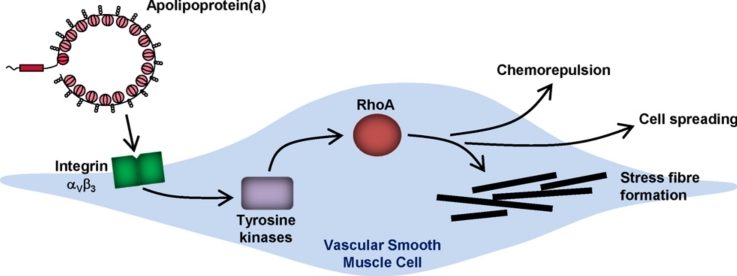
Graphical abstract
Abbreviations: Ao, aortic; α-SMA, alpha smooth muscle actin; apo(a), apolipoprotein(a); DMEM, Dulbecco's modified eagle medium; ERK, extracellular signal-regulated kinase; FCS, foetal calf serum; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HP, high power; Lp(a), lipoprotein(a); MAPK, mitogen activated protein kinase; NFκB, nuclear factor kappa B; PDGF, platelet-derived growth factor-BB; ROCK, Rho kinase; SV, saphenous vein; SMC, smooth muscle cell; TGFβ, transforming growth factor beta
Keywords: Lipoprotein(a), Vascular smooth muscle cells, Migration, RhoA, Remodelling
Highlights
-
•
Acute application of apo(a) to smooth muscle cells induced chemorepulsion.
-
•
Chronic application (>24 h) induced stress fibre formation and cell spreading.
-
•
Effects of apo(a) were mediated by integrin αVβ3, tyrosine kinases and RhoA/ROCK.
-
•
Apo(a) impaired SMC motility which potentially contributes to vascular dysfunction.
Abstract
Lipoprotein(a) (Lp(a)) is an independent risk factor for the development of cardiovascular disease. Vascular smooth muscle cell (SMC) motility and plasticity, functions that are influenced by environmental cues, are vital to adaptation and remodelling in vascular physiology and pathophysiology. Lp(a) is reportedly damaging to SMC function via unknown molecular mechanisms. Apolipoprotein(a) (apo(a)), a unique glycoprotein moiety of Lp(a), has been demonstrated as its active component. The aims of this study were to determine functional effects of recombinant apo(a) on human vascular SMC motility and explore the underlying mechanism(s). Exposure of SMC to apo(a) in migration assays induced a potent, concentration-dependent chemorepulsion that was RhoA and integrin αVβ3-dependent, but transforming growth factor β-independent. SMC manipulation through RhoA gene silencing, Rho kinase inhibition, statin pre-treatment, αVβ3 neutralising antibody and tyrosine kinase inhibition all markedly inhibited apo(a)-mediated SMC migration. Our data reveal unique and potent activities of apo(a) that may negatively influence SMC remodelling in cardiovascular disease. Circulating levels of Lp(a) are resistant to lipid-lowering strategies and hence a greater understanding of the mechanisms underlying its functional effects on SMC may provide alternative therapeutic targets.
1. Introduction
Lipoprotein(a) (Lp(a)) is a plasma glycoprotein expressed only in humans and some primates, and Lp(a) concentration is an independent risk factor for cardiovascular disease (Marcovina et al., 2003). Plasma concentrations of Lp(a) are genetically determined and although there is considerable variation in circulating levels within the population (<10 to >100 mg/dL), levels remain relatively constant throughout an individual's lifetime. Unlike cholesterol, Lp(a) is resistant to modulation by lipid-lowering strategies such as statins or to lifestyle changes including diet, exercise or smoking (Pena-Diaz et al., 2000). There are currently no specific and effective pharmacological therapeutics for Lp(a), indicating a clear rationale for their development (reviewed in Tsimikas and Hall, 2012). Plasma concentrations greater than 20 mg/dL are indicative of a ∼2-fold increased risk of developing cardiovascular disease such as atherosclerosis and vein graft failure, and importantly these levels are evident in approximately 25% of the population (Dahlen et al., 1986; Danesh et al., 2000; Koschinsky et al., 1991). Importantly, plasma concentrations of Lp(a) are elevated in patients with familial hypercholesterolaemia (FH) (Kraft et al., 2000) and Lp(a) is an independent predictor for the development of coronary heart disease in patients with FH (Seed et al., 1990).
The structure of Lp(a) has been thoroughly described (Pena-Diaz et al., 2000), comprising an LDL-apoB-100 core covalently linked to apolipoprotein(a) (apo(a)). The copy number of kringle IV type 2 in apo(a) is variable and inversely correlated with Lp(a) plasma concentration (Frank et al., 1996; Koschinsky et al., 1991). In addition to copy number, single nucleotide polymorphisms within the apo(a) gene associate with the variation in circulating Lp(a) levels (Clarke et al., 2009).
A cognate receptor for Lp(a) has not been identified although it has been shown to signal through the integrins αVβ3 and αMβ2 in some cell types (Liu et al., 2009; Sotiriou et al., 2006). Another possible mode of action of Lp(a) (through the apo(a) moiety) is by decreasing the formation of active transforming growth factor beta (TGFβ) (Liu et al., 2009; O’Neil et al., 2004). Additionally, activation of intracellular signalling pathways such as extracellular signal-regulated kinase (ERK), p38 mitogen activated protein kinase (MAPK) (Liu et al., 2009), nuclear factor kappa B (NFκB) (Sotiriou et al., 2006) and RhoA (Cho et al., 2008; Pellegrino et al., 2004) have been reported. Functionally, this can manifest as modulation of cell proliferation and migration (Komai et al., 2002; Liu et al., 2009; O’Neil et al., 2004; Syrovets et al., 1997; Yu et al., 2003).
