Abstract
Object
The purpose of this study is to analyze the data in terms of the number of channels employed to examine the usefulness of multi-channels in intraoperative spinal cord monitoring.
Methods
The prerequisites for inclusion in the baseline data were as follows: (1) cases in which only CMAP monitoring was conducted; (2) cases in which monitoring was conducted under the same stimulation condition and the recording condition. Cases where inhalation anesthesia was used or muscle relaxants were used as maintenance anesthesia was excluded from the baseline data. Of the 6,887 cases, 884 cases met the criteria. The items examined for each of the different numbers of channels were the sensitivity and specificity, the false positive rate, the false negative rate, and the coverage rate of postoperative motor deficit muscles.
Result
To examine these two items in terms of the number of channels, the 4-channel group had lower sensitivity and specificity scores compared with the 8- and 16-channel groups (4 channels 73/93 %, 8 channels 100/97 %, 16 channels 100/95 %). Only four channels were derived for these cases and the coverage of postoperative motor deficit muscles was 38 % with only 30 out of the 80 postoperative motor deficit muscles in total being monitored. In the 8-channel group, it was 60 % with 12 of the 20 postoperative motor deficit muscles being monitored. The 16-channel group had 100 % coverage rate of postoperative motor deficit muscles.
Conclusion
We suggest that multi-channel monitoring of at least eight channels is desirable for intraoperative spinal cord monitoring.
Keywords: Compound muscle action potentials, Sensitivity, Specificity, Multi-channels, Intraoperative spinal cord monitoring
Introduction
Somatosensory-evoked potential (SSEP) has been used as spinal surgery monitoring since the 1980s [1–5], and cord-evoked potential after stimulation to the brain (Br-SCEP, D-wave) has also been used as motor pathway monitoring since the 1990s [6–11]. Other monitoring techniques were subsequently developed, including free running electromyography (EMG) [12, 13], spinal cord evoked potential after stimulation to the spinal cord (Sp-SCEP), spinal cord evoked potential after stimulation to the peripheral nerve (Pn-SCEP), and compound muscle action potential (CMAP) [14–18]. CMAP is regarded as the most sensitive method of monitoring as it accurately indicates the invasiveness of the surgery on a real-time basis. Accordingly, its usefulness has been reported by a large number of authors. Additionally, the importance of multi-modality monitoring or combinations of at least two of the above methods rather than single-modality approaches has been pointed out in numerous reports [9, 19]. No previous report, however, has focused on the number of channels used in CMAP. We at the Monitoring Committee of the Japanese Society for Spine Surgery and Related Research conducted a nationwide multi-institution survey in 2007 and collected data on about 6,887 cases of monitoring carried out at numerous institutions during the preceding 5 years [20]. The purpose of this study is to analyze the data in terms of the number of channels employed in order to examine the usefulness of multi-channels in intraoperative spinal cord monitoring.
Materials and methods
Subjects
In 2007, the Monitoring Committee of the Japanese Society for Spine Surgery and Related Research conducted a nationwide multi-institution survey to determine the manner in which intraoperative spinal cord monitoring was conducted. A questionnaire was sent to a total of 72 institutions consisting of the training institutions and the institutions of the ossification of posterior longitudinal ligament (OPLL) study group of the Japanese Society for Spine Surgery and Related Research to analyze and compile data about cases of monitoring that had been conducted during the preceding 5 years. The questionnaire was returned from 60 institutions (80 %), in which 47 institutions replied that they were conducting monitoring while the remaining 13 institutions (22 %) replied that they were not. A total of 6,887 cases of monitoring were compiled.
The items of the questionnaire were: (1) the types of monitoring; (2) the names and number of disease; (3) the conditions of anesthesia; (4) the condition of stimulation, the monitored muscle and its number (for CMAP only), the elicited rate, and the alarm points for each type of monitoring conducted; (5) complications; and (6) details of the cases of true positives and false negatives [the diagnosis and number of disease, the operating method, the derivation areas, the number of muscles, the preoperative and postoperative manual muscle test (MMT), presence of dysesthesia, the duration of postoperative motor deficit, and copies of intraoperative waveforms].
