Abstract
Purpose
Primary intraspinal facet cysts in the lumbar spine are uncommon, but it is unclear whether cyst incidence increases following decompression surgery and if these cysts negatively impact clinical outcome. We examined the prevalence, clinical characteristics, and the risk factors associated with intraspinal facet cysts after microsurgical bilateral decompression via a unilateral approach (MBDU).
Methods
We studied 230 patients treated using MBDU for lumbar degenerative disease (133 men and 97 women; mean age 70.3 years). Clinical status, as assessed by the Japanese Orthopedic Association (JOA) score and findings on X-ray and magnetic resonance images, was evaluated prior to surgery and at both 3 months and 1 year after surgery. The prevalence of intraspinal facet cysts was determined and preoperative risk factors were defined by comparing presurgical findings with clinical outcomes.
Results
Thirty-eight patients (16.5 %) developed intraspinal facet cysts within 1 year postoperatively, and 24 exhibited cysts within 3 months. In 10 patients, the cysts resolved spontaneously 1 year postoperatively. In total, 28 patients (12.2 %) had facet cysts 1 year postoperatively. The mean JOA score of patients with cysts 1 year postoperatively was significantly lower than that of patients without cysts. This poor clinical outcome resulted from low back pain that was not improved by conservative treatment. Most cases with spontaneous cyst disappearance were symptom-free 1 year later. The preoperative risk factors for postoperative intraspinal facet cyst formation were instability (OR 2.47, P = 0.26), scoliotic disc wedging (OR 2.23, P = 0.048), and sagittal imbalance (OR 2.22, P = 0.045).
Conclusions
Postoperative intraspinal facet cyst formation is a common cause of poor clinical outcome in patients treated using MBDU.
Keywords: Decompression microsurgery, Minimally invasive surgery, Lumbar spinal stenosis, Lumbar spine
Introduction
Surgical management of degenerative lumbar disease has become progressively less invasive. Preservation of the posterior spinal elements is the most important factor for successful decompression surgery of lumbar canal stenosis (LCS). Some authors have reported good clinical outcomes following microsurgical bilateral decompression via a unilateral approach (MBDU), a less invasive technique preserving the posterior elements, including the paravertebral muscle, facet joints, and lamina [1–6]. Some studies have also reported the clinical results of MBDU for LCS in mild instability cases, such as spondylolisthesis and complications from haemodialysis [7, 8], but there are few reports examining complications after MBDU or risk factors that might preclude certain patients from MBDU.
Improved diagnostic imaging techniques, especially magnetic resonance imaging (MRI), have greatly facilitated the diagnosis of intraspinal lesions. Intraspinal facet cysts in the lumbar spine, including synovial and ganglion cysts, are considered an uncommon cause of low back pain, radicular pain, and cauda equina syndrome. Although many cases of primary intraspinal facet cysts in the lumbar spine have been reported [9–12], few have documented the incidence of postoperative intraspinal facet cysts after decompression surgery or identified preoperative risk factors [13, 14]. Therefore, we conducted a retrospective analysis of 230 patients treated for lumbar degenerative diseases using MBDU, focusing on the incidence, clinical characteristics, and risk factors for postoperative intraspinal facet cysts. We suggest that postoperative facet cyst formation is a significant cause of poor clinical outcome following MBDU. The purpose of this study was to examine the prevalence, clinical characteristics, and the risk factors associated with intraspinal facet cysts after MBDU.
Materials and methods
Patients
This study included 230 patients (334 discs; 133 men, 97 women; age range 34–92 years; mean age 70.3 years) treated in our institution from 2006 to 2010 by MBDU. Clinical indications for this surgery were leg pain and/or leg numbness inducing intermittent claudication (rather than back pain). The radiological indications were lumbar spinal stenosis, degenerative lumbar spondylolisthesis with slippage <Meyerding Grade I, or degenerative lumbar scoliosis with <20° of Cobb’s angle. The preoperative diagnoses were lumbar spinal stenosis (129 patients), degenerative lumbar spondylolisthesis (50 patients), and degenerative lumbar scoliosis (51 patients). Patients with primary intraspinal facet cysts were excluded. Level of surgery was L2–3 in 18 discs, L3–4 in 104 discs, L4–5 in 204 discs, and L5–S1 in 8 discs.
Surgical procedure
Microscopic bilateral decompression via a unilateral approach was modified from the method reported previously for complete decompression on the contralateral side [6]. The laminotomy was performed on the side of approach in the area of the ligamentum flavum insertion, and resection of the articular process was performed in a trumpeted fashion to the inner aspect of the pedicle, with slight lateral tilting of the microscope. After the side of approach had been completely decompressed, the operating table and microscope were tilted about 15° to observe the contralateral side. The basal part of the spinous process of the caudal half of the cranial lamina and a small cranial portion of the caudal lamina were removed with a high-speed drill. Then, the contralateral lamina was undercut with a high-speed air drill, leaving the ligamentum flavum in place as protection for the dural sac and nerve root. Following sufficient resection of the bony segment, the ligamentum flavum was removed en bloc with a curette while protecting the dural sac and contralateral nerve root with a patty. Adequate decompression of the contralateral side was confirmed by recognition of the inner aspect of the contralateral pedicle (Fig. 1).
