Abstract
Purpose
To examine the time needed from a surgeon’s viewpoint to treat a patient operated for lumbar spinal stenosis. We firstly aimed to give evidence of the wide ranging duration of standardized procedure. Secondly, we investigated factors affecting the time allocated to each patient.
Methods
438 medical records of patients operated on for lumbar decompression without fusion (2005–2011) were retrospectively examined. Primary data were operative time (OT, min), length of stay (LoS, days) and number of postoperative visits. A fourth parameter was calculated, the time spent per patient (TSPP, min) by summing the time spent in surgery, during inpatient and outpatient follow-up visits. Factors that influenced these medical resources were examined.
Results
Median (5th–95th percentile) LoS was 5 days (2–15), OT 106 min (60–194), number of medical visits 5 (2–11) and TSPP 329 min (206–533). In descending order, factors predicting LoS were age, no. of levels, sex, operative technique, cardiovascular risk index, dural tear and haematoma. Factors predicting OT were number of levels, dural tear, foraminotomy, synovial cyst and body mass index. The statistical model could predict 36 % of the TSPP variance. We recommend that surgeons add 35 min for each level, 29 min for patients over 65 years, 30 min for women, 132 min for dural tear and 108 min for epidural haematoma.
Conclusion
TSPP treated for lumbar spinal stenosis is highly variable, yet partially predictable. These data may help individual surgeons or heads of departments to plan their activities.
Keywords: Time, Length of stay, Outpatients
Introduction
Lumbar spinal stenosis (LSS) is a common condition requiring spine surgery [1], in which patients complain of symptoms relating to anatomical reduction of the lumbar spinal canal. The incidence of LSS has significantly increased over the last decade due to the aging population [1–4]. The aim of surgical visits is to confirm, clinically and by means of imaging, the diagnosis of LSS and to assess its degree of severity. After unsuccessful medical management, a surgical intervention should be considered [5]. Several surgical techniques exist including uni- or bilateral decompression, associated or not with a posterior or circumferential fusion [6–9]. Experienced surgeons appreciate the large variability of time required for inpatient and outpatient visits and surgical procedures. For this reason, the overall time spent per patient (TSPP) calculated in our study could prove useful for health care providers as well as department management teams.
Two hypotheses can be made when considering LSS surgery from the point of view of the surgeon:
TSPP is highly variable.
TSPP can be efficiently predicted using specific factors.
Methods
Data source
Between January 2005 and August 2011, all patients operated on for LSS by a single surgeon in a single institution were selected. The institution database was compared with patients’ medical records. Lumbar fusion (instrumented or not) was excluded and only decompression without fusion was considered. Two surgical techniques were used: bilateral decompression with sparing of posterior structures (spinous process, interspinous and supraspinous ligaments) and the unilateral variant with a single laminotomy and microscopic circumferential decompression. The latter approach is the least invasive, preserving the facet joints and neural arch of the contralateral side thus limiting postoperative destabilization [10, 11]. Surgical interventions can be conducted on one to five levels. Perioperatively, some additional techniques may be performed such as the release of the dural sac by removal of a synovial cyst or root decompression by foraminotomy. After discharge, patients were followed-up at 1 and 3 months and if necessary more in cases of poor outcome.
The remaining 438 patients were retrospectively examined using medical records, in which we exhaustively reviewed resource parameters (i.e. dependant variables, Table 1) and factors able to interact with these (i.e. independent variables, Table 2). Patient-related risk factors, drug use, operative time and operating room time were extracted using anaesthesiology records, and surgery-related risk factors with complications were obtained from the operating protocol, hospital reports and postoperative visit records. For the number of pre- and postoperative consultations, we performed a data selection to ensure a minimum follow-up of 2 years and included all visits related to LSS. Iterative surgery was reported when other levels had been operated on in previous surgical interventions. None of the operated levels considered in this study had been operated on previously. Regarding drug use, we dissociated antiplatelets, not stopped or stopped 7 days before surgery, from clopidogrel [stopped 5 days before surgery, following guidelines (12)] and acenocoumarol.
Table 1.
List of medical resources (dependent variables)
| Number of pre- and postoperative consultations (No) |
| Operating time (OT) and operating room time (min) |
| Length of hospital stay (LoS) (days) |
| Time spent per patient (TSPP) (min) |
Table 2.