Criteria for selecting cases
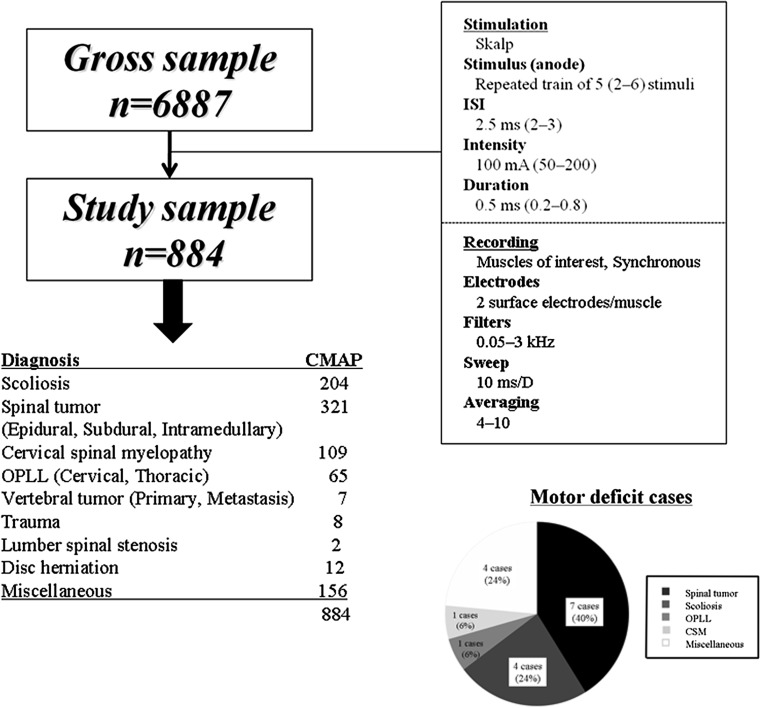
The prerequisites for inclusion in the baseline data were as follows: (1) cases in which only CMAP monitoring was conducted; (2) cases in which monitoring was conducted under the stimulation condition and the recording condition shown in Fig. 1; and (3) cases recorded at institutions where loss of amplitude was used as the alarm point. Cases where inhalation anesthesia was used or muscle relaxants were used as maintenance anesthesia was excluded from the baseline data [21].
Fig. 1.
The flowchart of the subjects of this study and the condition of stimulation and recording and the breakdown and number for true positive and false negative cases
Of the 6,887 cases, 884 cases (13 institutions) met the criteria (Fig. 1).
The disease for which monitoring was conducted included 321 cases (36 %) of spinal cord tumor (including extramedullary and intramedullary tumor), 204 cases (23 %) of scoliosis, 109 cases (12 %) of cervical myelopathy, and 65 cases (7 %) of cervical and thoracic OPLL.
Examined items
The items examined for each of the different numbers of channels were sensitivity and specificity, false positive rate, false negative rate, and coverage rate of postoperative motor deficit muscles, i.e., (the number of muscles with reduced waveforms)/(the total number of postoperative motor deficit muscles).
Result
The breakdown of the cases (institutions) in terms of the number of monitoring channels were; 4-channel monitoring conducted in 663 cases (75 %) at eight institutions, 8-channel monitoring conducted in 35 cases (4 %) at three institutions, and 16-channel monitoring conducted in 186 cases (21 %) at two institutions. Table 1 shows the “recorded muscles”. Normally each of all muscles were recorded bilaterally (counted two channels). According to the institution, different muscles were recorded. In total, 17 cases (1.9 %) of postoperative motor deficit were identified, consisting of 14 cases of true positives and three cases of false negatives (Fig. 1). There were 56 cases of false positives with an overall sensitivity of 82 % and an overall specificity of 94 %.
Table 1.
The recording muscles
| Group | Upper extremity | Lower extremity |
|---|---|---|
| 4 channel (N = 661) | Abductor pollicis brevis | Tibialis anterior |
| Adductor digiti minimi | Gastrocnemius | |
| Abductor hallucis | ||
| 8 channel (N = 38) | Deltoid | Quadriceps |
| APB | TA | |
| ADM | GC | |
| AH | ||
| 16 channel (N = 145) | Deltoid | Quadriceps |
| Biceps | Hamstring | |
| Triceps | TA | |
| APB | GC | |
| ADM | Flexor hallucis longus | |
| AH | ||
| Anal |
Sensitivity and specificity in terms of the number of channels (Table 2)
Table 2.
