Abstract
Purpose
A comparative immunolocalisation study of perlecan, HS, FGF-18 and FGFR-3 in the 12–20-week gestational age human foetal spine was undertaken to identify spatiotemporal associations between these components to provide insights into prospective roles in spinal development.
Methods
Comparative immunolocalisations of matrix and cell associated components in Histochoice-fixed paraffin-embedded human foetal spinal tissues.
Results
The 12–14-week-old human foetal spine was a predominantly cartilaginous structure with the discs displaying a relative paucity of proteoglycan compared to the adjacent cartilaginous vertebral rudiments, notochordal remnants were also observed. HS and perlecan had a widespread distribution throughout the spine at 12 weeks, however, FGF-18 was only localised to the outer AF margins and hypertrophic cell condensations in the vertebral bodies. This contrasted with HS distributions at 14–20 weeks, which were prominent in the developing intervertebral disc (IVD). Ossification centres were also evident centrally within the vertebral rudiments surrounded by small columns of hypertrophic chondrocytes which expressed FGFR-3 and FGF-18 and upregulated levels of perlecan. FGF-18 also had a prominent localisation pattern in the developing IVD and the cartilaginous endplate while FGFR-3 was expressed throughout the disc interspace. This suggested roles for perlecan, FGF-18 and FGFR-3 in chondrogenic and osteogenic events which drive discal development and ossification of the vertebral bodies.
Conclusions
The above data supported a role for FGF-18 in discal development and in the terminal osteogenic differentiation of chondroprogenitor cell populations, which promote vertebral ossification during spinal development.
Keywords: Fibroblast growth factor (FGF)-18, Fibroblast growth factor receptor (FGFR)-3, Perlecan, Heparin sulphate (HS)
Introduction
The normal adult human spine contains 24 articulating vertebrae and nine fused vertebrae in the sacrum and coccyx. The 23 intervertebral discs (IVDs) of the human spine contribute to approximately one quarter of its total length, and have major roles to play in weight bearing, and in the provision of spinal flexibility. The spinal column is a readily identified structure in the human foetus and consequently its anatomy and morphology have been extensively documented using a variety of imaging methodologies for over two decades. These include magnetic resonance imaging [1], ultrasound [2], electron microscopy [3] and radiography [4]. Collation of biometric data from these imaging studies has enabled the tabulation of reference values for the non-invasive assessment of normal human foetal spinal growth [2]. Histochemistry and immunohistochemistry has demonstrated the distribution of collagen types [5], including the alternatively spliced type IIA and IIB collagens [6], elastic microfibrillar and matrix proteins [7] and proteoglycans [8, 9].
During early human foetal development (gastrulation), three somatic germ cell layers are initially laid down as an outer ectodermal, middle mesodermal and inner endodermal layer [10]. A mid-line longitudinal rod shaped column of mesoderm, the notochord, subsequently develops from cell aggregates located between the ectoderm and endoderm and establishes cranial/caudal and posterior/anterior axes in the developing embryo [10, 11]. Ectoderm posterior to the notochord gives rise to the neuroectoderm from which the neural tube develops, adjacent mesodermal tissue develops into discrete tissue units termed the somites [12]. The somites consist of three tissue types: (1) the dermatome which gives rise to the dermis, (2) the myotome which gives rise to the axial musculature and (3) the sclerotome from which vertebral structures arise. Cells of the sclerotome migrate medially and ventrally to form a continuous tube of mesenchymal cells (perichordal sheath), which surround the notochord. Increased proliferation of cells at regular lengths along the perichordal tube creates areas of low and high cell density [12] from which the vertebrae and annulus fibrosus (AF), transitional zone and spinal ligaments develop. Formation of the vertebral bodies results in notochordal segmentation and its persistence in central regions of the developing IVD, which gives rise to the nucleus pulposus (NP) [11]. Thus, during embryonic development, cells of the AF are derived from the sclerotome and the NP from the notochord [11]. The PAX genes encoding the Pax transcription factor family has critical roles to play in directing the formation of the human foetal spinal column [13] and together with the Hox gene family these specify the identity of particular spinal segments.