List of factors (independent variables) investigated
| Patient-related risk factors |
| Age (years) |
| Sex |
| Body mass index (BMI) [kg/m²] |
| History of cardiac disease |
| History of hypertension |
| History of diabetes mellitus |
| History of respiratory disease |
| History of renal disease |
| Drug use |
| Antiplatelet (acetylsalicylic acid, clopidogrel) |
| Anticoagulant (acenocoumarol) |
| Preoperative scores |
| Revised cardiac risk index (RCRI) |
| Severe postoperative pain score |
| American Society of Anaesthesiologists (ASA) score |
| Surgery-related risk factors |
| Surgical technique (uni- or bilateral) |
| Iterative surgery at the same site |
| Number of operated levels (No.) |
| Associated foraminotomy |
| Surgical resection of an intracanalar synovial cyst |
| Surgical complications |
| Dural tear |
| Compressive epidural haematoma |
| Wound sepsis |
| Neurological damage |
Complications encountered were dural tear reported with or without leak of cerebrospinal fluid and managed with suture and/or glue, compressive epidural haematoma found on MR-imaging a few days after surgery and requiring in most cases further surgery and wound sepsis and neurological damage found in the hospital reports and postoperative visit records.
Three preoperative scores were investigated: revised cardiac risk index (RCRI), severe postoperative pain score and American Society of Anaesthesiologists (ASA) score. The RCRI identifies patients at higher risk for cardiovascular complications during noncardiac surgery [13]. It takes into account six independent predictive factors of complications, and it is a useful tool in risk stratification [13, 14]. The severe postoperative pain score is a preoperative assessment of pain caused by the procedure based on a patient’s previous history. It includes 10 different weighted factors for which points are assigned. The total score is 15 and the risk of postoperative pain is considered significant if the score is >4. Preoperative tests may be predictive of postoperative pain [15], and thus could have an impact on use of resources. The ASA score [16] is a subjective assessment of a patient’s overall health that is based on five classes (I to V). This score is predictive of perioperative morbidity and mortality, and patients in classes III to V are at greater risk of surgical infection.
Time spent per patient is a composite variable which includes daily visits in the hospitalization period including paperwork, operating room time (accounting for operative time—OT, as well as that spent on other perioperative procedures such as installation) and postoperative visits. TSPP is an estimation of the time accorded to a patient by a surgeon since the end of the last preoperative visit. It was calculated by considering 20 min per visit (inpatient or outpatient).
Statistical analysis
To explore the data, we sequentially performed a univariate and bivariate analysis, and then focused on inference and modelling. Resources data were analysed in terms of central tendency and dispersion. For continuous variables, their normal distribution was tested using a Kolmogorov–Smirnov test. Scale variables, such as medical resources or BMI, did not have a Gaussian distribution and were presented as median (5th–95th percentile). These variables were normalized with logarithmic transformation. Most variables did not meet the usual transformations. Concerning these, we used non-parametric tests for inference (Kruskal–Wallis). All data were bilaterally tested with a level of significance of 0.05, two tailed.
Due to a large number of independent variables, we first searched for a model susceptible to detect any link between these variables and the dependent ones. The statistical modelling was based on multiple mixed linear regression models with a log-transformed independent variable. For each resource variable, a linear regression model with top-down method was created and then adjusted. Factors with a level of significance of 0.10 were then selected to obtain a more realistic and simplified model. Each of them met conditions of the absence of multicollinearity, independence of errors (Durbin-Watson) and approached a normal distribution of residues, providing three reliable models (LoS, OT, TSPP). Standardized beta coefficients were used to compare the strength of predictors, in terms of weight and regression coefficients, at predicting variation of the dependent variable by change in the independent one. To ensure the absence of interacting factors in our models, we used an analysis of covariance with a general linear model, which permit to reveal the effect of one predictor by controlling the others.
All statistics were performed using SPSS software (v.19, SPSS, Inc., Chicago, IL, USA).
Results
Univariate analysis
From January 2005 to August 2011, 438 patients underwent surgery for LSS refractory to medical treatment, i.e. an average of 67 patients per year. There was a slight preponderance of women (52 %) with a mean age of 69 years (range 24–90 years) at the time of surgical intervention. The average BMI was 28. The medical histories of our patients were numerous with 53.9 % hypertension, 30.7 % cardiovascular disease, 18.4 % diabetes, 20.1 % respiratory disease and 13 % renal disease. Before surgery, 31.7 % of patients took acetylsalicylic acid and 7.1 % of whom were unable to stop it, 5 % took clopidogrel and 6.1 % acenocoumarol. The preoperative scores highlighted the fragility of the population affected by this disease. Indeed, 30 % of patients were at a higher risk for perioperative cardiovascular complications (RCRI) and 97 % of patients had an ASA score of 2 or 3. The severe postoperative pain score showed that 75.3 % of patients were at risk since their score was >4. Over the 6 year study period, 72.6 % of operations were performed unilaterally and 10.3 % were iterative surgeries at the same site, regardless of the hospital where they underwent the primary procedure. The number of operated levels varied between 1 and 5 though 73.5 % of procedures were performed on 1 or 2 levels. Synovial cysts and foraminal stenosis were identified preoperatively by MRI in 12.6 and 12.3 %, respectively, and surgically treated. There were no intraoperative deaths. Of the complications encountered, dural tear was at the top of the list with a rate of 6.6 %, followed by compressive epidural haematoma (1.1 %), wound sepsis (0.9 %) and neurological damage (0.2 %, i.e. only one patient), respectively. We found no association between drug use and haematoma as only one patient took acenocoumarol and five patients had epidural haematomas (p = 0.282).
