Abstract
Purpose
The incidence of gram-negative bacterial haematogenous vertebral osteomyelitis (GNB HVO) is increasing. We performed a retrospective cohort study of patients with this type of infection in an effort to gain an improved understanding of the current clinical presentation, management and outcome.
Methods
Between May 2007 and May 2010, all patients, over the age of 18 years, suffering from GNB HVO were identified and their microbiological diagnoses were evaluated.
Results
This study identified seventy-nine patients with haematogenous vertebral osteomyelitis (HVO). Of these seventy-nine patients, 10 patients (12.66 %) had Gram-negative organisms isolated. These organisms included Escherichia coli (4), Pseudomonas aeruginosa (3), Klebsiella pneumonia (1), Haemophilus influenza (1) and Enterobacter cloacae (1). Eight patients were successfully treated with antibiotics and/or surgery. Of the eight patients whose HVO was cured, five had Ciprofloxacin as part of their definitive antibiotic regime.
Conclusion
The treatment of GNB HVO is often challenging because of unpredictable resistance patterns and limited published data on effective treatment regimens. Our study has highlighted the need for prompt microbiological sampling and initiation of early appropriate antibiotic regime. The most effective treatment for GNB HVO was with oral Ciprofloxacin over a period of 6–8 weeks.
Keywords: Discitis, Gram-negative bacterial, Haematogenous, Vertebral osteomyelitis, Infection
Introduction
Vertebral osteomyelitis and discitis (VO), also termed spondylodiscitis, are common manifestations of osteomyelitis in adults [1]. Infection affects the vertebral body and adjacent intervertebral disc(s) but can involve the epidural space, paraspinal soft tissues and neural arch [2]. The prevalence in males is slightly more frequent than in females, with a male predominance of 55 to 75 % [2] and the mean age of presentation is approximately 60 years [3]. Most infections are bacterial but fungi are occasionally implicated [2].
In recent years, there has been an increase in the incidence of spinal infections in the United States (US) but data in this regard from the UK is lacking [4]. This increase has been attributed to a variety of factors including an increasing proportion of individuals with predisposing factors such as advanced age, diabetes mellitus, intravenous drug use, HIV infection, malignancy, long-term steroid use, increasing use of indwelling devices, increase in orthopaedic prosthesis implantation and better diagnostic techniques [4, 5]. A recent study by Bhavan et al. [1] in the US found chronic renal insufficiency and diabetes mellitus to be the most frequent co-morbidities among patients with haematogenous vertebral osteomyelitis (HVO) [1].
HVO results from the spread of infection to the spine following a transient bacteraemia or following a bacteraemia from a distant primary focus of infection. It is associated with significant mortality and morbidity including: prolonged antimicrobial therapy, need for monitoring, problems with vascular access, development of resistance, failure of eradication, risk of recurrence, decreased functional status, painful, progressive deformity and neurological compromise, including paralysis [2]. Evidence from previous studies over the last 20 years confirms Staphylococcus aureus as the most common causative organism in haematogenous spinal infections, accounting for 40–60 % of cases [6, 7]. Gram negative bacilli (GNB) cause a significant proportion of all cases of HVO, ranging between 15 and 23 % [7, 8]. The proportion of HVO cases caused by GNB is greater in recent studies suggesting that the incidence may be increasing [7, 8].
At present there is no standardised national consensus for treatment of HVO. We were concerned about the treatment of HVO caused by GNB in our hospital and therefore performed a retrospective cohort study of patients with this infection in an effort to gain an improved understanding of the current clinical presentation, management and outcome.
Methodology
Between May 2007 and May 2010, all patients, over the age of 18 years, suffering from GNB HVO were identified within Leeds Teaching Hospital Trust. None of the patients were tertiary referrals from other centres. Patients were identified from Magnetic Resonance Images (MRI) and Computed Tomography scans (CT), by identifying hospital codes for patients shown to have VO/disk space infection or the request cards stating the patient may have VO/disk space infection.
Clinical features indicating spinal infection included back pain, which was not relieved by simple analgesia or rest, in combination with fever and neurological deficit. Radiological features of spinal infection included CT or MRI scans demonstrating infection in ≥1 vertebrae, intervertebral disk, epidural space and/or paraspinal tissues. Patients were included if they had clinical, radiological and microbiological evidence of GNB spinal infection. The diagnosis was defined as “definite” when a micro-organism was located from the involved disk space, vertebrae or paravertebral/epidural abscess or ≥2 or more blood cultures that were positive during the patient’s illness; or “probable” when an organism was isolated from only one blood culture. All patients with no positive microbiological results as stated above were excluded.
Medical records of all the patients who met our inclusion criteria were reviewed. The following were collected from all medical records: demographic information, history of presenting complaint, details of presentation to hospital, co-morbidities, antibiotic history, vital signs on admission, physical examination, diagnostic procedures, radiological findings, microbiology, preceding spinal surgery, medical and surgical treatment, length of stay and outcome.
