Abstract
Purpose
Schmorl’s nodes (SNs) are commonly seen in vertebral imaging of the normal adult population referred for different reasons and are duly noted by the radiologist. However, little is known about their etiology: either SNs are perceived as largely inert developmental or congenital herniations of disc tissue into weak areas of the vertebral end-plates, or they are perceived as a common pathological pathway of different adverse and general factors such as malignancy, trauma, infection, osteoporosis, Paget’s disease and so forth. A commonly accepted morphological definition of what precisely constitute SNs does not exist, and consequently prevalences vary wildly in the literature. In the present study of 4,151 standardized lateral radiographs of the lumbar spine in an adult, Caucasian population between 22 and 93 years (median age 63 years, M 1,533, W 2,618).
Methods
We investigated prevalence, distribution and epidemiologic relationships of SNs.
Results
SNs occur primarily in the upper part of the lumbar spine, and usually there are multiple lesions in the same individual. We could not establish any significant correlation between SNs and gender, age, BMI, height, weight or occupational exposure for heavy lifting. The overall prevalence was 3.8 %. We did not find any significant correlations between SNs and overall degeneration of the lumbar spine.
Conclusion
We found a prevalence of SNs in the lower end of the spectrum than hitherto reported.
Keywords: Schmorl’s nodes, Spinal diseases, Disc herniation, Lumbar spine, Epidemiology, Radiography
Introduction
Although 85 years have passed since Schmorl [1] first described herniations of the nucleus pulposus into the vertebral end-plates of the thoracic and lumbar spine, controversy still exists regarding the pathogenesis, etiology, epidemiology and clinical significance of these lesions [2–6]. It is still debated whether Schmorl’s nodes (SNs) are symptomatically silent developmental or congenital aberrations of the normal vertebral anatomy or represent a common histopathological pathway for various and adverse metabolic, traumatic, infectious, degenerative or malignant factors. SNs’ relationships with common low back pain, degeneration, regeneration, or epidemiological characteristics such as race, gender, age, body weight or exposure to heavy, manual labor are also uncertain.
The purposes of the present study were (1) to investigate the radiographic prevalence and distribution of SN of the Copenhagen Heart Study Cohort (CHSC) of 4,151 individuals investigated with standardized lateral, lumbar spine radiographs and (2) to identify relationships between SNs and radiographic signs of degeneration of the lumbar spine, and epidemiological factors such as gender, age, BMI, and exposure to heavy lifting.
Materials and methods
The Copenhagen City Heart Study cohort
The CCHS is a longitudinal survey of an adult, almost completely Caucasian cohort selected from the county of Österbro in Copenhagen using a random social security number algorithm. The county is a typical white, middle-class and affluent district of greater Copenhagen. The survey has registered life style and health parameters since its beginning in 1976 [7]. From 1991 to 1994 lateral, weight-bearing lumbar radiographs were recorded in 4,151 participants. There were 1,533 men with an average age of 62 years (range 23–93 years), and 2,618 women with an average age of 65 years (range 22–92 years). Radiographs were obtained standing. Feet pointed straight forward, lower extremities were positioned in neutral abduction–adduction. The X-ray beam was centered 10 cm cranially for the apex of the iliac crest. Radiographs recorded the vertebrae from Th 11 to the cranial surface of the sacral promontory. Tube to film distance was 120 cm. The magnification was 1.2. Two radiography technicians obtained all radiographs. Age, gender, height, weight and BMI were registered at the time of recording. Additional radiographs were recorded of hands, wrists, hips and knees. The sample was dictated and limited by considerations of radiation exposure. A complete recording of the entire spine was not feasible within these considerations, and the lumbar spine was chosen to investigate as much as possible the correlations of socio-economic factors to low back pain as such. From the beginning in 1976, written consent for investigating and publishing test results was given by all participants and projects were approved by the local ethical committee for the health sciences for greater Copenhagen.
