Abstract
Cholangiocarcinomas are the second most frequent primary hepatic malignancy, and make up from 5% to 30% of malignant hepatic tumours. Hilar cholangiocarcinoma (HCC) is the most common type, and accounts for approximately 60% to 67% of all cholangiocarcinoma cases. There is not a staging system that permits us to compare all series and extract some conclusions to increase the long-survival rate in this dismal disease. Neither the extension of resection, according to the sort of HCC, is a closed topic. Some authors defend limited resection (mesohepatectomy with S1, S1 plus S4b-S5, local excision for papillary tumours, etc.) while others insist in the compulsoriness of an extended hepatic resection with portal vein bifurcation removed to reach cure. As there is not an ideal adjuvant therapy, R1 resection can be justified to prolong the survival rate. Morbidity and mortality rates changed along the last decade, but variability is the rule, with morbidity and mortality rates ranging from 14% to 76% and from 0% to 19%, respectively. Conclusion: Surgical resection continues to be the main treatment of HCC. Negative resection margins achieved with major hepatic resections are associated with improved outcome. Preresectional management with biliary drainage, portal vein embolization and staging laparoscopy should be considered in selected patients. Additional evidence is needed to fully define the role of orthotopic liver transplant. Portal and lymph node involvement worsen the prognosis and long-term survival, and surgery is the only option that can lengthen it. Improvements in adjuvant therapy are essential for improving long-term outcome. Furthermore, the lack of effective chemotherapy drugs and radiotherapy approaches leads us to can consider R1 resection as an option, because operated patients have a longer survival rate than those who not undergo surgery.
Keywords: Cholangiocarcinoma, Klatskin tumor, Outcome, Pronostic factors, Survival rate
Core tip: Klatskin described the specific clinical characteristics in 1965, and the tumor is often referred to as Klatskin tumor. Cholangiocarcinomas (CC) are the second most frequent primary hepatic malignancy. Hilar cholangiocarcinoma (HCC) is the most common type, and accounts most of CC cases. These tumors are slowly growing, and have a tendency to local spread and infrequent distant metastases. The most common presentation is with the onset of jaundice. The majority of HCC are small infiltrating tumors. Long-term survival in patients with HCC depends critically on complete tumor resection. This work is an important update concerning outcome of surgical management in Klatskin tumors.
INTRODUCTION
Carcinomas arising from the confluence of the hepatic ducts were first described by Altemeier et al[1]. Klatskin[2] described the specific clinical characteristics in 1965, and the tumor is often referred to as Klatskin tumor. Cholangiocarcinomas (CC) are the second most frequent primary hepatic malignancy and make up from 5% to 30% of malignant hepatic tumors. Hilar cholangiocarcinoma (HCC) is the most common type, and accounts for approximately 60% to 67% of all CC cases (intrahepatic, hilar and distal)[3,4]. These tumors are slowly growing, and have a tendency to local spread and infrequent distant metastases. The most common presentation is with the onset of jaundice. The majority of HCC are small infiltrating tumors. Approximately 90% of malignant-appearing hilar strictures prove to be HCC[5].
Adenocarcinoma is the most common histologic subtype. Three morphologic subtypes of cholangiocarcinoma have been described: sclerosing (70%), nodular (20%), and papillary (5%)[6]. Characteristics of nodular and sclerosing types may coexist.
Long-term survival in patients with HCC depends critically on complete tumor resection. In the absence of widespread disease, the likelihood of achieving a complete resection requires examination of all factors related to local tumor extent, which increasingly has become possible with non invasive imaging studies[7,8]. Tumor location and extent within the biliary tree is only one component. Additional factors that must be addressed relate to radial tumor growth and its impact on adjacent structures, specifically portal venous involvement and consequent hepatic lobar atrophy. Perihilar CC’s are focused on because liver resection is required in most cases.
STAGING AND RESECTABILITY
The TNM staging system of the American Joint Committee on Cancer (AJCC) (Table 1) is the most commonly used for staging of HCC. However, this system is based on histological criteria and does not provide information on the potential for resectability. de Jong et al[9] conclude that the AJCC T-classification criteria did not stratify patients with regard to prognosis and that depth of tumor invasion is a better predictor of long-term outcome. Besides that, the histologic type of tumor may also modify the staging and type of surgery required[10].
Table 1.
Staging of perihilar cholangiocarcinoma (Green FL 2002)
| Tumor, nodes and metastases definitions |
| Primary tumor |
| Tis Carcinoma in situ |
| T1 Tumor confined to the bile duct histologically |
| T2 Tumor invades beyond the wall of the bile duct |
| T3 Tumor invades the liver, gallbladder, pancreas, and/or ipsilateral branches of the portal vein or hepatic artery |
| T4 Tumor invades any of the following: main portal vein or its branches bilaterally, common hepatic artery, or other adjacent structures, such as the colon, stomach, duodenum, or abdominal wall. |
| Regional lymph nodes |
| N0 No regional lymph node metastasis |
| N1 Regional lymph node metastases |
| Metastasis |
| M0 No distant metastasis |
| M1 Distant metastasis |
| Stage grouping |
| Stage 0 Tis, N0, M0 |
| Stage IA T1, N0, M0 |
| Stage IB T2, N0, M0 |
| Stage IIA T3, N0, M0 |
| Stage IIB T1, N1, M0 T2, N1, M0 T3, N1, M0 |
| Stage III T4, any N, M0 |
| Stage IV Any T, any N, M1 |
Therefore, other staging systems have been used to predict resectability and evaluate the extent of resection. The modified Bismuth-Corlette (B-C) classification stratifies patients according to the extent of biliary involvement by tumor[11-13]. Although it does not incorporate radial tumor extension, it provides a useful preoperative terminology to describe the extension of the hepatic resection that will be necessary to encompass the longitudinal intraductal extension of HCC.
The preoperative clinical T-staging system of the Memorial Sloan Kettering Cancer Centre (MSKCC) (Table 2), as proposed by Jarnagin and Blumgart (MSKCC), defines both the longitudinal and radial extension of HCC, which are critical factors in the determination of resectability[14,15]. This staging system incorporates three factors based on preoperative imaging studies: (1) location and extent of ductal involvement; (2) presence or absence of portal vein invasion; and (3) presence or absence of hepatic lobar atrophy. Criteria for unresectable disease include: locally advanced tumor extending to secondary biliary radicles bilaterally, unilateral sectional bile ducts with contralateral portal vein branch involvement, encasement or occlusion of the main portal vein proximal to its bifurcation, and atrophy of one hepatic lobe with contralateral tumor extension to sectional bile ducts. Of note, the right bile duct is shorter and therefore more likely to be involved when the tumor appears at the confluence. Patients who have distant metastases, including metastases to lymph node groups beyond the hepatoduodenal ligament are also unresectable. By incorporating these criteria of resectability, the MSKCC staging system has been shown to correlate with both surgical resectability and survival, but it still is not the ideal staging[14].
Table 2.
Memorial Sloan Kettering Cancer Centre stage
| Stage | Hilar involvement | Portal vein | Lobar atrophy |
| T1 | Biliary confluence ± 1/2 unilateral extension to second-order biliary radicles | No | No |
| T2 | Biliary confluence ± unilateral extension to second-order biliary radicles | + Ipsilateral | + Ipsilateral |
| Biliary confluence + bilateral extension to second-order biliary radicles | Yes/No | Yes/No | |
| Biliary confluence + unilateral extension to second-order biliary radicles | + Contralateral | Yes/No | |
| T3 | Biliary confluence + unilateral extension to second-order biliary radicles with contralateral hepatic lobar atrophy; | Yes/No | + Contralateral |
| Biliary confluence + unilateral/bilateral | Bilateral | Yes/No |
Consequently, the staging systems are not uniform and the prognostic factors that can be obtained do not allow a rigorous comparison between series. Furthermore, many series extend over a prolonged period, frequently longer than 20 years. Indeed, these reports lack a uniform approach to diagnosis, assessment of disease extent and resection, and the evaluation of the results is hence complicated. Also, most studies come from surgical departments and tend to appraise the operating findings and their results, whereas they do not contribute data of all the valued patients, which makes drawing conclusions difficult.