Decompression and reconstruction surgery was performed if there was any intractable pain, failure of medical treatment, neurological impairment, destruction of vertebrae causing spinal instability and/or segmental kyphosis or rarely, to obtain open tissue biopsies when other sampling methods had failed.
The clinical, laboratory, and radiological findings were followed to assess the response to antimicrobial therapy. Inflammatory markers, such as full blood cell count, including white blood cell count (WBC) and C-reactive protein (CRP) level, were followed weekly during admission until discharge. We defined the normalisation of values of WBC and CRP as beneath 10,000/L and 0.5 mg/dL, respectively.
Outcome was evaluated following completion of antimicrobial therapy, and at 6 months and 12 months. All patients were followed up for a minimum of 12 months. Outcomes were classified as “cured” when patients had no signs and symptoms of infection on completion of antimicrobial therapy, normalised inflammatory markers and no relapses in the next 12 months. Where the symptoms and inflammatory markers did not resolve with the initial antibiotic regimen but went on to fully recover after a change in antibiotics, they were classified as: “initial failure of therapy”. The classification “failure of therapy” was applied when patients died due to infection or there was relapse of infection (recurrence of symptoms with microbiological evidence of the same organism within 12 months following completion of therapy). Antimicrobial therapy was considered “appropriate” if the causative organism was susceptible to chosen agent in vitro and was prescribed at the higher end of the dosing range.
Results
This study identified seventy-nine patients, over the age of 18 years, with HVO. Of these, ten patients (12.66 %) had a diagnosis of GNB HVO (Table 1). The average age of patients suffering from GNB HVO was 76.5 years (range 64–88 years) and there was a male to female ratio of 7:3. All patients were diagnosed, treated and followed up for at least 12 months within Leeds Teaching Hospital NHS Trust.
Table 1.
Overview of cases
| Patient no. | Sex/age | Symptoms on presentation | Predisposing condition | Site of infection | Diagnosis | Organism | Inflammatory markers on admission (white blood cells: WBC, C-reactive protein: CRP) | Surgery | Duration of admission (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F/76 | Back pain Fever Lower limb weakness |
Malignancy | L3/L4 | ≥2 +ve blood cultures | E. coli | WBC: 7.7 CRP: 78 |
No | 76 |
| 2 | M/81 | Lower limb weakness Fever |
Diabetes malignancy | L5/S1 | ≥2 +ve blood cultures | P. aeruginosa | WBC: 19.14 CRP: 183 |
No | 49 |
| 3 | M/68 | Back pain Lower limb weakness Fever Rigors Weight loss |
Malignancy Immunosuppresion |
L3/L4 | 1 +ve blood culture | H. influenzae | WBC: 5.38 CRP: 369 |
No | 23 |
| 4 | M/73 | Back pain Rigors |
Malignancy Immunosuppresion |
L5/S1 | Spinal biopsy + 1 +ve blood culture | P. aeruginosa | WBC: 8.5 CRP: 353 |
No | 12 |
| 5 | M/73 | Back pain Lower limb weakness |
Diabetes | L3/L4 | Spinal biopsy + ≥ 2 +ve blood cultures | K. pneumoniae | WBC: 9.0 CRP: 299 |
Yes | 156 |
| 6 | F/86 | Back pain Fever Rigors |
Malignancy | L3/L4 | Intraoperative sample | P. aeruginosa | WBC: 7.3 CRP: 147 |
Yes | 76 |
| 7 | M/78 | Back pain Fever Rigors Lower limb weakness |
Diabetes Malignancy Long-term uninary catheter |
T9/T10 | Intraoperative tissue | E. coli | WBC: 6.78 CRP: 149 |
Yes | 84 |
| 8 | M/78 | Back pain Fever Rigors Confusion Weight loss |
Malignancy EVAR |
T10/T11 & L4/5 | ≥2 +ve blood cultures | E. coli | WBC: 10.66 CRP: 121 |
No | 26 |
| 9 | M/88 | Back pain Fever Rigors Weight loss |
Long-term urinary catheter Malignancy |
T10/T11 | ≥2 +ve blood cultures | E. cloacae | WBC: 10.06 CRP: 227 |
No | 44 |
| 10 | F/64 | Back pain Fever |
Diabetes Pylonephritis 6/52 prior |
T8/T9 | Intraoperative sample | E. coli | WBC: 8.77 CRP: 9.1 |
Yes | 24 |
| Patient no. | Sex/age | Length of time before MRI performed (days) | Length of time to from admission first +ve micro sample (days) | Length of time from admission to directed antibiotics (days) | Length of time from MRI to directed antibiotics commenced (days) | Directed antibiotics and duration (weeks) | Outcome | Comment: Delay in directed antibiotics | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F/76 | 25 | 1 | 51 | 26 | Ciprofloxacin oral (10) | Resolved:initial failure of therapy | ||
| 2 | M/81 | 9 | 2 | 2 | −7* | Ciprofloxacin oral (8) | Resolved | ||
| 3 | M/68 | 11 | 1 | 3 | −8* | Amoxicillin IV (2) Ciprofloxacin oral (4) |
Resolved | ||
| 4 | M/73 | 28 | 0 | 6 | −22* | Ciprofloxacin oral (8) | Resolved | ||