Sensitivity and specificity in terms of the number of channels and false negative cases in 4 channel
| Motor deficit + | Motor deficit − | ||||||
|---|---|---|---|---|---|---|---|
| 4-channel | |||||||
| Waveform change + | 8 | 48 | PPV 14 % | ||||
| Waveform change − | 3 | 602 | NPV 99 % | ||||
| Sensitivity 73 % | Specificity 93 % | False positive 7.3 % | |||||
| False negative 0.5 % | |||||||
| 8-channel | |||||||
| Waveform change + | 2 | 1 | PPV 66 % | ||||
| Waveform change − | 0 | 35 | NPV 100 % | ||||
| Sensitivity 100 % | Specificity 97 % | False positive 2.6 % | |||||
| False negative 0 % | |||||||
| 16-channel | |||||||
| Waveform change + | 4 | 7 | PPV 36 % | ||||
| Waveform change − | 0 | 134 | NPV 100 % | ||||
| Sensitivity 100 % | Specificity 95 % | False positive 4.8 % | |||||
| False negative 0 % | |||||||
| Patient | Pathology | Surgery | Recording muscle | Neurological deterioration | Cover rate (%) | Wave change | Duration |
|---|---|---|---|---|---|---|---|
| f, 12-year old | Scoliosis (T5–L3) | Correction and fusion | Bil APB, AH | Bil Quad, Ham, TA | 0 | No | 2 weeks |
| m, 11-year old | Kyphosis (T3–T6) | VCR and fusion | Bil APB, AH | Bil TA | 0 | No | 2 weeks |
| m, 49-year old | CSM | Laminoplasty | Bil Delt, ADM | Rt Delt, biceps | 50 | No | 3 months |
To examine these two items in terms of the number of channels, the 4-channel group had lower sensitivity and specificity scores compared with the 8- and 16-channel groups (4 channels 73/93 %, 8 channels 100/97 %, 16 channels 100/95 %).
False positive rate and false negative rate in terms of the number of channels (Table 2)
The overall false positive and false negative rates were 6.4 and 0.3 %, respectively. Although comparison of the false positive and false negative rates in terms of the number of channels revealed no significant difference, the four-channel group registered higher rates (7.3 and 0.5 %) (Table 2).
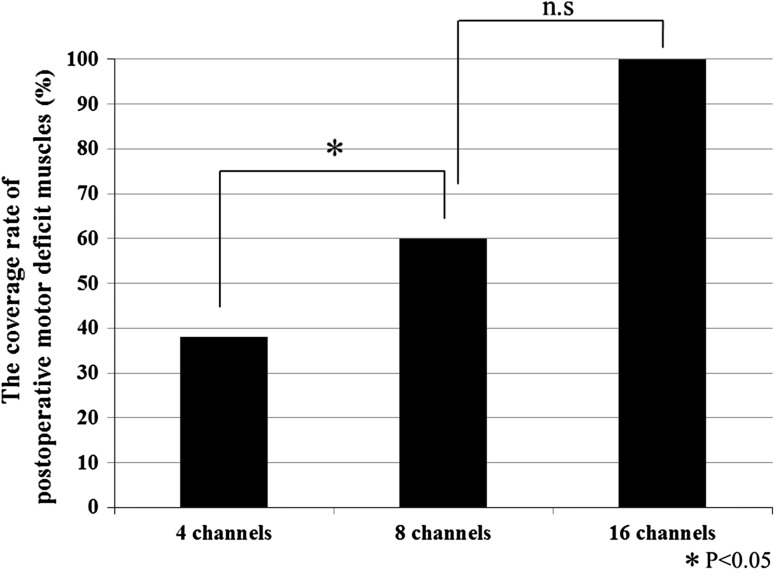
Coverage rate of postoperative motor deficit muscles in terms of the number of channels (Fig. 2)
Fig. 2.
The coverage rate of postoperative motor deficit muscles
In the four-channel group, 6 of the 11 cases of postoperative motor deficit exhibited reduction in MMT in the lower limbs from the quadriceps down. However, only four channels were derived for these cases and the coverage of postoperative motor deficit muscles was 38 % with only 30 out of the 80 postoperative motor deficit muscles in total being monitored. In the 8-channel group, the coverage rate of postoperative motor deficit muscles was 60 % with 12 of the 20 postoperative motor deficit muscles being monitored. The 16-channel group had a full or 100 % coverage rate of postoperative motor deficit muscles with all the 28 postoperative motor deficit muscles being monitored. This means that both 8-channel and 16-channel groups had significantly higher coverage rate of postoperative motor deficit muscles than the 4-channel group (p < 0.05).