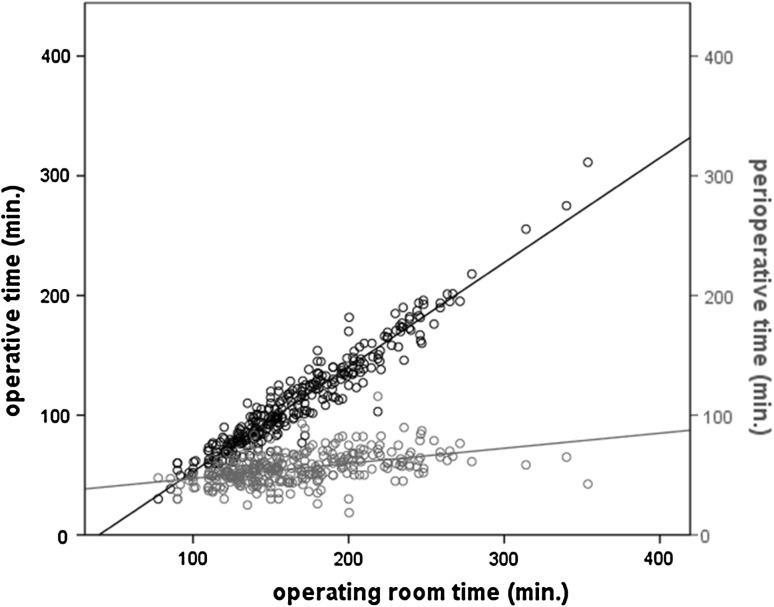
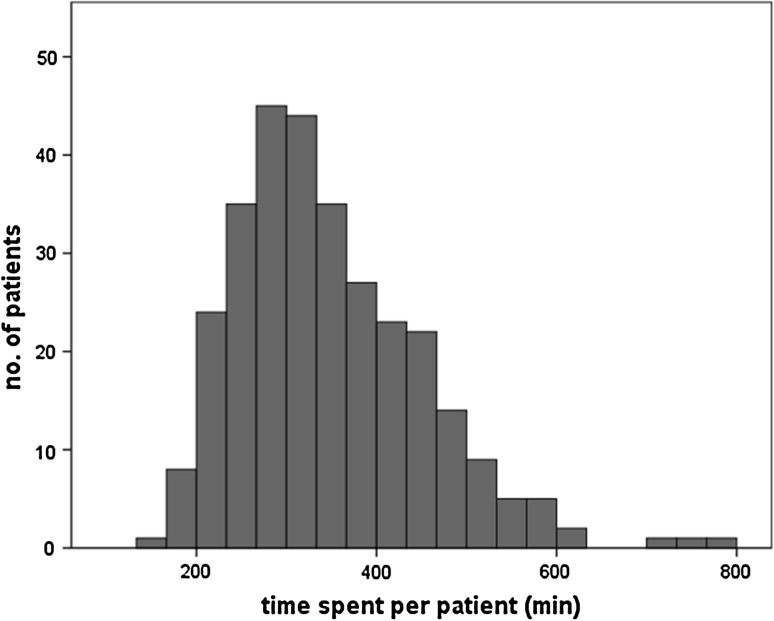
The use of medical resources is outlined in Table 3 alongside the scale independent variables. There was a significant gender difference in LoS (p < 0.01) with women being hospitalized longer (median = 6 days) than men (median = 4 days). The operating room time was mainly correlated with OT (R² = 0.922) and much less with procedures of anaesthesia and installation (R² = 0.198), (Fig. 1). Considering the out-patient visits, 306 patients were followed-up for a minimum of 2 years and attended a total of 1,709 consultations, including 598 preoperative and 1,129 postoperative. In general, each operated patient needed five consultations, including two preoperative and three postoperative. 38.4 % of patients required more than five consultations. It should be noted that the wide distribution of visits depicted in Fig. 2 essentially concerned the postoperative ones. Finally, the distribution of TSPP is largely skewed (Fig. 3) with a median (range) of 330 min (153–783).
Table 3.