| 5 | M/73 | 66 | 1 | 62 | −4* | Meropenum IV (7) | Resolved:initial failure of therapy | ||
| 6 | F/86 | 5 | 17 | 19 | 14 | Tazocin IV (5) and Ciprofloxacin PO (2) | Resolved | 12 day delay in surgery from positive MRI. Hence delay in microbiological diagnosis | |
| 7 | M/78 | 3 | 3 | 7 | 4 | Amox and Gent IV (2) and Amox PO (4) | Resolved | ||
| 8 | M/78 | 55 | 1 | 2 | 2 | IV Amox and Gent (2) patient SD after IV ceftriaxone (4) in the community | Resolved | ||
| 9 | M/88 | 4 | 12 | 19 | 15 | Meropenum IV (3) and Ciprofloxacin (LT) | Failure to therapy: Patient refused surgical intervention | Patient commenced on empirical treatment prior to blood cultures, hence delay in microbiological diagnosis | |
| 10 | F/64 | 6 days prior to admission | 3 | 189 | 195 | Meropenum (6) | Failure of therapy - Patient died |
Delay in microbiological diagnosis, after initial empirical treatment |
* already commenced on definitive antibiotics before MRI
Of the ten patients diagnosed with GNB HVO all patients had a condition that predisposed to the infection. Four patients had more than one such condition, including diabetes mellitus (n = 4), underlying malignancy (n = 8), immunosuppression (n = 2), previous infection (outside the spine) with same organism during the previous year (n = 1) and permanent indwelling catheter (n = 2).
Clinical presentation
All but one patient had localised back pain corresponding to their site of infection. Five (50 %) patients presented with weakness of their lower limbs, eight (80 %) patients had a fever at presentation and two (20 %) reported weight loss. No patients presented with upper limb weakness or loss of bladder or bowel control. The majority of patients (six) had symptoms for 3 weeks or more.
Laboratory results and microbiology
Of the ten patients diagnosed with GNB HVO the following organisms were isolated; Escherichia coli (4), Pseudomonas aeruginosa (3), Klebsiella pneumoniae (1), Haemophilus influenzae (1) and Enterobacter cloacae (1). Four patients had the causative organism isolated on ≥2 positive blood cultures; two from CT guided spinal biopsy and ≥1 positive blood culture; three from specimens of spinal tissue taken during operation (one of which also had positive blood cultures) and one was diagnosed from a single positive blood culture.
Surgical treatment
Four patients required surgical intervention in the form of decompressive surgery with an instrumented fusion. All had intraoperative samples sent for microbiological analysis. None of the patients required any further surgery. Hence, the primary surgery was the definitive treatment.
Antibiotic treatment
Six (60 %) patients were started on empirical therapy (i.e. prior to identification of the causative organism) and one patient was already on oral antibiotics at the time of presentation. Empirical treatment included Cefuroxime (n = 2), Piperacillin/Tazobactam (n = 2), Meropenum (n = 1) and Co-amoxiclav (n = 1). Initial empirical therapy was “appropriate” (according to study definition) in all of these patients. The empirical regimen was modified in all patients when the diagnosis of HVO was made, and the average length of time it took from microbiological diagnosis to commencing ‘definitive’ antibiotics in all patients was 36 days. The modified regimen (i.e. once a diagnosis of HVO had been made and the causative organism identified) therapy was curative in six cases and failed in four cases. However, in two of these ‘failed’ cases, curative treatment was established following an additional alteration change in antibiotic regime and after a year of follow up the patients did not relapse.
Seven (70 %) of the causative GNB were resistant to Amoxicillin; five (50 %) were resistant to Cefuroxime and two (20 %) were resistant to Ciprofloxacin. Seven (70 %) of the isolates were sensitive to the 3rd generation cephalosporin ceftazidime.
The most successful treatment regime involved the use of Ciprofloxacin. Of the eight patients whose HVO was cured, five had Ciprofloxacin as part of their definitive antibiotic regime. Three received Ciprofloxacin monotherapy while two patients received Ciprofloxacin in combination with another antimicrobial. Numerous changes to antibiotics were often made, with the involvement of the microbiology team. The mean number of antibiotics used was four (range 2–7).
Complications of antibiotic treatment
One patient developed a Hickman line infection during their inpatient treatment. This resolved with removal and re-siting of the device. The same patient developed a Clostridium difficile infection 4 weeks after admission and following initial unsuccessful treatment of the spinal infection with Cefuroxime.