False negative cases (Table 2)
Three cases of false negative were identified. These were all from the 4-channel group. Case 1 was one in which posterior correction and fusion was performed for scoliosis. While the APB and AH muscles were both monitored, the quad, ham, and TA had postoperative motor deficit for 2 weeks after the surgery. Case 2 was one in which vertebral column resection osteotomy was performed for thoracic kyphosis. While the APB and AH muscles were both monitored, the TA had postoperative motor deficit for 2 weeks after the surgery.
Case 3 was one in which laminoplasty was performed for cervical myelopathy. While both deltoid muscles and both ADM muscles were both monitored, the right deltoid and biceps remained paralyzed for more than 3 months after the surgery.
Discussion
Numerous papers have been published that state the importance of spinal monitoring and CMAP is regarded as the most sensitive monitoring method of all [22–27]. In addition, monitoring is possible from any muscle of the upper limb, the trunk, and the lower limb and laterality comparison is also possible. No previous report, however, has examined the significance of the number of channels employed for monitoring as well as the importance of multi-channels.
While recognizing no significant difference in sensitivity, this study does show the 8- and 16-channel groups had a higher sensitivity (100 %) than the 4-channel group (73 %), thus confirming the usefulness of multi-channel monitoring. Likewise, the 4-channel group had higher scores in false positive and false negative rates (7.3 and 0.5 %). Moreover, the three cases of false negative were all from the four-channel group, in which very few motor deficit muscles had electrodes inserted therein.
Sutter et al. [19] employed CMAP in 1,006 of the 1,017 monitored cases, reporting the average number of channels to be 2.6 pairs (5.2 channels). Eggspuehler et al. [28] employed CMAP in 216 cases of spinal deformity, reporting the average number of channels to be 2.8 pairs (5.6 channels). Eggspuehler et al. [29] also employed CMAP in 241 cases of cervical spine surgery, reporting the average number of channels to be 2.6 pairs (5.2 channels). Multimodal intraoperative monitoring (MIOM) was performed in the cases in all of the reports to employ other monitoring methods to detect paralysis. However, the numbers of channels were insufficient for CMAP and no comparison with multi-channel monitoring was made in the reports. This is the first report to focus on multi-channels in CMAP and compare and examine different groups of cases in terms of the number of channels employed. Based on the result of the examination, it is believed that multi-channel monitoring of at least 8-channels should be used to minimize false negative cases and maximize the detection rate of motor deficit muscles.
One limitation of this study is that since it is a retrospective and multicenter study, the monitoring conditions (the anesthesia condition, the stimulation condition, the recording condition, and the alarm point) were not strictly standardized and the number of eight-channel group is so small. Although only the cases meeting the conditions of Table 1 were selected for this study, we believe there is a need for a nationwide prospective study with a standardized set of monitoring conditions, surgery and large number. Another limitation is that after the cases were sorted by the number of channels, there were insufficient number of cases of paralysis in each category to make a statistically satisfactory comparison in sensitivity and specificity as well as false positive and negative rates. It is thought, therefore, that studies of larger scales are needed. Our sample size analysis carried out with G*Power software (version3.1.3, Heinrich-Heine-University, Dusseldorf, Germany) showed that statistical power for all group was 47.4 %. Although adequate sample size was not mandatory, because the present study was an exploratory study, calculated statistical power over 80 % was generally optimal for a significant result.
Final limitation is the insufficiency of the functional assessment since our analysis was based only on MMT measurements. We are considering that variability in MMT scoring may account for some of the alterations in the specificity and sensitivity scores. Indeed if the MMT is used for paralysis then it is reliable for grades of <4.
Conclusion
We suggest that multi-channel monitoring of at least eight channels is desirable for intraoperative spinal cord monitoring.
Conflict of interest
None.