Heavy lifting
The CCHS questionnaires have recorded occupation since leaving primary school or high school. For each occupation reported, the CCHS have registered frequency of different levels of lifting during a typical working day. The questions were formulated along the guidelines of The Danish National Board of Industrial Injuries, using the following categories: (1) primarily seated occupation, (2) standing, walking occupation, no repeated lifting, (3) daily repeated lifting equivalent to 50 × 20 or 20 × 50 kg, (4) repeated daily lifting equivalent to 50–100 × 20, or 20–50 × 50 kg, (5) repeated daily lifting equivalent to 100–250 × 20, or 50–100 × 50 kg, and (6) repeated daily lifting equivalent to 250–500 × 20, or 100–250 × 50 kg or above. Exposition for different levels of heavy lifting by years was recorded.
Radiographic analysis
Radiographs were analyzed by two senior radiologists (HR and HM). The presence of SN was recorded from Th 11 to L5. An SN was defined as a distinct, focal indentation centrally in the vertebral end-plate delineated by a thin sclerotic boundary and in an otherwise intact vertebra (Fig. 1). A central localization of the indentation was chosen as not to confuse SNs with residual notochords in the anterior portion of the vertebral end-plates. The height of each disk interspace was measured and overall narrowing of the disc space was graded from 0 (no narrowing) to 2 (marked narrowing), the presence of osteophytosis was recorded at each level and graded from 0 (no osteophytes) to 4 (large claw- or traction osteophytes, and overall end-plate sclerosis was recorded and graded from 0 (no sclerosis) to 2 (marked sclerosis) as recommended by Kellgren and Lawrence [8]. Observations were integrated into the classification of spinal osteoarthritis by Kellgren and Lawrence and individual lumbar spines were assigned an overall grade of radiographic degeneration from grade 0 (no osteoarthritic degeneration of the lumbar spine) to grade 4 (severe degeneration).
Fig. 1.
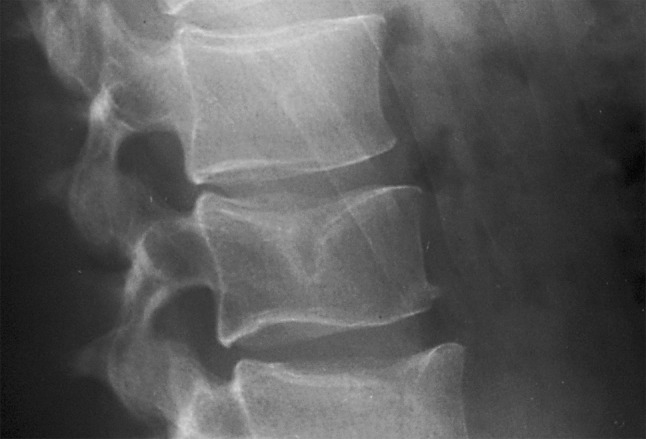
Schmorl’s node in the cranial end plate of the third lumbar vertebrae
All radiographs were re-read in a blinded cross-over fashion 6 months after initial observations and intra-class reliability coefficients were computed. Since it was not feasible to re-read all radiographs at individual levels the question of presence or non-presence of SNs in the lumbar/lower thoracic spine as such was formulated.
Statistical analysis
The relationship between the presence of SNs and gender was investigated using a Chi-square analysis. The relationship between SN and continuous variables such as age, BMI, height, weight and years occupied by various levels of physical labor were investigated by multiple logistic regression analysis, and the relationship between SN and overall grades of lumbar spine degeneration was investigated by Mann–Whitney non-parametric U test. With the lumbar spine dichotomized into a lower and an upper part, statistical differences between distribution of SNs in the lower part of the thoraco-lumbar spine and upper thoraco-lumbar spine was investigated by Chi-square tests. Inter- and intra-observer reliabilities were investigated by intraclass coefficients and Kendall and Spearman linear analysis.
The statistical significance level was set at p < 0.05.
All statistical analyses were performed using SPSS v. 19.0 statistical software (IBM, USA).