In one patient, resistance to Ciprofloxacin developed when new specimens were cultured following a relapse. This followed a 6-week course of oral Ciprofloxacin. One patient also developed nephrotoxicity from gentamicin, which was promptly stopped, with subsequent improvement in renal function.
Time taken until microbiological diagnosis and treatment with appropriate antibiotics
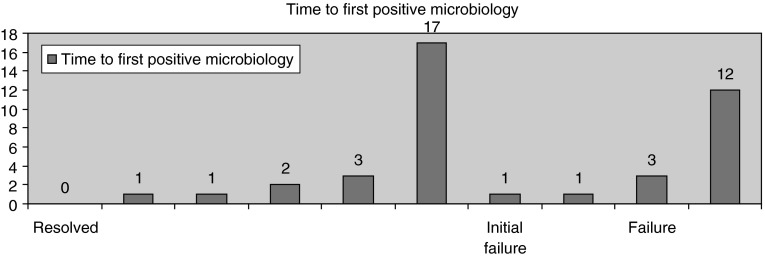
The mean duration of time taken to achieve a correct tissue or blood microbiological diagnosis was 4 days (from admission to first positive microbiology). Figure 1 demonstrates that of the six patients who had an initial successful antibiotic regime, five patients had a positive microbiological diagnosis within 0–3 days.
Fig. 1.
Bar chart showing the outcome versus the time to first positive microbiology, in each of the 10 patients in the case series
In contrast, if the two failed regimes were excluded, the mean time taken from admission to appropriate commencement of successful antibiotic treatment regimen was 19 days (range 2–62 days).
Outcome
Resolved
Of the ten patients identified with GNB HVO, eight patients were deemed cured at 12 months follow up. Of these eight, six were solely treated with antibiotics and two received both surgery and antibiotics. Two of these eight patients had initial failure of therapy and under went a revision of their initial antibiotic regime. Following this change in antibiotics their condition had resolved at 12 months.
Failure of therapy
Two patients were deemed to have a failure of therapy within or at 12 months. Of these, one was due to unsuccessful primary treatment. This patient developed signs and symptoms of VO 6 months following an E. coli sepsis from a urinary tract infection. At the time of initial presentation a spinal biopsy was preformed and an Acinetobacter species isolated. This was thought to be a contaminant and the patient was treated empirically with oral Amoxicillin and Ciprofloxacin for 6-weeks. However blood cultures and urine taken during the initial inpatient episode with a urinary tract infection were both resistant to Amoxicillin, hence treatment with this antibiotic was inappropriate. The patient re-presented 6 months later with acute spinal cord compression and required emergency surgery. Tissue sample taken at the time of surgery cultured an E. coli with was resistant to Amoxicillin and Ciprofloxacin. The patient was commenced on Meropenem as per sensitivities some 195 days after initial presentation, but later died.
The remaining patient was diagnosed with discitis, but declined an operative intervention. They were receiving palliative treatment for an underlying malignancy and refused operative intervention. The patient was discharged with oral antibiotics and subsequently died while in community care.
Discussion
Osteomyelitis is a relatively common infection, but a surprisingly small number of comparative trials about its treatment have been published. Moreover, most of the studies involve relatively few patients and are not randomized [9]. The available literature published solely on VO is even less, particularly concerning gram-negative infection. Therefore, our cohort of ten patients represents one of the largest, single centre studies of GNB HVO in current literature. Osenbach et al. [10] only identified seven patients with GNB HVO over a 7-year period.
Given the Leeds population of 700,000, the prevalence of GNB is around 1.4/100 000. Research has previously demonstrated that HVO usually affects older men which are consistent with our findings, with a mean age at diagnosis of 76.5 years and a male preponderance (M7:F3) [3]. The most common site of infection was the lumbar spine (n = 6) followed by the thoracic spine (n = 3). Consistent with previous reviews of HVO, the most common presenting symptoms were back pain (90 %) and fever (80 %) [10, 11].
In recent years there has been an increase in the incidence of spinal infections [3, 12, 13], this has been attributed to an increasing proportion of individuals with predisposing factors, such as underlying malignancy, diabetes and immune deficiency [3, 7, 14]. All patients identified in this study had been diagnosed with one or more of the above-mentioned conditions. This signifies the importance of thorough history taking in patients presenting with signs and symptoms of HVO.
Beronius et al. reported duration ranging between 2 weeks and 9 months from the onset of symptoms and diagnosis of VO, whereas other researches have demonstrated a delay of between 2 and 4 months [15–17]. Our study demonstrates a similar pattern and emphasises the fact that it is often difficult to establish an accurate diagnosis of HVO [18, 19]. Sixty percent of patients in this study had their symptoms for more than 3–4 weeks prior to presentation. Furthermore, following admission we observed a delay in MRI imaging (mean + 23 days) and hence diagnosis. However, most patients (n = 6) already had a positive microbiology result and had been commenced on antibiotic therapy. This highlights the importance of blood culture sampling before commencing empirical antibiotic therapy. Obtaining a microbiological diagnosis is imperative given the need for prolonged antibiotic treatment and the unpredictable resistance profiles of GNB. In this study 50 % of diagnoses were made solely on the basis of positive blood cultures. This is consistent with the previously positive blood cultures rate of 20–59 % [2].