Fig. 1.
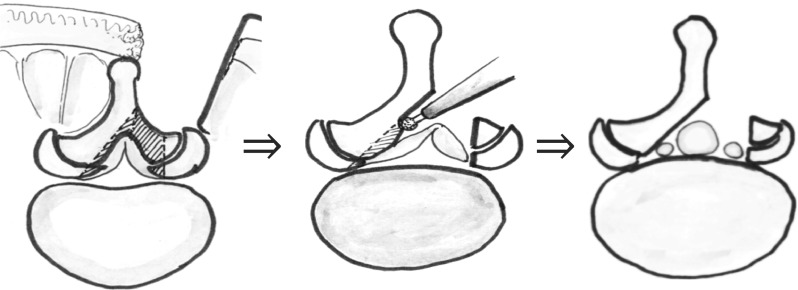
Schema showing our modified microsurgical bilateral decompression via a unilateral approach (MBDU) method (see “Materials and methods” and “Surgical procedure” for details)
Clinical and radiographic evaluation
Clinical outcomes were evaluated by comparing the preoperative Japanese Orthopedic Association (JOA) scores and X-ray findings (anterioposterior and lateral plain X-rays) to the postoperative JOA scores, X-ray findings, and MRI results at 3 months and 1 year. The ratio of JOA score improvement was calculated using the formula proposed by Hirabayashi [15, 16]. Slippage ratio was measured by the Boxall method from lateral view X-rays [17]. The angle of scoliosis was measured as the Cobb’s angle of L1–5 from anteroposterior view X-rays. The development of postoperative juxtafacet cysts was revealed by MRI. Cysts were defined as round or oval lesions of material, fluid, or blood signals arising from the facet joint. Synovial juxtafacet cysts (non-intraspinal canal cysts) were not included in this study. Typical acquisition settings for T1-weighted images were TR = 580 ms, TE = 12 ms, and slice thickness of 4 mm. For T2-weighted images, typical settings were TR 4,000–7,500 ms, TE = 120 ms, and slice thickness of 4 mm. Typical fields of view were 300 mm × 225 mm for sagittal images and 200 mm × 150 mm for axial images.
Statistical analysis
Statistical correlations were examined using the Mann–Whitney U, one-way analysis of variance (ANOVA), and the Chi-squared (χ2) tests. Probability values <0.05 were considered statistically significant.
Results
Prevalence of intraspinal facet cysts
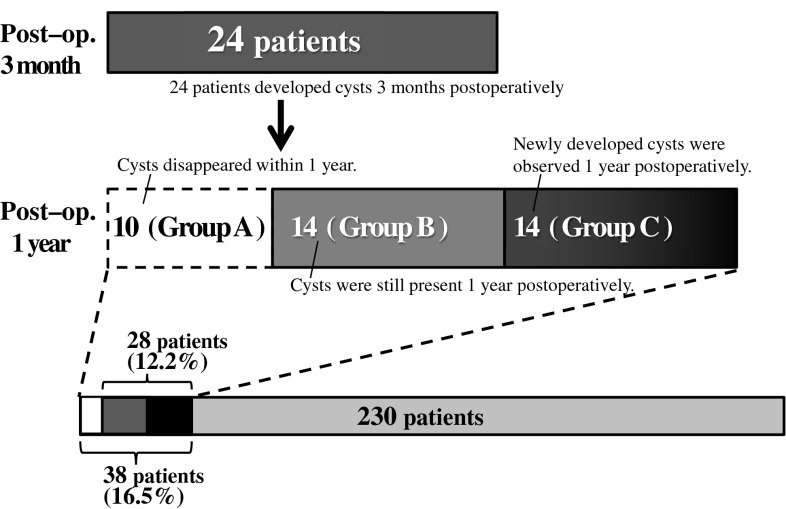
Intraspinal facet cysts were observed in 24 patients (26 cysts) 3 months postoperatively. One year postoperatively, 10 of these early cysts had disappeared, while 16 cysts in 14 patients remained. Fourteen newly developed cysts were observed in 14 patients 1 year postoperatively. In total, 40 postoperative intraspinal facet cysts developed in 38 patients during the first postoperative year (40/334 or 11.9 % of treated discs in 38/230 or 16.5 % of patients). Twenty-eight patients had facet cysts (30 in total) 1 year postoperatively (12.2 % of patients, 8.9 % of treated discs) (Fig. 2).
Fig. 2.
