Abstract
Purpose
Clinically, neuropathic pain is frequent and intense following brachial plexus injury. It is thought that brachial plexus pain is not generated by avulsed roots, but rather by non-avulsed roots, since the avulsed root could not possibly transmit action potentials to central nerves. The aim of this study was to evaluate pain behavior and activation of sensory neurons in a brachial plexus avulsion (BPA) model in rats.
Methods
Fifteen male Wistar rats were used. In the BPA group, the C8–T1 roots were avulsed from the spinal cord with forceps at the lower trunk level (n = 5). In the naïve group, rats did not receive any procedures (n = 5). In the sham-operated group, the lower trunk was simply exposed (n = 5). Mechanical hyperalgesia of forelimbs corresponding to C6 and C7 dermatomes was measured using von Frey filaments every third day for 3 weeks. Activation of DRG neurons was immunohistochemically examined using anti-ATF3 (a marker for neuron activation) antibodies 21 days after surgery. Von Frey and immunohistochemical data between groups were analyzed using a Kruskal–Wallis test, followed by Mann–Whitney U tests. Bonferroni corrections were performed.
Results
Animals in the BPA group displayed significant mechanical hyperalgesia at the dermatome innervated by uninjured nerves continuing through day 21 compared with animals in the sham-operated group. ATF3-immunoreactive small and large DRG neurons were significantly activated in the BPA group (10.6 ± 9.5 and 5.2 ± 4.1 %, 39.7 ± 6.7 and 25.2 ± 10.3 %, 78.0 ± 9.1 and 53.7 ± 29.3 %) compared with the sham-operated group (0.7 ± 0.9 and 0 ± 0 %, 2.8 ± 2.0 and 1.0 ± 2.0 %, 3.9 ± 2.7 and 8.6 ± 10.1 %) at every level of C5, 6, and 7. In the naïve group, no DRG neurons were activated. ATF3-immunoreactive small and large DRG neurons were significantly activated at the level of C7 compared with C6 and C5, and significantly activated at the level of C6 compared with C5 in the BPA group.
Conclusions
Expression of ATF3 in uninjured DRG neurons may contribute to pain following brachial plexus avulsion injury. Consequently, spared spinal sensory nerves may represent therapeutic targets for treatment of this pain.
Keywords: Brachial plexus avulsion, Pain, ATF3, Immunohistochemistry, Rat model
Introduction
Brachial plexus injury is a common event in humans caused by traction of spinal cord roots. It is generally caused by a motorcycle traffic accident or a fall. Individual or multiple roots are typically avulsed from the spinal cord and/or ruptured from C5 to T1. Patients display various symptoms depending on injury level and/or injury type, such as avulsion, postganglionic injury, and neurapraxia. Brachial plexus injury produces a characteristic constant crushing and intermittent shooting pain that is often intractable [1, 2]. This lesion may lead to important pathological changes responsible for increased pain sensations [3]. The main characteristics of brachial plexus injury are sensory paralysis, motor palsy, and the rapid onset of pain, which occurs immediately after the trauma [4, 5].
In almost 80 % of patients with complete brachial plexus palsy, at least one root is not avulsed [6, 7]. Hence, it is believed that brachial plexus pain is not generated by avulsed roots, but rather by non-avulsed roots. In fact, it has been reported that the injection of anesthetics close to the non-avulsed roots controls pain temporarily [8].
Rodrigues-Filho [9] reported that avulsion of the brachial plexus in the rat produces persistent mechanical allodynia and mechanical hyperalgesia, while Paszcuk [10] demonstrated that cannabinoid receptors in DRGs were increased after brachial plexus avulsion. However, no reports have evaluated DRGs compared with hyperalgesia rostral to the injury level. We hypothesize that sensory neurons rostral to the injury-level dorsal root ganglions are activated and transmit nociceptive signals in brachial plexus injury-associated pain. The aim of this study was to evaluate pain behavior and activation of sensory neurons in a BPA model in rats.
Materials and methods
All protocols for the animal procedures were approved by the ethics committees of our institutions following the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (1996 revision). A total of ten male Wistar rats weighing 170–200 g were used in this study. All animals were kept in cages with free access to food and water. They were maintained in a temperature-controlled room (22 ± 2 °C) under a 12 h light cycle (lights on 0600 hours).