Characteristics of the growth pattern of HCC include: transmural invasion of bile ducts, radial extension into periductal tissue and adjacent structures, and longitudinal extension along the bile ducts in the submucosa[16]. The papillary phenotype is associated with better prognosis[17]. In contrast, longitudinal spread along the duct wall with microscopic submucosal extension is characteristic of mass-forming and periductal-infiltrating subtypes; this biologic feature often impedes obtaining histologically negative margins[18]. These tumors are often accompanied by both direct and lymphatic invasion into the periductal tissues, causing marked fibrosis and infiltration of inflammatory cells. These histologic changes give a macroscopic similarity between the tumor and peritumoral inflammatory changes that make preoperative and intraoperative biopsies diagnostically challenging. Radial extension of HCC is also common, often resulting in invasion of the portal vein, hepatic arteries and the hepatic parenchyma adjacent to the hilar plate.
When analysing survival according to staging, Li et al[19] in their audit of 215 patients found that the results from univariate analyses suggest that histological grade, lymph node metastasis, vascular invasion, neuroinvasion, R1 resection and T2 or T3 stage were significant predictors for poor survival rates; by multivariate analysis, only lymph node metastasis and R1 resection were significantly associated with poor survival rates.
Series with more than 100 cases in consulted literature are scarce, and those ones that fulfil this condition cover a prolonged period of time and are retrospective. The resectability rates were highly variable, ranging between 28% and 95%, and curative resection rates ranged between 14% and 95%[4,14,15,17,20-42]. Such wide variability of resectability is probably due to heterogeneous methods of patient selection, differences in preoperative imaging techniques, and the broad range of data for inclusion in these studies. The report of DeOliveira et al[43] where 282 HCC patients are assessed, is one of the biggest published series of only one institution, together with that one of Nagoya group, but it covers a 31-year period and is retrospective[44]. Apart from the changes in management over the course of a long period of time, as can be seen in Figure 1, the resectability rate in that study was 62% and R0 resection was achieved only in 19% of cases[43].
Figure 1.
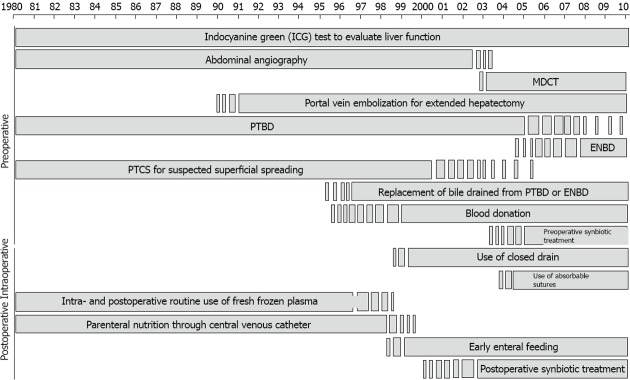
Changes in pre-, intra-, and postoperative management over the course of the study period (With permission. Courtesy of Professor Nimura). ENBD: Indicates endoscopic naso-biliary drainage; MDCT: Multidetector-row computed tomography; PTBD: Percutaneous transhepatic biliary drainage; PTCS: Percutaneous transhepatic cholangioscopy.
Even in high-volume centres, the resectability rate is about 30% of all patients with HCC, with the operative mortality rate ranging from 0% to 15%. After curative resection, the 1-, 3- and 5-year survival rates range from 50% to 70%, 30% to 40%, and 10% to 40%, respectively (Figure 1)[14,20,43-48].
The major determinants of resectability include extent of vascular invasion, hepatic lobar atrophy, amount of hepatic parenchyma involved, and extent of spread within the biliary tree. Hepatic lobar atrophy with contralateral portal vein or hepatic artery encasement or contralateral tumor extension to secondary biliary radicles may preclude resection. Bilateral hepatic disease and presumed insufficient hepatic reserve preclude resection. Even with current imaging technology, accurate determination of tumor resectability pre-operatively may occur in as few as 60%-74% of patients[49,50]. Thus, a number of patients undergoing resection with curative intent will be left with a resultant R1 margin status.
ADJUVANT TREATMENT
Adjuvant therapy for CC has not been supported by clinical evidence. Recently, gemcitabine has been shown to be active, with response rates of 8%-60% and median survival of 6-16 mo. Therefore, further studies of gemcitabine and of 5-FU plus cisplatin are warranted. For HCC, Cheng et al[51] reported better survival for patients with Bismuth types III/IV tumors who received adjuvant radiotherapy after curative resection. Todoroki et al[41] also showed a statistically significance of radiotherapy for R1 radical resection of stage IVa HCC. Thus, radiotherapy is potentially beneficial in patients with positive resection margins or unresectable tumors. However, Vern-Gross concluded that there is no benefit with adjuvant therapy in postoperative setting[52].
PREOPERATIVE BILIARY DRAINAGE
The role of preoperative biliary drainage (PBD) in jaundiced patients remains controversial[22,45,53,54]. Actually, most patients undergo biliary drainage prior to referral for resection, despite the lack of data showing a benefit. Clearly, the presence of cholangitis mandates biliary decompression, but there is no proof that routine biliary drainage in all patients facilitates resection or reduces postsurgical morbidity[55,56]. On the contrary, the available data would suggest that biliary stents are associated with greater postoperative infection complications[57,58]. Previous studies investigating this issue have been criticized for several design flaws, and whether major hepatic resection in the face of biliary obstruction is associated with a greater risk of liver failure or other complications remains an open question[59].
Cherqui et al[53] reported the results of major hepatobiliary resection without PBD in 20 patients with biliary cancer. Postoperative liver failure rate was 5%, and mortality was documented in the same patients.
PBD is associated with an increased risk of cholangitis and prolonged postoperative hospital stay, and can impede the ability to determine the extent of tumor during surgery. Cholangitis after PBD has been reported in 20%-60% of cases and may compromise subsequent surgery with patient dropout. Intraoperative bile cultures have been found to be positive in 65% of patients with PBD, while the rate was 8% in patients without PBD. This may be associated with increased postoperative infections such as wound or intraperitoneal abscesses[60].
However, unrelieved biliary obstruction is associated with hepatic and renal dysfunction and coagulopathy. Most patients with HCC will benefit from PBD of remnant liver to increase post-resection hypertrophy ability. Reported complications in transhepatic percutaneous catheter placement include: haemobilia, pseudoaneurysm of hepatic artery, fistula between hepatic artery and bile duct or between hepatic artery and portal vein, and catheter tract implantation metastases.
Some randomized controlled trials have revealed that biliary diversion does not improve perioperative results and increases infectious complications. But, also, none of these trials has managed to clarify the safety of major hepatic reaction for cholestatic patients with HCC[53,54]. The report of Laurent et al[58] states some conditions to avoid PBD: onset of jaundice < 2-3 wk, total bilirubin < 200 μmol/L, functional remnant liver (FRL) > 40%, neither endoscopic retrograde cholagiopancreatography nor percutaneous transhepatic cholangiography, and no sepsis. Although the results are not modified for not to drain, in agreement with other authors, undrained patients have a higher postoperative morbidity rate and transfusion requirements, and both facts are important factors of tumor recurrence. Thus, it may depend on each group’s experience to determine whether to use PDB or not. It will be taken into consideration that, if the conditions described by Laurent et al[58] are not fulfilled, there will be more perioperative transfusion and morbidity if the patient is not drained, which could affect the overall survival and disease-free survival rate.