References
- 1.Ben-david B, Haller G, Taylor P. Anterior spinal fusion complicated by paraplegia; a case report of a false-negative somatosensory-evoked potential. Spine. 1987;12:536–539. doi: 10.1097/00007632-198707000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Ginsburg HH, Shetter AG, Raudzens PA. Postoperative paraplegia with preserved intraoperative somatosensory evoked potentials. Case report. Neurosurgery. 1985;63:296–300. doi: 10.3171/jns.1985.63.2.0296. [DOI] [PubMed] [Google Scholar]
- 3.Lesser RP, Raudzens P, Lüders H, Nuwer MR, Goldie WD, Morris HH, 3rd, et al. Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann Neurol. 1986;19:22–25. doi: 10.1002/ana.410190105. [DOI] [PubMed] [Google Scholar]
- 4.Zileli M, Coşkun E, Ozdamar N, Ovül I, Tunçbay E, Oner K, et al. Surgery of intramedullary spinal cord tumors. Eur Spine. 1996;J5:243–250. doi: 10.1007/BF00301327. [DOI] [PubMed] [Google Scholar]
- 5.Zornow MH, Grafe MR, Tybor C, Swenson MR, et al. Preservation of evoked potentials in a case of anterior spinal artery syndrome. Electroencephalogr Clin Neurophysiol. 1990;77:137–139. doi: 10.1016/0168-5597(90)90028-C. [DOI] [PubMed] [Google Scholar]
- 6.Karl FK, Vedran D, Fred JE. Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical neurophysiological data in a series of 100 consecutive procedures. Neurosurgical Focus. 1998;4:e1. doi: 10.3171/foc.1998.4.5.4. [DOI] [PubMed] [Google Scholar]
- 7.Kothbauer K, Deletis V, Epstein FJ. Intraoperative spinal cord monitoring for intramedullary surgery; an essential adjunct. Pediatr Neurosurg. 1997;26:247–254. doi: 10.1159/000121199. [DOI] [PubMed] [Google Scholar]
- 8.Fujiki M, Furukawa Y, Kamida T, Anan M, Inoue R, Abe T, Kobayashi H. Intraoperative corticomuscular motor evoked potentials for evaluation of motor function: a comparison with corticospinal D and I waves. J Neurosurg. 2006;104:85–92. doi: 10.3171/jns.2006.104.1.85. [DOI] [PubMed] [Google Scholar]
- 9.Sala F, Giorgio P, Elisabetta B, Paola L, vedran D, Franco F, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery. 2006;58:1129–1143. doi: 10.1227/01.NEU.0000215948.97195.58. [DOI] [PubMed] [Google Scholar]
- 10.Sala F, Albino B, Franco F, Paola L, Massimo G. Surgery for intramedullary spinal cord tumors: the role of intraoperative (neurophysiological) monitoring. Eur Spine. 2007;J16:S130–S139. doi: 10.1007/s00586-007-0423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulkatan S, Neuwirth M, Bitan F, Minardi C, Kokoszka A, Deletis V. Monitoring of scoliosis surgery with epidurally recorded motor evoked potentials (D wave) revealed false results. Clin Neurosurg. 2006;117:2093–2101. doi: 10.1016/j.clinph.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Juan CJ, Sepehr S, Berton B, Harel D, Jhon KR. Palsies of the fifth cervical nerve root after cervical decompression: prevention using continuous intraoperative electromyography monitoring. J Neurosurg Spine. 2005;3:92–97. doi: 10.3171/spi.2005.3.2.0092. [DOI] [PubMed] [Google Scholar]
- 13.Thorsteinn G, Andrei VK, Roger S, Michael GF. Real time continuous intraoperative electromyographic and somatosensory evoked potential recordings in spinal surgery: correlation of clinical and electrophysiologic findings in a prospective, consecutive series of 213 cases. Spine. 2004;29:677–684. doi: 10.1097/01.BRS.0000115144.30607.E9. [DOI] [PubMed] [Google Scholar]
- 14.Bartley K, Woodforth IJ, Stephen JP, Burke D. Corticospinal volleys and compound muscle action potentials produced by repetitive transcranial stimulation during spinal surgery. Clin Neurophysiol. 2002;113:78–90. doi: 10.1016/S1388-2457(01)00711-8. [DOI] [PubMed] [Google Scholar]
- 15.Langeloo DD, Lelivelt A, Louis Journée H, Slappendel R, de Kleuver M. Transcranial electrical motor-evoked potential monitoring during surgery for spinal deformity. Spine. 2003;28:1043–1050. doi: 10.1097/01.BRS.0000061995.75709.78. [DOI] [PubMed] [Google Scholar]