Only three patients had both a raised white blood cell count and CRP above the ‘normal limits’, but ninety percent of patients had a significantly raised CRP at the time of presentation/investigation. As shown in studies by Carragee et al. and Osenbach et al. [10], we confirm that WBC measurement is not useful in the diagnosis of HVO because of a lack of specificity. In contrast, CRP does have a role in initial investigation of patients presenting with symptoms of HVO [2].
The causative micro-organisms isolated in this study were consistent with those in preceding research, with E. coli (n = 3) and P. aeruginosa (n = 3) being the most common [3, 10, [20]. GNB infections represent a minor proportion of all diagnosed cases of HVO, however, these infections are often more complicated, due to the virulence of the organisms, their increasing resistance to antimicrobials, lack of alternative treatments and associated co-morbidities of these patients [20]. Furthermore, there are no consensus guidelines for the treatment of GNB HVO. A second-generation cephalosporin is often used, as reported by Norden et al. [21] and Lew et al. [22] Although both of these studies were not solely assessing the management of VO. They concluded that oral Ciprofloxacin has been as effective and as safe as standard parenteral regimens, with success rates often greater than 80 % for both regimens [22, 23]. Eighty percent of the GNB infections isolated in our study were sensitive to Ciprofloxacin, and 70 % were sensitive to ceftazidime. Further research is required to investigate the epidemiology and susceptibility of GNB causing HVO.
Moreover, results demonstrate that Ciprofloxacin alone (n = 3) or in conjunction (n = 2) with another co-antibiotic was the most successful regime used in this study. The average duration of treatment was 8.6 weeks when used alone and 3 weeks when used in conjunction with another antibiotic, giving an overall average of 6.4 weeks (alone and in duel therapy). Indeed, of the eight patients whose discitis resolved, five received Ciprofloxacin as part of their ‘definitive’ antibiotic regime. The dosage, route, and duration of antibiotic therapy advocated by various investigators for the treatment of gram-positive and gram-negative osteomyeltis have been extremely contentious. Some authors advocated 6–8 weeks of parenteral therapy alone, while others proposed 6–8 weeks parenteral therapy followed by 2 months or more of oral therapy [10, 18, 24–27]. Our study suggests treatment with Ciprofloxacin alone or in conjunction with other antibiotics for 8 and 6 weeks, respectively, demonstrated the most successful form of antibiotic therapy ± surgical intervention.
The adult intervertebral disk is the largest avascular structure in the body. Therefore penetration of the tissue by antibiotics must be carefully considered when choosing an appropriate antibiotic regime. Both in vivo and in vitro [28, 29] research has been conducted to examine the bone penetration of many antibiotics, but due to the lack of standardisation between studies, results are not always comparable [30]. However, it has been suggested that clindamycin, fluoroquinolones, macrolides, rifampicin, fusidic acid, metronidazole and linezolid reach therapeutic levels in bone tissue [31]. Whereas, Beta-lactam antibiotics and glycopeptides achieve moderate levels and aminoglycosides diffuse poorly into the bone [31].
Within infected tissue there is a low vascularity in the necrotic bone. This leads to areas of poor penetration and low oxygen tension. This can then further compromise the activity of certain antibiotics, such as gentamicin and vancomycin [32, 33]. In contrast, Rifampicin and cephalosporins still appear to function well in this environment. With regard to Ciprofloxacin, research has shown that it has positively charged ionisable groups at a lower pH; hence there is increased penetration in the anaerobic infected vertebral disc [34].
Fluoroquinolones such as Ciprofloxacin are commonly used for the treatment of bone and joint infections, due to their activity against a broad spectrum of bacteria. Fluroquinolones have an excellent in vitro activity against both gram-positive and gram-negative bacteria [35]; including the treatment of gram-negative osteomyelitis [36]. The bactericidal action of Ciprofloxacin results from inhibition of topoisomerase IV and DNA gyrase, which are essential for bacterial DNA replication, transcription, repair and recombination. This results in rapid and concentration-dependent killing [37]. Furthermore, Fluoroquinolones have been shown to have excellent oral bioavailability and reach adequate bone concentrations to treat osteomyelitis with oral administration [38] therefore, making outpatient treatment more accessible, and potentially increasing compliance rates.
Another Fluoroquinolone which may be useful in the treatment of VO is Moxifloxacin. Moxifloxacin has been shown to be highly active against gram-positive microorganisms, anaerobes, and Mycobacterium tuberculosis [39–44]. Hence, Moxifloxacin may be particularly helpful in the treatment of tuberculosis spondylitis. Research has also demonstrated that oral Moxifloxacin has excellent bioavailability, with good bone and plasma concentrations [45].