Fibroblast growth factor (FGF)-18 is a member of the FGF family of growth factors. These polypeptide growth factors signal through four tyrosine kinase FGF receptors (FGFR 1-4) to regulate skeletogenesis [14]. The FGFRs each occur as three alternatively spliced forms, FGF-18 signals predominantly through the FGFR-3IIIc isoform [15]. In vitro studies conducted using FGF-18- and Baf32-engineered cells specifically expressing the FGFR-3IIIc isoform have demonstrated that FGF-18 and the HS-proteoglycan perlecan (HSPG-2), potently promote cellular proliferation [15]. FGF-18 is required for early chondrocyte proliferation and co-ordinates chondrocyte differentiation during chondrogenic and osteogenic processes. The HS chains on perlecan equip it as a low affinity co-receptor, which sequesters and presents FGF-18 to FGFR-3IIIc to effect cell signalling. Perlecan is an early chondrogenic marker [16] and a prominent component of the cartilaginous rudiments of the developmental spine [17]. A crucial role exists for the control of FGF signalling to regulate the transition from condensed mesenchyme to cartilage during foetal human spinal development and to define the boundary of the skeletal elements. This involves complex interactions between FGFs, HS and FGFRs [18], however, despite the clear importance of perlecan, FGF-18 and FGFR-3 in such developmental processes no specific human studies have so far been undertaken. The present study is the first to undertake comparative immunolocalisations of these components in human foetal spinal tissues.
Materials and methods
Materials
Chondroitinase-ABC was obtained from Sigma-Aldrich, Castle Hill, NSW, Australia. Menzel and Glaser SuperFrost ultraPlus, positively charged microscope sides were obtained from Fisher Scientific, Braunschweig, GmbH. Biotinylated anti-mouse IgG secondary antibody and horseradish peroxidase conjugate were obtained from Dako (Botany, NSW, Australia). Histochoice® was an Amresco product (Solon, OH, USA). Mouse MAb A76 to perlecan domain I was obtained from abcam through Sapphire Biosciences (Redfern, Australia). NovaRED substrate was obtained from Vector laboratories (Burlingame, CA, USA). Mono-specific polyclonal antibodies to FGFR-3 were purchased from Sigma-Aldrich, Sydney, Australia. Anti-FGF-18 was purchased from Santa Cruz Biotechnology, CA, USA. Heparitinase-III (EC 4.2.2.8) and MAb 3-G-10 to the unsaturated stub epitopes of HS generated by heparitinase III were purchased from The Seikagaku Corporation through Sapphire Bioscience (Sydney, Australia). A mouse monoclonal antibody to deer antler type X collagen [19] was kindly supplied by Dr. G. Gibson, Henry Ford Hospital, Detroit, MI.
Tissues
Two 12- and one 14- and 20-week-old human foetal spine were harvested at termination of pregnancy with ethical approval from The Human Care and Ethics Review Board of The North Sydney and Central Coast Human Research and Ethics Committee of The Royal North Shore Hospital.
Methods
Preparation of human foetal spinal segments for immunohistology
The 12- and 14-week spinal segments did not require de-calcification and were fixed in Histochoice for 24 h. The 20-week spine was fixed in 10 % neutral formalin prior to decalcification in 10 % formic acid 5 % neutral buffered formalin for 4 days. The fixed spines were bisected longitudinally in the mid-sagittal plane. The spine halves were dehydrated in graded ethanol solutions and embedded in paraffin blocks. Four micron microtome sections were cut in the mid or para-sagittal longitudinal planes and attached to Superfrost Plus glass microscope slides (Menzel-Glaser, Germany), de-paraffinised in xylene (2 changes × 5 min), and re-hydrated through graded ethanol washes (100–70 %, v/v) to water.
Histochemistry
Anionic proteoglycans were localised in tissue sections by staining for 10 min with 0.04 % w/v toluidine blue in 0.1 M sodium acetate buffer, pH 4.0 followed by a 2-min counterstain in 0.1 % w/v fast green FCF. Mineral deposition in the vertebral centre of ossification was visualised in non-decalcified mid-sagittal sections of human foetal spinal tissues using a modification of the Von Kossa procedure described by Drury and Wallington 1967 [20]. Initially, tissue sections were stained with silver nitrate (1.5 %, w/v) in the dark for 90–120 s, the sections were then incubated with hydroquinone (1 %, w/v) for 1–2 min, and finally with sodium thiosulphate (5 %, w/v) for 5 min and the sections were counterstained with haematoxylin and eosin and mounted in Eukitt.