Surgical procedure
We used the technique that Rodrigues-Filho [9] reported to model avulsion of the brachial plexus in the rat. Animals were anesthetized with sodium pentobarbital (40 mg/kg i.p.) and treated aseptically throughout the experiments. The right brachial plexus was approached through a horizontal incision parallel to the clavicle, running from the sternum to the axillary region in the BPA and sham groups. The pectoralis major muscle was displaced, leaving the cephalic vein intact. The subclavian vessels were located and the lower trunk of the right brachial plexus was isolated from surrounding tissues. In the BPA group, the right C8–T1 roots were avulsed from the spinal cord with forceps at the lower trunk level (n = 5). In the sham-operated group the lower trunk was simply exposed (n = 5). The cut tissue layers were then approximated and the skin closed with 4–0 nylon sutures. In the naïve group (n = 5), rats did not receive any procedures (n = 5).
Evaluation of tactile hyperalgesia
Mechanical withdrawal thresholds (von Frey)
We evaluated 15 rats for tactile hyperalgesia (BPA group, n = 5; sham group n = 5; naive group n = 5). There were no animal dropouts on any day. Mechanical thresholds of the right front paw were assessed using a series of von Frey filaments (Stoelting, Wood Dale, IL), ranging from 0.008 to 180 g before operation and at 3, 6, 9, 12, 15, 18, and 21 days after operation in the BPA and sham groups. In the naïve group, thresholds were assessed only at the first test. The von Frey filaments were applied to right front paws corresponding to the C6 and C7 dermatomes (area from the 1st digit to the 3rd digit) for five trials at approximately 5 min intervals [11]. Each probe was applied to the paw until it just bent, and was kept in this position for 6–8 s [12]. Filaments were applied in ascending order, and the smallest filament that elicited a foot withdrawal response was considered the threshold stimulus. The average threshold stimulus was calculated.
Immunohistochemistry for ATF3 (a marker of DRG neuron activation)
At 21 days after the operation, rats were anesthetized with sodium pentobarbital (40 mg/kg i.p.) and transcardially perfused with 0.9 % saline, followed by 500 ml of 4 % paraformaldehyde in phosphate buffer (0.1 M, pH 7.4). Next, dorsal root ganglia from C5 to C7 levels were resected and the specimens immersed in the same fixative solution overnight at 4 °C. After storage in 0.01 M phosphate-buffered saline (PBS) containing 20 % sucrose for 20 h at 4 °C, each ganglion was sectioned at 12 μm thickness on a cryostat and mounted on poly-l-lysine-coated slides. Endogenous tissue peroxidase activity was quenched by soaking the sections in 0.3 % hydrogen peroxide solution in 0.01 M PBS for 30 min. The specimens were then treated for 90 min at room temperature in blocking solution consisting of 0.01 M PBS containing 0.3 % Triton X-100 and 3 % skim milk. For staining of DRGs, the sections were processed for ATF3 immunohistochemistry using a rabbit antibody to ATF3 (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted in blocking solution and incubated for 20 h at 4 °C. Sections were incubated with goat anti-mouse and rabbit Alexa 488 fluorescent antibody conjugate for visualization (1:1,000; Molecular Probes, Eugene, OR, USA). After each step, the sections were rinsed three times in 0.01 M PBS.
Ten sections (two sections of each DRG) in each group were examined using a fluorescence microscope. The number of ATF3-immunoreactive (IR) neurons and total neurons were counted at 400× magnification using a counting grid. DRG neurons were classified as small (<700 µm2) and large (>700 µm2) according to the measured cross-sectional area [13, 14]. Positive or negative staining was blinded and observed by three orthopedic hand surgeons. If at least two of the observers were in agreement, their evaluation was used. These observers were blind to the classification of the groups. A percentage of ATF3-IR neurons were calculated and compared among groups.
Statistical analysis
Von Frey measurements and immunohistochemical data between groups were analyzed using a Kruskal–Wallis test, followed by Mann–Whitney U tests. Bonferroni corrections were performed.