Characteristics of scale variables
| Variable | No. (missing) | Median (P5–P95) |
|---|---|---|
| Age | 438 (0) | 71 (49.9–84.1) |
| Body mass index | 421 (17) | 27.5 (21.0–36.8) |
| Length of stay | 438 (0) | 5 (2–15) |
| Operative time | 424 (14) | 106 (60–194) |
| Operating room time | 306 (132) | 157 (110–246) |
| No. of visits (pre and post op) | 306 (132) | 5 (2–11) |
| Time spent per patient | 302 (136) | 329 (206–533) |
Fig. 1.
Relations between OT and perioperative time on operative room time
Fig. 2.
Observed dispersion of outpatients visits
Fig. 3.
Observed dispersion of TSPP
Bivariate analysis was performed to identify the relationship between a pair of dependant–independent variables (Table 4).
Table 4.
Bivariate analysis of potential predictors and test of significance
| No. | Length of stay (days) | Operative time (min) | Outpatient surgical visits (no.) | Time spent per patient (min) | |
|---|---|---|---|---|---|
| Median (P5–P95) | Median (P5–P95) | Median (P5–P95) | Median (P5-P95) | ||
| Age | |||||
| <61 | 113 | 4 (1–9)* | 105 (54–182) | 5 (3–11) | 300 (177–470)* |
| 61–71 | 114 | 5 (2–12) | 118 (61–196) | 5 (2–10) | 316 (210–506)$ |
| 71–78 | 119 | 6 (2–13) | 100 (60–193) | 5 (2–12) | 362 (236–510) |
| >78 | 92 | 7 (3–19) | 104 (66–201) | 5 (2–8) | 351 (234–585) |
| Sex | |||||
| women | 229 | 6 (2–16)* | 105 (60–201) | 5 (2–12) | 340 (212–572)* |
| men | 209 | 4 (2–12) | 112 (60–182) | 5 (2–10) | 320 (203–506)$ |
| BMI (kg/m²) | |||||
| <25 | 123 | 5 (1–14) | 98 (60–185)* | 5 (2–10) | 303 (210–504)* |
| >25 | 298 | 5 (2–14) | 115 (60–195) | 5 (2–11) | 341 (203–556) |
| RCRI | |||||
| 1 | 210 | 5 (1–12)* | 104 (58–193) | 5 (2–10) | 317 (201–514) |
| 2 | 63 | 5 (3–17) | 97 (67–160) | 5 (3–9) | 335 (230–476) |
| 3 | 17 | 5 (3–13) | 115 (70–183) | 5 (1–10) | 370 (230–575) |
| 4 | 8 | 9 (6–16) | 125 (64–182) | 4 (2–8) | 408 (306–585) |
| Post op pain score | |||||
| <4 | 55 | 5 (2–12) | 101 (65–187) | 4 (2–9)* | 325 (212–514) |
| >4 | 168 | 5 (1–12) | 97 (58–176) | 5 (3–10) | 316 (201–499) |
| ASA | |||||
| 1 | 10 | 3 (1–12)* | 137 (59–185) | 8 (4–13)* | 399 (193–517) |
| 2 | 291 | 5 (2–13) | 105 (60–201) | 5 (3–11) | 317 (212–515) |
| 3 | 116 | 6 (3–16) | 109 (56–180) | 5 (2–9) | 355 (210–573) |
| 4 | 2 | 8 (7–9) | 121 (92–151) | 5 (5–5) | 412 (365–459) |
| Acetylsalicylic acid | |||||
| No | 290 | 5 (2–13) | 105 (60–194) | 5 (2–11) | 317 (210–506) |
| Stop day-7 | 104 | 5 (2–16) | 114 (66–201) | 5 (2–11) | 355 (203–585) |
| No stop | 30 | 6 (3–18) | 94 (56–149) | 5 (3–9) | 357 (218–558) |
| Clopidogrel | |||||
| No | 403 | 5 (2–14) | 107 (60–195) | 5 (2–11) | 327 (203–527) |
| Yes | 21 | 6 (3–11) | 100 (64–157) | 5 (3–9) | 367 (242–719) |
| Acenocoumarol | |||||
| No | 397 | 5 (2–14)* | 106 (60–194) | 5 (2–11) | 329 (203–527) |
| Yes | 27 | 6 (3–14) | 103 (70–182) | 5 (2–9) | 330 (230–608) |
| No. of levels | |||||
| 1 | 147 | 4 (1–15)* | 80 (53–144)* | 5 (2–12) | 275 (180–485)* |
| 2 | 175 | 5 (3–14) | 110 (70–188) | 5 (2–11) | 353 (238–558)$ |
| 3 | 83 | 6 (3–18) | 130 (77–194) | 5 (3–10) | 361 (273–517) |
| 4 | 29 | 8 (4–12) | 167 (120–310) | 4 (2–8) | 420 (340–514) |
| 5 | 4 | 8 (5–8) | 238 (150–300) | 5 (4–6) | 472 (403–540) |
| Surgical technique | |||||
| Unilateral | 318 | 5 (2–14)* | 98 (58–182)* | 5 (2–10) | 314 (203–527)* |
| Bilateral | 120 | 7 (3–18) | 127 (75–210) | 5 (2–11) | 414 (250–540)$ |
| Synovial cyst | |||||
| No | 383 | 5 (2–15)* | 108 (60–190) | 5 (2–11) | 333 (212–517)* |