Vascular remodelling (through increased cellular proliferation and/or migration) plays a key role in the development of cardiovascular disease and vein graft adaptation. Whilst the causes of atherosclerosis are complex and multifactorial, plasma Lp(a) levels are correlated with the degree of carotid atherosclerosis in stroke patients (Nasr et al., 2011). Furthermore, immunoreactivity for apo(a) has been demonstrated in human coronary and carotid atherosclerotic lesions and the adjacent intima (Cushing et al., 1989; Haque et al., 2000). Lp(a) has been shown to influence multiple cell types including inflammatory cells, platelets, endothelial cells and smooth muscle cells (SMC) by modulating their function in a manner that compromises vascular health (reviewed in Riches and Porter, 2012). Although pro-inflammatory (Sotiriou et al., 2006; Tsimikas et al., 2003) and mitogenic (Kojima et al., 1991; Komai et al., 2002) actions of apo(a) on human aortic SMC function have been described, a full characterisation of its effects on vascular SMC motility and phenotype have not been determined. In this study we used in vitro methods to reveal novel, but potentially adverse effects of recombinant human apo(a) on vascular SMC motility and elucidate the underlying molecular mechanisms.
2. Materials and methods
2.1. Reagents
Recombinant 17K-apo(a) was prepared in the laboratory of Prof. ML Koschinsky, University of Windsor, Ontario. In brief, the protein was purified from a stably expressing cell line, extensively characterised and found to be highly comparable to the apo(a) derived from Lp(a) (Koschinsky et al., 1991). Moreover, contamination from LPS has not been detected in routine testing. Cell culture reagents were from Invitrogen except foetal calf serum (FCS) which was from BioSera. Boyden chambers were from BD Biosciences and platelet-derived growth factor-BB (PDGF) was from Sigma–Aldrich. Primary antibodies were from Cell Signaling Technology (phospho-Smad2 (Ser465/467), phospho-ERK (Thr202/Tyr204) and total ERK), Santa Cruz (RhoA), AbCam (β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH)) and Sigma–Aldrich (alpha smooth muscle actin (α-SMA)). Neutralising antibodies against TGFβRII (AF-241-NA) and integrin αVβ3 (MAB1976 clone LM109) were from R&D Systems and Millipore respectively. Fluorescent nuclear, cytoplasmic and F-actin stains were from Invitrogen. G-LISA RhoA Activation Assay Biochem Kit was from Cytoskeleton Inc. RhoA siRNA was from Dharmacon and Lipofectamine 2000 transfection reagent from Invitrogen. Rho kinase (ROCK) inhibitor Y-27632 and fluvastatin were from Calbiochem.
2.2. Cell culture
Saphenous vein (SV) and aortic (Ao) specimens were obtained from patients undergoing elective coronary artery bypass graft surgery at the Leeds General Infirmary. Local research ethics committee approval (Leeds West, CA/01/040) and informed, written patient consent were obtained. The investigation conformed to the principles outlined in the Declaration of Helsinki. SMC were cultured by explant technique as described previously (Porter et al., 2002). Briefly, vessel fragments were diced within a 2 mL volume of Dulbecco's modified eagle medium (DMEM) containing 10% FCS, transferred to tissue culture flasks and maintained in a tissue culture incubator at 37 °C, 5% CO2 in air. Following 1–2 weeks of culture, SMC were observed migrating out from the tissue. Once confluent, SMC were cultured in media containing 10% FCS and passaged using trypsin/EDTA as required. All experiments were performed on early passage cells (P3-P6) from different patients.
2.3. Migration assays
In vitro migratory properties were assessed as described previously using a modified Boyden chamber technique (Porter et al., 2002) performed over 6 h. Serum-deprived cells (1 × 105) were loaded in the upper chamber in basal medium comprising DMEM with 0.4% FCS. The lower chamber contained basal medium alone (control) or was supplemented with PDGF (10 ng/mL) as chemoattractant. In further experiments the upper or lower chamber was supplemented with 0–50 nM apo(a) as required. After incubation for 6 h at 37 °C, duplicate membranes were fixed, processed, stained with Haematoxylin and Eosin and migrated cells were quantified by counting cells in 10 random fields under high power (HP) light microscopy (400×), as described previously (Porter et al., 2002).
2.4. Immunoblotting
Serum-starved cells were treated with 25 nM apo(a) for 5–30 min before preparing whole cell homogenates and immunoblotting for phospho-Smad2 (Ser465/467), phospho-ERK (Thr202/Tyr204), total ERK, RhoA or β-actin as described previously (Turner et al., 2001). In selected experiments, cells were pretreated with increasing concentrations (0.3–30 μg/mL) of a TGFβ RII neutralising antibody prior to stimulation with 5 ng/mL TGFβ for 20 min. In a separate series of experiments, SMC were exposed to vehicle or 25 nM apo(a) for 6–48 h before preparing whole cell homogenates and immunoblotting for α-SMA and GAPDH. Densitometric analysis was performed using a flatbed scanner and ImageQuant software (Amersham Life Sciences). Data are presented as the ratio of densitometric analyses (mean ± SEM).
2.5. Immunofluorescence microscopy
SMC were seeded in glass-bottomed 96-well plates at a density of 2 × 103/well in triplicate in full growth medium for 24 h before serum depriving in basal medium for an additional 24 h. They were then exposed ± 50 nM apo(a) for 24 h before fixation in 4% paraformaldehyde.
Fixed cells were treated with fluorescent markers to stain the nucleus (DAPI, 2 μg/mL), cytoplasm (TOTO3, 1 μM) and the F-actin cytoskeleton (rhodamine phalloidin, 1:40) in PBS. Stained cells were visualised using 20× objective on a Perkin Elmer Operetta high throughput fluorescence microscope. Five images were taken for each well (field dimensions 675 μm × 510 μm). Images were analysed using Perkin Elmer Harmony 3.0 software using custom made algorithms to define cell boundaries (accuracy of the defined cell boundaries was confirmed by two observers). Cells overlapping the edge of the image were excluded from the analysis. Cell debris was excluded from the analysis by filtering out objects of less than 1000 μm2. Cells that had partially detached and folded over, resulting in an artefactually high actin intensity, were removed from the analysis by filtering out objects with an actin intensity of greater than 800 arbitrary units. Read-outs were cell area and actin morphology (F-actin intensity and ridge frequency).