Results
Effects of brachial plexus injuries on mechanical hyperalgesia
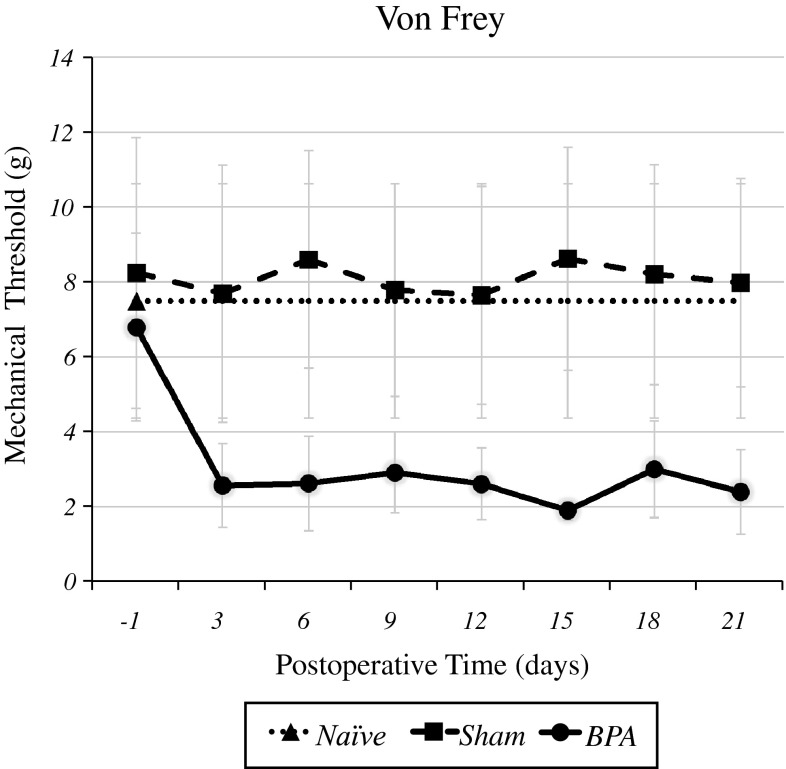
Figure 1 shows that there was no significant difference in the mechanical threshold between the naïve, sham-operated and pre-operated groups. BPA produced a marked decrease in the mechanical threshold at C6 and C7 dermatomes at all time points studied (3, 6, 9, 12, 15, 18, and 21 days) when compared with the sham and naïve groups. The statistical analysis of these data showed significant differences among the groups (p < 0.01).
Fig. 1.
Effects of brachial plexus injuries on mechanical hyperalgesia. BPA produced a marked decrease in the mechanical threshold at C6 and C7 dermatomes at all postoperative time points compared with the sham and naïve groups. Statistical analysis of these data showed significant differences between the groups (p < 0.01)
Immunohistochemistry (Figs. 2, 3)
Fig. 2.
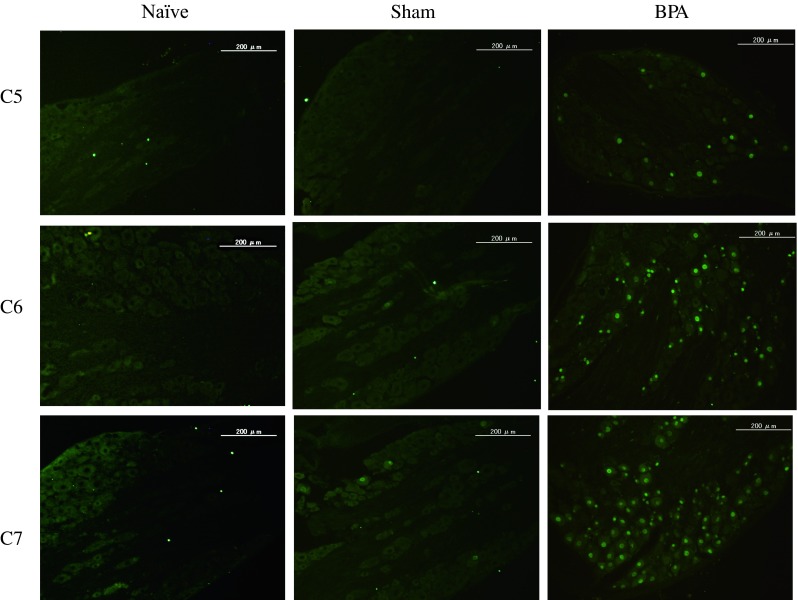
In the naïve group, no DRG small or large neurons expressed ATF3. In the sham-operated group, few DRG small and large neurons expressed ATF3. In the BPA group, many small and large neurons expressed ATF3
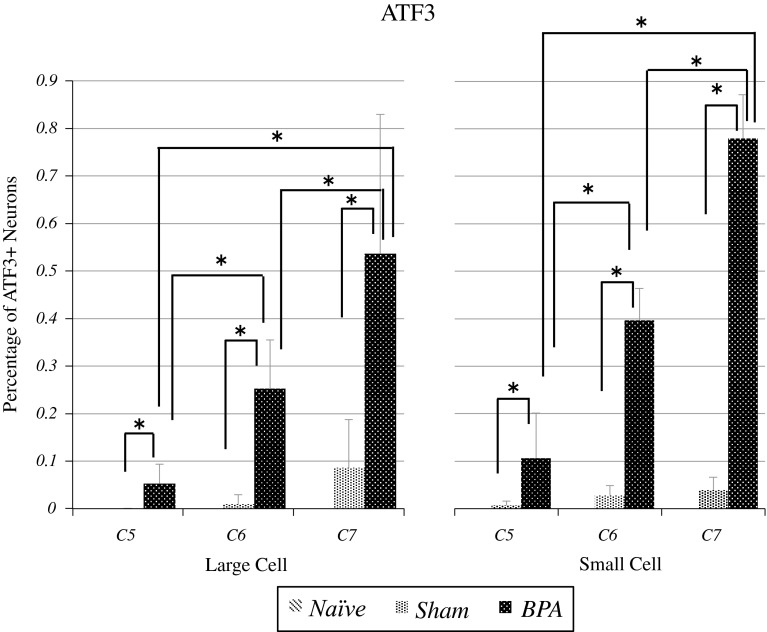
Fig. 3.
ATF3-immunoreactive small and large neurons were significantly elevated at the level of the C7 DRG compared with the C6 and C5 DRG (p < 0.01), and significantly elevated at the level of the C6 DRG compared with the C5 DRG in the BPA group (p < 0.01)
