement in US expectancy ratings during extinction training. In a comparison of Trial 6 with Trial 24, immediate extinguishers F(1, 10) = 4.48, p= .06; delayed extinguishers F(1, 15) = 8.98, p < .01. Dashed vertical lines demarcate the six blocks constitute the extinction test session and allow for direct comparison with the fear-potentiated startle data shown in Panel A.
Extinction training (within-session extinction): US expectancy
US expectancy ratings at the outset of extinction training indicated significant recall of the habituation phase (in which the CS was not reinforced) that preceded acquisition. This was evident by decreased US expectancy ratings on Trials 2 through 5 of the extinction training session (Figure 3A). This carry-over effect resulted in an increase in US expectancy on Trial 6 (an approximation of the point in the earlier acquisition session when the reinforced CSs were presented). Subjects in both the immediate and delayed groups displayed a significant within-session decrement in US expectancy ratings during extinction training (comparing Trial 6 with Trial 24, for immediate extinguishers, F(1, 10) = 4.48, p = .06; for delayed extinguishers, F(1, 15) = 8.98, p < .01 (see Figure 3B).
Extinction test: Fear-potentiated startle
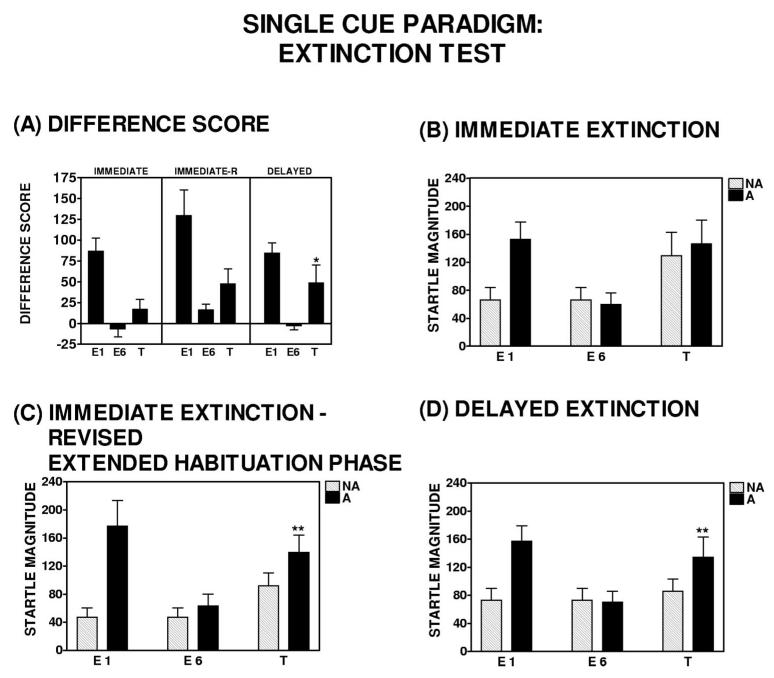
Subjects in both the immediate and delayed groups were presented with an extinction test 96 hr after acquisition (see Figure 1A). Spontaneous recovery was only assessed in those subjects displaying at least 50% extinction during extinction training (difference score to the CS in Block 1 vs. Block 6; i.e., the extinguishers). This was primarily due to the fact that any level of extinction above a 50% decrement would preclude the observation of spontaneous recovery. Subjects were presented with four presentations each of the previously extinguished CS and NA. In a comparison of the difference score from the final block of extinction training to the extinction test block (see Figure 4A), the delayed group showed a significant increase in fear-potentiated startle—a repeated measures ANOVA revealed a main effect of Block, F(1, 18) = 5.38, p < .05—whereas the immediate group did not show an increase in fearpotentiated startle between these two time points; a repeated measures ANOVA revealed no main effect of Block, F(1, 11) = 2.02, p = .18 (see Figure 4A). Thus, spontaneous recovery of fearpotentiated startle was not observed in the immediate group, but it was evident in the delayed group. At face value, this is consistent with the Myers et al. (2006) report showing that immediate extinction reduces spontaneous recovery.
Figure 4.
Fear-potentiated startle response during the extinction test in the single-cue paradigm. (Panel A) On the basis of difference scores from baseline, delayed extinguishers exhibited a significant increase in fearpotentiated startle from the end of extinction training (E6) to the time of the extinction test (T), whereas immediate extinguishers did not. However, there was a significant difference in baseline startle amplitude between the immediate (Panel B) and delayed (Panel D) extinguishers at the time of the extinction test. For this reason, a third experimental group (immediate-R; Panel C) was included, for which fear was extinguished 10 min after acquisition, and this group was tested for spontaneous recovery after 96 hr. Before the extinction test, this group received nine presentations of the startle probe to reduce the apparent context conditioning present in the original immediate group. The reduction of this context conditioning revealed spontaneous recovery in immediate-R extinguishers that was similar to the delayed extinguishers (Panel C vs. Panel D). **p < .05, repeated measures analysis of variance, significant effect of Trial Type during the extinction test. Note: the lack of a significant Block × Group interaction may have been due to a small number of individuals with low-end startle responses during the extinction test. If these lower startlers were removed from analysis (although not outliers by standard deviation), then a significant Block × Group interaction is present with a p = .05. E = extinction, T = test.
An important test for spontaneous recovery is the degree of fear-potentiated startle at the time of the extinction test relative to where it was at the end of extinction training. The finding that the delayed group displayed a greater degree of spontaneous recovery would be strengthened by a significant Block × Group interaction in addition to the significant main effect of Block within the delayed group but not the immediate group. Although the power in the present study limited the scope of follow-up analyses, the lack of a significant Block × Group interaction appears to be due to a small number of individuals with low-end startle responses during the extinction test. If these lower startlers were removed from analysis (although not outliers by standard deviation), then a significant Block × Group interaction is present with a p of .05.