2.6. RhoA activity assay
Cells were seeded at a density of 2 × 105 in 25 cm2 flasks and cultured for 3 days in DMEM with 10% FCS before being serum-starved for 48 h and then exposed to 25 nM apo(a) for 5–20 min. RhoA activity (i.e. GTP-bound RhoA) was measured using G-LISA RhoA Activation Assay according to manufacturer's instructions.
2.7. RhoA gene silencing
SV-SMC were transfected with 0 or 25 nM RhoA-targeted siRNA (ON-TARGET plus SMARTpool Human RhoA, L-003860-00) with the following sequences: CGACAGCCCUGAUAGUUUA; GACCAAAGAUGGAGUGAGA; GCAGAGAUAUGGCAAACAG; GGAAUGAUGAGCACACAAG, using Lipofectamine 2000 transfection reagent as described previously (Turner et al., 2007). The efficiency of gene silencing was assessed by western blotting for RhoA as described above.
2.8. Statistical analysis
Results are expressed as mean ± SEM with n representing cells from different patients except where otherwise indicated. Cell size and actin morphology were analysed using a paired ratio t-test, and all other data were analysed using one-way ratio ANOVA with Newman–Keuls post hoc test (GraphPad Prism software). P < 0.05 was considered statistically significant.
3. Results
3.1. Effect of apo(a) on SMC migration
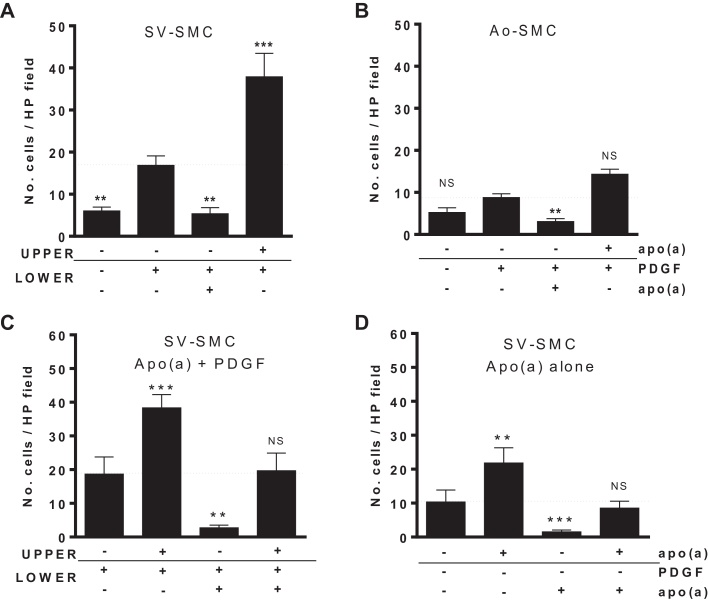
SV-SMC motility was investigated over a 6 h period using a modified Boyden chamber assay. PDGF added to the lower chamber significantly increased migration of cells from the upper chamber (Fig. 1A). Inclusion of 25 nM apo(a) in the lower chamber together with 10 ng/mL PDGF counteracted the chemotactic response. Conversely, when apo(a) was included in the upper chamber along with the cells, a markedly augmented PDGF response was observed, indicating that apo(a) was acting as a chemorepellent (Fig. 1A). This chemorepellent activity was apparent in SMC from both aortic and venous sources although the magnitude of response was smaller in Ao-SMC (Fig. 1A and B), and was independent of length of time in culture/passage number (Supplementary Fig. 1). Using PDGF as a chemoattractant, we performed a chequerboard analysis of apo(a) on SV-SMC and observed consistent chemorepulsion irrespective of its location (upper or lower chamber). However in the absence of a concentration gradient (i.e. apo(a) in both the upper and lower chambers, with PDGF in the lower chamber), the number of migrated cells was comparable to that observed with PDGF alone (Fig. 1C). Furthermore, this directional effect was apparent even in the absence of PDGF, indicating that the chemorepellent activity of apo(a) was independent of PDGF (Fig. 1D).
Fig. 1.
Effect of apo(a) on SMC migration in a Boyden chamber (6 h). Changes are compared to migration in the presence of PDGF in the lower chamber (horizontal dashed line). (A) Number of migrated cells per high power (HP) field for SV-SMC and (B) aortic SMC (n = 6 and 3 respectively, ***P < 0.001, **P < 0.01, *P < 0.05, NS = non-significant vs. PDGF in lower chamber). (C) Chequerboard analysis of SV-SMC migration in the presence of PDGF (all n = 6, ***P < 0.001, **P < 0.01, *P < 0.05, NS = non-significant vs. PDGF alone in lower chamber). (D) Chequerboard analysis in response to apo(a) alone (n = 6, ***P < 0.001, **P < 0.01 vs. control media in lower chamber).
Effect of passage number on SV-SMC migration. (A) Basal migration rates were collated for SV-SMC from all patient donors used in the study and separated according to passage number. (B) Fold change in migration in response to apo(a) in the upper chamber or (C) PDGF in the lower chamber according to passage number. All comparisons were non-significant (one-way ANOVA).