In the naïve group, no C5 DRG neurons were activated. In the sham-operated group, the percentage of C5 DRG small and large neurons expressing ATF3 was 0.7 ± 0.9 and 0 ± 0 %, respectively. In the BPA group, percentages were 10.6 ± 9.5 and 5.2 ± 4.1 %, respectively. There was a significant difference between sham and BPA groups (p < 0.01).
In the naïve group, no C6 DRG neurons were activated. In the sham-operated group, the percentage of C6 DRG small and large neurons expressing ATF3 was 2.8 ± 2.0 and 1.0 ± 2.0 %, respectively. In the BPA group, percentages were 39.7 ± 6.7 and 25.2 ± 10.3 %, respectively. There was a significant difference between sham and BPA groups (p < 0.01).
In the naïve group, no C7 DRG neurons were activated. In the sham-operated group, the percentage of C7 DRG small and large neurons expressing ATF3 was 3.9 ± 2.7 and 8.6 ± 10.1 %, respectively. In the BPA group, percentages were 78.0 ± 9.1 and 53.7 ± 29.3 %, respectively. There was a significant difference between sham and BPA groups (p < 0.01).
ATF3-immunoreactive small and large neurons were significantly activated at the level of C7 DRG compared with C6 and C5 DRG (p < 0.01), and were also significantly activated at the level of C6 DRG compared with C5 DRG in the BPA groups (p < 0.01).
Discussion
In this study, we report that mechanical hyperalgesia developed after BPA injury. This brachial plexus injury model engenders neuropathic pain at dermatomes corresponding to intact DRG neurons. Expression of ATF3-immunoreactive neurons was observed in non-injured DRG neurons.