However, there was a significant difference in baseline startle magnitude between the immediate and delayed groups at the time of the extinction test; a repeated measures ANOVA revealed a significant Block × Group interaction, F(1, 29) = 5.41, p < .05 (compare NA in Figures 4B and 4D). This difference in startle magnitude may have been due to greater acquisition or expression of context conditioning in the immediate group, as compared with the delayed group at the time of the extinction test. Earlier studies of fear-potentiated startle have argued that increased startle magnitude during non-CS trials is a marker of fear conditioning to the testing context (Grillon & Davis, 1997). This change in baseline startle could have confounded the results, artificially making it look as if there was less spontaneous recovery in the immediate group.
Because of the difference in startle magnitude between the immediate and delayed groups during the extinction test, a follow-up experiment was conducted with a third group, in which was fear was extinguished 10 min after acquisition (immediate-R) and presented with nine additional startle probes before the start of the extinction test. The additional noise probes were administered in an effort to reduce startle magnitude at the outset of the extinction test and thus address the apparent difference in startle magnitude between the immediate and delayed groups. Although baseline startle magnitude in the immediate-R group was somewhat reduced, compared with the immediate group, both the immediate and the immediate-R groups still showed a significant increase from the end of extinction training to the start of the extinction test (a repeated measures ANOVA revealed a significant Block effect: For the immediate group, F(1, 11) = 12.11, p < .01; and for the immediate-R group, F(1, 14) = 7.73, p < .05. The delayed group did not show this increase: A repeated measures ANOVA showed no significant Block effect, F(1, 18) = 1.00, p = .33 (see Figure 4, Panels B−D). However, the additional nine startle probes did bring the baseline startle level in the immediate-R group down to that of the delayed group, and under these conditions, there was a trend in the immediate-R group toward a significant Block × Trial Type interaction, F(1, 14) = 3.94, p = .07; and there was a significant effect of Trial Type during the extinction test, F(1, 14) = 7.07, p < .05. Viewed in this way, the results suggest that immediate extinction training did not prevent spontaneous recovery relative to delayed extinction training, inconsistent with Myers et al. (2006).
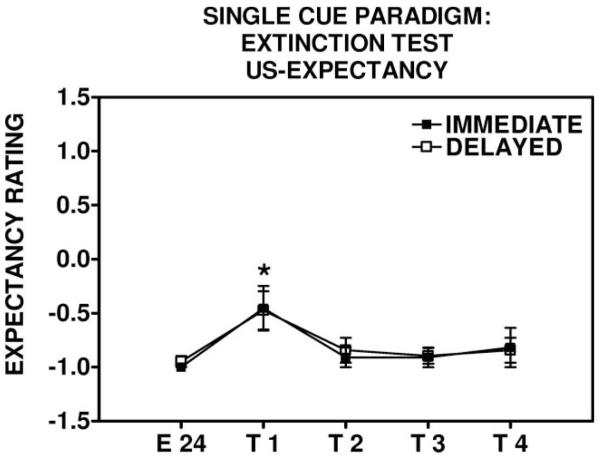
Extinction test: US expectancy rating
Subjects in both the immediate and delayed groups increased their US expectancy ratings from the end of extinction training to the extinction test: For the immediate group, F(1, 10) = 6.92, p < .05; and for the delayed group, F(1, 18) = 8.78, p < .01 (see Figure 5), and there was no difference in these ratings between the immediate and delayed groups.
Figure 5.
Unconditioned stimulus (US) expectancy responses during the extinction test in the single-cue paradigm. Immediate and delayed extinguishers increased their US expectancy ratings from the last trial of extinction training (E 24) to the first trial of the extinction test (T 1). *p < .05, repeated measures analysis of variance, significant Block effect. E = extinction, T = test.
Paradigm 2: Differential Conditioning (A+/B−): Immediate Versus Delayed Extinction
CS habituation and acquisition phases: Fear-potentiated startle
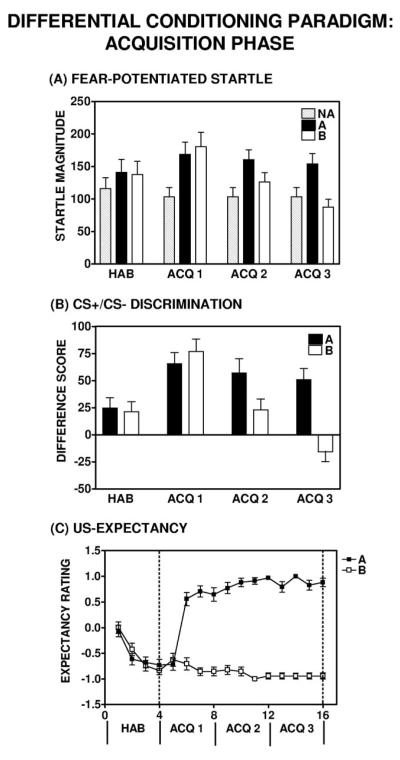
Participants were randomly assigned to the immediate (10-min acquisition to extinction interval) or the delayed (72-hr acquisition-to-extinction interval) group. All subjects received the same experimental procedures during the CS habituation and acquisition phases. There was no significant difference between the immediate and delayed groups with regard to baseline startle; There was no main effect of Group, F(1, 33) = 0.06, p = .80; and no significant Block × Group interaction comparing startle magnitude to NA trials during the CS habituation and acquisition phases of the experiment, F(1, 33) = 4.01, p > .05. Fearpotentiated startle developed rapidly in all subjects and across the acquisition session; a repeated measures ANOVA revealed a significant Block × Trial Type interaction, F(1, 34) = 13.39, p = .001 (see Figure 6A). There was no difference between the immediate and delayed groups with regard to the acquisition of fear-potentiated startle; a repeated measures ANOVA revealed no significant Block × Trial Type × Group interaction, F(1, 33) = 0.53, p = .47.
Figure 6.