- 16.Langeloo DD, Journée HL, de Kleuver M, Grotenhuis JA. Criteria for transcranial electrical motor evoked potential monitoring during spinal deformity surgery. A review and discussion of the literature. Neurophysiol Clin. 2007;37:431–439. doi: 10.1016/j.neucli.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Lang EW, Chesnut RM, Beutler AS, Kennelly NA, Renaudin JW. The utility of motor-evoked potential monitoring during intramedullary surgery. Anesth Analg. 1996;83:1337–1341. doi: 10.1097/00000539-199612000-00038. [DOI] [PubMed] [Google Scholar]
- 18.Luk KD, Hu Y, Wong YW, Cheung KM. Evaluation of various evoked potential techniques for spinal cord monitoring during scoliosis surgery. Spine. 2001;26:1772–1777. doi: 10.1097/00007632-200108150-00008. [DOI] [PubMed] [Google Scholar]
- 19.Sutter M, Eggspuehler A, Grob D, Jeszenszky D, Benini A, Porchet F, Mueller A, Dvorak J. The diagnostic value of multimodal intraoperative monitoring (MIOM) during spine surgery: a prospective study of 1,017 patients. Eur Spine J. 2007;16:S162–S170. doi: 10.1007/s00586-007-0418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuyama Y, Shimiya K, Ando M, Satomi K, Tani S, Ishiguro N. The current situation and alarm points of intraoperative spinal cord monitoring—a multi-facility survey conducted by the monitoring committee of the Japanese society for spine surgery and related research. Clin Brain Wave. 2009;51(5):286–291. [Google Scholar]
- 21.Sutter M, Eggspuehler A, Muller A, Dvorak J. Multimodal intraoperative monitoring: an overview and proposal of methodology based on 1,017 cases. Eur Spine J. 2007;16:S153–S161. doi: 10.1007/s00586-007-0417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginsburg HH, Shetter AG, Raudzens PA. Postoperative paraplegia with preserved intraoperative somatosensory evoked potentials. Case report. Neurosurgery. 1993;63:296–300. doi: 10.3171/jns.1985.63.2.0296. [DOI] [PubMed] [Google Scholar]
- 23.Taniguchi Cedzich C, Schramm J. Modification of cortical stimulation for motor evoked potentials under general anesthesia: technical description. Neurosurgery. 1993;32:219–226. doi: 10.1227/00006123-199302000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Calancie B, Harris W, Brindle GF, Green BA, Landy HJ. Threshold-level repetitive transcranial electrical stimulation for intraoperative monitoring of central motor conduction. J Neurosurg. 2001;95:161–168. doi: 10.3171/spi.2001.95.2.0161. [DOI] [PubMed] [Google Scholar]
- 25.Calancie B, Harris W, Broton JG, Alexeeva N, Green BA. Threshold-level multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tract; description of method and comparison to somatosensory evoked potential monitoring. J Neurosurg. 1998;88:457–470. doi: 10.3171/jns.1998.88.3.0457. [DOI] [PubMed] [Google Scholar]
- 26.Accadbled F, Henry P, de Gauzy JS, Cahuzac JP. Spinal cord monitoring in scoliosis surgery using an epidural electrode. Result of a prospective, consecutive series of 191 cases. Spine. 2006;31:2614–2623. doi: 10.1097/01.brs.0000240642.28495.99. [DOI] [PubMed] [Google Scholar]
- 27.Jones SJ, Harrison R, Koh KF, Mendoza N, Crockard HA. Motor evoked potential monitoring during spinal surgery; responses of distal limb muscles to transcranial cortical stimulation with pulse trains. Electroencephalogr Clin Neurophysiol. 1996;10:375–383. [PubMed] [Google Scholar]
- 28.Eggspuehler A, Sutter MA, Grob D, Jeszenszky D, Dvorak J. Multimodal intraoperative monitoring during surgery of spinal deformities in 217 patients. Eur Spine J. 2007;16:S188–S196. doi: 10.1007/s00586-007-0427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eggspuehler A, Sutter MA, Grob D, Jeszenszky D, Porchet F, Dvorak J. Multimodal intraoperative monitoring (MIOM) during cervical spine surgical procedures in 246 patients. Eur Spine J. 2007;16(Suppl 2):S209–S215. doi: 10.1007/s00586-007-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]