Brachial plexus injury, a common event in humans caused by spinal cord root avulsion, produces a characteristic constant crushing and intermittent shooting pain that is often intractable [1, 2]. Ciaramitaro [15] reported that the incidence of neuropathic pain in peripheral nerve injury was 52 % and following root avulsion injury was 100 %.
Although the injured roots are not connected to the spine, patients nevertheless suffer from intolerable pain. It is thought that brachial plexus pain is not generated by avulsed roots, but rather by non-avulsed roots. In fact, it is reported that injection of anesthetics close to the non-avulsed roots controls pain temporarily [8].
Rodrigues-Filho reported that avulsion of the brachial plexus in rat produces persistent mechanical allodynia and mechanical hyperalgesia in the hind paw [9]. Paszcuk described the development of long-lasting mechanical allodynia in the hind paw of mice submitted to BPA until the 30th day after surgery [10]. However, the authors did not reveal that the DRG activation was relevant to hyperalgesia at levels rostral to the injury level. We demonstrated that sensory neurons in the dorsal root ganglions rostral to the injury level were activated and mechanical hyperalgesia occurred after BPA rostral to the injury level.
Several authors have reported studies of neuropathic pain models incorporating paw measures. The plantar surface of the rat forepaw is innervated by the C6–C8 spinal nerves and the rat hindpaw is innervated by the L3–L5 spinal nerves [10]. Regarding cervical nerve injury, C7/8 dorsal rhizotomy [19], C7 spinal nerve compression [20], spinal nerve ligation [20], and BPA [15] are all capable of contributing to neuropathic plantar pain in the rat. In these studies, injury-level DRG neurons were evaluated and were activated by nerve injury, but uninjured-level DRG neurons were not evaluated.
Among the three major neuropathic [10, 16, 17] hind paw plantar pain models in rats [18–20], the L5 and L6 spinal nerve ligation (SNL) model is unique because the uninjured L4 DRG neurons are clearly separated from the axotomized L5 and L6 DRG neurons. Thus, the activated L4 spinal nerve should be the main route through which the impulses evoked in the periphery are transferred to the spinal dorsal horn in this model [21]. Considering previous reports and the current study, we conclude that activation of intact spinal nerves transmits pain from the foot pad to the spinal dorsal horn in the current BPA injury model.
In the current study, we measured ATF3-positive DRG neurons in small and large neurons in a BPA model. ATF3 is a commonly used marker of DRG neuron activation, and it is also known that ATF3 regulates neurite outgrowth [22–27]. Lindwall reported that ATF3 is a c-Jun dimerization partner and that JNK-mediated c-Jun activation is associated with axonal outgrowth following axotomy of adult rat sensory neurons [25]. Seijffers demonstrated that ATF3 contributes to neurite outgrowth in injured neurons [26]. Woolf showed that the central terminals of primary afferents sprouting into lamina of the dorsal horn contribute to neuropathic pain [27]. ATF3 expression might not only represent a neural marker of injury, but may also functionally contribute to neuropathic pain.
The mechanisms associated with mechanical allodynia can include the sensitization of C fibers; however, recent evidence points to a major contribution of large-diameter myelinated Ab fibers. In previous studies [25], the expression of calcitonin gene-related peptide (CGRP) mRNA and preprotachykinin (PPT; a gene encoding substance P) mRNA increased in large DRG neurons (which do not transmit pain under physiological conditions) in rat lumbar neuropathic pain models.
Furthermore, after nerve injury-induced central sensitization of spinal cord neurons, large diameter, low-threshold Ab mechanoreceptors begin to sprout into lamina II of the dorsal horn and an area that normally receives only noxious information then receives input from non-noxious tactile stimuli, thus becoming capable of generating pain [26, 27]. Such sprouting of Ab fibers into lamina II of the dorsal horn has been clearly observed following sciatic axotomy, partial nerve ligation and, more recently, after sciatic chronic constriction injury [28–30]. It is proposed that Ab inputs can trigger withdrawal reflexes to previously innocuous mechanical stimuli, presumably by activating sensitized pain-responsive neurons [31]. Spread of hyperalgesia is likely due to central sensitization of nociceptive neurons in the spinal cord by primary nociceptive afferent input (neurogenic hyperalgesia) [32]. The current data related to ATF3 activation of large DRG neurons may explain mechanical allodynia commonly seen following BPA in humans. However, further studies are needed to explore pathological mechanisms related to BPA pain.