Prevalence of intraspinal facet cysts. Thirty-eight patients (16.5 %) developed postoperative intraspinal facet cysts developed at some point during the first postoperative year. In 28 patients, the facet cysts persisted 1 year after surgery (12.2 %)
Patients with cysts were categorised into three groups: Group A patients developed cysts 3 months postoperatively that disappeared within 1 year (10 patients, 10 cysts), Group B patients developed cysts within 3 months that were still present 1 year postoperatively (14 patients, 16 cysts), and Group C patients developed cysts >3 months postoperatively that remained 1 year later (14 patients, 14 cysts). Table 1 presents patient demographics, including data on Group N, i.e. patients without postoperative facet cysts. Mean age, operative time, blood loss, and gender ratio did not differ significantly between the groups. Locations of postoperative intraspinal facet cysts are presented in Table 2. The most common sites in all groups were L3–4 and L4–5; few cysts developed at L2–3 and L5–S1. Cyst incidence on the approach side was nearly equal to that on the contralateral side in Groups A and B with early cyst development. The later developing cysts of Group C were more frequently on the approach side. In Groups B and C, bilateral cysts developed more frequently than in Group A.
Table 1.
Patients’ demographics
| Group A | Group B | Group C | Group N (patients without cyst) | |
|---|---|---|---|---|
| Number | 10 | 14 | 14 | 192 |
| Age (years) | 75.8 | 69.2 | 70.7 | 70.1 |
| Gender (men/women) | 8/2 | 11/3 | 9/5 | 105/87 |
| Time of operation (min) | 187 | 173 | 174 | 168 |
| Blood loss (ml) | 64 | 92 | 88 | 80 |
| Approach side (right/left) | 5/5 | 6/8 | 3/11 | 111/119 |
| Diagnosis | ||||
| (LCS/DS/DLS) | 7/2/1 | 6/6/2 | 8/2/4 | 108/40/44 |
| No. of levels decompressed | ||||
| (1/2/3) | 3/7/0 | 4/9/1 | 6/7/1 | 121/65/6 |
LCS lumbar canal stenosis, DS degenerative lumbar spondylolisthesis, DLS degenerative lumbar scoliosis
Table 2.
Location of postoperative intraspinal facet cyst
| Total (Groups A, B, and C) | Group A | Group B | Group C | |
|---|---|---|---|---|
| Level | ||||
| L2–3 | 1 | 1 | 0 | 0 |
| L3–4 | 17 | 5 | 6 | 6 |
| L4–5 | 18 | 4 | 6 | 8 |
| L3–4, L4–5 | 2 | 0 | 2 | 0 |
| Side | ||||
| Approach side | 16 | 4 | 4 | 8 |
| Contralateral side | 15 | 6 | 5 | 4 |
| Bilateral side | 7 | 0 | 5 | 2 |
Clinical results
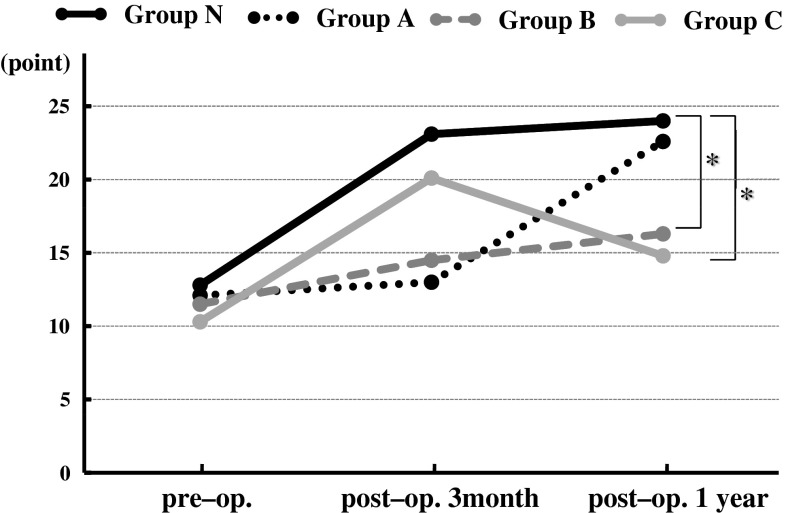
Mean operative time for all patients was 169.3 min (range 59–391 min) and mean operative time per level was 116 min. Mean total blood loss was 80.3 ml (range 0–480 ml) and 55.2 ml per level. Mean JOA score was 12.5 preoperatively, 21.9 at 3 months postoperatively, and 22.9 at 1 year postoperatively. No patients presented with neurological deficits postoperatively, and none required a second surgery because of worsening symptoms. We compared the clinical results among the four groups (Fig. 3). Mean preoperative JOA scores were 12.8 in Group N, 12.1 in Group A, 11.5 in Group B, and 10.3 in Group C. Three months postoperatively, mean JOA scores improved significantly to 23.1 in Group N and to 20.1 in Group C; Groups A and B demonstrated more modest improvements (13 and 14.5, respectively). However, the mean JOA score of Group A improved to 22.6 points, while that of Group C regressed to 14.8 at 1 year postoperatively. Mean recovery ratios of JOA scores 1 year postoperatively were 69.1 % in Group N, 62.1 % in Group A, 27.4 % in Group B, and 24 % in Group C. Recovery ratios of Groups B and C were significantly lower than that of Group N.