Results
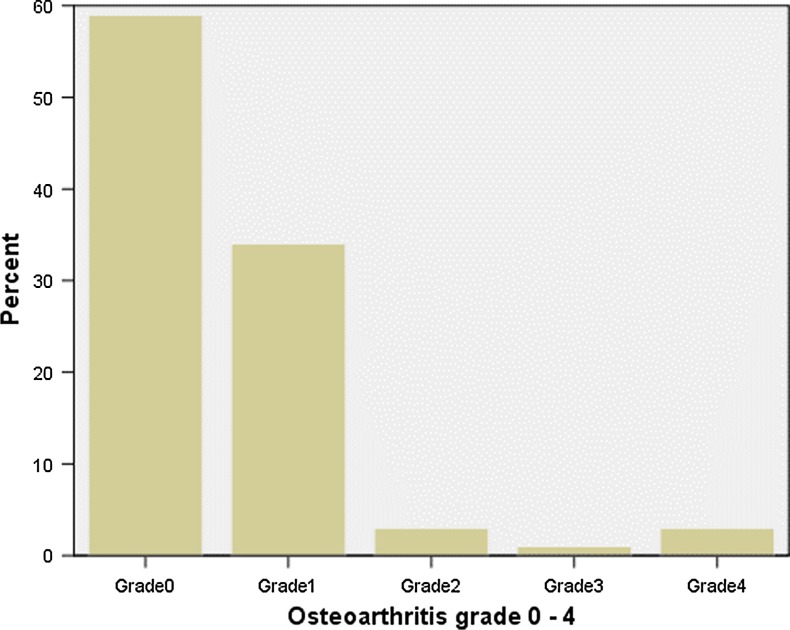
We found excellent inter and intra-observer agreement with regard to the presence or absence of SNs in the entire spine radiographs of 0.90. Intra class reliability was 0.907 95 % CI (0.90–0.95). The study included 1,538 men and 2,613 women. The distribution of study subjects in sex and age-groups is presented in Fig. 2, and the physical characteristics of the cohort are summarized in Table 1. The distribution of SN on lumbar level is presented in Tables 2 and 3. If dichotomized between the upper part of the thoraco-lumbar spine from Th 11 to and including L2, and a lower part from L3 to and including L5, a Chi-square analysis found a significant difference between the upper and lower vertebrae with predilection of SNs in the upper part of the thoraco-lumbar spine (p = 0.02). We found no significant difference between localization in the cranial or caudal end-plates. Five per cent of male subjects had SN on one or more levels (n = 78), and 3 % of the women were affected (n = 80). There was no significant difference between sexes (p = 0.15) (Table 4). The overall cross-sectional prevalence of SN was 3.8 %. We found no significant relationships between the presence of SNs and age, weight, height, BMI or years with different levels of heavy manual labor, since adolescence (p ranging from 0.34 to 0.62). Furthermore, we found no statistically significant relationship of SN with overall radiographic degeneration of the thoraco-lumbar spine (p = 0.29) (Figs. 3, 4).
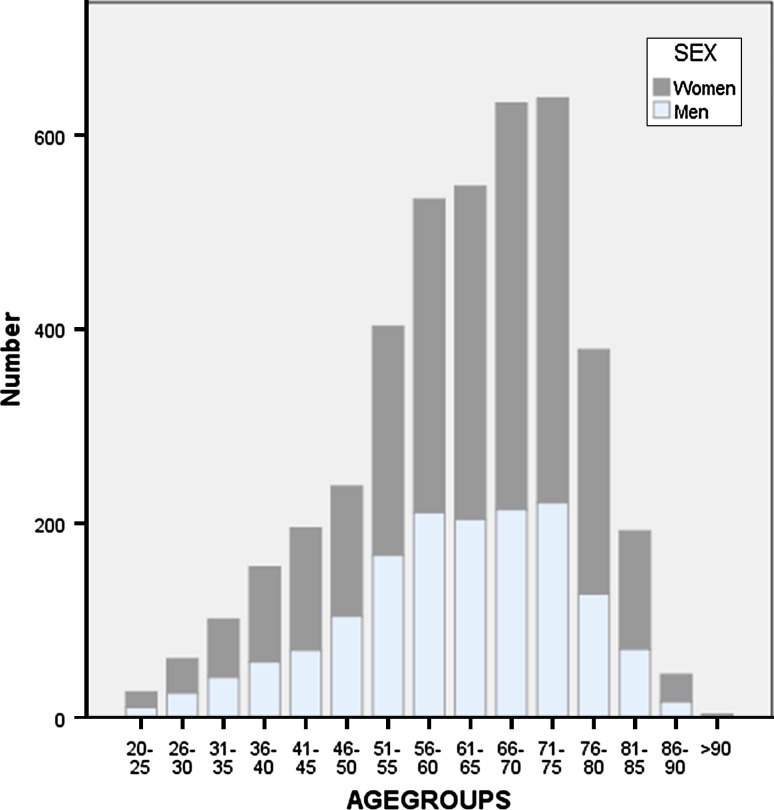
Fig. 2.