Summary of the acquisition phase in the differential conditioning paradigm. Significant (Panel A) fear-potentiated startle and (Panel B) discrimination between the conditioned stimuli (CS+ [Light A] and CS− [Light B]) developed in all subjects by the end of acquisition (ACQ 3). Acquisition: repeated measures analysis of variance, significant Block × Trial Type interaction, F(1, 34) = 13.39, p = .001. Discrimination: repeated measures analysis of variance, significant Block × Trial Type interaction, F(1, 34) = 32.41, p < .001. (Panel C) Subjects’ unconditioned stimulus (US) expectancy ratings demonstrated rapid learning of the CS–US contingency as significant discrimination was evident by the third trial of the acquisition phase (ACQ). HAB = habituation phase.
All subjects showed significant discrimination between the CS+ (Light A) and CS− (Light B) during the acquisition phase, as evident by greater potentiated startle in the presence of Light A, as compared with Light B. A repeated measures ANOVA revealed a significant Block × Trial Type interaction, F(1, 34) = 32.41, p < .001 (see Figure 6B). There was no significant difference between the immediate and delayed groups with regard to CS+/CS− discrimination between during the acquisition session; and there was no significant Block × Trial Type × Group interaction, F(1, 33) = 0.57, p = .46.
CS habituation and acquisition phases: US expectancy ratings
Similar to startle measures, US expectancy ratings also indicated rapid development of declarative knowledge for the CS–US contingency in all subjects; a repeated measures ANOVA revealed a significant effect of Trial from the last habituation trial to the second trial of acquisition, F(1, 31) = 61.52, p < .001 (see Figure 6C). During acquisition, subject responses on the keypad indicated successful declarative knowledge for the CS−US contingency and discrimination between the CS+ and CS− on the basis of expectancy ratings of danger on A trials and safety on B trials. A repeated measures ANOVA revealed a significant Trial × Trial Type interaction, F(1, 26) = 345, p < .001 (see Figure 6C). There was no significant difference between subjects in the immediate and delayed groups with regard to US expectancy ratings during the CS habituation and acquisition phases; there was no significant Trial × Trial Type × Group interaction, F(1, 24) = 0.35, p = .56.
Extinction training (within-session extinction): Fear-potentiated startle
Subjects were randomly assigned to one of two experimental conditions for the extinction training phase: (a) a 10-min acquisition-to-extinction interval (immediate) or (b) a 72-hr acquisition-to-extinction interval (delayed). As in the previously described single-cue experiments, we calculated difference scores using an overall mean startle magnitude to the noise probe alone for the entire extinction training session similar to previous studies (Jovanovic et al., 2005).
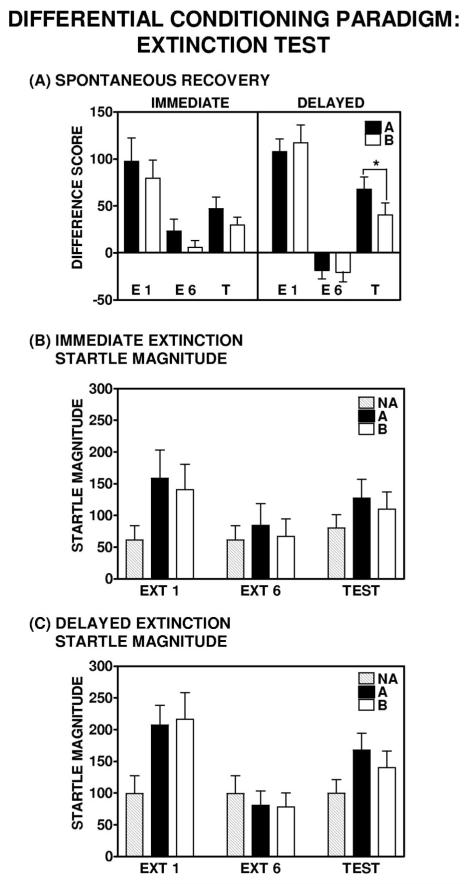
Similar to the aforementioned single-cue paradigm and our previous work (Norrholm et al., 2006), significant within-session extinction was not observed in all participants. Again, participants who met the 50% extinction criterion were referred to as extinguishers. The following analyses were conducted only on the extinguishers subset of the immediate and delayed groups (see Figure 1B). Extinguishers in the immediate (n = 13) and delayed (n = 14) groups displayed a mean within-session extinction decrement of 108% ± 15% and 124% ± 12%, respectively. Difference scores on A− trials during extinction training revealed a main effect of Block for the A Trial Type: For the immediate group, F(1, 12) = 31.58, p < .001; for the delayed group, F(1, 13) = 62.19, p = .001 (see Figure 8, Panels A and B). There was a significant difference between the immediate and delayed groups with respect to the degree of within-session extinction; a repeated measures ANOVA revealed a significant Block × Group interaction, F(1, 25) = 5.61, p < .05. Furthermore, the delayed group displayed a greater degree of terminal extinction, compared with the immediate group; there was a main effect of Group for Block 6 of extinction training, F(1, 25) = 7.51, p = .01.
Figure 8.