| Yes | 55 | 5 (1–15) | 105 (60–240) | 4 (3–10) | 304 (202–574) |
| Foraminotomy | |||||
| No | 384 | 5 (2–14) | 105 (60–193)* | 5 (2–11) | 329 (212–517) |
| Yes | 54 | 5 (1–18) | 120 (67–200) | 5 (2–11) | 355 (168–608) |
| Dural tear | |||||
| No | 409 | 5 (2–14)* | 104 (60–182)* | 5 (2–10) | 320 (206–506)* |
| Yes | 29 | 8 (4–19) | 162 (77–300) | 6 (2–14) | 472 (335–747)$ |
| Epidural haematoma | |||||
| No | 433 | 5 (2–15)* | 107 (60–194) | 5 (2–11) | 329 (206–517)* |
| Yes | 5 | 13 (7–17) | 90 (78–130) | 4 (3–14) | 500 (309–572)$ |
| Wound sepsis | |||||
| No | 434 | 5 (2–15) | 106 (60–193) | 5 (2–11) | 329 (208–522) |
| Yes | 4 | 10 (5–19) | 144 (78–201) | 6 (4–8) | 565 (384–747) |
| Neurological damage | |||||
| No | 437 | 5 (2–15) | 107 (60–194) | 5 (2–11) | 330 (210–527) |
| Yes | 1 | 4 (4–4) | 98 (98–98) | 5 (5–5) | 325 (325–325) |
Significant bold values indicate bivariate analysis and/or modelling
* for p value <0.05 according to Kruskal–Wallis test
$for predictor for the general model TSPP
Inference and modelling
The model for LoS was significant and efficient with R² = 0.416. It took seven variables into account. Relative weights of variables were represented in the model (cf. standardized beta, Table 5). Age, number of operated levels and type of surgery (uni- or bilateral) were, with sex, the best predictors of LoS. The RCRI was better than the ASA score (even when it was also independently significant according to Kruskal–Wallis test), and these five variables accounted for most of the LoS. It is also interesting to note that the non-significant variables BMI, history of respiratory disease, drug use (antiplatelets or anticoagulants), iterative surgery, the presence of associated synovial cyst or foraminal stenosis, local sepsis and neurological damage increased very little or not at all the LoS.
Table 5.
Predictors from multivariate regression models, that are significantly associated with resources
| Predictor | Model for LoS | Model for OT | Model for TSPP | |||
|---|---|---|---|---|---|---|
| β coef. (95 % CI) | Sig. | β coef. (95 % CI) | Sig. | β coef. (95 % CI) | Sig. | |
| Age | 0.274 (0.01–0.02) | 0.000 | 0.141 (0.028–0.142) | 0.003 | ||
| Sex (W0, M1) | −0.232 (−0.384–0.168) | 0.000 | −0.151 (−0.142–0.033) | 0.002 | ||
| No. of levels | 0.241 (0.095–0.223) | 0.000 | 0.549 (0.183–0.245) | 0.000 | 0.346 (0.08–0.144) | 0.000 |
| Uni- bilateral (0.1) | 0.23 (0.204–0.498) | 0.000 | 0.157 (0.045–0.196) | 0.002 | ||
| RCRI | 0.193 (0.084–0.235) | 0.000 | ||||
| Dural tear (0.1) | 0.112 (0.063–0.567) | 0.014 | 0.198 (0.18–0.409) | 0.000 | 0.278 (0.221–0.443) | 0.000 |
| Haematoma (0.1) | 0.11 (0.106–1.031) | 0.016 | 0.121 (0.066–0.485) | 0.01 | ||
| Foraminotomy (0.1) | 0.1 (0.027–0.202) | 0.011 | ||||
| Synovial cyst (0.1) | 0.089 (0.013–0.185) | 0.024 | ||||
| BMI | 0.071 (0–0.011) | 0.074 | ||||
RCRI revised cardiac risk index
BMI body mass index
The model for OT was significant and efficient with R² = 0.396. It took five variables into account (cf. standardized beta, Table 5). Two main parameters (number of operated levels and occurrence of a dural tear) accounted for most of the variance explained by the model, whereas the associated synovial cyst, associated foraminal stenosis and BMI contributed less. Since the main determining factor was the number of operated levels, it was also possible to model the operating time according to this single variable (Fig. 4, CI 95 %). The regression equation was time (min) = 60.9 + 26.6 (no. of operated levels).