Immunohistochemistry
Endogenous peroxidase activity was blocked by incubating the tissue sections with 3 % H2O2 for 5 min and after washing in water non-specific-binding sites were blocked with 10 % swine serum for 10 min. Selected sections were pre-digested with chondroitinase ABC (0.25 U/ml) for 1 h at 37 °C in 0.1 M Tris–HCl, 0.03 M sodium acetate buffer pH 8.0 to remove aggrecan and improve antibody accessability. The Δ-4,5 uronate HS stub epitopes identified by MAb 3-G-10, were generated using a 2-h pre-digestion with heparitinase III (2.5 mU/ml) in 50 mM HEPES buffer, pH 7.0, containing 100 mM NaCl, 3 mM CaCl2, and 1 mg/ml bovine serum albumin. Tissue sections destined for FGFR-3 immunolocalisation were pre-digested with proteinase K (Dako) for 10 min at room temp. The tissues were then blocked 2 h at room temp with Dako non-protein blocking agent. The primary, anti-perlecan MAb A76 (1/1,000 dilution), FGF-18 (1/50 dilution), anti FGFR-3 (1/150 dilution), MAb 3-G-10 (1/1,000 dilution) or anti type X collagen (1/200 dilution) were diluted in 50 mM Tris–HCl buffer pH 7.2, 0.15 M BSA applied to slides and the specimens were incubated at 4 °C overnight. Sections destined for localisation of type X collagen were pre-digested with bovine testicular hyaluronidase (1,000 U/ml) for 1 h at 37 °C in phosphate buffer pH 5.0 prior to incubation with primary antibody. The primary Abs were subsequently localised using biotinylated anti-mouse IgG antibodies and horse-radish peroxidase-conjugated streptavidin for the visualisation of the tissue immune complexes using NovaRED or diaminobenzidene substrates for colour development. Control sections were also prepared where the authentic primary antibody was either omitted or it was replaced with an irrelevant isotype matched mouse IgG. The stained tissue specimens were then examined using a Leica photomicroscope linked to a DFC 480 digital camera.
Results
Toluidine blue staining of mid-sagittal longitudinal sections demonstrated the cartilaginous nature of the human foetal spine at 12–20-week gestational age (Fig. 1). Condensations of hypertrophic cells were prominent in the developing vertebral rudiments at 12 weeks and notochordal remnants in the nucleus pulposus (Fig. 1a) and in some cases tracking through the vertebral body of adjacent IVDs (Fig. 1b, c). Prominent ossification centres were evident by 20-week gestational age along with notochordal remnants within the NP in some cases (Fig. 1d, e). Mineralisation was demonstrated using the Von Kossa procedure (Fig. 2a, b) and was evident as small spicules within the developing ossification centre (Fig. 2b). Accumulation of haemopoietic cells at 14 weeks in the marrow space was also evident in H&E-stained sections based on their characteristic cellular morphologies (Fig. 2c, d). The hypertrophic chondrocytes of the ossification centres expressed type X collagen (Fig. 2d, e).
Fig. 1.
Toluidine blue-stained spinal segments from 12- (a, b, c) and 20-week (d, e) spines depicting areas of hypertrophic chondrocytes in the cartilaginous vertebral bodies and the intervening developing intervertebral IVDs (arrows) and notochordal remnants (N). In the 12-week specimens, two central notochordal remnants are evident centrally in the NP in the L2L3 and L1L2 IVDs (a) in another 12-week spine the notochord is evident as a tube like structure within the L3 vertebral rudiment linking the notochordal remnants in the L3L4 and L2L3 IVDs (b). A prominent notochord is also evident in the L3L4 IVD of a 20-week spine with the adjacent ossification centres evident occupying a major proportion of the L3 and L4 vertebral rudiments (d). A higher power view of the notochord within the boxed area in (d) is also provided in photosegment (e)
Fig. 2.