Fear-potentiated startle response during the extinction test in the differential conditioning paradigm. (Panel A) These data illustrate three critical factors to consider with regard to the return of fear in humans as it relates to the time elapsed since acquisition. First, as compared with the immediate group, the delayed group shows a greater degree of within-session extinction; repeated measures analysis of variance (ANOVA), significant Block × Group interaction, F(1, 25) = 5.61, p < .05. Second, both the immediate and delayed groups display a robust return of fear relative to their levels of fear-potentiated startle at the end of extinction; immediate group, F(1, 11) = 4.74, p = .05; delayed group, F(1, 13) = 36.7, p < .001. Third, as compared with the immediate group, the delayed group shows better discrimination between the CS+ and CS− at the extinction test: For the delayed group repeated measures ANOVA, there was a main effect of Trial Type, F(1, 13) = 5.26, p < .05; for the immediate group repeated measures ANOVA, there was no main effect of Trial Type, F(1, 11) = 2.58, p = .14). Unlike the single-cue paradigm, there was no evidence of context conditioning at the time of the extinction test in the (Panel B) immediate or (Panel C) delayed extinguishers in the differential conditioning paradigm. Note: As with previous fear-potentiated startle studies (e.g., Jovanovic et al., 2005), the difference in trial type (e.g., CS+ vs. CS−) is not always apparent when presented as overall startle magnitude (Panels B and C). However, the crucial analysis is the startle response to Lights A (previously reinforced CS+) and B (CS−) as a function of noise alone (NA), or baseline. When expressed as the difference score from NA or baseline (Panel A), responses to Lights A and B were significantly different. The same NA values were used to compute difference scores for Lights A and B, as the NA trials were interwoven throughout the extinction test session.
Although there was no overall significant Block × Trial Type interaction over the entire six blocks of extinction training—for the immediate group, F(1, 12) = 0.30, p = .86; for the delayed group, F(1, 13) = 0.33, p = .58—discrimination between the previously reinforced CS+ and the CS– was evident during the later blocks of the extinction training session in both immediate and delayed extinguishers. There was a main effect of Trial Type during Blocks 5 and 6 of extinction training for the immediate group, F(1, 12) = 6.64, p < .05; and there was a main effect of Trial Type during Block 5 of extinction training for the delayed group, F(1, 13) = 6.29, p < .05 (see Figure 7, Panels A and B).
Figure 7.
Summary of extinction training in the differential conditioning paradigm. Extinguishers in the (Panel A) immediate (n = 13) and (Panel B) delayed (n = 14) groups displayed a mean within-session extinction decrement of 108% ± 15% and 124% ± 12%, respectively (difference scores on A trials during extinction training: Immediate group, F(1, 12) = 31.58, p < .001; delayed group, F(1, 13) = 62.19, p = .001). Immediate and delayed extinguishers displayed a significant difference in the degree of within-session extinction during extinction training; repeated measures analysis of variance, significant Block × Group interaction, F(1, 25) = 5.61, p < .05. Discrimination between the conditioned stimuli (CS+ [Light A] and CS− [Light B]), as measured by difference scores, did not emerge until the later blocks of extinction training. (Panel C) Immediate and (Panel D) delayed extinguishers differed in their US expectancy ratings on the first presentation of Light A (the previously reinforced CS) at the outset of extinction training; one-way analysis of variance, F(1, 23) = 9.48, p < .01. Immediate extinguishers, who were extinguished 10 min after acquisition, showed significant recall of the CS–US contingency at the outset of extinction training, whereas delayed extinguishers, for whom fear was extinguished 72 hr after acquisition, exhibited greater uncertainty.
Extinction training (within-session extinction): US expectancy
Immediate and delayed extinguishers displayed a significant within-session decrement in US expectancy ratings during extinction training. A repeated measures ANOVA revealed a main effect of Trial: For the immediate group, F(1, 10) = 36.97, p < .001; for the delayed group, F(1, 10) = 21.09, p = .001 (see Figure 7, Panels C and D). The immediate and delayed groups differed in their US expectancy ratings on the first presentation of Light A (the previously reinforced CS) at the outset of extinction training; as revealed in a one-way ANOVA, F(1, 23) = 9.48, p < .01. However, there was no difference between the immediate and delayed groups with regard to within-session decrement in US expectancy ratings on A trials during extinction training, F(1, 20) = 1.02, p = .33. The immediate and delayed groups exhibited significant discrimination between the CS+ (Light A) and CS− (Light B) during extinction training. There was a significant Trial × Trial Type interaction: For the immediate group, F(1, 10) = 16.67, p < .01; for the delayed group, F(1, 9) = 5.09, p = .05 (see Figure 7, Panels C and D). There was no difference between the immediate and delayed groups with regard to discrimination between the CSs during extinction training, F(1, 19) = 1.71, p = .21.
Extinction test: Fear-potentiated startle
Subjects in both the immediate and delayed groups were presented with an extinction test 96 hr after acquisition (see Figure 1A). Spontaneous recovery was only assessed in those subjects displaying at least 50% extinction during extinction training (difference score to the CS in Block 1 vs. Block 6). Subjects were presented with four presentations each of the previously extinguished CS and the NA. When we compared the difference score from the end of extinction training to the extinction test block, both the immediate and delayed groups showed an increase in fear-potentiated startle in the presence of the previously reinforced CS (Light A); for the immediate group, F(1, 11) = 4.74, p = .05; for the delayed group, F(1, 13) = 36.7, p < .001 (see Figure 8A). However, the magnitude of this recovery was considerably larger in the delayed group versus the immediate group; a repeated measures ANOVA revealed a significant Block × Group interaction, F(1, 24) = 11.40, p < .01. The delayed group showed significant discrimination between the CSs during the extinction test; a repeated measures ANOVA revealed a main effect of Trial Type, F(1, 13) = 5.26, p < .05; whereas the immediate group did not discriminate between the previously reinforced CS+ and nonreinforced CS−; a repeated measures ANOVA revealed no main effect of Trial Type, F(1, 11) = 2.58, p = .14 (see Figure 8A). It is important to note that, in contrast to the single-cue experiment, there was no difference in baseline startle magnitude between the immediate and delayed groups during extinction training and the extinction test. A repeated measures ANOVA revealed no significant Block × Group interaction, F(1, 24) = 0.68, p = .42; so the observed difference in spontaneous recovery between the two groups was not an artifact of changes in baseline startle.
As with our previous fear-potentiated startle studies (e.g., Jovanovic et al., 2005), the difference in trial type (e.g., CS+ vs. CS−) is not always visually apparent when presented as overall startle magnitude (see Figure 8, Panels B and C). However, the crucial analysis is the startle response to Lights A (previously reinforced CS+) and B (CS−) as a function of NA or baseline. When expressed as the difference score from NA or baseline (see Figure 8A), the difference was more evident and responses to Lights A and B were significantly different. The same NA values were used to compute difference scores for Lights A and B, as the NA trials were interwoven throughout the extinction test session.