Fig. 3.
Graph showing clinical outcome according to the Japanese Orthopedic Association scoring system (JOA score). The asterisk indicates the recovery ratio of JOA score in Groups B and C were significantly lower than that in Group N (P < 0.05)
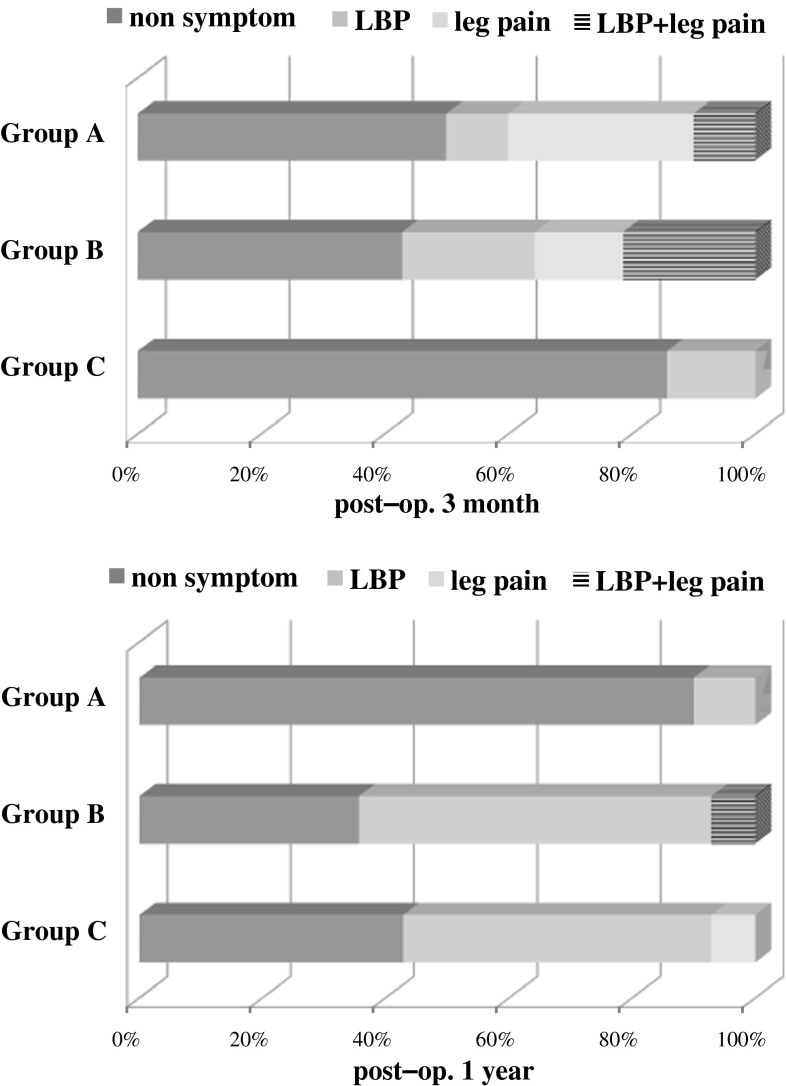
We analysed the symptoms of patients with cysts by group (Fig. 4). Patients with radicular symptoms (leg pain) used a steroid injection to the nerve roots and those with low back pain used medication, physical therapy, and facet joint block. More than half of Group A and B patients reported symptoms associated with cysts, whereas there were no symptoms in the majority of Group C patients (12/14, 86 %) 3 months postoperatively. About half of the patients in Group A had radicular pain that was relieved by the nerve block. One year postoperatively, there were no symptoms in 9 patients (90 %) in Group A; however, more than half of the patients in Groups B and C with persistent or late developing cysts reported low back pain that did not improve with conservative treatment resulting in poor clinical outcome.
Fig. 4.
Graph showing the symptoms of patients with cysts. Three months postoperatively, about half of the patients in GroupsA and B had several symptoms. One year postoperatively, most patients in GroupA were symptom-free, while more than half of the patients in GroupsB and C had low back pain
Radiographic factors related to cyst development
We examined the relationship between radiographic findings that may predict postsurgical complications (instability, scoliosis, scoliotic disc wedging, and sagittal imbalance) and the presence of cysts (n = 28) 1 year postoperatively (Table 3). Preoperative anteroposterior instability was defined as anteroposterior translation of >3 mm by flexion–extension. Scoliosis was defined as having a Cobb’s angle of >10° at L1–5. Preoperative scoliotic disc wedging was defined as wedging of >5° on anteroposterior X-rays in supine position (Fig. 5). Sagittal imbalance was defined as >50 mm distance between the C7 plum line and sacral promontorium on lateral view of the spine in standing. Postoperative exacerbation of instability was defined as a translational increase of ≥3 mm 1 year postoperatively. Postoperative progress of scoliosis and scoliotic disc wedging were defined as an increase in scoliosis to ≥5° and an increase of ≥3° for wedging. Exacerbation of sagittal imbalance was defined as an increase in anterior displacement of ≥50 mm (Fig. 6). In total, there were 59 patients of preoperative instability, 51 of scoliosis, 77 of scoliotic disc wedging, and 84 of preoperative sagittal imbalance. There was an association between preoperative instability and postoperative cyst formation (OR 2.47, P = 0.26). Preoperative scoliotic disc wedging (OR 2.23, P = 0.048) and preoperative sagittal imbalance (OR 2.22, P = 0.045) were significantly associated with postoperative cyst formation. Postoperative increases in instability, scoliosis, scoliotic disc wedging, and sagittal imbalance were not associated with postoperative cyst development.