Demonstration of mineralisation in an ossification centre of a 14-week-old human foetal lumbar spinal segment using the Von Kossa procedure (a), a higher power view is also provided of the central boxed area in the ossification centre (b), an H&E-stained photosegment of a similar area to that depicted in (b) is also presented in (c). Immunolocalisation of type X collagen produced by the hypertrophic terminally differentiated chondrocytes surrounding an ossification centre (d), a higher power view of the boxed area in (d) is presented in (e). Positive Von Kossa staining is indicated by a black colour on a pink background. The brown staining for type X collagen is due to the NovaRED chromogen
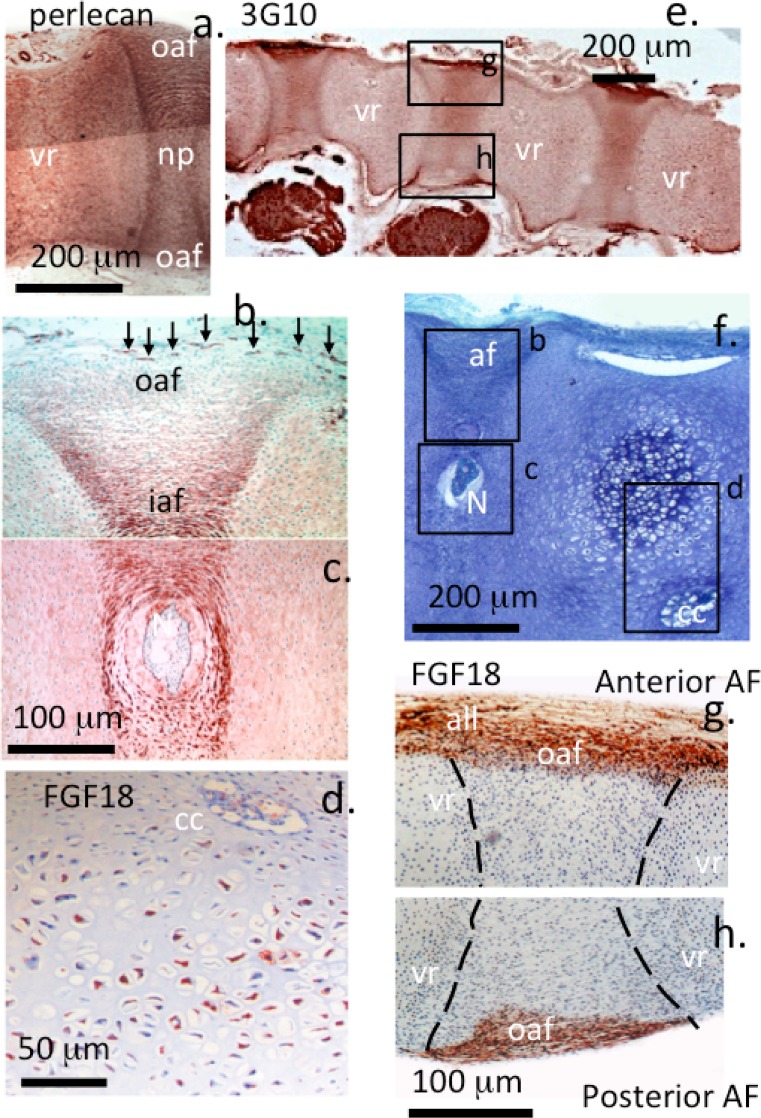
Comparative immunolocalisations on the 12-week gestational age spines demonstrated a widespread localisation of perlecan (Fig. 3a–c) and HS (Fig. 3e). Perlecan was also localised to small blood vessels in the outer AF (Fig. 3b). FGF-18 was prominently immunolocalised to hypertrophic cells in the vertebral rudiment (Fig. 3d) and in the outer AF and anterior longitudinal ligament (Fig. 3g, h). FGFR-3 staining was very weak throughout the IVD and cartilaginous rudiments at 12 weeks of spinal development with faint reactivity detectable in the accumulations of hypertophic cells, which eventually formed the ossification centres at later stages of spinal development (Table 1).
Fig. 3.
Immunolocalisation of perlecan (a, b, c), FGF-18 expression by hypertrophic cells within a vertebral rudiment (d), HS [MAb 3G10 (e) toluidine blue-stained proteoglycan (f)] and Immunolocalisation of FGF-18 expression (g, h) in the outer AF in 12-week-old human foetal spinal segments. Photosegments (a, e, g, h) are parasagittal sections, photosegments (b–d, f) are mid-saggital sections. vr vertebral rudiment, oaf outer annulus fibrosus, np nucleus pulposus, N notochord, cc cartilage canal. The dotted outlines in (g, h) depict the extent of the IVD. Photosegments (b–d) are higher power views of the boxed areas indicated in (f). Photosegments (g, h) are higher power views of the boxed areas in (e). Positive staining is indicated by the red–brown staining of the NovaRED chromogen
Table 1.