PORTAL VEIN EMBOLIZATION
Resection greater than 80% of total liver volume is associated with major complications and prolonged hospital stay in patients with normal liver function, and resection greater than 60% is associated with an increase of major complications, postoperative hepatic insufficiency and mortality in patients with impaired liver function due to chronic liver disease, chronic biliary obstruction or high-dose chemotherapy[61-64]. Preoperative portal vein embolization (PVE) was first described in 1986 and is currently used to increase FRL volume and function[65].
Randomized controlled trials and individual institutional series support the safety and efficiency of preoperative PVE[20,61,66-69]. A potential disadvantage of PVE is that it may be difficult to determine preoperatively whether a right or left hepatectomy will be required if the tumor is located centrally in the hilum. At present, there is no evidence to support the routine use of PVE for HCC, but PVE should be considered for potentially resectable patients with normal liver function when anticipated FRL is less than 20% of the total liver volume, or for patients with compromised liver function when anticipated FRL is less than 40% of the total liver volume. Most patients with HCC present with jaundice and are considered to have cholestasis-induced compromised liver function. There are not many data on the impact and real volume of PVE on FRL liver function increase, associated or not to biliary drainage. Only in the second period in which their series is divided do Cannon et al[70] use PVE in 9.1% of cases, which means 4.5% out of a total of 110 patients, and despite that use they achieve only 62% of R0 resections.
As a consequence, PVE must be assessed and chosen with precaution to avoid the frightening postoperative hepatic insufficiency, one of the main causes of mortality in these patients. Also, its application must be evaluated in accordance with a previous surgical plan, which, if uncertain, could lead us to use another type of tactic, such as associating liver partition and portal vein ligation for staged hepatectomy (ALPPS)[71].
LIVER RESECTION: HOW MUCH IS ENOUGH?
In the last 20 years the use of hepatic resection in patients with HCC has risen. The objective of all the techniques and of the tendency to major resection with or without resection of vessels is to obtain free resection margins. The 5-year survival rate in patients undergoing non-curative resection for HCC is below 10%[4]. The 5-year survival rate for operated patients is with curative intention 11%-41% (Figure 1). All scientific community agrees that surgical resection is the only potentially curative treatment for CC, but the disease is usually advanced at the time of diagnosis and mostly treated by chemoradiotherapy or palliative therapy, including biliary drainage or stenting. Resectability rates are low because of early infiltration of the tumor into adjacent structures such as hepatic artery, portal vein and caudate lobe. In patients treated with curative intent, an extended hemihepatectomy is often needed to achieve negative margins. Preoperative jaundice and extended procedures are important risk factors for postoperative complications[57].
The aims of surgery in HCC are: (1) to achieve macroscopic removal of the tumor; (2) to restore satisfactorily the bile flow to the gut; and (3) to minimize postoperative liver failure or death. At the beginning of last decade, resection was possible only in 20% of cases, and the operative mortality was 10%. The median survival was only 20 mo, but resected patients enjoyed a good quality of life[3,4]. Last decade saw an aggressive approach to HCC with an increasing use of major hepatic resections[5,14,20,27,43-46,66]. The resectability rate increased to 80% with the addition of hepatic resection to bile duct resection without increasing the postoperative death rate. Bismuth et al[13] and Pichlmayr et al[72] suggested a stagewise management strategy with the prime objective of achieving complete surgical resection of the tumor without leaving behind macroscopic residual disease. Patients with Bismuth typesI and II were treated by bile duct resection. For Bismuth stage IIIa/IIIb lesions, resection of the corresponding hemiliver was recommended. However, major hepatic resection is a formidable operation in patients with a cholestatic liver and carries a high complication rate, with a morbidity of up to 81% and mortality rates of between 6% and 10% in the most advanced centres.
Vascular encasement with or without biliary obstruction may result in segment or lobar atrophy. Long-standing biliary obstruction can cause moderate atrophy, whereas concomitant portal venous compromise usually produces rapid and severe atrophy of the involved segments[3]. Approximately 30% of patients subjected to surgical exploration show evidence of lobar atrophy[15]. It is one of the problems of the PVE, together with vascular involvement not detected before embolization.
The caudate lobe is frequently involved by either direct invasion or ductal extension. Caudate bile ducts can drain to both the right and left hepatic ducts; in fact, some series have identified microscopic tumor infiltration into the caudate lobe in nearly all patients with HCC[21]. In general, the primary drainage of the caudate lobe is into the left hepatic duct[73]. For this reason, it has been alleged the necessity to resect the caudate lobe in Bismuth type II from now on.
Ikeyama et al[10], in their audit of 54 consecutive type I and II HCC resected patients, concluded that for nodular and infiltrating tumors right hepatectomy is essential; for papillary tumors, bile duct resection with or without limited hepatectomy is adequate. But the problem is that it is very difficult to know these issues preoperatively and intraoperatively. Nuzzo et al[74] reached the same conclusion in their audit of 440 patients, showing that pathologic factors independently predicted overall and disease-free survival at multivariate analysis.
Major hepatic resections have increased the proportion of R0 resections[4,14,29,37], improved the outcome of disease-free survival, and decreased the prevalence of hepatic recurrence[75]. Surgical results improved in the 1990s thanks to a better ability to perform R0 resections, which is likely due to increasing use of major hepatic resection and portal resections, as well as the improvement of preoperative management concerning both prognosis and FRL preparation and care[44,75]. Recent studies have also reported an improvement in morbidity and mortality in comparison with previous decades, which probably responds to advances in overall perioperative care. Also, the improvement of preoperative management has had a consequence, as can be seen in the report of Nagino et al[44].
Nonetheless, it is uncertain whether the major hepatic resection may improve the survival of patients with B-C typesI or II HCC. Ikeyama et al[10] and Jang et al[76] showed survival benefit in right hepatectomy with caudate lobectomy for nodular and sclerosing tumors, but not for papillary ones. However, others have reported a non-significant difference between hepatectomy and isolated bile duct resection in B-C typesI and II tumors[77].
Regarding proximal margin, it can be stated nowadays that survival outcomes improve when bile duct resection is associated with hepatectomy, even in patients with B-C typesI and II tumors[14,26]. In the series published by Jarnagin et al[14] in 2001, the 5-year survival was 37% when a hepatic resection was performed (84% of R0 resections) and 0% when only a bile duct excision was performed (56% of R0 resections). The best results are obtained with a right hepatectomy, probably because this surgical technique facilitates en-bloc resection of the tumor and surrounding tissues and thereby increases radicality[26]. In the series of Neuhaus et al[24,77], the worst outcomes after hepatectomy with curative intent were obtained in patients undergoing left hepatectomy. Although Nimura defended the radical surgery of left-sided Klatskin tumors by performing a left trisectionectomy, this is characterized by high morbidity rates and by mortality rates superior to 10%[78,79]. The analysis of recurrence after R0 resection with hepatectomy shows a low frequency of local recurrence, but a high frequency of peritoneal seeding recurrence[26]. Then, manipulation of the tumor as well as biopsies may favour local recurrence, and this is the reason why some authors advise en-bloc resection including surrounding vessels, a “non-touch technique”, in order to avoid this cause of recurrence.
The hepatectomy must include the caudate lobe, since this is a frequent site of tumor recurrence when it is not included in the resection piece. However, as it happens with other “evidences” related to Klatskin tumor treatment, there are no controlled studies that support this recommendation[32,80]. Performing a perioperative biopsy of the biliary resection margin in the liver remnant is common practice for most surgeons.
In a recent report of Ribero et al[81], in the analysis of 82 cases, the group of patients who had primary R0 was compared with those patients who achieved a secondary R0 after an intraoperative additional resection, and also with the patients who were R1. The 1-, 3- and 5-year survival rates were similar in the groups with primary R0 and secondary R0, but different in R1 patients (5-year survival rate: 50%, 30.8% and 0% respectively). The authors concluded that an additional resection of a positive proximal bile duct margin, albeit associated with an increased risk of biliary fistula, offers a significant survival benefit and should be attempted whenever possible. But this Italian group does not re-operate on those patients who the pathologist changes to R1 resection in the postoperative study, and thus, although they only have 13 cases that underwent re-resection, they do not defend re-operations on patients when this occurs. However, it is necessary to take into consideration that frozen biopsy is often not concluding and that resection extension, when the biopsy is positive, is frequently impracticable[26]. This explains why perioperative biopsies in this location have low profitability. Furthermore, such resection of margin-positive proximal duct does improve survival even when a negative margin can be achieved with additional resection[82].