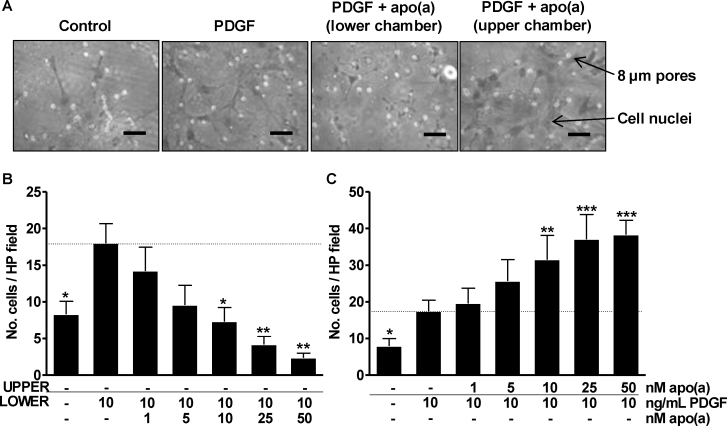
To explore the concentration-dependency of the apo(a) effect, SV-SMC were studied in Boyden assays with increasing concentrations of apo(a) (1–50 nM) in the upper or lower wells. Fig. 2A shows representative images of Boyden chamber membranes with cell nuclei indicated. When apo(a) was included in the lower chamber, PDGF-induced chemotaxis was attenuated in a concentration-dependent manner (Fig. 2A and B). Inclusion of 10 nM apo(a) was sufficient to completely negate the chemotactic effect of PDGF, which was reduced further (below basal levels) at higher concentrations of apo(a) (Fig. 2B). Conversely, inclusion of apo(a) in the upper chamber enhanced the chemotactic response to PDGF by two-fold at 25 nM apo(a) (Fig. 2A and C).
Fig. 2.
Concentration dependency of the effect of apo(a) on SMC migration. Changes are compared to migration in the presence of PDGF in the lower chamber (horizontal dashed line). (A) Representative images of Boyden chamber assays (PDGF = 10 ng/mL, apo(a) = 25 nM, scale bar = 50 μm). Number of migrated cells per HP field when (B) apo(a) was included in the lower chambers (**P < 0.01 ANOVA for effect of apo(a)) or (C) upper chamber (all n = 5–6,***P < 0.001 ANOVA for effect of apo(a). Post hoc *P < 0.05, **P < 0.01, ***P < 0.001 vs. PDGF alone in lower chamber).
3.2. Apo(a) effects are independent of TGFβ and ERK signalling
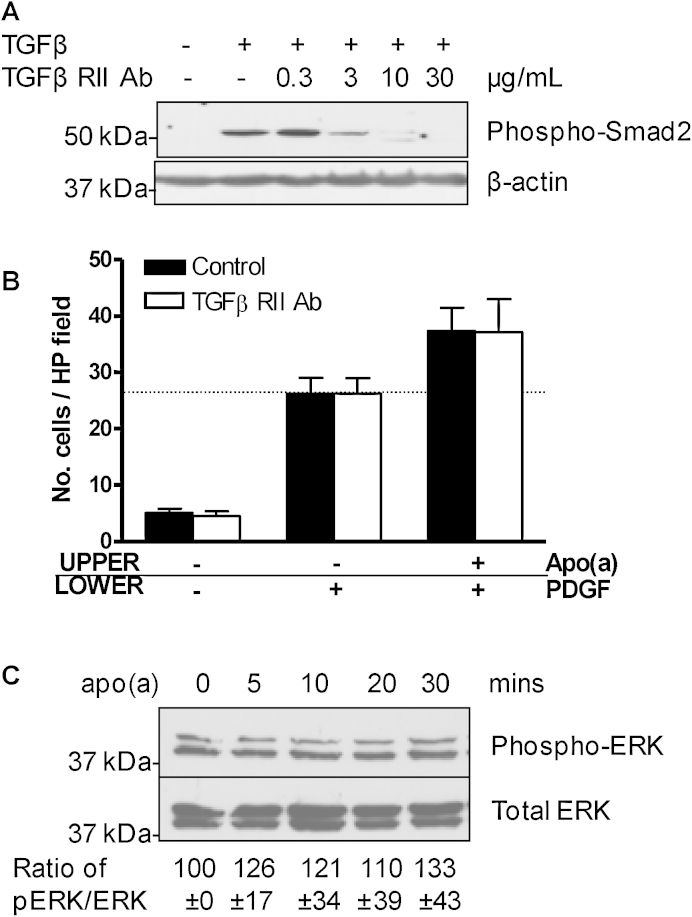
Apo(a) is reported to induce functional cellular effects via inhibition of the TGFβ activation pathway (Liu et al., 2009; O’Neil et al., 2004). Pre-treatment of SV-SMC with 10 μg/mL TGFβ RII neutralising antibody prevented TGFβ signalling, as demonstrated by a loss of TGFβ-induced Smad2 phosphorylation (Fig. 3A). Inclusion of the neutralising antibody in the Boyden chamber assay failed to modulate SMC migration, irrespective of the stimulus (Fig. 3B), indicating that the effects of apo(a) were TGFβ-independent. Apo(a) is also reported to activate ERK signalling, yet in SV-SMC exposure to apo(a) alone consistently failed to increase ERK-1/2 phosphorylation above basal levels (Fig. 3C). Importantly, PDGF induced robust phosphorylation of ERK-1/2, confirming functionality of this pathway in SV-SMC (Supplementary Fig. 2). However, apo(a) (1–50 nM) did not modulate this PDGF-induced ERK phosphorylation (Supplementary Fig. 2).
Fig. 3.
Effects of apo(a) are TGFβ- and ERK-independent. (A) Cells were pre-treated with 0.3–30 μg/mL TGFβ RII neutralising antibody (Ab) for 1 h, stimulated with 5 ng/mL TGFβ for 20 min and lysates immunoblotted for phospho-Smad2 and β-actin. (B) Number of migrated cells per HP field in response to PDGF and apo(a) following pre-treatment with 10 μg/mL TGFβ RII neutralising antibody (n = 3). Horizontal dashed line represents effect of PDGF alone. (C) Cells were treated with apo(a) for 5–30 min and lysates immunoblotted for phospho-ERK and total ERK (n = 3). Numbers refer to densitometry data (mean ± SEM).