Distributions of relative immunohistochemical staining for matrix and cellular components in human foetal spinal tissues
| Parameter time point | Outer AF | Inner AF | NP | CEP | Vertebral rudiment | Ossification centre |
|---|---|---|---|---|---|---|
| 12 weeks | ||||||
| Perlecan | −/+ | −/+ | + | − | −/+ | + |
| HS | ++ | ++ | ++ | ++ | + | + |
| FGF-18 | +++ | − | − | − | − | − |
| FGFR-3 | −/+ | −/+ | − | −/+ | −/+ | + |
| 14 weeks | ||||||
| Perlecan | + | + | ++ | + | ++ | +++ |
| HS | +++ | +++ | +++ | −/+ | − | + |
| FGF-18 | +++ | ++ | + | ++ | −/+ | + |
| FGFR-3 | ++ | + | + | + | + | +++ |
| 20 weeks | ||||||
| Perlecan | + | ++ | ++ | − | +++ | ++++ |
| HS | +++ | ++ | + | ++ | + | + |
| FGF-18 | + | +++ | ++ | +++ | + | ++ |
| FGFR-3 | ++ | ++ | + | + | + | +++ |
AF annulus fibrosus, NP nucleus pulposus, CEP cartilaginous end plate, HS heparin sulphate, FGF fibroblast growth factor, FGFR fibroblast growth factor receptor
− no detectable staining, −/+ weak patchy or incomplete staining, + moderate staining, ++ elevated staining but less than +++, +++ heavy staining
At 14-week gestational age, the spine was still a glycosaminoglycan rich tissue (Fig. 4a), perlecan also had a widespread distribution and was highly expressed by the hypertrophic cells surrounding the ossification centres (Fig. 4b). HS was immunolocalised in the IVD with little signal evident in the adjacent cartilaginous vertebral rudiments (Fig. 4c). At 14 weeks FGF-18 was immunolocalised in the anterior AF, cartilaginous endplates notochordal remnant in the NP but relatively weak expression in the hypertrophic cells surrounding the ossification centres (Fig. 4d, h, i). FGFR-3 also had a similar widespread distribution as FGF-18 at 14 weeks (Fig. 4e, f).
Fig. 4.
Localisation of toluidine blue-stained proteoglycan (a), and immunolocalisation of perlecan (b), HS (MAb 3G10) (c), FGF-18 (d) and FGFR-3 (e, f) in defined areas of a 14-week-old human foetal spinal segment. FGFR-3 negative control (g). The boxed areas in segment (b) are provided at higher magnification in (c, e, f), respectively. FGF-18 is expressed by cells in the notochord (N) and ossification centre in the mid-saggital section provided in (d). Ossification centres are clearly evident in each spinal level in the mid-saggital sections provided in (a, b). Photosegments (c, e) are parasaggital sections. Positive staining is indicated by the red-brown staining of the NovaRED chromogen. Immunolocalisation of FGF-18 in longitudinal mid-saggital spinal sections of another 14-week human foetal spine (h). The boxed area (i) is also presented at higher magnification at the bottom of the figure along with negative control. Positive staining is indicated by red staining check chromogen used
At 20 weeks, the spines were still predominantly cartilaginous (Fig. 5a). FGFR-3 was prominently and widely expressed by cells in the IVD (Fig. 5b), and ossification centres (Fig. 5b, f). FGF-18 was expressed by cells at the periphery of the vertebral rudiments, within the developing IVD including the cartilaginous endplate, margins of the cartilage canals (Fig. 5c) and the hypertrophic cells at the margins of the ossification centres (Fig. 5g). HS was immunolocalised predominantly to the developing IVD (Fig. 5d). Perlecan had a widespread distribution not only in the vertebral rudiments and around the ossification centres within it but by cells throughout the disc interspace (Fig. 5e).
Fig. 5.