Extinction test: US expectancy rating
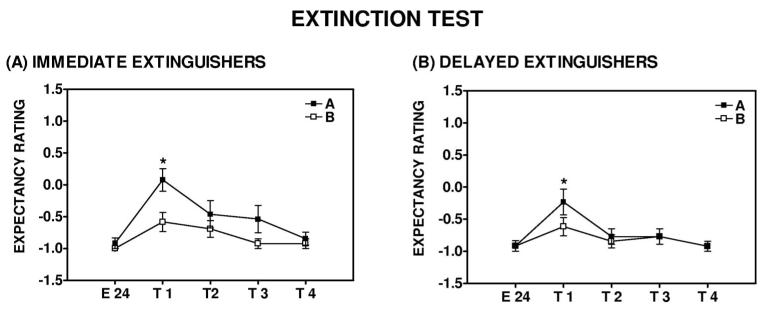
Subjects in both the immediate and delayed groups increased their US expectancy ratings on A and B trials from the end of extinction training to the extinction test. For the immediate group, there was a main effect of Trial, F(1, 10) = 39.05, p < .001; and there was a significant Trial × Trial Type interaction, F(1, 10) = 5.21, p = .05 (see Figure 9A). For the delayed group, there was a main effect of Trial, F(1, 11) = 9.43, p = .01; but there was no Trial × Trial Type interaction, F(1, 11) = 3.14, p > .05 (see Figure 9B). Immediate and delayed groups showed significant discrimination between A and B trials during the extinction test phase: For the immediate group, F(1, 11) = 6.77, p < .05; for the delayed group, F(1, 12) = 4.55, p = .05 (see Figure 9, Panels A and B).
Figure 9.
Unconditioned stimulus (US) expectancy responses during the extinction test in the differential conditioning paradigm. (Panel A) Immediate and (Panel B) delayed extinguishers increased their US expectancy ratings from the end of extinction training to the extinction test For the immediate group: repeated measures analysis of variance (ANOVA), main effect of Trial, F(1, 10) = 39.05, p < .001; for the delayed group, repeated measures ANOVA, main effect of Trial, F(1, 11) = 9.43, p = .01. Immediate and delayed extinguishers also showed significant discrimination between A and B trials during the extinction test phase: Repeated measures ANOVA, significant Trial × Trial Type interaction for the immediate group, F(1, 11) = 6.77, p < .05; and for the delayed group, F(1, 12) = 4.55, p = .05.
Discussion
Main Findings
Single-cue study
In the single-cue experiments, conditioned fear developed rapidly to the reinforced light CS as evidenced by fear-potentiated startle and US expectancy ratings in both the immediate and delayed groups. As in our previous extinction work, within-session extinction was only observed in a subset of participants (based on a 50% criterion for the within-session decrement in fear-potentiated startle during extinction training). Those subjects who met the 50% within-session criterion were termed extinguishers. Near-complete within-session extinction of fear-potentiated startle was observed by extinguishers in both the immediate and delayed groups.
During extinction training, US expectancy ratings from extinguishers in both groups indicated significant recall of the habituation phase that occurred during acquisition. In addition, US expectancy ratings from extinguishers in both groups revealed uncertainty at the point in the extinction training session at which they would have expected the US during acquisition. However, these initial uncertain responses decreased over the course of extinction training. At the end of the extinction training session, conditioned fear to the previously reinforced CS was completely extinguished, as indicated by startle and US expectancy measures.
The carry-over effect of the habituation phase into extinction training was present in the majority of subjects in both the immediate and delayed groups. This effect highlights the confusion that is becoming increasingly prevalent in our human studies of extinction. There was a subset of subjects in both the immediate (5 of 12) and delayed (8 of 19) groups that showed clear expectancy of the US at the time of the extinction test, no expectancy at the end of extinction training (score of −1) and spontaneous recovery at the extinction test (score at or near 1; data not shown, as there was an insufficient number of subjects for statistical analysis).
As mentioned previously, Myers et al. (2006), using a single-cue paradigm similar to that used in the present study, demonstrated that spontaneous recovery of fear-potentiated startle could be abolished by extinguishing fear in rats 10 min after, but not 72 hr after, acquisition. In the present human study, there was a significant increase in fear-potentiated startle in the delayed group, but not in the immediate group, when assessed by difference score from baseline startle. However, this apparent difference in spontaneous recovery of fear-potentiated startle resulted from an increase in baseline startle (startle to the noise probe in the absence of a CS) in the immediate group that was not observed in the delayed group.
It is not clear why the immediate group showed more context conditioning than the delayed group. One possibility is that context conditioning is retained more poorly than cue conditioning. Thus, a test of context conditioning 96 hr after fear conditioning in the delayed group would show less memory of context conditioning than would be seen in the immediate group tested 10 min after fear conditioning. A second possibility is that the increase in startle in the immediate group was due to sensitization of startle by the US (Davis, 1989), in which startle can be elevated shortly after a series of US presentations, that may or may not reflect rapid context conditioning. Another possibility is that in the single-cue paradigm, subjects were presented with only one CS, and this stimulus exhibited both excitatory and inhibitory properties within a short period of time. It is plausible that the use of a single CS coupled with the short duration between acquisition and extinction training introduced a greater degree of unpredictability in the immediate group. As discussed by Grillon (2002), unpredictability can lead to greater context conditioning (Grillon, 2002). Whatever the precise mechanism, it is clear that a significant shift in baseline startle coupled with a potential ceiling effect can potentially confound the measure of spontaneous recovery using fear-potentiated startle techniques. As discussed later with respect to Paradigm 2, it appears possible to avoid the observed shift in baseline startle through the use of differential conditioning.