There are some limitations to this study. First, we did not evaluate contralateral (uninjured) DRGs in either the BPA or sham rats. Regarding previous investigations, although there are no reports describing changes in contralateral DRGs in the BPA model, Hatashita [33] reported contralateral DRG neuron and spinal glia activation following hemilateral spinal nerve injury in rats. It is possible that contralateral DRG neurons are activated by brachial plexus avulsion. Second, we did not evaluate neurons at all DRG levels. Therefore, we do not know how neurons were activated at all DRG levels.
Our results suggest that heightened expression of ATF3 in uninjured spinal nerves may contribute to neuropathic pain after BPA injury. The current findings suggest that uninjured nerves are therapeutic targets for pain relief following brachial plexus damage.
Conflict of interest
None.
References
- 1.Anand P, Birch R. Restoration of sensory function and lack of long-term chronic pain syndromes after brachial plexus injury in human. Brain. 2002;125:113–122. doi: 10.1093/brain/awf017. [DOI] [PubMed] [Google Scholar]
- 2.Berman JS, Birch R, Anand P. Pain following human brachial plexus injury with spinal cord root avulsion and the effect of surgery. Pain. 1998;75:199–207. doi: 10.1016/S0304-3959(97)00220-0. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho GA, Nikkhah G, Samii M. Pain management after post-traumatic brachial plexus lesions. Conservative and surgical therapy possibilities. Orthopede. 1997;26:621–625. doi: 10.1007/s001320050132. [DOI] [PubMed] [Google Scholar]
- 4.Parry CB. Pain in avulsion lesions of the brachial plexus. Pain. 1980;9:41–53. doi: 10.1016/0304-3959(80)90027-5. [DOI] [PubMed] [Google Scholar]
- 5.Parry CB. Brachial plexus injuries. Br J Hosp Med. 1984;32:130–139. [PubMed] [Google Scholar]
- 6.Alnot JY. Traumatic brachial plexus lesions in the adult. Indications and results. Hand Clin. 1995;4:623–631. [PubMed] [Google Scholar]
- 7.Berman J, Anand P, Chen L, Taggart M, Birch R. Pain relief from preganglionic injury to the brachial plexus by late intercostal nerve transfer. J Bone Jt Surg. 1996;78:759–760. [PubMed] [Google Scholar]
- 8.Bertelli JA, Ghizoni MF. Pain after avulsion injuries and complete palsy of the brachial plexus: the possible role of nonavulsed roots in pain generation. Neurosurgery. 2008;62:1104–1114. doi: 10.1227/01.neu.0000325872.37258.12. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues-Filho R, Santos AR, Bertelli JA, Calixto JB. Avulsion injury of the rat brachial plexus triggers hyperalgesia and allodynia in the hindpaws: a new model for the study of neuropathic pain. Brain Res. 2003;982:186–194. doi: 10.1016/S0006-8993(03)03007-5. [DOI] [PubMed] [Google Scholar]
- 10.Paszcuk AF, Dutra RC, da Silva KA, Quintão NL, Campos MM, Calixto JB. Cannabinoid agonists inhibit neuropathic pain induced by BPA in mice by affecting glial cells and MAP kinases. PLoS One. 2011;6:e24034. doi: 10.1371/journal.pone.0024034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi Y, Nakajima Y. Dermatomes in the rat limbs as determined bv antidromic stimulation of sensory C-fibers in spinal nerves. Pain. 1996;67:197–202. doi: 10.1016/0304-3959(96)03116-8. [DOI] [PubMed] [Google Scholar]
- 12.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 13.Novakovic SD, Tzoumaka E, McGivern JG, Haraguchi M, Sangameswaran L, Gogas KR, et al. Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. J Neurosci. 1998;18:2174–2187. doi: 10.1523/JNEUROSCI.18-06-02174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harper AA, Lawson SN. Conduction velocity is related to morphogical cell type in rat dorsal root ganglion neurones. J Physiol. 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciaramitaro P, Mondelli M, Logullo F, Grimaldi S, Battiston B, Sard A, et al. Traumatic peripheral nerve injuries: epidemiological findings, neuropathic pain and quality of life in 158 patients. J Peripher Nerv Syst. 2010;15:120–127. doi: 10.1111/j.1529-8027.2010.00260.x. [DOI] [PubMed] [Google Scholar]