Localisation of toluidine blue-stained proteoglycan in a 20-week-old human foetal spine (a), FGFR-3 is also immunolocalised in the same spine in (b). Selected boxed areas in a, b are also provided at higher magnification in (c–e), respectively, depicting immunolocalisation of FGF-18, HS and perlecan. FGFR-3 is also immunolocalised surrounding the ossification centres (f). Positive staining is indicated by the red-brown staining of the NovaRED chromogen. FGF-18 immunolocalisation in a longitudinal section of another 20-week spine (g). Positive staining is indicated by brown staining marked with asterisks
We also undertook high power immunolocalisations of FGF-18 and FGFR-3 in the developing IVD and vertebral rudiments at 20-week gestational age in order to demonstrate their cellular distributions (Fig. 6). This demonstrated that FGF-18 was prominently expressed by cells of a more flattened morphology particularly in the outer AF but was also expressed by cells in the inner AF and NP (Fig. 6a–c). FGFR-3 also had a widespread expression pattern by cells throughout the foetal IVD, particularly those located in the outer AF (Fig. 6d). The more sparsely distributed vertebral rudiment cells of a rounded morphology also expressed FGFR-3 (Fig. 6e) thus FGFR-3 was ubiquitously expressed throughout the developing cartilaginous spine at 20-week gestational age. A summation of the spatiotemporal immunolocalisation patterns for perlecan, FGF-18 and FGFR-3 observed in this study are given schematically in Fig. 7 and the relative intensities of the immunolocalisations are given in Table 1.
Fig. 6.
Higher power immunolocalisation of FGF-18 (a–c) and FGFR-3 (d, e) in selected disc regions of a lumbar IVD from a 20-week-old human foetal spinal segment depicting their strong and widespread cellular localisations. Positive staining is indicated by brown staining of the diaminobenzidene chromogen in (a–c) and red chromogen of NovaRED in (d, e). Representative FGF-18 positive cells are labelled with arrows
Fig. 7.
Idealised schematic depicting the relative localisation patterns of perlecan, FGF-18 and FGFR-3 in 12-, 14- and 20-week-foetal spinal segments. The intervertebral disc and adjacent cartilaginous vertebral rudiment is depicted in each case with relative changes in the area occupied by the ossification centres within the rudiment. Cartilage canals within the rudiment are also depicted
Discussion
The 12–20-week gestational age human foetal spine is predominantly cartilaginous in nature, ossification centres within the vertebral rudiments and remnants of the notochord in the NP are also evident at 14–20 weeks. Mineralisation of the ossification centres and expression of type X collagen by hypertrophic chondrocytes also occurred at 14–20 weeks. Haematopoietic cells populated regions of the ossification centres at 12–14 weeks providing evidence of marrow development. Perlecan was a prominent matrix proteoglycan in the vertebral rudiments and IVD at all time points examined in this study. HS was ubiquitously distributed in spinal tissues at 12 weeks but by 14–20 weeks was predominantly localised to the developing IVD. The presence of HS chains on perlecan is critical to growth factor and HS-mediated matrix stabilising interactions [15]. At 12 weeks, FGF-18 was detectable in the outer AF and to a lesser extent the hypertrophic cells of the mesenchymal condensations in the vertebral rudiments. By 14 weeks, FGF-18 was expressed throughout the anterior AF, cartilaginous endplates and hypertrophic cells surrounding the ossification centres. FGFR-3 was also widely expressed in spinal development, however, the vertebral rudiments had lower cell densities than the disc and consequently displayed comparatively lower levels of FGF-18 and FGFR-3 expression. The spatiotemporal immunolocalisations of perlecan, HS, FGF-18 and FGFR-3 evident in the present study were, however, consistent with roles in chondrogenic differentiation of the IVD cells and osteogenic differentiation of hypertrophic chondrocytes surrounding the ossification centres and consistent with a number of studies where FGF-18 and FGFR-3 have been shown to have dominant roles in chondrogenic and osteogenic events during skeletogenesis. Fgfr3 is expressed by proliferating chondrocytes in the reserve and proliferating zone of growth plates, suggesting a direct role for FGFR-3 in the regulation of chondrocyte proliferation and