Fig. 4.
Equation of OT based on number of operated levels
The number of overall medical visits and postoperative visits were analysed in 306 patients. The model was significant but ineffective with R² = 0.02. Bivariate analysis highlighted the association between no. of visits and ASA score, however, in the model created, ASA was not significant (p = 0.129). Severe postoperative pain score was the only variable that was significantly associated with number of consultations (p = 0.038), however, it remained impossible to predict the number of visits based on this variable.
The model of overall resources attempts to explain TSPP in terms of predictors. It is significant, effective and identified six factors of which two were main predictors: no. of levels and dural tear (Table 5). Since it explains 36 % of the variance (R² = 0.361), it is possible to highlight some relations (Table 6) between change in predictor values and effects on TSPP.
Table 6.
Effects of predictors on TSPP
| Change in predictor value | Effect on TSPP (min.) (IC 95 %) |
|---|---|
| No. of levels +1 | +34.4 (22.8/46) |
| Occurrence of dural tear | +132 (92.5/172) |
| Unilateral surgical technique | −45.9 (−18.6/−73.2) |
| Age >65 | +28.8 (8.4/49.3) |
| Occurrence of epidural haematoma | +108 (32.1/183) |
| Female sex | +29.7 (10.2/49.2) |
Discussion
The primary objective of this study was to test two hypotheses on data collected over the last 6 years. As expected, we found a large variance (from 1 to 5) in the time spent by the same surgeon in treating patients with LSS. The main predictors of TSPP were no. of operated levels and dural tear; second predictors were surgical technique (uni/bilateral laminotomy), haematoma, age and sex.
Are our data solid and reliable?
The data used in the present study can be considered as solid being based on a large sample size, however, information was collected retrospectively which could affect their reliability. The use of an administrative database in screening patients who underwent surgery may constitute a bias by the lack of data accuracy [17]. Moreover, our study was based in a single institute with a single surgeon thus rendering the generalization of the data obtained impossible. Our results must, therefore, be considered as preliminary on which further studies designing a prospective method for use on registry data should be based. Finally, the model for TSPP depends on arbitrary data and the value of 20 min for inpatient or outpatient visits reflects our daily practice and can be adapted.
From another point of view, TSPP could be useful when comparing surgeons and/or different institutions. Multicentric studies may provide less specific results by providing a mean of the differences between surgeons on the basis of the patients selected, type of surgery, time to discharge and access to medical visits.
Are the findings interesting for the surgeon?
This study emphasizes the importance of the invasiveness of surgery (as assessed by the no. of levels) in estimating the time allocated by surgeons to each patient. Our results also put into perspective the effects that patient characteristics have on TSPP. Moreover, if TSPP is found to reflect the standard of care of surgeons or institutions, one additional goal would be to stimulate comparisons with other surgical teams.
Comparison of data
The factors identified considering the model for TSPP (no. of levels, dural tear, surgical technique, haematoma, age and sex) had to be compared with the main predictors of use of resources found in the literature. The rate of complications was previously found to be related to the increasing LoS and OT [6, 18], and patients with more operated levels had more complications [19]. By contrast, less invasive techniques were found associated with low rates [6, 20] and had the potential to decrease treatment costs and LoS [9]. Some studies [20–22] reported an association between dural tear and increasing LoS and OT.
Age is a factor in the model of TSPP which can be used as a predictor for use of resources, and while it is not related to surgical complications [23], it is positively correlated to OT [7].
One could consider sex as a surprising factor. In our study, the LoS for women was 2 days longer compared to men, as has been found in other studies [4, 6].
Considering the age and gender variables, the result showed 29 min longer for over 65 years and 30 min longer for women. We can logically ask whether this is because of the average age of women was higher than men. To answer this point, we need to see if there is still a significant gender effect on TSPP when the effect of age is controlled.On the first hand, it is useful to note that age is gender-dependant in our data and women are significantly older than men (p = 0.006). On the second hand, by performing an analysis of covariance, we can state that, given two people with similar age, you can expect longer TSPP for females (+23.3 min, p = 0.047). Therefore, gender is an independent predictor for TSPP, even when considering people with similar age.
Some authors report that a high BMI is associated with poorer outcome [24] and longer OT [17, 25]. BMI, even though significantly associated with TSPP was not found as a predictor in the last model for TSPP. Concerning the duration, our regression equation for time predicted 87.5 min. Khoo and Fessler [26] reported an OT of 109 min for one level microscopic unilateral laminotomy and 88 min for open laminectomy. Other authors [27] reported a mean OT of 153 min.