LYMPHATIC SPREAD
In addition to extension along the bile ducts, HCC often metastasizes via the lymphatics. Lymphatic metastases are found in 30% to 50% of patients undergoing resection[14,83,84]. Hilar and pericholedochal lymph nodes (LN) are the most commonly involved, followed by periportal, common hepatic, posterior pancreoticoduodenal, celiac and preaortic ones[85]. Metastasis in regional LN is an important prognostic factor that affects survival after the resection of an HCC[36]. Kitagawa et al[73] evaluated 110 patients that underwent resection for HCC with LN dissection, including both the regional and para-aortic ones, and found that 47% of patients had no involved LN, 35% had metastases in regional LN and 17% had metastases in regional and para-aortic LN. The 5-year survival was 30% for patients with negative LN, 15% for patients with metastases in regional LN and 12% for patients with metastases in regional and para-aortic LN. Other studies have reported a worse survival in patients with LN involvement beyond the hepatoduodenal ligament, with a 5-year survival rate ranging from 0% to 6%[42]. Consequently, routine LN dissection beyond hepatoduodenal ligament is not recommended. Patients with macroscopically involved LN beyond hepatoduodenal ligament are considered to have unresectable disease, even though some surgeons resect them if they find them intraoperatively.
Only one study has presented the number of affected LN as a variable than worsens survival[86].
VASCULAR RESECTION
Radial growth of the tumor may infiltrate the surrounding vessels. Right hepatic artery involvement is more frequent due to its proximity to the biliary bifurcation. Contralateral artery infiltration to the hepatic resection that is to be performed is a reason for contraindication of surgical treatment. Portal involvement is present in 20%-30% of R0 resections and its preoperative identification is achieved with a precision of 85%. In the experience of Nagoya University, in approximately one third of the patients whose portal vein is resected because of apparent infiltration, this is not histologically confirmed[66]. However, most of these patients had a tumor infiltration adjacent to the vein, and the margin would have been positive without vein resection. On the other hand, vascular resection was not associated with a significant increase of morbimortality. Anyhow, resection can improve survival in some patients when R0 resection is achieved.
Encasement or occlusion of the main portal vein or vessels supplying the hepatic remnant is considered a contraindication to surgery[14]. Recent reports have shown that en-bloc resection with vascular reconstruction can achieve negative margins with a 10% perioperative mortality in selected patients.
Portal vein resection and reconstruction has been carried out in HCC with conflictive results[24,87]. Although several retrospective series have not shown difference in operative mortality between the patients that underwent portal vein resection and those ones that did not[24], the impact of portal vein resection on long-term survival is less clear. Neuhaus et al[24] proposed portal vein resection as part of a “non-touch” resection of the tumor and surrounding tissue. Portal vein resection was identified as a positive independent prognostic factor in their multivariate analysis of patients undergoing R0 resections, when mortality within the first 60 d was excluded. Nevertheless, overall mortality within 60 d after portal vein resection was 17%, in comparison with 5% in patients without portal resection, and all the deaths occurred after non-curative resections. Other studies have reported similar or worse survival in patients undergoing portal vein en-bloc resection[22,75,88]. The role of routine portal vein resection (as stated by Neuhaus) is not likely to be clearly designed unless a randomized clinical trial is completed. However, Hemmings rejects the routine performance of this procedure and in 2012 the Nagoya group reported a 5-year survival rate of 40% in the last period of portal resection, but a morbidity of 57.3%[42-44].
Portal resection must be recommended whenever the tumor cannot be freed from it, since the microscopic invasion of the portal vein does not seem to influence on survival when a vascular resection is carried out, whereas the macroscopic invasion does have negative results on survival.
Nishio et al[89] concluded that although lymph node metastasis and macroscopic portal vein involvement were independent negative prognostic factors, the 5-year survival rate obtained in patients with portal vein resection or lymph node metastasis still was about 10% (Table 3). Even in patients with both cancer invasion of the portal vein and regional lymph node metastasis, or with para-aortic lymph node metastasis, curative resection resulted in significantly longer survival than the one found in unresected patients.
Table 3.
Prognostic factors and 5-year survival rate
| Hilar cholangiocarcinoma | No. of patients | Prognostic factors | Operative mortality(%) | 5-yr SV (%) |
| Jarnagin et al[14] | 80 | Margin, hepatectomy, differentiation | 10 | 27 |
| Seyama et al[20] | 58 | Lymph nodes | 0 | 40 |
| Dinant et al[27] | 99 | Margin, resection period, lymph nodes | 15 | 27 |
| DeOliveira et al[43] | 281 | Margin, lymph nodes | 5 | 10 |
| Rea et al[45] | 46 | Lymph nodes, tumor grade, bilirubin | 9 | 26 |
| Silva et al[46] | 45 | Tumor stage, margin | 9 | 11 |
| Witzigmann et al[47] | 60 | Residual tumor status, grading | 8 | 22 |
| Baton et al[48] | 59 | Chemotherapy, margin, lymph nodes | 5 | 20 |
| Wahab et al[55] | 243 | Margin, S1 resection, lymph nodes, grading | 7 | 16 |
| de Jong et al[90] | 305 | Lymph nodes, margin | 5 | 20 |
SV: Survival rate.
Some groups had 100% morbidity and mortality in arterial resections, although in arterial and portal combined resections mortality was 43%, and the overall percentage of positive margins was 32%[29]. de Jong et al[90] reported in a recent paper that combined hepatectomy, extrahepatic biliary duct resection and portal vein resection can offer long-term survival in some patients with advanced HCC, with 17.6% mortality rate and 28% 5-year survival rate.
Some authors recommend hepatectomy with simultaneous arterial and portal vein resection. They reach 66% of R0 resection with 2% mortality rate, 54% morbidity rate and 1-, 3-, and 5-year survival rates of 78.9%, 36.3%, and 30.3%, respectively, but these data are not reproducible[86].
Su et al[39], Miyazaki et al[87] and Muñoz et al[91] reported as a conclusion that, although both portal vein and hepatic artery resection are independent poor prognostic factors after curative operative resection for locally advanced HCC, portal vein resection is acceptable from an operative risk perspective and might improve the prognosis in the selected patients, but combined hepatic artery resection cannot be justified because the 3-year survival rate is 0%.
LIVER TRANSPLANT
Orthotopic liver transplant (OLT) is contraindicated in HCC because of disappointing long-term outcomes. However, a recent multi-institutional study in the United States, including 280 patients with earlier-stage tumors who received aggressive neoadjuvant chemoradiation, has reported that transplantation remarkably improves survival: the 1- and 5-year survival rates were 74% and 38%, respectively[92]. The Mayo Clinic protocol sets a strict selection of the patients candidates to liver transplant. Although the selection is highly rigorous and biased for patients with biologically favourable disease, the early results published by the Mayo group showed an 82% 5-year survival rate[93]. The histological analysis of resected pieces confirmed N0 and R0 state in all the patients. However, only 58% of the patients had histologically confirmed cancer.
Liver transplantation is currently done only in the setting of clinical trials. It offers the advantage of resection of all structures that may be involved by the tumor, including portal vein, bilateral hepatic ducts and atrophic hepatic lobes. Thus, total hepatectomy may permit R0 resection even in locally advanced tumors, which are beyond resection criteria. Efficacy of neoadjuvant therapy and transplantation is demonstrated by comparing results with the natural history of the disease. Untreated HCC has a 50%-70% mortality rate within 12 mo, which is much worse than 55% 5-year survival for patients who entered the Mayo Clinic protocol and 71% 5-year survival after transplantation[94,95].