differentiation [21]. Another growth factor we are interested in and which we have also examined previously in the Baf32 cell system is FGF-2 [22], however, despite its widespread expression, targeted deletion of FGF-2 causes a relatively mild phenotype, leading to decreased bone density, but no defects in skeletal size, patterning or in chondrogenesis. Fgf18 is also expressed around the condensing mesenchyme and genetic studies have identified a defect in chondrogenesis and osteogenesis in mice lacking FGF-18 [23, 24]. Mice lacking Fgf18 display a more severe phenotype than mice lacking Fgfr3 indicating a dominant role in skeletal development [23, 25]. FGFs have important roles in many biological processes, including development, pattern formation, cellular differentiation, tissue remodeling and mitogenesis. FGFs are involved in all stages of skeletogenesis, from limb bud development to the growth and remodeling of bone. A major difficulty in deciphering the contributions of individual FGF members and FGFRs to the process of skeletogenesis is that of redundancy. A total of 23 FGFs have so far been characterised and these signal through four highly homologous FGFRs, which each occur as three alternatively spliced isoforms. Many of the FGFs and FGFRs have overlapping spatiotemporal distributions during skeletal development and display similar properties with regard to how they influence cellular proliferation and differentiation. One approach which has been utilised previously is to use engineered Baf32 cell lines which express only one of the FGFR isoforms and this allows the effects of defined combinations of FGFs and HS proteoglycans on cellular proliferation, differentiation and matrix production to be tested [15, 22]. The Baf cell lines do not synthesise HS-proteoglycans, heparin or a HS-proteoglycan must be supplied to maintain cellular viability. Thus, using Baf32 cells specifically expressing FGFR-3IIIc, and variable amounts of FGF-18 and perlecan we previously demonstrated cell signalling and a potent effect on cellular proliferation [15]. Of the FGFR splice variants, we examined FGFR-3IIIc provided optimal results with FGF-18 consistent with published studies on its roles in skeletal development [24, 26, 27]. Thus, it was logical to further examine the spatiotemporal localisation of these components in the present study. Moreover, FGF-18 is currently undergoing phase III clinical trials (Merck-Serono) for the repair of osteoarthritic lesions thus a greater understanding of its role in spinal development may provide valuable insights as to how this important growth factor may be harnessed to promote regenerative strategies on the spine.
Conflict of interest
None.
References
- 1.Glenn OA, Barkovich AJ. Magnetic resonance imaging of the fetal brain and spine: an increasingly important tool in prenatal diagnosis, part 1. AJNR Am J Neuroradiol. 2006;27:1604–1611. [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng PJ, Huang SY, Shaw SW, Chueh HY, Soong YK. Evaluation of fetal spine biometry between 11 and 14 weeks of gestation. Ultrasound Med Biol. 2010;36:1060–1065. doi: 10.1016/j.ultrasmedbio.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Babic MS. Development of the notochord in normal and malformed human embryos and fetuses. Int J Dev Biol. 1991;35:345–352. [PubMed] [Google Scholar]
- 4.Bagnall KM, Harris PF, Jones PR. A radiographic study of the growth in width of the human fetal vertebral column. Anat Rec. 1982;204:265–270. doi: 10.1002/ar.1092040311. [DOI] [PubMed] [Google Scholar]
- 5.Nerlich AG, Boos N, Wiest I, Aebi M. Immunolocalization of major interstitial collagen types in human lumbar intervertebral discs of various ages. Virchows Arch. 1998;432:67–76. doi: 10.1007/s004280050136. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, McAlinden A, Sandell LJ. Type IIA procollagen in development of the human intervertebral disc: regulated expression of the NH(2)-propeptide by enzymic processing reveals a unique developmental pathway. Dev Dyn. 2001;220:350–362. doi: 10.1002/dvdy.1115. [DOI] [PubMed] [Google Scholar]
- 7.Hayes AJ, Smith SM, Gibson MA, Melrose J. Comparative immunolocalization of the elastin fiber-associated proteins fibrillin-1, LTBP-2, and MAGP-1 with components of the collagenous and proteoglycan matrix of the fetal human intervertebral disc. Spine (Phila Pa, 1976) 2011;36:E1365–E1372. doi: 10.1097/BRS.0b013e31821fd23e. [DOI] [PubMed] [Google Scholar]