Distribution of cohort in sex- and age-matched groups
Table 1.
Characteristics of the cohort
| Men | Women | |
|---|---|---|
| n | 1,538 (37 %) | 2,613 (63 %) |
| Mean weight (kg) | 80 (42–147) | 67 (37–135) |
| Mean height (cm) | 174 (153–199) | 161 (134–186) |
| Mean BMI (kg/m2) | 26 (15–44) | 25 (15–51) |
| Mean age (years) | 60 (18–92) | 61 (21–90) |
Table 2.
Distribution of Scmorl’s nodes on spinal level
| Men (n = 1,538) | Women (n = 2,613) | |
|---|---|---|
| n (%) | n (%) | |
| L1 | 35 (23.9) | 42 (28.3) |
| L2 | 46 (31.5) | 38 (25.6) |
| L3 | 22 (15.0) | 26 (17.5) |
| L4 | 11 (7.5) | 8 (5.5) |
| L5 | 4 (2.7) | 0 (0.0) |
| Th 12 | 21 (14.3) | 22 (14.8) |
| Th 11 | 7 (4.7) | 12 (8.2) |
| Total | 146 (100) | 148 (100) |
Table 3.
Localisation of Schmorl’s nodes
| Vertebral body | L1 n (%) |
L2 n (%) |
L3 n (%) |
L4 n (%) |
L5 n (%) |
Th 12 n (%) |
Th 11 n (%) |
|---|---|---|---|---|---|---|---|
| Cranial endplate | 17 (22) | 39 (46) | 25 (52) | 11 (58) | 2 (50) | 8 (19) | 1 (6) |
| Caudal endplate | 40 (52) | 22 (26) | 13 (27) | 6 (31) | 2 (50) | 26 (60) | 15 (78) |
| Both | 20 (26) | 23 (28) | 10 (21) | 2 (11) | 0 (0) | 9 (21) | 3 (16) |
Table 4.
Prevalence of Schmorl’s nodes
| Men | Women | |
|---|---|---|
| n | 1,538 | 2,613 |
| Individuals affected | 78 | 80 |
| Prevalence (%) | 5.0 | 3.0 |
p = 0.15
Fig. 3.
Distribution of Schmorl’s nodes in sex- and age-matched groups
Fig. 4.
Grading of osteoarthritis of the lower spine 0–4
Discussion
Since there is no universal consensus regarding the morphological, histological, radiographic or macroscopic characteristics of SNs, and the subject stratum for individual researchers varies between excavated skeletons from the thirteenth century to modern-day patients investigated using MRI, it is hardly surprising that prevalences of SN varies wildly in the literature from 2 to 76 % [2–4, 9–12]. Furthermore, a golden standard in regard to investigative techniques and modalities has never been established. Some researchers have used consecutive MRI scans in establishing the presence of SN [10, 11] in small groups of highly selected patients referred for back pain, others have investigated historical osteologic collections [3, 4] and still others have investigated fresh spinal specimens from newly deceased [2, 5]. In the latter cases, the spinal column has been sagittally sectioned and individual slabs have been radiographed using fine grain X-ray films. It is often postulated that more SNs are visible on MRI than on conventional radiographs or that specimen slab radiographs are more sensitive in detecting SNs than the conventional radiographs of the spine—in situ, so to speak—but we do not know if these postulates are correct. To our knowledge, no comparative analyses of sensitivity and specificity of detecting SNs have even been carried out on the same stratum. Due to the absence of radiographic or anatomic definitions, different authors have had difficulties in differentiating between different end plate configurations. Some authors have included every indentation of the end plates in the SN category and subsequently have arrived at very high prevalences [2]. Using CT-scans might be an even better modality for observing and detecting SNs. However, it has not been used extensively for epidemiological or morphological investigations to our knowledge and considerations of radiation exposure would probably prove prohibitive in an epidemiological setting. Again, there has been no comparative analysis regarding specificity and sensitivity of different radiographic modalities.