We found that multiple antibiotic regimes were often used in the treatment of VO. However, this was because a definitive diagnosis was not initially found and patients were often commenced on a broad-spectrum antibiotic. All patients received more than one antibiotic during their treatment regime. Multiple antibiotics were often used, with one patient receiving seven different antibiotics during the course of their treatment. Given the current concerns regarding antibiotic resistance, treatment regimes need to be produced so to prevent the use of multiple antibiotics. Indeed even in this small sample, one patient became resistant to Ciprofloxacin at the time of spinal biopsy, following a 6-week course of oral Ciprofloxacin.
The addition of co-antibiotic regimes has been shown on occasions to aid the treatment of HVO. In our study patients treated with a co-antibiotic regime appeared to have a shortened length of treatment (6.25 weeks), when compared with single antibiotic regimes (7.8 weeks). This is excluding the patient who was treated with Meropenum and discharged on long term Ciprofloxacin treatment. Lazzarini et al. [9] demonstrated that Rifampicin has excellent oral bioavailability and a very good tissue penetration index. However, it is always used as a dual therapy regime, rather than monotherapy, due to the rapid development of resistance. Indeed, research has shown combination therapy with Fluoroquinolones to be effective in the treatment of bone infections [12].
Our study demonstrates that the treatment of GNB HVO is often very challenging, requiring multiple changes in antibiotic therapy and prolonged hospital stays (mean = 57 days). This study found that organisms are often isolated quickly (mean 4 days), yet the time taken for commencement of successful and appropriate antibiotic treatment is much longer, with a mean time of 19 days extrapolated from our data. In this study only four of the patients required a surgical intervention, thus signifying that in many case antibiotics alone are the most important form of treatment. Therefore, correct and timely prescription of antibiotics is the most important factor in the treatment of GNB HVO.
Limitation
One of the main limitation of this study is the fact that all patients were identified solely from MRI and CT scans coded as patients identified to have VO/disk space infection or the request card stated they may have VO/disk space infection. These were not cross-referenced with the medical records of patients coded in the medical records department with the discharge diagnosis of VO or disk-space infection. Therefore there is potential that some patients treated for GNB HVO in our institute were not included in the study.
Additionally, because only a small number of GNB were identified it is not possible to draw statistically significant conclusions. Therefore, our study group feel there is a need to perform a large case series study using a large number of patients so that our finding can be clarified using a large cohort of patients.
Conclusion
In summary, we report descriptive data from a retrospective cohort of GNB HVO over a 3-year period. The treatment of GNB HVO is often challenging because of unpredictable resistance patterns and limited published data on effective treatment regimens. In addition, these patients are often elderly with underlying medical co-morbidities. We highlighted the need for prompt microbiological sampling and hence an early appropriate antibiotic regime being initiated. Our results demonstrate that the most effective treatment for GNB HVO was with oral Ciprofloxacin over a period of 6–8 weeks, depending if used alone or in conjunction with another antibiotic.
Conflict of interest
The authors have no conflicts of interests.
Footnotes
S. M. Graham and A. Fishlock contributed equally to the paper.
References
- 1.Bhavan KP, Marschall J, Olsen MA, Fraser VJ, Wright NM, Warren DK. The epidemiology of hematogenous vertebral osteomyelitis: a cohort study in a tertiary care hospital. BMC Infect Dis. 2010;10:158. doi: 10.1186/1471-2334-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaramillo-de la Torre JJ, Bohinski RJ, Kuntz C 4th (2006) Vertebral osteomyelitis. Neurosurg Clin N Am 17(3) pp 339–51, vii [DOI] [PubMed]
- 3.Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79(6):874–880. doi: 10.2106/00004623-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Priest DH, Peacock JE., Jr Hematogenous vertebral osteomyelitis due to Staphylococcus aureus in the adult: clinical features and therapeutic outcomes. South Med J. 2005;98(9):854–862. doi: 10.1097/01.smj.0000168666.98129.33. [DOI] [PubMed] [Google Scholar]