Considering outpatients data, only a few studies have reported the utilization of health care resources. The mean number of medical visits has been reported as 5 ± 2 [28], with generally three postoperative visits [6, 29]. These findings correlate well with our data showing, respectively, 5 days and three postoperative visits. To the best of our knowledge, no reports have concerned predictors of number of medical visits. In the present study, the most significant variable associated with number of medical visits was severe postoperative pain score.
Conclusion
The identification of factors related to and influencing the use of resources is useful if modifying them would reduce such use. According to our data, surgeons can partly reduce the use of medical resources by improving surgical techniques that are either more efficient or associated with reduced rates of complications. The remaining factors are patient-related and cannot be modulated although surgeons could use them to select patients. Knowledge of the TSPP with regards to medical resources may be helpful for surgical teams as an assessment of their daily practice and for comparing standards of care in different institutions, as well as financial costs [30].
Conflict of interest
None.
References
- 1.Du Bois M, Szpalski M, Donceel P (2012) A decade’s experience in lumbar spine surgery in Belgium: sickness fund beneficiaries, 2000-2009. European Spine Journal: Official Publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society [DOI] [PMC free article] [PubMed]
- 2.Kalichman L, Cole R, Kim DH, Li L, Suri P, Guermazi A, Hunter DJ. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J. 2009;9:545–550. doi: 10.1016/j.spinee.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciol MA, Deyo RA, Howell E, Kreif S. An assessment of surgery for spinal stenosis: time trends, geographic variations, complications, and reoperations. J Am Geriatr Soc. 1996;44:285–290. doi: 10.1111/j.1532-5415.1996.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 4.Deyo RA, Mirza SK. Trends and variations in the use of spine surgery. Clin Orthop Relat Res. 2006;443:139–146. doi: 10.1097/01.blo.0000198726.62514.75. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein JN, Tosteson TD, Lurie JD, Tosteson ANA, Blood E, Hanscom B, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goldberg H, Berven S, An H. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358:794–810. doi: 10.1056/NEJMoa0707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303:1259–1265. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu Y-S, Zeng B-F, Xu J-G. Long-term outcomes of two different decompressive techniques for lumbar spinal stenosis. Spine. 2008;33:514–518. doi: 10.1097/BRS.0b013e3181657dde. [DOI] [PubMed] [Google Scholar]
- 8.Müslüman AM, Cansever T, Yılmaz A, Çavuşoğlu H, Yüce İ, Aydın Y. Midterm outcome after a microsurgical unilateral approach for bilateral decompression of lumbar degenerative spondylolisthesis. J Neurosurg Spine. 2012;16:68–76. doi: 10.3171/2011.7.SPINE11222. [DOI] [PubMed] [Google Scholar]
- 9.Yagi M, Okada E, Ninomiya K, Kihara M. Postoperative outcome after modified unilateral-approach microendoscopic midline decompression for degenerative spinal stenosis. J Neurosurg Spine. 2009;10:293–299. doi: 10.3171/2009.1.SPINE08288. [DOI] [PubMed] [Google Scholar]
- 10.Sasai K, Umeda M, Maruyama T, Wakabayashi E, Iida H. Microsurgical bilateral decompression via a unilateral approach for lumbar spinal canal stenosis including degenerative spondylolisthesis. J Neurosurg Spine. 2008;9:554–559. doi: 10.3171/SPI.2008.8.08122. [DOI] [PubMed] [Google Scholar]
- 11.Cavuşoğlu H, Kaya RA, Türkmenoglu ON, Tuncer C, Colak I, Aydin Y. Midterm outcome after unilateral approach for bilateral decompression of lumbar spinal stenosis: 5-year prospective study. Eur Spine J. 2007;16:2133–2142. doi: 10.1007/s00586-007-0471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nandi S, Aghazadeh M, Talmo C, Robbins C, Bono J. Perioperative clopidogrel and postoperative events after hip and knee arthroplasties. Clin Orthop Relat Res. 2012;470:1436–1441. doi: 10.1007/s11999-012-2306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KK, Ludwig LE, Pedan A, Goldman L. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. doi: 10.1161/01.CIR.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 14.Rao JY, Yeriswamy MC, Santhosh MJ, Shetty GG, Varghese K, Patil CB, Iyengar SS. A look into Lee’s score: peri-operative cardiovascular risk assessment in non-cardiac surgeries-usefulness of revised cardiac risk index. Indian Heart J. 2012;64:134–138. doi: 10.1016/S0019-4832(12)60047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rago R, Forfori F, Materazzi G, Abramo A, Collareta M, Miccoli P, Giunta F. Evaluation of a preoperative pain score in response to pressure as a marker of postoperative pain and drugs consumption in surgical thyroidectomy. Clin J Pain. 2012;28:382–386. doi: 10.1097/AJP.0b013e3182326495. [DOI] [PubMed] [Google Scholar]