The Cincinnati Transplant Tumor Registry reported 28% 5-year survival, with a tumor recurrence rate of 51%[88]. The Spanish liver transplant centres provided similar results, with 30% 5-year survival rate and 53% tumor recurrence rate, in 36 patients with unresectable, non-disseminated HCC[96]. As a consequence of such initial results and the limited availability of organs, HCC was perceived as a relative contraindication to OLT. Also, it is a well-known fact that 55% of HCC even in T2 stages have affected LN, which is one of the contraindications to transplant[97].
A further complication to transplants in HCC is that, as response to postoperative radiotherapy and chemotherapy is low both in R1 and recurrence, tumors must have a more favourable biological behaviour, and if sizes bigger than 2 cm are rejected for rescue with liver transplant, then very few patients can be candidates to be transplanted[52]. It is important to remember that, out of the 281 cases analysed by DeOliveira, 58% were > 2 cm and hilar involvement occurred in 28%[43].
Schüle et al[98] concluded that an acceptable survival rate could be achieved by transplantation for HCC with LN metastases as the only exclusion criterion, even if they use living donors. In this article, the authors got a 5-year survival rate of 50% in those patients with negative LN.
Nowadays, OLT cannot be considered as a standard therapy for HCC in patients with resectable disease, but it offers a potential option to patients with underlying primary sclerosing cholangitis. Additional studies are necessary to define the role of OLT in depth.
MORBIDITY AND MORTALITY
Due to the complex biliary and liver resections required to obtain complete tumor removal, the risks of perioperative morbidity and mortality are significant. Morbidity and mortality rates range from 14% to 76% and from 0% to 19%, respectively. Perioperative morbidity includes haemorrhage, biliary fistula, hepatic insufficiency and infectious complications. Among them, infectious complications are particularly common and account for 50% to 80% of all complications[14,42]. The postoperative liver failure and its morbidity have been joined with the extension of hepatic resection[23]. However, recent publications suggest a decrease in morbidity and mortality with the use of preoperative PVE, even in extended hepatectomies[33,42,61,69](Table 4).
Table 4.
Morbidity and mortality rate and R0 resections
| Ref. | Resections | R0(%) | Morbidity | Mortality | 5-yr Survival rate |
| Burke et al[3] | 30 | 83 | NA | 6 | 45 |
| Nakeeb et al[4] | 109 | 26 | 47 | 4 | 11 |
| Jarnagin et al[14] | 80 | 78 | 64 | 10 | 26 |
| Nimura et al[15] | 55 | 84 | 41 | 6 | 41 |
| Jarnagin et al[17] | 106 | 77 | 62 | 8 | NA |
| Seyama et al[20] | 87 | 64 | 43 | 0 | 40 |
| Kosuge et al[23] | 65 | 52 | 37 | 9 | 33 |
| Neuhaus et al[24] | 80 | 61 | 55 | 8 | 22 |
| Launois et al[25] | 131 | NA | NA | 19 | NA |
| Kondo et al[26] | 40 | 95 | 48 | 0 | NA |
| Dinant et al[27] | 99 | 31 | 66 | 15 | 27 |
| Gerhards et al[29] | 112 | 14 | 65 | 18 | NA |
| Hemming et al[32] | 53 | 80 | 40 | 9 | 35 |
| IJitsma et al[33] | 42 | 65 | 76 | 12 | 19 |
| Kawarada et al[34] | 65 | 64 | 28 | 2.3 | 26 |
| Klempnauer et al[35] | 151 | 77 | NA | 10 | 28 |
| Miyazaki et al[37] | 76 | 71 | 33 | 13 | 26 |
| Nimura et al[38] | 142 | 61 | 49 | 9 | 26 |
| Su et al[39] | 49 | 49 | 47 | 10 | 15 |
| Todoroki et al[41] | 101 | 14 | 14 | 4 | 28 |
| DeOliveira et al[43] | 281 | 62 | 60 | 5 | 30 |
| Nagino et al[44] | 574 | 76.5 | 43.1 | 4.7 | 32.5 |
| Rea et al[45] | 46 | 80 | 52 | 9 | 26 |
| Nuzzo et al[74] | 440 | 77.3 | 47.5 | 8.6 | 25.5 |
| Ito et al[85] | 38 | 63 | 32 | 0 | 33 |
| Kawasaki et al | 79 | 68 | 14 | 1.3 | 22 |
NA: Not available.
OUTCOME OF RESECTION
Published 5-year survival rates range from 25% to 40% in recent series, and, even, it has been reported that many clinical and histological factors have a positive impact on long-term outcome, including negative histologic margin status[99,100], concomitant hepatic resection[30], absence of nodal involvement[14,23,48,101], low TNM status[36], well-differentiated tumor grade[68], papillary tumor morphology[36,44,80], and lack of perineural invasion[23]. Complete resection with negative histologic margins is the only modifiable factor and, for that reason, the primary aim of surgical therapy. There is a close association between hepatic resection and negative margins[24,37,99]. The effect of R1 resection vs no resection on outcome has been object of discussion and analysis in surgical literature, with some recent studies that report improvement in survival after R1 resection in comparison with patients with unresectable disease[42].
Recurrence after resection occurs quite frequently, in up to 50%-75% of cases[10,22,76]. The median recurrence time ranges from 12 to 43 mo[10,22,42,76]. Prognostic factors for recurrence-free survival include histologic grade, T and N stages, and margin status[10,22,76,102]. Since patients with recurrent disease are not candidates for curative therapy, advances in adjuvant therapy are essential to improve long-term outcome. However, the effectiveness of radiotherapy and chemotherapy is still very limited. In the report of Cherqui et al[53], the authors concluded that adjuvant radiotherapy was not associated with an improvement in long-term overall survival in patients with resected HCC.
CONCLUSION
Surgical resection continues to be the main treatment of HCC. Negative resection margins enhanced by major hepatic resections are associated with improved outcome. Pre-resectional management with biliary drainage, PVE and staging laparoscopy should be considered in selected patients. Additional evidence is needed to fully define the role of OLT. Improvements in adjuvant therapy are essential for improving long-term outcome. Portal and node involvement worsens the prognosis and long-term survival, and surgery is the only option that can lengthen it. Furthermore, the lack of effective chemotherapy and radiotherapy treatments, at this moment, leads us to consider R1 resection as an option because these patients have a longer survival rate than patients who do not undergo resection.