- 5.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362(19):1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia A, Jr, Grantham SA. Hematogenous pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1960;42-A:429–436. [PubMed] [Google Scholar]
- 7.Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976) 2000;25(13):1668–1679. doi: 10.1097/00007632-200007010-00010. [DOI] [PubMed] [Google Scholar]
- 8.McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342–1350. doi: 10.1086/340102. [DOI] [PubMed] [Google Scholar]
- 9.Lazzarini L, Lipsky BA, Mader JT. Antibiotic treatment of osteomyelitis: what have we learned from 30 years of clinical trials? Int J Infect Dis. 2005;9(3):127–138. doi: 10.1016/j.ijid.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Osenbach RK, Hitchon PW, Menezes AH. Diagnosis and management of pyogenic vertebral osteomyelitis in adults. Surg Neurol. 1990;33(4):266–275. doi: 10.1016/0090-3019(90)90047-S. [DOI] [PubMed] [Google Scholar]
- 11.Chen WH, Jiang LS, Dai LY. Surgical treatment of pyogenic vertebral osteomyelitis with spinal instrumentation. Eur Spine J. 2007;16(9):1307–1316. doi: 10.1007/s00586-006-0251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cottle L, Riordan T. Infectious spondylodiscitis. J Infect. 2008;56(6):401–412. doi: 10.1016/j.jinf.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Tsiodras S, Falagas ME. Clinical assessment and medical treatment of spine infections. Clin Orthop Relat Res. 2006;444:38–50. doi: 10.1097/01.blo.0000203454.82264.cd. [DOI] [PubMed] [Google Scholar]
- 14.Krogsgaard MR, Wagn P, Bengtsson J. Epidemiology of acute vertebral osteomyelitis in Denmark: 137 cases in Denmark 1978–1982, compared to cases reported to the National Patient Register 1991–1993. Acta Orthop Scand. 1998;69(5):513–517. doi: 10.3109/17453679808997789. [DOI] [PubMed] [Google Scholar]
- 15.Bonfiglio M, Lange TA, Kim YM. Pyogenic vertebral osteomyelitis. Disk space infections. Clin Orthop Relat Res. 1973;96:234–247. doi: 10.1097/00003086-197310000-00033. [DOI] [PubMed] [Google Scholar]
- 16.Digby JM, Kersley JB. Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br. 1979;61(1):47–55. doi: 10.1302/0301-620X.61B1.370121. [DOI] [PubMed] [Google Scholar]
- 17.Jones NS, Anderson DJ. Stiles PJ Osteomyelitis in a general hospital. A five-year study showing an increase in subacute osteomyelitis. J Bone Joint Surg Br. 1987;69(5):779–783. doi: 10.1302/0301-620X.69B5.3680342. [DOI] [PubMed] [Google Scholar]
- 18.Beronius M, Bergman B, Andersson R. Vertebral osteomyelitis in Goteborg, Sweden: a retrospective study of patients during 1990–95. Scand J Infect Dis. 2001;33(7):527–532. doi: 10.1080/00365540110026566. [DOI] [PubMed] [Google Scholar]
- 19.Wisneski RJ. Infectious disease of the spine. Diagnostic and treatment considerations. Orthop Clin North Am. 1991;22(3):491–501. [PubMed] [Google Scholar]
- 20.Schurman DJ, Wheeler R. Gram negative bone and joint infection: sixty patients treated with amikacin. Clin Orthop Relat Res. 1978;134:268–274. [PubMed] [Google Scholar]
- 21.Norden CW, Shinners E. Ciprofloxacin as therapy for experimental osteomyelitis caused by Pseudomonas aeruginosa. J Infect Dis. 1985;151(2):291–294. doi: 10.1093/infdis/151.2.291. [DOI] [PubMed] [Google Scholar]
- 22.Lew DP, Waldvogel FA. Quinolones and osteomyelitis: state-of-the-art. Drugs. 1995;49(Suppl 2):100–111. doi: 10.2165/00003495-199500492-00016. [DOI] [PubMed] [Google Scholar]
- 23.Gentry LO, Rodriguez GG. Oral Ciprofloxacin compared with parenteral antibiotics in the treatment of osteomyelitis. Antimicrob Agents Chemother. 1990;34(1):40–43. doi: 10.1128/AAC.34.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carragee EJ. Instrumentation of the infected and unstable spine: a review of 17 cases from the thoracic and lumbar spine with pyogenic infections. J Spinal Disord. 1997;10(4):317–324. doi: 10.1097/00002517-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Dietze DD, Fessler RG, Jr, Jacob RP. Primary reconstruction for spinal infections. J Neurosurg. 1997;86(6):981–989. doi: 10.3171/jns.1997.86.6.0981. [DOI] [PubMed] [Google Scholar]
- 26.Dimar JR, Carreon LY, Glassman SD, Campbell MJ, Hartman MJ, Johnson JR. Treatment of pyogenic vertebral osteomyelitis with anterior debridement and fusion followed by delayed posterior spinal fusion. Spine (Phila Pa 1976) 2004;29(3):326–332. doi: 10.1097/01.BRS.0000109410.46538.74. [DOI] [PubMed] [Google Scholar]
- 27.Emery SE, Chan DP, Woodward HR. Treatment of hematogenous pyogenic vertebral osteomyelitis with anterior debridement and primary bone grafting. Spine (Phila Pa 1976) 1989;14(3):284–289. [PubMed] [Google Scholar]