Another source of disagreement arises from different views of the etiology of SN which of course affects the observations made. Traditionally, SNs have been perceived as histologically inert and idiopathic herniation of disk tissue through congenitally weak areas of the vertebral end-plate [12], while other investigators have emphasized a relationship of SNs to trauma, osteoporosis, Paget’s disease, malignancy, infection, inflammation, hyperparathyroidism, Scheuermann’s disease and the subsequent weakening of the vertebral architecture. If SN’s are viewed in this light, they would represent dynamic lesions which vary over time in depth and size according to the severity of the underlying disease and the inherent healing potential of the affected individual in question. The hypothesis might explain some of the findings associated with consecutive MRI scans where some of the SNs are found to be vascularized, edematous and variable in regard to size and depth over time [9, 11, 12]. However, SNs are usually found at the thoracolumbar junction, they are usually not solitary lesions, they are usually found in otherwise intact vertebrae and they are usually not found to be associated to any overall degeneration of the spine. It is, therefore, difficult to see them as dynamic lesions tightly associated with general or focal disease. On the other hand, the present study, cross-sectionally as it is, does not permit us to conclude anything about the pathogenesis of the nodes.
So, consensus in this field is somewhat scarce—to say the least and we cannot be certain what we or other investigators are really talking about discussing SNs. For the present study, we defined SNs as bell shaped, clearly delineated lesions in the central portion of the vertebral endplate in an otherwise normally looking vertebral body, radiographically speaking. Centrally, so as not to confuse SNs with the anterior remains of the notochord. Clearly delineated in the sense that the outline of the SN was represented as a sclerotic condensation of the lesion’s osseous margin (Fig. 1).
We analyzed a total of 29,057 vertebral bodies and found an overall prevalence of SNs of 3.8 %. Usually, subjects had more than one lesion. There was no difference in the incidence of lesions in the cranial end-plate compared to the caudal. We found no gender-specific differences contrary to most authors that concludes that SNs are predominantly a male phenomenon [2, 5]. In accordance with most other authors, we found a predilection of SNs to the upper rather than the lower lumbar spine. We could not establish any significant correlations between the presence of SNs and the weight, height or BMI of the subjects. We did not find a relationship between SN and occupational exposure to heavy, manual labor. We did not find any correlation between increasing age groups and an increasing or decreasing incidence of SN, and we did not find a significant relationship between the occurrence of SN and overall radiographic degeneration of the lumbar spine.
The present study represents, to our knowledge, the first extensive radiographic study of SNs in a large, unselected cohort using standardized, lateral lumbar radiographs recorded for this purpose. The study has several limitations, to be sure: it is purely cross-sectional, so we have no way of following individual SNs over time to see if they in reality are subject to alterations, morphologically speaking (“filling out”). Our suggested prevalence of about 3 % is also tentative. We have no way of knowing whether all SNs are, in fact, visible on plain radiographs, but then nobody really knows. However, there is a long way from 3.8 % to frankly whopping prevalences of 76 % as suggested by Hilton et al. [2] (cadaver study), or 20 (sic) to 75 % found by Saluja et al. [3] (excavated skeletons from the thirteenth to the nineteenth century) or 58 % as found by Pfirrman and Resnick [5] (a cadaver study). We did not have radiographs of the entire thoraco-lumbar spine at our disposal and cannot make any valid suggestions about prevalence or incidence of SNs in the upper part of the spine.