- 16.Ramer LM, Borisoff JF, Ramer MS. Rho-Kinase Inhibition Enhances Axonal Plasticity and Attenuates Cold Hyperalgesia after Dorsal Rhizotomy. J Neurosci. 2004;24:10796–10805. doi: 10.1523/JNEUROSCI.3337-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang YW, Winkelstein BA. Schwann cell proliferation and macrophage infiltration are evident at day 14 after painful cervical nerve root compression in the rat. J Neurotrauma. 2011;28:2429–2438. doi: 10.1089/neu.2011.1918. [DOI] [PubMed] [Google Scholar]
- 18.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 19.Yi H, Kim MA, Back SK, Eun JS, Na HS. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Dorsi MJ, Meyer RA, Belzberg AJ. Mechanical hyperalgesia after an L5 spinal nerve lesion in the rat is not dependent on input from injured nerve fibers. Pain. 2000;85:493–502. doi: 10.1016/S0304-3959(00)00250-5. [DOI] [PubMed] [Google Scholar]
- 22.Lindwall C, Kanje M. The Janus role of c-Jun: cell death versus survival and regeneration of neonatal sympathetic and sensory neurons. Exp Neurol. 2005;196:184–194. doi: 10.1016/j.expneurol.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Seijffers R, Allchorne AJ, Woolf CJ. The transcription factor ATF-3 promotes neurite outgrowth. Mol Cell Neurosci. 2006;32:143–154. doi: 10.1016/j.mcn.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355(2):75–78. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- 25.Fukuoka T, Tokunaga A, Kondo E, Miki K, Tachibana T, Noguchi K. Change in mRNAs for neuropeptides and the GABA(A) receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain. 1998;78:13–26. doi: 10.1016/S0304-3959(98)00111-0. [DOI] [PubMed] [Google Scholar]
- 26.Koltzenburg M, Lundberg LE, Torebjörk HE. Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain. 1992;51:207–219. doi: 10.1016/0304-3959(92)90262-A. [DOI] [PubMed] [Google Scholar]
- 27.Vrinten DH, Kalkman CJ, Adan RA, Gispen WH. Neuropathic pain: a possible role for the melanocortin system? Eur J Pharmacol. 2001;429:61–69. doi: 10.1016/S0014-2999(01)01306-1. [DOI] [PubMed] [Google Scholar]
- 28.Lekan HA, Carlton SM, Coggeshall RE. Sprouting of Ab fibers into laminae II of the rat dorsal horn in peripheral neuropathy. Neurosci Lett. 1996;208:147–150. doi: 10.1016/0304-3940(96)12566-0. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura S, Myers RR. Myelinated afferents sprout into laminae II of L3–5 dorsal horn following chronic constriction nerve injury in rats. Brain Res. 1999;818:285–290. doi: 10.1016/S0006-8993(98)01291-8. [DOI] [PubMed] [Google Scholar]
- 30.Shortland P, Kinman E, Molander C. Sprouting of A-fiber primary afferents into laminae II in two rat models of neuropathic pain. . Eur J Pain. 1997;1:215–227. doi: 10.1016/S1090-3801(97)90107-5. [DOI] [PubMed] [Google Scholar]
- 31.Malmberg AB, Basbaum AI. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain. 1998;76:215–222. doi: 10.1016/S0304-3959(98)00045-1. [DOI] [PubMed] [Google Scholar]
- 32.Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-C. [DOI] [PubMed] [Google Scholar]
- 33.Hatashita S, Sekiguchi M, Kobayashi H, Konno S, Kikuchi S. Contralateral neuropathic pain and neuropathology in dorsal root ganglion and spinal cord following hemilateral nerve injury in rats. Spine. 2008;33:1344–1351. doi: 10.1097/BRS.0b013e3181733188. [DOI] [PubMed] [Google Scholar]