To address this difference in baseline startle during the extinction test, we performed a follow-up experiment in which subject fear was extinguished 10 min after acquisition and then tested for spontaneous recovery 96 hr after extinction. Immediately before the extinction test session, subjects received nine presentations of NA to reduce the baseline startle magnitude (context conditioning) that was evident in the initial immediate group. The follow-up immediate group (immediate-R) exhibited both an increase in baseline startle and significant spontaneous recovery at the time of the extinction test. The results from this single cue study indicated that the time interval between fear acquisition to extinction learning may have affected either the acquisition or expression context conditioning but not the degree to which extinguished fear-potentiated startle recovers after extinction training. These results are not consistent with work in rodents (Myers et al., 2006).
Differential conditioning (A+/B−)
In the differential conditioning experiments (A+/B−), subjects from both the immediate and delayed groups showed robust fear-potentiated startle in the presence of the reinforced light CS+ and significant discrimination between the CS+ and CS−. Similar to the single-cue paradigm and our previous work, there was a subset of extinguishers in the immediate and delayed groups. With regard to fear-potentiated startle, subjects in both time groups displayed significant within-session extinction of fear-potentiated startle in the presence of the previously reinforced CS (Light A). There was less discrimination between Lights A and B during the initial blocks of extinction training in both groups, an effect that was observed in our previous study of extinction in humans (Norrholm et al., 2006). Last, extinguishers in the delayed group showed a greater degree of within-session extinction during extinction training compared with extinguishers in the immediate group, consistent with work in rodents (Myers et al., 2006).
Expectancy ratings
The interval between acquisition and extinction appears to be an important determinant in subject recall of the CS–US contingency from acquisition and/or their expectations regarding the extinction training session. For example, extinguishers in the immediate group showed clear discrimination between the previously reinforced CS (Light A) and nonreinforced CS (Light B). Extinguishers in the delayed group, however, displayed greater uncertainty and a lack of discrimination between the CSs during the initial block of extinction training. This does not appear to be due to subjects’ forgetting, as the delayed group showed discrimination between Lights A and B later in the extinction training session. It may be that the immediate group expects the extinction training session, which occurs only 10 min after acquisition, to be an additional acquisition session, whereas the delayed group experiences enough time during the 72-hr period between sessions for uncertainty to emerge.
The test for spontaneous recovery revealed an increasingly familiar phenomenon in human fear conditioning studies. There was an apparent dissociation between startle measures and US expectancy ratings (Norrholm et al., 2006). With regard to fear-potentiated startle, both the immediate and delayed groups showed an increase between the end of extinction training and the extinction test. However, only the delayed group discriminated between Lights A and B. With respect to US expectancy ratings, the immediate group, which did not discriminate between Lights A and B on startle measures, showed greater discrimination between the two CSs during the extinction test. This counterintuitive finding underscores the complexity of translating animal fear conditioning studies to the human arena.
Unlike the findings from the single-cue paradigm, there was no apparent effect of acquisition-to-extinction interval on context conditioning at the time of the extinction test, as there was no difference in baseline startle between the immediate and delayed groups at this time point. It may be that the presence of a safety signal (CS−) was protective against context conditioning. As mentioned earlier, in the single-cue paradigm, subjects were presented with only one CS, and this stimulus exhibited both excitatory and inhibitory properties within a short period of time, potentially introducing more unpredictability in the immediate group, resulting in context conditioning (Grillon, 2002). The delayed group may have experienced less unpredictability, given that their most recent exposure to the single cue was inhibitory in nature (extinction training). In addition, the use of a defined CS+ and CS− may protect against context conditioning, because there is less uncertainty with regard to the properties of each CS.
Timing of Extinction and the Return of Conditioned Fear
The similarities and differences between the findings of the present study and those reported by Myers et al. (2006) may be strongly related to the unique characteristics of human fear conditioning studies. For example, humans, unlike rats, possess the capacity to anticipate an expansive range of outcomes and contingencies with the presentation of each experimental session. This is evident in fear-potentiated startle responses during extinction training in the differential conditioning paradigm. In the present study as well as our previous work (Norrholm et al., 2006), subjects did not show discrimination between the previously reinforced CS (Light A) and the nonreinforced CS (Light B) during the first half of the extinction training session. On the basis of participant responses during the exit interview for each of the aforementioned studies, this lack of discrimination reflects fear acquisition to Light A as well as an expected contingency shift during extinction training. In other words, a large number of subjects expected extinction training to be an additional acquisition session in which the CS–US contingency is reversed.
Consistent with the subjects’ apparent expectation that extinction training would be an additional, albeit modified, acquisition session, extinguishers in the immediate and delayed groups in the differential conditioning paradigm displayed a significant difference in the degree of within-session extinction. This difference may indicate that the immediate group expected paired presentations of Light A and the airblast US within extinction training, given its close temporal proximity to acquisition. For example, the immediate group displayed an increase in fear-potentiated startle near the end of extinction training that the delayed extinguishers did not exhibit (see Figure 7, Panels A and B, extinction Block 5).
Timing of Extinction and Context Conditioning
In the present study, we found a significant difference in context conditioning between the immediate and delayed groups in the single-cue paradigm. Startle magnitude during the interstimulus interval (or NA trials in the present study) is a sensitive index of contextual anxiety in the experimental environment (Grillon & Davis, 1997). Our measurement of startle magnitude during the period between CS presentations indicated greater context conditioning in the immediate group, compared with the delayed group. The basis for this difference may lie in the unpredictability associated with each time group and their experience within the experimental protocol. The immediate group experienced acquisition and extinction training on a single day during a single visit to the laboratory. The delayed group experienced extinction training (and the lack of US presentations) on the day immediately preceding the extinction test. It is quite possible that the immediate group, despite the 10-min interval in which they were both physically and temporally removed from the acquisition context, recalls the acquisition and extinction training phases as a single session. As such, subject confusion may lead to an interference effect in which startle responses reflect both generalized anxiety (because of increased unpredictability) and cue-specific fear.