Table 3.
Preoperative radiographic factors related to postoperative cyst development
| Cyst (−) | Cyst (+) | OR | P value | |
|---|---|---|---|---|
| Instability (−) | 155 | 16 | 1 | |
| Instability (+) | 47 | 12 | 2.47 | 0.026 |
| Scoliosis (−) | 157 | 22 | ||
| Scoliosis (+) | 45 | 6 | ||
| Scoliotic disc wedging (−) | 139 | 14 | 1 | |
| Scoliotic disc wedging (+) | 63 | 14 | 2.23 | 0.048 |
| Sagittal imbalance (−) | 133 | 13 | 1 | |
| Sagittal imbalance (+) | 69 | 15 | 2.22 | 0.045 |
OR odds ratio, Cyst intraspinal facet cyst which existed 1 year postoperatively
Fig. 5.
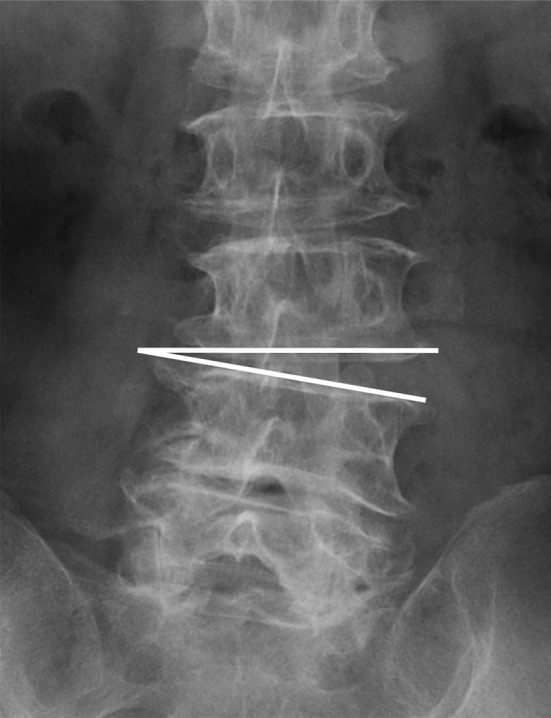
Schema showing scoliotic disc wedging represented by the line. Preoperative scoliotic disc wedging was defined as wedging of >5° on anteroposterior X-rays in supine position
Fig. 6.
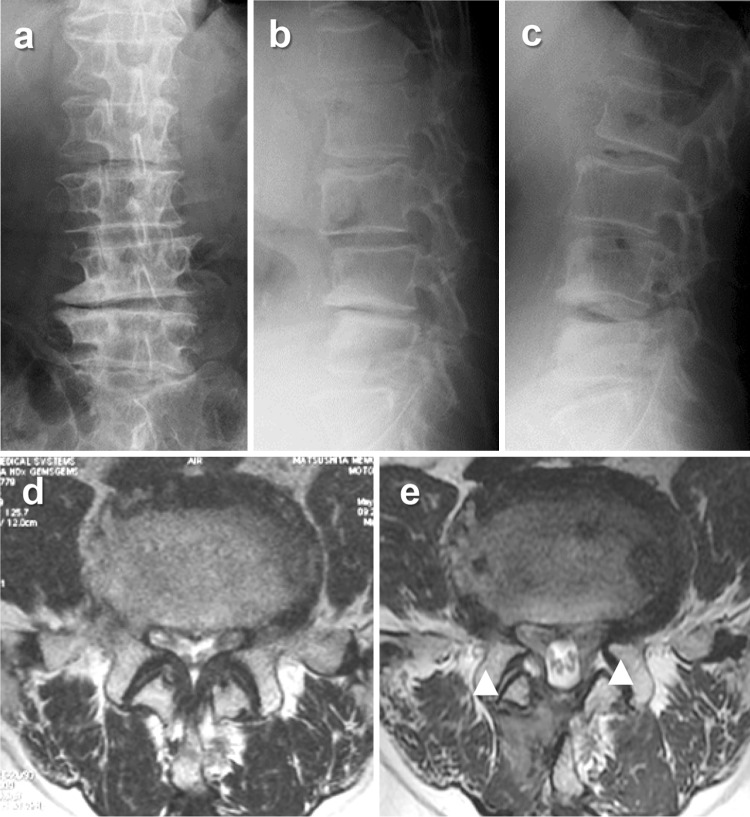
Representative case. A 68-year-old man with L4–5 degenerative lumbar spondylolisthesis and disc wedging reported intermitted claudication due to leg pain (JOA score of 13). After MBDU, intermitted claudication disappeared, but disabling lower back pain appeared 2 months postoperatively that resisted conservative treatment (1 year JOA score 15). a–c Preoperative X-rays: a anteroposterior view, b lateral view (flexion), c lateral view (extension). d, e Axial T2-weighted magnetic resonance imaging of L4–5: d preoperative, e 1 year postoperatively (arrowheads indicate intraspinal facet cysts)
Discussion
Primary lumbar intraspinal facet cysts are rare, observed in only 2.3 % of patients referred for back pain or radiculopathy [11]. Postoperative lumbar intraspinal facet cysts also appear to be uncommon, but may be more frequent following lumbar decompression surgery. Ikuta et al. [13] reported an 8.6 % prevalence of postoperative cysts after decompression surgery. In the present study, 16.5 % of patients developed postoperative cysts at some point during the first postoperative year, with 10.4 % developing cysts within the first 3 months and 12.2 % exhibiting cysts 1 year later. MBDU was performed on many of our patients presenting with severe degenerative changes of the facet joints, including lumbar spondylolisthesis and degenerative lumbar scoliosis, which may account for the higher frequency of postoperative cysts.
Spinal segmental instability associated with facet joint osteoarthritis is a common cause of juxtafacet cysts [18–21]. The subsequent defect in the intervertebral joint capsule becomes surrounded by myxoid degeneration and cyst formation in the collagenous connective tissue. The cysts then lead to inflammatory cell infiltration and synovial cell proliferation, which result in the formation of a cystic cavity that can communicate with the joint. Therefore, primary synovial cysts are typically observed in the mobile segments of the lumbar spine where spondylosis most frequently occurs. The most common sites, in descending order of incidence, are L4–5 and L3–4 [9, 20]. These lumbar levels appear in association with the degeneration of the facet joint and are probably caused by repetitive trauma and a microinstability that produce areas of focal weakness in the facet capsule. In this study, postoperative juxtafacet cysts were most common at L3–4 and L4–5, where degenerative spondylotic changes in facet joints were most frequent which is characteristically similar to those of primary facet joints. The pathogenic mechanisms for postoperative intraspinal cysts development remain unknown, but are similar among postoperative and primary intraspinal cysts. The segmental instability and degenerative joint changes in the lumbar spine might contribute to both postoperative and primary juxtafacet cyst development.