Effect of apo(a) on PDGF-induced ERK phosphorylation. Cells were treated with increasing concentrations of apo(a) either alone or in combination with 10 ng/mL PDGF for 20 min, and lysates blotted for phospho-ERK-1/2 and total ERK-1/2 (upper panel). Densitometry data were normalised to the lane containing PDGF alone, and analysed using ratio repeated measures one-way ANOVA with Newman–Keuls post-hoc test (lower panel n = 4, **P < 0.01).
3.3. RhoA/Rho kinase mediate the effects of apo(a)
The influence of apo(a) on SV-SMC morphology was determined using high throughput immunofluorescence microscopy. Application of apo(a) caused a significant 1.3-fold increase in spread cell area (Fig. 4A and B). Furthermore, apo(a) induced the formation of stress fibres with a 1.2-fold increase in both the intensity of F-actin fibres (Fig. 4C) and frequency of F-actin fibre bundles (Fig. 4D). Alterations in cell area are often associated with a switch in phenotype from differentiated (spindle shaped) to dedifferentiated (rhomboid shaped); mirrored by reduced expression of α-SMA (Owens et al., 2004). Therefore, to determine whether apo(a) influenced SMC phenotype per se we measured levels of α-SMA in cells treated over a 6–48 h time period; however no differences were observed (Fig. 4E). RhoA is involved in stress fibre formation and apo(a) has previously been reported to induce RhoA activation in endothelial cells (Pellegrino et al., 2004; Cho et al., 2008), so we examined whether a similar effect was seen in SV-SMC. Apo(a) induced rapid activation of RhoA, which was significant within 20 min after application (Fig. 4F).
Fig. 4.
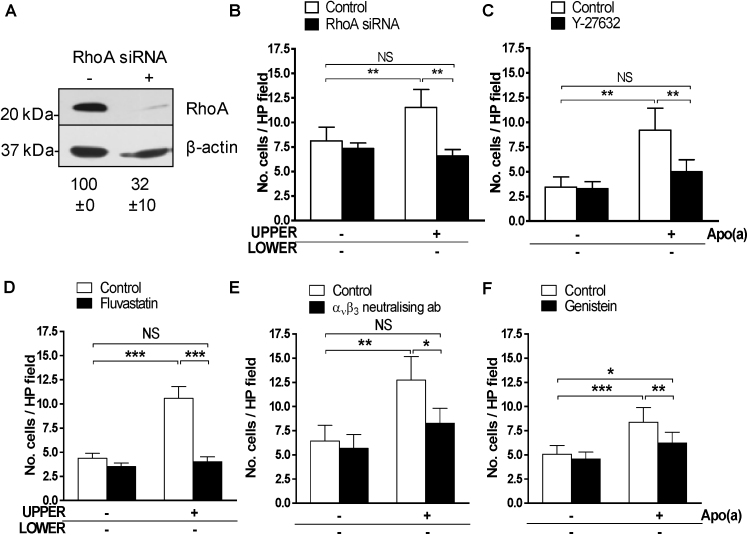
Effect of apo(a) on cell morphology, α-SMA expression and RhoA activation. (A) SV-SMC were treated with apo(a) for 24 h, fixed and stained with rhodamine phalloidin (F-actin, yellow) and TOTO-3 (cytoplasm/nuclei, red). Actin fibres were detected using Harmony software. Scale bar = 20 μm. (B) Spread cell area measurements, (C) F-actin stain intensity and (D) morphology. Ridges indicate actin bundling (all n = 200 cells, ***P < 0.001). (E) Cells were treated with 25 nM apo(a) for 6–48 h and lysates immunoblotted for α-SMA and GAPDH (n = 4). Numbers refer to densitometry data (mean ± SEM). (F) RhoA activation in response to apo(a) (n = 8, **P < 0.01 ANOVA for effect of apo(a). Post hoc *P < 0.05 vs 0 min). Mean activity levels were 10.9 ± 2.5 pg GTP-bound RhoA per μg total cellular protein in unstimulated samples and 16.3 ± 3.9 pg following 20 min apo(a) exposure. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To determine whether activation of the RhoA/ROCK pathway was responsible for the chemorepellent effects of apo(a), we employed RhoA gene silencing (siRNA). Efficient RhoA protein knock-down in SV-SMC was confirmed by immunoblotting (Fig. 5A). RhoA silencing had no effect on basal migration, but fully overcame the modulatory effect of apo(a) (Fig. 5B). Similar results of RhoA siRNA were obtained for apo(a)-induced migration in the presence of PDGF (Supplementary Fig. 3A), in keeping with the chequerboard analysis that demonstrated apo(a) chemorepellent activity was independent of PDGF (Fig. 1C). To examine the role of ROCK, SV-SMC were pre-treated with 10 μM Y-27632 (pharmacological ROCK inhibitor), before performing Boyden chamber assays (Fig. 5C). Consistent with the RhoA knock down experiments, inhibition of ROCK had no effect on basal migration but prevented the effects of apo(a). Again, similar results were obtained for apo(a)-induced migration in the presence of PDGF (Supplementary Fig. 3B).
Fig. 5.
Mechanism of apo(a)-induced effects. (A) SV-SMC were transfected with 25 nM RhoA siRNA; knockdown was confirmed via immunoblotting. Numbers represent densitometry data for RhoA (n = 6). (B) Number of migrated cells per HP field under basal conditions and in response to apo(a) following RhoA silencing (closed bars) compared to mock transfected cells (open bars, n = 5). (C). Migration under basal conditions and in response to apo(a) following pre-treatment with vehicle control (0.002% dH2O; open bars) or 10 μM ROCK inhibitor, Y-27632 (closed bars) for 1 h (n = 6). (D) Migration in response to apo(a) following pre-treatment with vehicle control (0.001% dH2O; open bars) or 1 μM fluvastatin (closed bars) for 2 h (n = 5). (E). Migration in response to apo(a) following pre-treatment with vehicle control (0.001% PBS; open bars) or 5 μg/mL αvβ3 integrin neutralising antibody (closed bars) for 5 min (n = 5). (F) Migration in response to apo(a) following pre-treatment with vehicle control (0.001% DMSO; open bars) or 100 μM genistein (closed bars) for 1 h (n = 5). ***P < 0.001, **P < 0.01, *P < 0.05, NS = non-significant.