Footnotes
P- Reviewers Koike N, Leardkamolkarn V, Kumar PD S- Editor Wen LL L- Editor A E- Editor Yan JL
References
- 1.Altemeier WA, GALL EA, ZINNINGER MM, HOXWORTH PI. Sclerosing carcinoma of the major intrahepatic bile ducts. AMA Arch Surg. 1957;75:450–460; discussion 460-461. doi: 10.1001/archsurg.1957.01280150140015. [DOI] [PubMed] [Google Scholar]
- 2.Klatskin G. Adenocarcinoma of the Hepatic Duct at Its Bifurcation Within the Porta Hepatis. An Unusual Tumor with Distinctive Clinical and Pathological Features. Am J Med. 1965;38:241–256. doi: 10.1016/0002-9343(65)90178-6. [DOI] [PubMed] [Google Scholar]
- 3.Burke EC, Jarnagin WR, Hochwald SN, Pisters PW, Fong Y, Blumgart LH. Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228:385–394. doi: 10.1097/00000658-199809000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473; discussion 473-475. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buc E, Lesurtel M, Belghiti J. Is preoperative histological diagnosis necessary before referral to major surgery for cholangiocarcinoma? HPB (Oxford) 2008;10:98–105. doi: 10.1080/13651820802014585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinbren K, Mutum SS. Pathological aspects of cholangiocarcinoma. J Pathol. 1983;139:217–238. doi: 10.1002/path.1711390210. [DOI] [PubMed] [Google Scholar]
- 7.Hann LE, Greatrex KV, Bach AM, Fong Y, Blumgart LH. Cholangiocarcinoma at the hepatic hilus: sonographic findings. AJR Am J Roentgenol. 1997;168:985–989. doi: 10.2214/ajr.168.4.9124155. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz LH, Coakley FV, Sun Y, Blumgart LH, Fong Y, Panicek DM. Neoplastic pancreaticobiliary duct obstruction: evaluation with breath-hold MR cholangiopancreatography. AJR Am J Roentgenol. 1998;170:1491–1495. doi: 10.2214/ajr.170.6.9609160. [DOI] [PubMed] [Google Scholar]
- 9.de Jong MC, Hong SM, Augustine MM, Goggins MG, Wolfgang CL, Hirose K, Schulick RD, Choti MA, Anders RA, Pawlik TM. Hilar cholangiocarcinoma: tumor depth as a predictor of outcome. Arch Surg. 2011;146:697–703. doi: 10.1001/archsurg.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeyama T, Nagino M, Oda K, Ebata T, Nishio H, Nimura Y. Surgical approach to bismuth Type I and II hilar cholangiocarcinomas: audit of 54 consecutive cases. Ann Surg. 2007;246:1052–1057. doi: 10.1097/SLA.0b013e318142d97e. [DOI] [PubMed] [Google Scholar]
- 11.Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170–178. [PubMed] [Google Scholar]
- 12.Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg. 1988;12:39–47. doi: 10.1007/BF01658484. [DOI] [PubMed] [Google Scholar]
- 13.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31–38. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517; discussion 517-519. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nimura Y, Hayakawa N, Kamiya J, Kondo S, Shionoya S. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14:535–543; discussion 544. doi: 10.1007/BF01658686. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi S, Miyazaki M, Kondo Y, Nakajima N. Invasive growth patterns of hepatic hilar ductal carcinoma. A histologic analysis of 18 surgical cases. Cancer. 1994;73:2922–2929. doi: 10.1002/1097-0142(19940615)73:12<2922::aid-cncr2820731208>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 17.Jarnagin WR, Bowne W, Klimstra DS, Ben-Porat L, Roggin K, Cymes K, Fong Y, DeMatteo RP, D’Angelica M, Koea J, et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg. 2005;241:703–712; discussion 712-714. doi: 10.1097/01.sla.0000160817.94472.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimada H, Niimoto S, Matsuba A, Nakagawara G, Kobayashi M, Tsuchiya S. The infiltration of bile duct carcinoma along the bile duct wall. Int Surg. 1988;73:87–90. [PubMed] [Google Scholar]
- 19.Li H, Qin Y, Cui Y, Chen H, Hao X, Li Q. Analysis of the surgical outcome and prognostic factors for hilar cholangiocarcinoma: a Chinese experience. Dig Surg. 2011;28:226–231. doi: 10.1159/000327361. [DOI] [PubMed] [Google Scholar]
- 20.Seyama Y, Kubota K, Sano K, Noie T, Takayama T, Kosuge T, Makuuchi M. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238:73–83. doi: 10.1097/01.SLA.0000074960.55004.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizumoto R, Kawarada Y, Suzuki H. Surgical treatment of hilar carcinoma of the bile duct. Surg Gynecol Obstet. 1986;162:153–158. [PubMed] [Google Scholar]
- 22.Lai EC, Mok FP, Fan ST, Lo CM, Chu KM, Liu CL, Wong J. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994;81:1195–1198. doi: 10.1002/bjs.1800810839. [DOI] [PubMed] [Google Scholar]
- 23.Kosuge T, Yamamoto J, Shimada K, Yamasaki S, Makuuchi M. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230:663–671. doi: 10.1097/00000658-199911000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, Wex C, Lobeck H, Hintze R. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–818; discussion 819. doi: 10.1097/00000658-199912000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Launois B, Reding R, Lebeau G, Buard JL. Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers. J Hepatobiliary Pancreat Surg. 2000;7:128–134. doi: 10.1007/s005340050166. [DOI] [PubMed] [Google Scholar]
- 26.Kondo S, Hirano S, Ambo Y, Tanaka E, Okushiba S, Morikawa T, Katoh H. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg. 2004;240:95–101. doi: 10.1097/01.sla.0000129491.43855.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinant S, Gerhards MF, Rauws EA, Busch OR, Gouma DJ, van Gulik TM. Improved outcome of resection of hilar cholangiocarcinoma (Klatskin tumor) Ann Surg Oncol. 2006;13:872–880. doi: 10.1245/ASO.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 28.Gazzaniga GM, Filauro M, Bagarolo C, Mori L. Surgery for hilar cholangiocarcinoma: an Italian experience. J Hepatobiliary Pancreat Surg. 2000;7:122–127. doi: 10.1007/s005340050165. [DOI] [PubMed] [Google Scholar]
- 29.Gerhards MF, van Gulik TM, de Wit LT, Obertop H, Gouma DJ. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma--a single center experience. Surgery. 2000;127:395–404. doi: 10.1067/msy.2000.104250. [DOI] [PubMed] [Google Scholar]
- 30.Hadjis NS, Blenkharn JI, Alexander N, Benjamin IS, Blumgart LH. Outcome of radical surgery in hilar cholangiocarcinoma. Surgery. 1990;107:597–604. [PubMed] [Google Scholar]
- 31.Hasegawa S, Ikai I, Fujii H, Hatano E, Shimahara Y. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg. 2007;31:1256–1263. doi: 10.1007/s00268-007-9001-y. [DOI] [PubMed] [Google Scholar]
- 32.Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241:693–699; discussion 699-702. doi: 10.1097/01.sla.0000160701.38945.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.IJitsma AJ, Appeltans BM, de Jong KP, Porte RJ, Peeters PM, Slooff MJ. Extrahepatic bile duct resection in combination with liver resection for hilar cholangiocarcinoma: a report of 42 cases. J Gastrointest Surg. 2004;8:686–694. doi: 10.1016/j.gassur.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Kawarada Y, Das BC, Naganuma T, Tabata M, Taoka H. Surgical treatment of hilar bile duct carcinoma: experience with 25 consecutive hepatectomies. J Gastrointest Surg. 2002;6:617–624. doi: 10.1016/s1091-255x(01)00008-7. [DOI] [PubMed] [Google Scholar]
- 35.Klempnauer J, Ridder GJ, von Wasielewski R, Werner M, Weimann A, Pichlmayr R. Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J Clin Oncol. 1997;15:947–954. doi: 10.1200/JCO.1997.15.3.947. [DOI] [PubMed] [Google Scholar]