Single-Cue Versus Differential Conditioning
A recent study by Alvarez and colleagues (2007) examined renewal of fear-potentiated startle after short interval extinction. In the latter study, the authors found that short interval extinction did not abolish renewal of fear-potentiated startle. Alvarez and others suggest that the discrepancy between their findings and those of Myers et al. (2006) may reflect the use of differential conditioning as opposed to single cue conditioning. The differential conditioning experiment yielded three striking findings that are relevant to extinction learning and the return of fear as a function of the time elapsed since fear acquisition. In the delayed group, as compared with the immediate group, (a) there was a greater degree of within-session extinction, (b) there was a robust return of fear at the time of extinction test relative to the terminal level of extinction, and (c) there was significant discrimination between the previously reinforced CS+ and CS−.
It is interesting to note that Rescorla (2004), in an appetitive conditioning paradigm in rats, found that the degree of spontaneous recovery was inversely related to the interval between acquisition and extinction (Rescorla, 2004). In both the single-cue and differential conditioning paradigms from the present study, we found that the degree of spontaneous recovery was less in the immediate group, compared with the delayed group. The difference between our findings and those of Rescorla (2004) most likely reflect factors such as the longer acquisition-to-extinction interval in the latter study, the species examined, and the underlying neural circuitry mediating appetitive versus fear conditioning. Nevertheless, the findings reported by Rescorla (2004) provides further emphasis on the importance of the time elapsed between initial learning of a CS–US association and its subsequent extinction and return.
Extinguishers Versus Nonextinguishers
The data presented here as well as that which we have previously reported (Norrholm et al., 2006) demonstrate that individual differences exist with regard to the rate at which conditioned fear is extinguished. In the present study, approximately 20% of subjects in each paradigm did not meet the extinction criterion of a decrement of less than 50% during extinction training. An impaired ability to extinguish fear may represent a trait marker for increased vulnerability to developing PTSD after exposure to trauma and, as such, this phenomenon warrants further study through prospective analyses in populations likely to experience trauma (e.g., predeployment of military personnel, emergency first-responders, or firefighters; see Guthrie & Bryant, 2006). It has been suggested that animal models of PTSD should incorporate deficits in fear extinction (Milad, Rauch, Pitman, & Quirk, 2006), and a recent study by Bush, Sotres-Bayon, and LeDoux (2007) emphasized the importance of examining individual differences, as opposed to central tendencies, in rat studies of fear extinction designed to model human disorders (Bush et al., 2007). The study of individual responses in fear reactivity and extinction has only recently been initiated in humans (Hettema, Annas, Neale, Kendler & Fredrikson, 2003) and may identify an important physiological predictor for the development of fear disorders in people.
In summary, the present study demonstrated that the effect of short interval extinction on the return of fear that has been observed in rats may or may not be present in humans. There was little support for this conclusion using single cue conditioning but the use of a discrimination paradigm revealed some support. The acquisition-to-extinction interval may also be an important determinant of context conditioning in single cue conditioning paradigms. The fact that this did not occur using a discrimination paradigm suggests the presence of a safety cue (i.e., the CS−) may be protective against the development of context conditioning. The current parametric analysis underscores the complexity of translating effective animal fear conditioning paradigms into the human arena. Despite the idiosyncrasies associated with human extinction (e.g., individual perception, intentional distortion, demand characteristics), we now have the capability to reliably assess the acquisition, within-session extinction, between-session extinction, and return (through reinstatement and spontaneous recovery) of conditioned fear in humans.
Acknowledgments
This work was supported by the Mental Health Service, Atlanta VAMC; the STC Program, the Center for Behavioral Neuroscience of the National Science Foundation under Agreement No. IBN-9876754 (a Venture Grant to Erica J. Duncan); a grant from the American Psychiatric Association/GlaxoSmithKline (to Erica J. Duncan); National Institute of Mental Health (NIMH) Grant 1R24MH067314-01A1 (to Barbara Rothbaum) and NIMH Grants 2P50 MH058922 and R37 MH47840 (to Michael Davis); and by the Woodruff Foundation, the Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine; and a Yerkes National Primate Center base grant.
Contributor Information
Seth D. Norrholm, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, and Veterans Affairs Medical Center (VAMC), Atlanta, GA
Bram Vervliet, Social and Behavioral Sciences, University of Amsterdam, Amsterdam, the Netherlands, and Department of Psychology, University of Leuven, Belgium.
Tanja Jovanovic, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, and Veterans Affairs Medical Center (VAMC), Atlanta, GA.
William Boshoven, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, and Veterans Affairs Medical Center (VAMC), Atlanta, GA.
Karyn M. Myers, Center for Behavioral Neuroscience, Atlanta, GA
Michael Davis, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine.
Barbara Rothbaum, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine.