- 16.Keats AS. The ASA classification of physical status–a recapitulation. Anesthesiology. 1978;49:233–236. doi: 10.1097/00000542-197810000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Faciszewski T, Broste SK, Fardon D. Quality of data regarding diagnoses of spinal disorders in administrative databases. A multicenter study. J Bone Joint Surg Am. 1997;79:1481–1488. doi: 10.2106/00004623-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Raffo CS, Lauerman WC. Predicting morbidity and mortality of lumbar spine arthrodesis in patients in their ninth decade. Spine. 2006;31:99–103. doi: 10.1097/01.brs.0000192678.25586.e5. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Patil CG, Lad SP, Ho C, Tian W, Boakye M. Effects of age and comorbidities on complication rates and adverse outcomes after lumbar laminectomy in elderly patients. Spine. 2008;33:1250–1255. doi: 10.1097/BRS.0b013e3181714a44. [DOI] [PubMed] [Google Scholar]
- 20.Thomé C, Zevgaridis D, Leheta O, Bäzner H, Pöckler-Schöniger C, Wöhrle J, Schmiedek P. Outcome after less-invasive decompression of lumbar spinal stenosis: a randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J Neurosurg Spine. 2005;3:129–141. doi: 10.3171/spi.2005.3.2.0129. [DOI] [PubMed] [Google Scholar]
- 21.Desai A, Ball PA, Bekelis K, Lurie J, Mirza SK, Tosteson TD, Weinstein JN. SPORT: does incidental durotomy affect long-term outcomes in cases of spinal stenosis? Neurosurgery. 2011;69:38–44. doi: 10.1227/NEU.0b013e3182134171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strömqvist F, Jönsson B, Strömqvist B. Dural lesions in decompression for lumbar spinal stenosis: incidence, risk factors and effect on outcome. Eur Spine J. 2012;21:825–828. doi: 10.1007/s00586-011-2101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobottke R, Aghayev E, Röder C, Eysel P, Delank SK, Zweig T. Predictors of surgical, general and follow-up complications in lumbar spinal stenosis relative to patient age as emerged from the Spine Tango Registry. Eur Spine J. 2012;21:411–417. doi: 10.1007/s00586-011-2016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Athiviraham A, Wali ZA, Yen D. Predictive factors influencing clinical outcome with operative management of lumbar spinal stenosis. Spine J. 2011;11:613–617. doi: 10.1016/j.spinee.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Zheng F, Cammisa FP, Jr, Sandhu HS, Girardi FP, Khan SN. Factors predicting hospital stay, operative time, blood loss, and transfusion in patients undergoing revision posterior lumbar spine decompression, fusion, and segmental instrumentation. Spine. 2002;27:818–824. doi: 10.1097/00007632-200204150-00008. [DOI] [PubMed] [Google Scholar]
- 26.Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. 2002;51:S146–S154. [PubMed] [Google Scholar]
- 27.Mirza SK, Deyo RA, Heagerty PJ, Konodi MA, Lee LA, Turner JA, Goodkin R. Development of an index to characterize the “invasiveness” of spine surgery: validation by comparison to blood loss and operative time. Spine. 2008;33:2651–2661. doi: 10.1097/BRS.0b013e31818dad07. [DOI] [PubMed] [Google Scholar]
- 28.Adogwa O, Parker SL, Shau DN, Mendenhall SK, Aaronson O, Cheng JS, Devin CJ, McGirt MJ. Cost per quality-adjusted life year gained of revision neural decompression and instrumented fusion for same-level recurrent lumbar stenosis: defining the value of surgical intervention. J Neurosurg Spine. 2012;16:135–140. doi: 10.3171/2011.9.SPINE11308. [DOI] [PubMed] [Google Scholar]
- 29.Parker SL, Fulchiero EC, Davis BJ, Adogwa O, Aaronson OS, Cheng JS, Devin CJ, McGirt MJ. Cost-effectiveness of multilevel hemilaminectomy for lumbar stenosis-associated radiculopathy. Spine J. 2011;11:705–711. doi: 10.1016/j.spinee.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 30.Lotter O, Amr A, Chiarello P, Bihler M, Schaller H-E, Stahl S (2012) Diagnosis-Related Groups in Hand Surgery–a comparison of six European countries. Ger Med Sci 10: Doc08 [DOI] [PMC free article] [PubMed]