Effect of RhoA siRNA and ROCK inhibition on apo(a) migration in the presence of PDGF. (A) Number of migrated cells per HP field in response to PDGF and apo(a) following RhoA silencing (closed bars) compared to mock transfected cells (open bars, n = 7). (B) Migration in response to PDGF and apo(a) following pre-treatment with vehicle control (0.002% dH2O; open bars) or 10 μM ROCK inhibitor, Y-27632 (closed bars) for 1 h (n = 5). **P < 0.01, NS = non-significant.
3.4. Statins attenuate the effects of apo(a)
Statins exhibit multiple pleiotropic effects, one of which is inhibition of RhoA prenylation and activity, as a downstream consequence of HMG CoA reductase inhibition (Porter and Turner, 2011). As SV-SMC migration in response to PDGF is known to be modulated by statins (Corpataux et al., 2005), and we had consistently demonstrated that apo(a) exhibited chemorepellent activity independently of PDGF (Fig. 1C), we explored the effects of statins on SV-SMC migration in the absence of PDGF. SV-SMC were pre-treated with 1 μM fluvastatin for 2 h prior to use in Boyden chamber assays. Fluvastatin had no effect on basal migration, but markedly attenuated the directional effects of apo(a) (Fig. 5D).
3.5. Apo(a) acts via integrin αVβ3 and tyrosine kinases
To determine whether αVβ3 integrin activity accounted for the effects of apo(a) in SV-SMC, cells were pre-treated with 5 μg/mL mouse anti-human neutralising antibody against integrin αVβ3 prior to the Boyden chamber assay. Blocking αVβ3 integrin had no effect on basal cell migration, however it reduced the directional effects of apo(a) by 72% (Fig. 5E). Tyrosine kinase signalling pathways lie downstream of αVβ3 integrin, and accordingly pre-treatment with a general tyrosine kinase inhibitor (genistein, 100 μM) also reduced the directional effects of apo(a) by 65% (Fig. 5F), analogous to that of αVβ3 inhibition.
4. Discussion
SMC migration is central to the pathogenesis of numerous cardiovascular processes, including vessel wall remodelling/adaptation and atherosclerosis (Gerthoffer, 2007). This is the first report of a chemorepellent effect of apo(a) on vascular SMC. Whilst chemoattraction is common in SMC, the contribution of chemorepellent cues has been largely overlooked although it is not without precedent (Liu et al., 2006).
Apo(a) has previously been reported in vitro to enhance SMC migration (O’Neil et al., 2004). This is in contrast to our results, an inconsistency that may be explained by differences in experimental protocol. O’Neil et al. exposed SMC to apo(a) for 96 h prior to (but not during) migration assays whereas we exposed cells to apo(a) only at the point of, and during the assay. Our data indicate that apo(a) acted as a chemorepellent in both aortic and venous SMC from different patients, however O’Neil et al. used commercially available SMC from a single source. Nevertheless the Boyden chamber assays clearly demonstrate that SMC motility is dysfunctional in the presence of apo(a), an effect that would potentially impair vascular function through increasing vessel wall stiffness. Apo(a) alone acted in a potent chemorepellent manner. In support of its actions as a chemorepellent, the only occasion where apo(a) enhanced migration to PDGF was when apo(a) and PDGF were in opposing chambers of Boyden assays. The increased migration observed in this case was likely the net effect of chemorepulsion from apo(a) in the upper chamber in addition to the chemoattractant cues of PDGF in the lower chamber.
The functional effect of apo(a) appears to vary depending on the vascular bed, for example its ability to induce divergent adhesion molecule expression in endothelial cells (recently reviewed in Riches and Porter, 2012). Our data herein suggest that acute exposure to apo(a) is chemorepulsive to human SMC of both arterial and venous origin although the mechanism of action is undoubtedly complex and involves multiple signalling pathways, including integrin αVβ3. It is feasible that the magnitude of apo(a) effect could be governed by differential expression and activation of various signalling pathways in particular subsets (phenotypes) of SMC within vascular walls. The validity of such a hypothesis is upheld by studies in which SMC diversity in atherosclerotic plaques and restenotic lesions has been demonstrated (Adams et al., 2006; Zhang et al., 2002).
Effective adaptation to arterial environments early after implantation is a key determinant of the long-term patency of vein grafts (Owens, 2010), where the consequences of increased vessel wall stiffness may be deleterious. Following surgical grafting, the SV must adapt to a new role in a process often referred to as ‘arterialisation’. This process requires significant expansive remodelling; a process in which SMC phenotypic modulation is key. SMC exhibit increased motility and decreased contractility during this initial adaptive process (Borin et al., 2009; Mehta et al., 1998). The effects of apo(a) on SMC that we revealed in this study are therefore of potential significance to the fate of the adapting graft. We propose that transient increases in exposure to apo(a) following CABG, would act as a chemorepellent to SMC and compromise their capacity to remodel and “arterialise” the venous graft. Prolonged exposure to high circulating apo(a) levels would render the cells more stationary and contractile, exacerbating their inability to form an arterial conduit. Importantly, this adaptive phase is temporally distinct from subsequent maladaptive intimal thickening and occlusion (Wong et al., 2008). SV grafts are prone to early lipid retention and it is already well established that LDL plays a key role in the failure of grafts to remodel (Shi et al., 2001). Histological studies have reported deposition of Lp(a) in resected SV grafts (Cushing et al., 1989), suggesting a similar functional role to that of native LDL. Two further studies have quantified plasma levels of Lp(a) in patients with vein grafts and observed elevated levels in those with stenosis versus non-occluded grafts. One of these documented levels of 32 mg/dL versus 17 mg/dL respectively (Hoff et al., 1988); and the other 24 mg/dL versus 12 mg/dL respectively (Pokrovsky et al., 2003). The maximum concentration of apo(a) used in our study was 50 nM (equivalent to 1.4 mg/dL apo(a) or approx. 0.5 mg/dL whole Lp(a)), indicating potent effects directly upon SV-SMC.