- 36.Lee SG, Lee YJ, Park KM, Hwang S, Min PC. One hundred and eleven liver resections for hilar bile duct cancer. J Hepatobiliary Pancreat Surg. 2000;7:135–141. doi: 10.1007/s005340050167. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki M, Ito H, Nakagawa K, Ambiru S, Shimizu H, Shimizu Y, Kato A, Nakamura S, Omoto H, Nakajima N, et al. Aggressive surgical approaches to hilar cholangiocarcinoma: hepatic or local resection? Surgery. 1998;123:131–136. [PubMed] [Google Scholar]
- 38.Nimura Y, Kamiya J, Kondo S, Nagino M, Uesaka K, Oda K, Sano T, Yamamoto H, Hayakawa N. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7:155–162. doi: 10.1007/s005340050170. [DOI] [PubMed] [Google Scholar]
- 39.Su CH, Tsay SH, Wu CC, Shyr YM, King KL, Lee CH, Lui WY, Liu TJ, P’eng FK. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223:384–394. doi: 10.1097/00000658-199604000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, Nagakawa T, Nakayama T. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685–1695. doi: 10.1002/cncr.10831. [DOI] [PubMed] [Google Scholar]
- 41.Todoroki T, Kawamoto T, Koike N, Takahashi H, Yoshida S, Kashiwagi H, Takada Y, Otsuka M, Fukao K. Radical resection of hilar bile duct carcinoma and predictors of survival. Br J Surg. 2000;87:306–313. doi: 10.1046/j.1365-2168.2000.01343.x. [DOI] [PubMed] [Google Scholar]
- 42.Hemming AW, Kim RD, Mekeel KL, Fujita S, Reed AI, Foley DP, Howard RJ. Portal vein resection for hilar cholangiocarcinoma. Am Surg. 2006;72:599–604; discussion 604-605. [PubMed] [Google Scholar]
- 43.DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258:129–140. doi: 10.1097/SLA.0b013e3182708b57. [DOI] [PubMed] [Google Scholar]
- 45.Rea DJ, Munoz-Juarez M, Farnell MB, Donohue JH, Que FG, Crownhart B, Larson D, Nagorney DM. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg. 2004;139:514–523; discussion 523-525. doi: 10.1001/archsurg.139.5.514. [DOI] [PubMed] [Google Scholar]
- 46.Silva MA, Tekin K, Aytekin F, Bramhall SR, Buckels JA, Mirza DF. Surgery for hilar cholangiocarcinoma; a 10 year experience of a tertiary referral centre in the UK. Eur J Surg Oncol. 2005;31:533–539. doi: 10.1016/j.ejso.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 47.Witzigmann H, Berr F, Ringel U, Caca K, Uhlmann D, Schoppmeyer K, Tannapfel A, Wittekind C, Mossner J, Hauss J, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg. 2006;244:230–239. doi: 10.1097/01.sla.0000217639.10331.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baton O, Azoulay D, Adam DV, Castaing D. Major hepatectomy for hilar cholangiocarcinoma type 3 and 4: prognostic factors and longterm outcomes. J Am Coll Surg. 2007;204:250–260. doi: 10.1016/j.jamcollsurg.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 49.Lee HY, Kim SH, Lee JM, Kim SW, Jang JY, Han JK, Choi BI. Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: combined CT and cholangiography with revised criteria. Radiology. 2006;239:113–121. doi: 10.1148/radiol.2383050419. [DOI] [PubMed] [Google Scholar]
- 50.Yin L, Song B, Xu J, Li Y, Yang Y, Chen X, Li Z, Changxian Li 1, Zhong K, Sun J. Hilar Cholangiocarcinoma: Diagnosis and Evaluation of Resectability with the Three-Dimensional Thin-section Contrast-enhanced Dynamic MR Imaging Sequence. Int J MRI. 2007;1:33–42. [Google Scholar]
- 51.Cheng Q, Luo X, Zhang B, Jiang X, Yi B, Wu M. Predictive factors for prognosis of hilar cholangiocarcinoma: postresection radiotherapy improves survival. Eur J Surg Oncol. 2007;33:202–207. doi: 10.1016/j.ejso.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 52.Vern-Gross TZ, Shivnani AT, Chen K, Lee CM, Tward JD, MacDonald OK, Crane CH, Talamonti MS, Munoz LL, Small W. Survival outcomes in resected extrahepatic cholangiocarcinoma: effect of adjuvant radiotherapy in a surveillance, epidemiology, and end results analysis. Int J Radiat Oncol Biol Phys. 2011;81:189–198. doi: 10.1016/j.ijrobp.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Cherqui D, Benoist S, Malassagne B, Humeres R, Rodriguez V, Fagniez PL. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch Surg. 2000;135:302–308. doi: 10.1001/archsurg.135.3.302. [DOI] [PubMed] [Google Scholar]
- 54.Figueras J, Llado L, Valls C, Serrano T, Ramos E, Fabregat J, Rafecas A, Torras J, Jaurrieta E. Changing strategies in diagnosis and management of hilar cholangiocarcinoma. Liver Transpl. 2000;6:786–794. doi: 10.1053/jlts.2000.18507. [DOI] [PubMed] [Google Scholar]
- 55.Wahab MA, Fathy O, Sultan AM, Salah T, Elshoubary M, Elyazid AYA, Anwar N, Sultan A. Hilar cholangiocarcinoma fifteen-year experience with 243 patients at a single Egyptian center. Journal of Solid Tumors. 2011;1:112–119. [Google Scholar]
- 56.Bortolasi L, Burgart LJ, Tsiotos GG, Luque-De León E, Sarr MG. Adenocarcinoma of the distal bile duct. A clinicopathologic outcome analysis after curative resection. Dig Surg. 2000;17:36–41. doi: 10.1159/000018798. [DOI] [PubMed] [Google Scholar]
- 57.Nimura Y. Preoperative biliary drainage before resection for cholangiocarcinoma (Pro) HPB (Oxford) 2008;10:130–133. doi: 10.1080/13651820801992666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laurent A, Tayar C, Cherqui D. Cholangiocarcinoma: preoperative biliary drainage (Con). HPB (Oxford. ) 2008;10:126–129. doi: 10.1080/13651820802007472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lieser MJ, Barry MK, Rowland C, Ilstrup DM, Nagorney DM. Surgical management of intrahepatic cholangiocarcinoma: a 31-year experience. J Hepatobiliary Pancreat Surg. 1998;5:41–47. doi: 10.1007/pl00009949. [DOI] [PubMed] [Google Scholar]
- 60.Seyama Y, Makuuchi M. Current surgical treatment for bile duct cancer. World J Gastroenterol. 2007;13:1505–1515. doi: 10.3748/wjg.v13.i10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hemming AW, Reed AI, Howard RJ, Fujita S, Hochwald SN, Caridi JG, Hawkins IF, Vauthey JN. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237:686–691; discussion 691-693. doi: 10.1097/01.SLA.0000065265.16728.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shoup M, Gonen M, D’Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, Tuorto S, Blumgart LH, Fong Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- 63.Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 64.Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, Sugimachi K. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304–309. doi: 10.1016/s1072-7515(98)00301-9. [DOI] [PubMed] [Google Scholar]
- 65.Ebata T, Nagino M, Kamiya J, Uesaka K, Nagasaka T, Nimura Y. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg. 2003;238:720–727. doi: 10.1097/01.sla.0000094437.68038.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364–372. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Denys A, Sauvanet A. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sano T, Shimada K, Sakamoto Y, Yamamoto J, Yamasaki S, Kosuge T. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006;244:240–247. doi: 10.1097/01.sla.0000217605.66519.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vauthey JN, Pawlik TM, Abdalla EK, Arens JF, Nemr RA, Wei SH, Kennamer DL, Ellis LM, Curley SA. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–730; discussion 730-732. doi: 10.1097/01.sla.0000124385.83887.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cannon RM, Brock G, Buell JF. Surgical resection for hilar cholangiocarcinoma: experience improves resectability. HPB (Oxford) 2012;14:142–149. doi: 10.1111/j.1477-2574.2011.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 72.Pichlmayr R, Weimann A, Klempnauer J, Oldhafer KJ, Maschek H, Tusch G, Ringe B. Surgical treatment in proximal bile duct cancer. A single-center experience. Ann Surg. 1996;224:628–638. doi: 10.1097/00000658-199611000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kitagawa Y, Nagino M, Kamiya J, Uesaka K, Sano T, Yamamoto H, Hayakawa N, Nimura Y. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233:385–392. doi: 10.1097/00000658-200103000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nuzzo G, Giuliante F, Ardito F, Giovannini I, Aldrighetti L, Belli G, Bresadola F, Calise F, Dalla Valle R, D’Amico DF, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012;147:26–34. doi: 10.1001/archsurg.2011.771. [DOI] [PubMed] [Google Scholar]