- 28.Cunha BA, Gossling HR, Pasternak HS, Nightingale CH, Quintiliani R. The penetration characteristics of cefazolin, cephalothin and cephradine into bone in patients undergoing total hip replacement. J Bone Joint Surg Am. 1977;59(7):856–860. [PubMed] [Google Scholar]
- 29.Summersgill JT, Schupp LG, Raff MJ. Comparative penetration of metronidazole, clindamycin, chloramphenicol, cefoxitin, ticaricillin and moxalactam into bone. Antimicrob Agents Chemother. 1982;21(4):601–603. doi: 10.1128/AAC.21.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landersdorfer CB, Bulitta JB, Kinzig M, Holzgrabe U, Sorgel F. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin Pharmacokinet. 2009;48(2):89–124. doi: 10.2165/00003088-200948020-00002. [DOI] [PubMed] [Google Scholar]
- 31.Grados F, Lescure FX, Senneville E, Flipo RM, Schmit JL, Fardellone P. Suggestions for managing pyogenic (non-tuberculous) discitis in adults. Jt Bone Spine. 2007;74(2):133–139. doi: 10.1016/j.jbspin.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Norden CW, Shaffer M. Treatment of experimental chronic osteomyelitis due to Staphylococcus aureus with vancomycin and rifampicin. J Infect Dis. 1983;147(2):352–357. doi: 10.1093/infdis/147.2.352. [DOI] [PubMed] [Google Scholar]
- 33.Verklin RM, Mandell GL. Alteration of effectiveness of antibiotics by anaerobiosis. J Lab Clin Med. 1976;89(1):65–71. [PubMed] [Google Scholar]
- 34.Holm SH. Nutrition of the intervertebral disc. In: Weinstein JN, Wiesel SW, editors. The lumbar spine. Philadelphia: W. B. Saunders Company; 1990. pp. 244–260. [Google Scholar]
- 35.Stein GE. Pharmacokinetics and pharmacodynamics of newer fluoroquinolones. Clin Infect Dis. 1996;23(Suppl l):S9–S24. doi: 10.1093/clinids/23.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 36.Fraimow HS. Systemic antimicrobial therapy in osteomyelitis. Semin Plast Surg. 2009;23(2):90–99. doi: 10.1055/s-0029-1214161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKinnon PS, Davis SL. Pharmacokinetic and pharmacodynamic issues in the treatment of bacterial infectious diseases. Eur J Clin Microbiol Infect Dis. 2004;23(4):271–288. doi: 10.1007/s10096-004-1107-7. [DOI] [PubMed] [Google Scholar]
- 38.Rissing JP. Antimicrobial therapy for chronic osteomyelitis in adults: role of the quinolones. Clin Infect Dis. 1997;25(6):1327–1333. doi: 10.1086/516150. [DOI] [PubMed] [Google Scholar]
- 39.Aldridge KE, Ashcraft D. Comparison of the in vitro activities of BAY 12–8039, a new quinolone, and other antimicrobials against clinically important anaerobes. Antimicrob Agents Chemother. 1997;41(7):709–711. doi: 10.1128/aac.41.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brueggemann AB, Kugler KC, Doern GV. In vitro activity of BAY 12–8039, a novel 8-methoxyquinolone, compared to activities of six fluoroquinolones against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob Agents Chemother. 1997;41(7):1594–1597. doi: 10.1128/aac.41.7.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalhoff A, Petersen U, Endermann R. In vitro activity of BAY 12–8039, a new 8-methoxyquinolone. Chemotherapy. 1996;42(6):410–425. doi: 10.1159/000239474. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein EJC, Citron DM, Hudspeth M, Gerardo SH, Merriam CV. In vitro activity of Bay 12–8039, a new 8-methoxyquinolone, compared to the activities of 11 other oral antimicrobial agents against 390 aerobic and anaerobic bacteria isolated from human and animal bite wound skin and soft tissue infections in humans. Antimicrob Agents Chemother. 1997;41(7):1552–1557. doi: 10.1128/aac.41.7.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Visalli MA, Jacobs MR, Appelbaum PC. Activity of CP 99,219 (trovafloxacin) compared with Ciprofloxacin, sparfloxacin, clinafloxacin, lomefloxacin and Cefuroxime against ten penicillin-susceptible and penicillin-resistant pneumococci by time-kill methodology. J Antimicrob Chemother. 1996;37(1):77–84. doi: 10.1093/jac/37.1.77. [DOI] [PubMed] [Google Scholar]
- 44.Ji B, Lounis N, Maslo C, Truffot-Pernot C, Bonnafous P, Grosset J. In vitro and in vivo activities of moxifloxacin and clinafloxacin against mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42(8):2066–2069. doi: 10.1128/aac.42.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malincarne L, Ghebregzabher M, Moretti MV, Egidi AM, Canovari B, Tavolieri G, Francisci D, Cerulli G, Baldelli F. Penetration of moxifloxacin into bone in patients undergoing total knee arthroplasty. J Antimicrob Chemother. 2006;57(5):950–954. doi: 10.1093/jac/dkl091. [DOI] [PubMed] [Google Scholar]