In native atherosclerosis, SMC are key constituents of fibrous plaques and are responsible for promoting plaque stability through proliferation and migration (Fernandez-Hernando et al., 2009). Aberrant SMC migration provoked by high circulating Lp(a) levels would plausibly lead to fewer SMC in atherosclerotic plaques, disrupting morphology and conferring vulnerability to rupture.
The lack of an identified Lp(a) receptor has not hindered research into the signalling pathways by which it operates. Its actions have sometimes been attributed to inhibition of TGFβ activation (e.g. stimulating cell proliferation (O’Neil et al., 2004; Liu et al., 2009)); however the novel chemorepellent effect that we observed was independent of this mechanism. Not only did the short time-frame of action (<6 h) of apo(a) suggest that this was unlikely, inclusion of a neutralising antibody to TGFβ RII did not modulate the migratory response. Similarly, apo(a) was previously shown to potently and transiently activate ERK phosphorylation (Liu et al., 2009), yet our data demonstrate that this pathway is not activated above basal levels in SV-SMC, nor did we observe any modulation of PDGF-induced ERK phosphorylation by apo(a). Whilst a cognate Lp(a) receptor remains elusive, apo(a) has been shown to signal via the αVβ3 integrin in HUVEC (Liu et al., 2009). Our study reveals that this pathway is also functional in SV-SMC, although the downstream effectors are different. In HUVEC, signalling through αVβ3 integrin induced activation of ERK, whereas in SV-SMC tyrosine kinase and RhoA pathways are apparently the predominant signalling components.
RhoA and its downstream effector, ROCK, are directly involved in stress fibre formation and accordingly, acute exposure of SV-SMC to apo(a) induced activation of RhoA. This concurs with previous studies in HUVEC (Cho et al., 2008). Exposure of SV-SMC to apo(a) resulted in an increase in the frequency of stress fibre bundles and a notable increase in spread cell area, however it is unlikely that apo(a) was altering SMC phenotype as we did not detect any change in α-SMA expression levels. Transfection of SMC with RhoA siRNA or pre-treatment with ROCK inhibitor prior to the Boyden chamber assay had no effect on basal or PDGF-induced migration. This is in keeping with previous studies in human and rat aortic SMC in which Rac rather than RhoA was important for PDGF-induced migration (Ryu et al., 2002). RhoA/ROCK inhibition completely negated the effects of apo(a), indicating that its chemorepellent effects on SV-SMC were, at least in part, mediated via RhoA/ROCK; both of which are well recognised to have negative effects on the vasculature in pathological conditions (Sawada et al., 2000).
Statins are routinely prescribed as cholesterol-lowering drugs, but are also known to exhibit properties that are independent of lipid-lowering (pleiotropism), many of which are attributable to inhibition of small G-protein prenylation (e.g. RhoA and Rac) (Laufs et al., 2002; Porter and Turner, 2011). The influence of statin treatment on absolute plasma Lp(a) is contentious, with no consistently reported effect on circulating levels (Hunninghake et al., 1993; Joy et al., 2008; Kostner et al., 1989). Therefore, although statins do not appear to modulate circulating levels of Lp(a) per se, they hold potential to negate the adverse functional effects of apo(a) on SMC, through inhibition of RhoA activity. Indeed, graft patency rates in patients with high serum Lp(a) receiving statin therapy were reportedly superior to those of patients not receiving statins (Pokrovsky et al., 2003).
Plasma concentrations of Lp(a) remain relatively constant throughout life, however levels are reported to rise acutely under pathological challenge for example after myocardial infarction (MBewu et al., 1993; Tsimikas et al., 2003), percutaneous coronary intervention (Tsimikas et al., 2004) and plausibly therefore after SV bypass grafting. Under these circumstances it appears that Lp(a), acting as an acute phase reactant, impairs the crucial ability of vascular SMC to remodel and adapt appropriately.
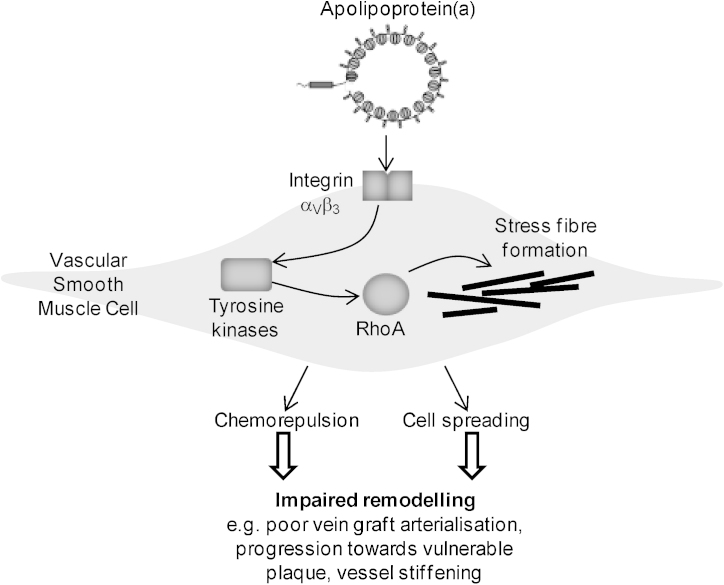
In conclusion, we have demonstrated that the apo(a) moiety of human Lp(a) confers distinct functional effects on SMC that may impair adaptive remodelling (summarised in Fig. 6). Our study has identified αVβ3 and RhoA as potential therapeutic targets to alleviate the perceived detrimental effects of Lp(a) on vascular SMC in cardiovascular pathologies. Development of specific therapeutics to Lp(a) is an area of focussed interest (Tsimikas and Hall, 2012), and statins may partly fulfil this role through their pleiotropic effects on small G-proteins.