- 8.Smith SM, Whitelock JM, Iozzo RV, Little CB, Melrose J. Topographical variation in the distributions of versican, aggrecan and perlecan in the foetal human spine reflects their diverse functional roles in spinal development. Histochem Cell Biol. 2009;132:491–503. doi: 10.1007/s00418-009-0623-z. [DOI] [PubMed] [Google Scholar]
- 9.Melrose J, Smith S, Ghosh P, Whitelock J. Perlecan, the multidomain heparan sulfate proteoglycan of basement membranes, is also a prominent component of the cartilaginous primordia in the developing human fetal spine. J Histochem Cytochem. 2003;51:1331–1341. doi: 10.1177/002215540305101010. [DOI] [PubMed] [Google Scholar]
- 10.Sinowatz F (2010) Musculo-skeletal system. In: Hyttel P, Sinowatz F, Vejlsted M (eds) Essentials of domestic animal embryology, 1st edn. Saunders Elsevier, Philadelphia, pp 286–316
- 11.Risbud MV, Schaer TP, Shapiro IM. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev Dyn. 2010;239:2141–2148. doi: 10.1002/dvdy.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGeady TA, Quinn PJ, Fitzpatrick ES (2006) Muscular and skeletal systems. In: McGeady TA, Quinn PJ, Fitzpatrick ES (eds) Veterinary embryology, 1st edn. Blackwell Publishing, Oxford, pp 184–204
- 13.Smith CA, Tuan RS. Human PAX gene expression and development of the vertebral column. Clin Orthop Relat Res. 1994;302:241–250. [PubMed] [Google Scholar]
- 14.Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- 15.Chuang CY, Lord MS, Melrose J, Rees MD, Knox SM, Freeman C, Iozzo RV, Whitelock JM. Heparan sulfate dependent signaling of fibroblast growth factor (FGF) 18 by chondrocyte-derived perlecan. Biochemistry. 2010 doi: 10.1021/bi1005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith SM, Shu C, Melrose J. Comparative immunolocalisation of perlecan with collagen II and aggrecan in human foetal, newborn and adult ovine joint tissues demonstrates perlecan as an early developmental chondrogenic marker. Histochem Cell Biol. 2010;134:251–263. doi: 10.1007/s00418-010-0730-x. [DOI] [PubMed] [Google Scholar]
- 17.Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- 18.Ornitz DM. FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays. 2000;22:108–112. doi: 10.1002/(SICI)1521-1878(200002)22:2<108::AID-BIES2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 19.Gibson G, Francki K, Caterson B, Foster B. Type X collagen is colocalized with a proteoglycan epitope to form distinct morphological structures in bovine growth cartilage. Bone. 1996;19:307–315. doi: 10.1016/S8756-3282(96)00222-0. [DOI] [PubMed] [Google Scholar]
- 20.Drury RAB, Wallington EA (1967) Carleton's histological technique, 4th edn. Oxford University Press, pp 150–151
- 21.Peters K, Ornitz D, Werner S, Williams L. Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev Biol. 1993;155:423–430. doi: 10.1006/dbio.1993.1040. [DOI] [PubMed] [Google Scholar]
- 22.Knox S, Merry C, Stringer S, Melrose J, Whitelock J. Not all perlecans are created equal: interactions with fibroblast growth factor (FGF) 2 and FGF receptors. J Biol Chem. 2002;277:14657–14665. doi: 10.1074/jbc.M111826200. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Xu J, Colvin JS, Ornitz DM. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 2002;16:859–869. doi: 10.1101/gad.965602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohbayashi N, Shibayama M, Kurotaki Y, Imanishi M, Fujimori T, Itoh N, Takada S. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 2002;16:870–879. doi: 10.1101/gad.965702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Lavine KJ, Hung IH, Ornitz DM. FGF18 is required for early chondrocyte proliferation, hypertrophy and vascular invasion of the growth plate. Dev Biol. 2007;302:80–91. doi: 10.1016/j.ydbio.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 26.Ellman MB, An HS, Muddasani P, Im HJ. Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene. 2008;420:82–89. doi: 10.1016/j.gene.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellsworth JL, Berry J, Bukowski T, Claus J, Feldhaus A, Holderman S, Holdren MS, Lum KD, Moore EE, Raymond F, Ren H, Shea P, Sprecher C, Storey H, Thompson DL, Waggie K, Yao L, Fernandes RJ, Eyre DR, Hughes SD. Fibroblast growth factor-18 is a trophic factor for mature chondrocytes and their progenitors. Osteoarthr Cartil. 2002;10:308–320. doi: 10.1053/joca.2002.0514. [DOI] [PubMed] [Google Scholar]