A few reports investigated the risk factors for postoperative cyst development. Ikuta et al. [13] reported that increased postoperative instability was a risk factor for facet cyst development. However, Minami et al. [14] found no correlation between postoperative instability and cyst development. The present study discovered evidence that preoperative instability, scoliotic disc wedging, and sagittal imbalance can contribute to postoperative intraspinal cyst development. The results of the present study therefore affirm the relationship between preoperative instability and postoperative cyst development. The authors suppose that an instable intervertebral joint can affect this change after surgery. Preoperative scoliotic disc wedging and sagittal imbalance can lead to postoperative cyst formation because they can produce excessive load or maldistributed load to the facet joints.
In a significant proportion (42 %) of our patients who developed cysts 3 months after surgery, the cysts disappeared spontaneously. A few previous reports have also described spontaneous regression or disappearance of primary intraspinal facet cysts [22, 23], whereas others have found that primary lumbar intraspinal facet cysts can spontaneously regress with corresponding improvements in clinical symptoms. One plausible explanation for regression is cyst rupture, which has been reported to occur in other regions of the body in conjunction with radiographically documented resolution of lesions. Alternatively, regression may be due to postoperative improvement in the local mechanical stress that initially drove the cystic formation. Postoperative and primary intraspinal cysts likely share a similar pathogenic mechanism. However, a postoperative cyst might be more fragile than a primary cyst, leading to more frequent rupturing under mechanical stress. The postoperative cysts were treated in the present study using a steroid injection with either a facet joint block or a nerve root block [24]. The authors speculate that either block helps decrease the irritation and that the steroid contributes to the cystic regression. Restoration of a damaged facet joint by surgical intervention and restabilisation of the spinal segment could cause cystic regression and associated histological changes.
Patients with juxtafacet cysts commonly report radicular pain, buttock pain, or lumbago, but the clinical presentation is not specific. A few reports found that low back pain preceded radicular symptoms in most patients [9, 11]. Because most intraspinal facet cysts are associated with spinal column arthropathy, non-localised back pain is usually not attributable solely to the cyst. Furthermore, many patients report symptoms of sciatica indistinguishable from those associated with a herniated disc. Over half of our patients with persistent cysts reported low back pain that was resistant to conservative therapy. Therefore, postoperative juxtafacet cysts should be recognised as a significant cause of postoperative symptom deterioration.