- 75.Havlik R, Sbisà E, Tullo A, Kelly MD, Mitry RR, Jiao LR, Mansour MR, Honda K, Habib NA. Results of resection for hilar cholangiocarcinoma with analysis of prognostic factors. Hepatogastroenterology. 2000;47:927–931. [PubMed] [Google Scholar]
- 76.Jang JY, Kim SW, Park DJ, Ahn YJ, Yoon YS, Choi MG, Suh KS, Lee KU, Park YH. Actual long-term outcome of extrahepatic bile duct cancer after surgical resection. Ann Surg. 2005;241:77–84. doi: 10.1097/01.sla.0000150166.94732.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Neuhaus P, Thelen A. Radical surgery for right-sided klatskin tumor. HPB (Oxford) 2008;10:171–173. doi: 10.1080/13651820801992708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nimura Y. Radical surgery of left-sided klatskin tumors. HPB (Oxford) 2008;10:168–170. doi: 10.1080/13651820801992674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ogura Y, Mizumoto R, Tabata M, Matsuda S, Kusuda T. Surgical treatment of carcinoma of the hepatic duct confluence: analysis of 55 resected carcinomas. World J Surg. 1993;17:85–92; discussion 92-93. doi: 10.1007/BF01655714. [DOI] [PubMed] [Google Scholar]
- 80.Clary B, Jarnigan W, Pitt H, Gores G, Busuttil R, Pappas T. Hilar cholangiocarcinoma. J Gastrointest Surg. 2004;8:298–302. doi: 10.1016/j.gassur.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 81.Ribero D, Amisano M, Lo Tesoriere R, Rosso S, Ferrero A, Capussotti L. Additional resection of an intraoperative margin-positive proximal bile duct improves survival in patients with hilar cholangiocarcinoma. Ann Surg. 2011;254:776–781; discussion 781-783. doi: 10.1097/SLA.0b013e3182368f85. [DOI] [PubMed] [Google Scholar]
- 82.Shingu Y, Ebata T, Nishio H, Igami T, Shimoyama Y, Nagino M. Clinical value of additional resection of a margin-positive proximal bile duct in hilar cholangiocarcinoma. Surgery. 2010;147:49–56. doi: 10.1016/j.surg.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 83.Verbeek PC, van Leeuwen DJ, de Wit LT, Reeders JW, Smits NJ, Bosma A, Huibregtse K, van der Heyde MN. Benign fibrosing disease at the hepatic confluence mimicking Klatskin tumors. Surgery. 1992;112:866–871. [PubMed] [Google Scholar]
- 84.Wetter LA, Ring EJ, Pellegrini CA, Way LW. Differential diagnosis of sclerosing cholangiocarcinomas of the common hepatic duct (Klatskin tumors) Am J Surg. 1991;161:57–62; discussion 62-63. doi: 10.1016/0002-9610(91)90361-g. [DOI] [PubMed] [Google Scholar]
- 85.Ito F, Agni R, Rettammel RJ, Been MJ, Cho CS, Mahvi DM, Rikkers LF, Weber SM. Resection of hilar cholangiocarcinoma: concomitant liver resection decreases hepatic recurrence. Ann Surg. 2008;248:273–279. doi: 10.1097/SLA.0b013e31817f2bfd. [DOI] [PubMed] [Google Scholar]
- 86.Nagino M, Nimura Y, Nishio H, Ebata T, Igami T, Matsushita M, Nishikimi N, Kamei Y. Hepatectomy with simultaneous resection of the portal vein and hepatic artery for advanced perihilar cholangiocarcinoma: an audit of 50 consecutive cases. Ann Surg. 2010;252:115–123. doi: 10.1097/SLA.0b013e3181e463a7. [DOI] [PubMed] [Google Scholar]
- 87.Miyazaki M, Kato A, Ito H, Kimura F, Shimizu H, Ohtsuka M, Yoshidome H, Yoshitomi H, Furukawa K, Nozawa S. Combined vascular resection in operative resection for hilar cholangiocarcinoma: does it work or not? Surgery. 2007;141:581–588. doi: 10.1016/j.surg.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 88.Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633–1637. doi: 10.1097/00007890-200004270-00019. [DOI] [PubMed] [Google Scholar]
- 89.Nishio H, Nagino M, Nimura Y. Surgical management of hilar cholangiocarcinoma: the Nagoya experience. HPB (Oxford) 2005;7:259–262. doi: 10.1080/13651820500373010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Jong MC, Marques H, Clary BM, Bauer TW, Marsh JW, Ribero D, Majno P, Hatzaras I, Walters DM, Barbas AS, et al. The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: a multi-institutional analysis of 305 cases. Cancer. 2012;118:4737–4747. doi: 10.1002/cncr.27492. [DOI] [PubMed] [Google Scholar]
- 91.Muñoz L, Roayaie S, Maman D, Fishbein T, Sheiner P, Emre S, Miller C, Schwartz ME. Hilar cholangiocarcinoma involving the portal vein bifurcation: long-term results after resection. J Hepatobiliary Pancreat Surg. 2002;9:237–241. doi: 10.1007/s005340200025. [DOI] [PubMed] [Google Scholar]
- 92.Becker NS, Rodriguez JA, Barshes NR, O’Mahony CA, Goss JA, Aloia TA. Outcomes analysis for 280 patients with cholangiocarcinoma treated with liver transplantation over an 18-year period. J Gastrointest Surg. 2008;12:117–122. doi: 10.1007/s11605-007-0335-4. [DOI] [PubMed] [Google Scholar]
- 93.Heimbach JK, Gores GJ, Haddock MG, Alberts SR, Pedersen R, Kremers W, Nyberg SL, Ishitani MB, Rosen CB. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. 2006;82:1703–1707. doi: 10.1097/01.tp.0000253551.43583.d1. [DOI] [PubMed] [Google Scholar]
- 94.Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts SR, Kremers WK, Gores GJ, Nagorney DM. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451–458; discussion 458-461. doi: 10.1097/01.sla.0000179678.13285.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosen CB, Heimbach JK, Gores GJ. Surgery for cholangiocarcinoma: the role of liver transplantation. HPB (Oxford) 2008;10:186–189. doi: 10.1080/13651820801992542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Robles R, Figueras J, Turrión VS, Margarit C, Moya A, Varo E, Calleja J, Valdivieso A, Valdecasas JC, López P, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265–271. doi: 10.1097/01.sla.0000108702.45715.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kurosaki I, Tsukada K, Hatakeyama K, Muto T. The mode of lymphatic spread in carcinoma of the bile duct. Am J Surg. 1996;172:239–243. doi: 10.1016/S0002-9610(96)00156-0. [DOI] [PubMed] [Google Scholar]
- 98.Schüle S, Altendorf-Hofmann A, Uteß F, Rauchfuß F, Freesmeyer M, Knösel T, Dittmar Y, Settmacher U. Liver transplantation for hilar cholangiocarcinoma--a single-centre experience. Langenbecks Arch Surg. 2013;398:71–77. doi: 10.1007/s00423-012-1007-8. [DOI] [PubMed] [Google Scholar]
- 99.Ito F, Cho CS, Rikkers LF, Weber SM. Hilar cholangiocarcinoma: current management. Ann Surg. 2009;250:210–218. doi: 10.1097/SLA.0b013e3181afe0ab. [DOI] [PubMed] [Google Scholar]
- 100.Kloek JJ, Ten Kate FJ, Busch OR, Gouma DJ, van Gulik TM. Surgery for extrahepatic cholangiocarcinoma: predictors of survival. HPB (Oxford) 2008;10:190–195. doi: 10.1080/13651820801992575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park SW, Park YS, Chung JB, Kang JK, Kim KS, Choi JS, Lee WJ, Kim BR, Song SY. Patterns and relevant factors of tumor recurrence for extrahepatic bile duct carcinoma after radical resection. Hepatogastroenterology. 2004;51:1612–1618. [PubMed] [Google Scholar]
- 102.Jarnagin WR, Shoup M. Surgical management of cholangiocarcinoma. Semin Liver Dis. 2004;24:189–199. doi: 10.1055/s-2004-828895. [DOI] [PubMed] [Google Scholar]