Again, the lack of consensus regarding SNs, their natural history and radiologic presentation does not permit us to draw any rigid conclusions about anything, really. Since the incidence of SNs were found to be independent of age, cross sectionally speaking, and since the nodes are usually found in legion in individual spines, it seems to us that they are not by themselves the result of underlying disease. After all, a concavity in the vertebral end-plate thought to be the result of infection, fracture or malignancy is probably just an acute lesion—not to be confused with the static developmental or congenital herniation into the body of the vertebrae as suggested by Schmorl himself.
Future study
In the next study of this series, the authors will investigate the relationships of SN to symptomatic low back pain and to lumbar Scheuermann’s disease.
Conflict of interest
None.
Footnotes
H. Rovsing: Deceased.
References
- 1.Schmorl G, Junghanns H. The human spine in health and disease (2nd American edition translated and edited by Besemann EF) New York: Grune and Strattión; 1971. [Google Scholar]
- 2.Hilton RC, Ball J, Benn RT. Vertebral end-plate lesions (Schmorl’s nodes) in the dorsolumbar spine. Ann Rheum Dis. 1976;35:127–132. doi: 10.1136/ard.35.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saluja G, Fitzpatrick K, Bruce M, Cross J. Schmorl’s nodes (intervertebral herniations of intervertebral disc tissue) in two historic British populations. J Anat. 1986;145:87–96. [PMC free article] [PubMed] [Google Scholar]
- 4.Dar G, Masharawi Y, Peleg S, Steinberg N, May H, Medlej B, Peled N, Hershkovitz A. Schmorl’s nodes distribution in the human spine and its possible etiology. Eur Spine J. 2010;19:670–675. doi: 10.1007/s00586-009-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfirrmann CWA, Resnick D. Schmorl nodes of the thoracic and lumbar spine: radiographic-pathological study of prevalence, characterization, and correlation with degenerative changes. Radiology. 2001;219:368–374. doi: 10.1148/radiology.219.2.r01ma21368. [DOI] [PubMed] [Google Scholar]
- 6.Chanchairujira K, Chung CB, Kim JY, Papakonstantinou O, Lee MH, Clopton P, Resnick D. Intervertebral disk calcification of the spine in an elderly population: radiographic prevalence, location, and distribution and correlation with spinal degeneration. Radiology. 2004;230:499–503. doi: 10.1148/radiol.2302011842. [DOI] [PubMed] [Google Scholar]
- 7.Schnohr P, Jensen G, Lange P, Scharling H, Appleyard M. The Copenhagen city heart study—Österbroundersøgelsen—tables with data from the third examination 1991–1994. Eur Heart J. 2001;3(Suppl. H):1–83. [Google Scholar]
- 8.Kellgren J, Lawrence JS. Radiological assessment of osteo-arthritis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi K, Miyazaki T, Ohnari H, Takino T, Tomita K. Schmorl’s nodes and low-back pain. Eur Spine J. 1995;4:56–59. doi: 10.1007/BF00298420. [DOI] [PubMed] [Google Scholar]
- 10.Benneker LM, Heini PF, Anderson SE, Alini M, Ito K. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur Spine J. 2005;14:27–35. doi: 10.1007/s00586-004-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu H-TH, Morrison WB, Schweitzer ME. Edematous Schmorl’s nodes on thoracolumbar MRI imaging: characteristic patterns and changes over time. Skeletal Radiol. 2006;35:212–219. doi: 10.1007/s00256-005-0068-y. [DOI] [PubMed] [Google Scholar]
- 12.Stäbler A, Bellan M, Weiss M, Gärtner C, Brossman J, Reiser MF. MR imaging of enhancing intraosseous disc herniation (Schmorl’s nodes) AJR. 1997;168:933–938. doi: 10.2214/ajr.168.4.9124143. [DOI] [PubMed] [Google Scholar]