Optimal treatments for intraspinal cysts remain a matter of debate. Conservative treatment consists of bed rest, analgesics plus anti-inflammatory drugs, physical therapy, bracing, transcutaneous electrical stimulation, epidural or intra-articular steroid injections, and computed tomography-guided fine needle aspiration and arthrography [25–27]. Surgical treatments include resection or fusion [28, 29]. A treatment regimen for postoperative cysts has not yet been established. Our institution uses steroid injection to the nerve root for patients with radiculopathy (leg pain) and an intervertebral joint injection for patients with lumbago. Almost all patients with radicular pain improved with steroid injections to the nerve root. However, injection to the facet joint was not effective for patients with low back pain. Persistent postoperative cysts might be more resistant to conservative treatment than primary intraspinal cysts are. When lumbago persists or is aggravated after surgery, especially in cases with cysts that cause lumbago 1 year after surgery, the authors recommend a second surgery (resection or fixation).
Postoperative intraspinal facet cysts appeared in only a minority of patients following minimally invasive lumbar decompression surgery, but often results in exacerbation of symptoms and poor clinical outcomes. These postoperative juxtafacet cysts manifest by several symptoms, but low back pain was the most difficult symptom to treat using conservative therapy. Postoperative intraspinal cysts should be recognised as a common cause of postoperative symptom deterioration following MBDU. We suggest caution in using MBDU for patients with instability, scoliotic disc wedging, or sagittal imbalance, because a fusion operation should be considered as an alternative in such situations.
Conclusions
Postoperative intraspinal facet cyst formation is a common cause of poor clinical outcome in patients treated using MBDU.
Conflict of interest
None.
References
- 1.Poletti CE. Central lumbar stenosis caused by ligamentum flavum: unilateral laminotomy for bilateral ligamentectomy: preliminary report of two cases. Neurosurgery. 1995;37(2):343–347. doi: 10.1227/00006123-199508000-00025. [DOI] [PubMed] [Google Scholar]
- 2.McCulloch JA, Young PA. Essentials of spinal microsurgery. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 3.Spetzger U, Bertalanffy H, Naujokat C, et al. Unilateral laminotomy for bilateral decompression of lumbar spinal stenosis. Part I: anatomical and surgical considerations. Acta Neurochir (Wien) 1997;139(5):392–396. doi: 10.1007/BF01808872. [DOI] [PubMed] [Google Scholar]
- 4.Spetzger U, Bertalanffy H, Reinges MH, et al. Unilateral laminotomy for bilateral decompression of lumbar spinal stenosis. Part II: clinical experiences. Acta Neurochir (Wien) 1997;139(5):397–403. doi: 10.1007/BF01808874. [DOI] [PubMed] [Google Scholar]
- 5.Weiner BK, Walker M, Brower RS, et al. Microdecompression for lumbar spinal canal stenosis. Spine. 1999;24(21):2268–2272. doi: 10.1097/00007632-199911010-00016. [DOI] [PubMed] [Google Scholar]
- 6.Toyoda H, Nakamura H, Kato M, et al. Clinical outcome of microsurgical bilateral decompression via unilateral approach for lumbar canal stenosis: minimum five-year follow-up. Spine. 2011;36(5):410–415. doi: 10.1097/BRS.0b013e3181d25829. [DOI] [PubMed] [Google Scholar]
- 7.Matsumura A, Namikawa T, Terai H, et al. The influence of approach side on facet preservation in microscopic bilateral decompression via a unilateral approach for degenerative lumbar scoliosis. J Neurosurg Spine. 2010;13(6):758–765. doi: 10.3171/2010.5.SPINE091001. [DOI] [PubMed] [Google Scholar]
- 8.Sasai K, Umeda M, Maruyama T, et al. Microsurgical bilateral decompression via a unilateral approach for lumbar spinal canal stenosis including degenerative spondylolisthesis. J Neurosurg Spine. 2008;9(6):554–559. doi: 10.3171/SPI.2008.8.08122. [DOI] [PubMed] [Google Scholar]
- 9.Lyons MK, Atkinson JL, Wharen RE, et al. Surgical evaluation and management of lumbar synovial cysts. J Neurosurg. 2000;93(1 Suppl):53–57. doi: 10.3171/spi.2000.93.1.0053. [DOI] [PubMed] [Google Scholar]