Erica J. Duncan, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, and Veterans Affairs Medical Center (VAMC), Atlanta, GA
References
- Alvarez RP, Johnson L, Grillon C. Contextual-specificity of short-delay extinction in humans: Renewal of fear-potentiated startle in a virtual environment. Learning & Memory. 2007;14:247–253. doi: 10.1101/lm.493707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameli R, Ip C, Grillon C. Contextual fear-potentiated startle conditioning in humans: Replication and extension. Psychophysiology. 2001;38:383–390. [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Context, event-memories, and extinction. Erlbaum; Hillsdale, NJ: 1985. [Google Scholar]
- Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychological Review. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- Bush DEA, Sotres-Bayon F, LeDoux JE. Individual differences in fear: Isolating fear reactivity and fear recovery phenotypes. Journal of Traumatic Stress. 2007;20:413–422. doi: 10.1002/jts.20261. [DOI] [PubMed] [Google Scholar]
- Cain CK, Godsil BP, Jami S, Barad M. The L-type calcium channel blocker nifedipine impairs extinction, but not reduced contingency effects, in mice. Learning & Memory. 2005;12:277–284. doi: 10.1101/lm.88805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Pharmacological and anatomical analysis of fear conditioning using the fear-potentiated startle paradigm. Behavioral Neuroscience. 1986;100:814–824. doi: 10.1037//0735-7044.100.6.814. [DOI] [PubMed] [Google Scholar]
- Davis M. Sensitization of the acoustic startle reflex by footshock. Behavioral Neuroscience. 1989;103:495–503. [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The amygdala. Vol. 2. Oxford University Publishing; Oxford, England: 2000. pp. 213–287. [Google Scholar]
- Davis M, Falls WA, Gewirtz J. Neural systems involved in fear inhibition: Extinction and conditioned inhibition. In: Myslobodsky M, Weiner I, editors. Contemporary issues in modeling psychopathology. Kluwer Academic; Boston: 2000. pp. 113–142. [Google Scholar]
- Falls WA, Davis M. Behavioral and physiological analysis of fear inhibition. In: Friedman MJ, Charney DS, Deutch AY, editors. Neurobiological and clinical consequences of stress: From normal adaptation to PTSD. Lippincott-Raven; Philadelphia: 1995. pp. 177–202. [Google Scholar]
- Foa EB. Psychosocial treatment of posttraumatic stress disorder. Journal of Clinical Psychiatry. 2000;61:43–48. [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Fear-potentiated startle conditioning in humans: Explicit and contextual cue conditioning following paired versus unpaired training. Psychophysiology. 1997;34:451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Fear-potentiated startle in adolescent offspring of parents with anxiety disorders. Biological Psychiatry. 1998;44:990–997. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Guthrie RM, Bryant RA. Extinction learning before trauma and subsequent posttraumatic stress. Psychosomatic Medicine. 2006;68:307–311. doi: 10.1097/01.psy.0000208629.67653.cc. [DOI] [PubMed] [Google Scholar]
- Hermans D, Dirikx T, Vansteenwegenin D, Baeyens F, Van den Bergh O, Eelen P. Reinstatement of fear responses in human aversive conditioning. Behaviour Research and Therapy. 2005;43:533–551. doi: 10.1016/j.brat.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Annas P, Neale MC, Kendler KS, Fredrikson M. A twin study of the genetics of fear conditioning. Archives of General Psychiatry. 2003;60:702–708. doi: 10.1001/archpsyc.60.7.702. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, Duncan EJ. Fear potentiation and fear inhibition in a human fearpotentiated startle paradigm. Biological Psychiatry. 2005;57:1559–1564. doi: 10.1016/j.biopsych.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Keyes M, Fiallos A, Jovanovic S, Myers KM, et al. Contingency awareness and fear inhibition in a human fear-potentiated startle paradigm. Behavioral Neuroscience. 2006;120:995–1004. doi: 10.1037/0735-7044.120.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee S, Park H, Song B, Hong I, Geum D, et al. Blockade of amygdala metabotropic glutamate receptor subtype 1 impairs fear extinction. Biochemical and Biophysical Research Communications. 2007;355:188–193. doi: 10.1016/j.bbrc.2007.01.125. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behavioral Neuroscience. 2005;119:677–686. doi: 10.1037/0735-7044.119.3.677. [DOI] [PubMed] [Google Scholar]
- Lin CH, Lee CC, Gean PW. Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Molecular Pharmacology. 2003;63:44–52. doi: 10.1124/mol.63.1.44. [DOI] [PubMed] [Google Scholar]
- Lin CH, Lee CC, Huang YC, Wang SJ, Gean PW. Activation of group II metabotropic glutamate receptors induces depotentiation in amygdala slices and reduces fear-potentiated startle in rats. Learning & Memory. 2005;12:130–137. doi: 10.1101/lm.85304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Leu WC, Chang WC, Wang ST, Gean PW. Identification of calcineurin as a key signal in the extinction of fear memory. Journal of Neuroscience. 2003;23:1574–1579. doi: 10.1523/JNEUROSCI.23-05-01574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao SC, Hsiao YH, Gean PW. Extinction training in conjunction with a partial agonist of the glycine site on the NMDA receptor erases memory trace. Journal of Neuroscience. 2006;26:8892–8899. doi: 10.1523/JNEUROSCI.0365-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Chang CH. Recent fear is resistant to extinction. Proceedings of the National Academy of Sciences, USA. 2006;103:18020–18025. doi: 10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biological Psychiatry. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learning & Memory. 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann DL, Lipp OV, Cory SE. Conducting extinction in multiple contexts does not necessarily attenuate the renewal of shock expectancy in a fear-conditioning procedure with humans. Behaviour Research and Therapy. 2007;45:385–394. doi: 10.1016/j.brat.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, Duncan EJ. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learning & Memory. 2006;13:681–685. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. Oxford University Press; Oxford, England: 1927. [Google Scholar]
- Quirk GJ. Memory for extinction of conditioned fear is longlasting and persists following spontaneous recovery. Learning & Memory. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Experimental extinction. In: Mowrer RR, Klein S, editors. Handbook of contemporary learning theories. Erlbaum; Mahwah, NJ: 2001. pp. 119–154. [Google Scholar]
- Rescorla RA. Spontaneous recovery varies inversely with the training–extinction interval. Learning & Behavior. 2004;32:401–408. doi: 10.3758/bf03196037. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes. 1975;104:88–96. [PubMed] [Google Scholar]
- Vansteenwegen D, Hermans D, Vervliet B, Francken G, Beckers T, Baeyens F, Eelen P. Return of fear in a human differential conditioning paradigm caused by a return to the original acquistion context. Behaviour Research and Therapy. 2005;43:323–336. doi: 10.1016/j.brat.2004.01.001. [DOI] [PubMed] [Google Scholar]