- 10.Wilby MJ, Fraser RD, Vernon-Roberts B, et al. The prevalence and pathogenesis of synovial cysts within the ligamentum flavum in patients with lumbar spinal stenosis and radiculopathy. Spine. 2009;34(23):2518–2524. doi: 10.1097/BRS.0b013e3181b22bd0. [DOI] [PubMed] [Google Scholar]
- 11.Doyle AJ, Merrilees M. Synovial cysts of the lumbar facet joints in a symptomatic population prevalence on MRI. Spine. 2004;29(8):874–878. doi: 10.1097/00007632-200404150-00010. [DOI] [PubMed] [Google Scholar]
- 12.Sachdev VP, Savitz MH, Hindi AI, et al. Synovial cysts of the lumbar facet joint. Mt Sinai J Med. 1991;58(2):125–128. [PubMed] [Google Scholar]
- 13.Ikuta K, Tono O, Oga M. Prevalence and clinical features of intraspinal facet cysts after decompression surgery for lumbar spinal stenosis. J Neurosurg Spine. 2009;10(6):617–622. doi: 10.3171/2009.2.SPINE08769. [DOI] [PubMed] [Google Scholar]
- 14.Minami N, Morinaga T, Ikeda S, et al. Facet cysts after hemi-laminectomy of the lumbar spine. J Spine Res. 2011;2(7):1241–1243. [Google Scholar]
- 15.Yone K, Sakou T, Kawauchi Y, et al. Indication of fusion for lumbar spinal stenosis in elderly patients and its significance. Spine. 1996;21(2):242–248. doi: 10.1097/00007632-199601150-00016. [DOI] [PubMed] [Google Scholar]
- 16.Hirabayashi K, Miyakawa J, Satomi K, et al. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine. 1981;6(4):354–364. doi: 10.1097/00007632-198107000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Boxall D, Bradford DS, Winter RB, et al. Management of severe spondylolisthesis in children and adolescents. J Bone Jt Surg Am. 1979;61(4):479–495. [PubMed] [Google Scholar]
- 18.Kurz LT, Garfin SR, Unger AS, et al. Intraspinal synovial cyst causing sciatica. J Bone Jt Surg Am. 1985;67(6):865–871. [PubMed] [Google Scholar]
- 19.Prestar FJ. Juxta facet cysts of the lumbar spine. Minim Invasive Neurosurg. 1996;39(2):45–49. doi: 10.1055/s-2008-1052215. [DOI] [PubMed] [Google Scholar]
- 20.Hsu KY, Zucherman JF, Shea WJ, et al. Lumbar intraspinal synovial and ganglion cyst (facet cyst). Ten-year experience in evaluation and treatment. Spine. 1995;20(1):80–89. doi: 10.1097/00007632-199501000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Kusakabe T, Kasama F, Aizawa T, et al. Facet cyst in the lumbar spine: radiological and histopathological findings and possible pathogenesis. J Neurosurg Spine. 2006;5(5):398–403. doi: 10.3171/spi.2006.5.5.398. [DOI] [PubMed] [Google Scholar]
- 22.Houten JK, Sanderson SP, Cooper PR. Spontaneous regression of symptomatic lumbar synovial cysts. J Neurosurg. 2003;99(2 Suppl):235–238. doi: 10.3171/spi.2003.99.2.0235. [DOI] [PubMed] [Google Scholar]
- 23.Hemminghytt S, Daniels DL, Williams AL, et al. Intraspinal synovial cysts: natural history and diagnosis by CT. Radiology. 1982;145(2):375–376. doi: 10.1148/radiology.145.2.7134440. [DOI] [PubMed] [Google Scholar]
- 24.Parlier-Cuau C, Wybier M, Nizard R, et al. Symptomatic lumbar facet joint synovial cysts: clinical assessment of facet joint steroid injection after 1 and 6 months and long-term follow-up in 30 patients. Radiology. 1999;210(2):509–513. doi: 10.1148/radiology.210.2.r99fe60509. [DOI] [PubMed] [Google Scholar]
- 25.Bjorkengren AG, Kurz LT, Resnick D, et al. Symptomatic intraspinal synovial cysts: opacification and treatment by percutaneous injection. Am J Roentgenol. 1987;149(1):105–107. doi: 10.2214/ajr.149.1.105. [DOI] [PubMed] [Google Scholar]
- 26.Howling SJ, Kessel D. Case report: acute radiculopathy due to a haemorrhagic lumbar synovial cyst. Clin Radiol. 1997;52(1):73–74. doi: 10.1016/S0009-9260(97)80313-3. [DOI] [PubMed] [Google Scholar]
- 27.Knox AM, Fon GT. The appearance of lumbar synovial cysts. Clin Radiol. 1991;44(6):397–401. doi: 10.1016/S0009-9260(05)80658-0. [DOI] [PubMed] [Google Scholar]
- 28.Boviatsis EJ, Stavrinou LC, Kouyialis AT, et al. Spinal synovial cysts: pathogenesis, diagnosis and surgical treatment in a series of seven cases and literature review. Eur Spine J. 2008;17(6):831–837. doi: 10.1007/s00586-007-0563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banning CS, Thorell WE, Leibrock LG. Patient outcome after resection of lumbar juxtafacet cysts. Spine. 2001;26(8):969–972. doi: 10.1097/00007632-200104150-00024. [DOI] [PubMed] [Google Scholar]