Fig. 6.
Summary figure. Our data suggest that apo(a) interacts with integrin αVβ3 on the surface of vascular SMC. This signals through tyrosine kinases to activate RhoA and induce stress fibre formation, cell spreading and chemorepulsion. These molecular effects may have a negative impact on smooth muscle cell remodelling contributing towards atherosclerosis, vessel stiffness, and impaired vein graft integration and arterialisation.
Acknowledgements
This work was supported by The British Heart Foundation (CH/92005). S.G. Ball is a British Heart Foundation Professor of Cardiology. We are grateful to J. Kaye for technical expertise in cell culture.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Adams L.D., Geary R.L., Li J., Rossini A., Schwartz S.M. Expression profiling identifies smooth muscle cell diversity within human intima and plaque fibrous cap: loss of RGS5 distinguishes the cap. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:319–325. doi: 10.1161/01.ATV.0000196647.45718.d6. [DOI] [PubMed] [Google Scholar]
- Borin T.F., Miyakawa A.A., Cardoso L., de Figueiredo B.L., Goncalves G.A., Krieger J.E. Apoptosis, cell proliferation and modulation of cyclin-dependent kinase inhibitor p21(cip1) in vascular remodelling during vein arterialization in the rat. International Journal of Experimental Pathology. 2009;90:328–337. doi: 10.1111/j.1365-2613.2009.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho T., Jung Y., Koschinsky M.L. Apolipoprotein(a), through its strong lysine-binding site in KIV(10’), mediates increased endothelial cell contraction and permeability via a Rho/Rho kinase/MYPT1-dependent pathway. Journal of Biological Chemistry. 2008;283:30503–30512. doi: 10.1074/jbc.M802648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R., Peden J.F., Hopewell J.C., Kyriakou T., Goel A., Heath S.C. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. New England Journal of Medicine. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- Corpataux J.M., Naik J., Porter K.E., London N.J. The effect of six different statins on the proliferation, migration, and invasion of human smooth muscle cells. Journal of Surgical Research. 2005;129:52–56. doi: 10.1016/j.jss.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Cushing G.L., Gaubatz J.W., Nava M.L., Burdick B.J., Bocan T.M., Guyton J.R. Quantitation and localization of apolipoproteins [a] and B in coronary artery bypass vein grafts resected at re-operation. Arteriosclerosis. 1989;9:593–603. doi: 10.1161/01.atv.9.5.593. [DOI] [PubMed] [Google Scholar]
- Dahlen G.H., Guyton J.R., Attar M., Farmer J.A., Kautz J.A., Gotto A.M., Jr. Association of levels of lipoprotein Lp(a), plasma lipids, and other lipoproteins with coronary artery disease documented by angiography. Circulation. 1986;74:758–765. doi: 10.1161/01.cir.74.4.758. [DOI] [PubMed] [Google Scholar]
- Danesh J., Collins R., Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 2000;102:1082–1085. doi: 10.1161/01.cir.102.10.1082. [DOI] [PubMed] [Google Scholar]
- Fernandez-Hernando C., Jozsef L., Jenkins D., Di L.A., Sessa W.C. Absence of Akt1 reduces vascular smooth muscle cell migration and survival and induces features of plaque vulnerability and cardiac dysfunction during atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:2033–2040. doi: 10.1161/ATVBAHA.109.196394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S., Durovic S., Kostner G.M. The assembly of lipoprotein Lp(a) European Journal of Clinical Investigation. 1996;26:109–114. doi: 10.1046/j.1365-2362.1996.112255.x. [DOI] [PubMed] [Google Scholar]
- Gerthoffer W.T. Mechanisms of vascular smooth muscle cell migration. Circulation Research. 2007;100:607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- Haque N.S., Zhang X., French D.L., Li J., Poon M., Fallon J.T. CC chemokine I-309 is the principal monocyte chemoattractant induced by apolipoprotein(a) in human vascular endothelial cells. Circulation. 2000;102:786–792. doi: 10.1161/01.cir.102.7.786. [DOI] [PubMed] [Google Scholar]
- Hoff H.F., Beck G.J., Skibinski C.I., Jurgens G., O’Neil J., Kramer J. Serum Lp(a) level as a predictor of vein graft stenosis after coronary artery bypass surgery in patients. Circulation. 1988;77:1238–1244. doi: 10.1161/01.cir.77.6.1238. [DOI] [PubMed] [Google Scholar]
- Hunninghake D.B., Stein E.A., Mellies M.J. Effects of one year of treatment with pravastatin, an HMG-CoA reductase inhibitor, on lipoprotein a. Journal of Clinical Pharmacology. 1993;33:574–580. doi: 10.1002/j.1552-4604.1993.tb04706.x. [DOI] [PubMed] [Google Scholar]
- Joy M.S., Dornbrook-Lavender K.A., Chin H., Hogan S.L., Denu-Ciocca C. Effects of atorvastatin on Lp(a) and lipoprotein profiles in hemodialysis patients. Annals of Pharmacotherapy. 2008;42:9–15. doi: 10.1345/aph.1K407. [DOI] [PubMed] [Google Scholar]
- Kojima S., Harpel P.C., Rifkin D.B. Lipoprotein (a) inhibits the generation of transforming growth factor beta: an endogenous inhibitor of smooth muscle cell migration. Journal of Cell Biology. 1991;113:1439–1445. doi: 10.1083/jcb.113.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai N., Morishita R., Yamada S., Oishi M., Iguchi S., Aoki M. Mitogenic activity of oxidized lipoprotein (a) on human vascular smooth muscle cells. Hypertension. 2002;40:310–314. doi: 10.1161/01.hyp.0000029974.50905.b4. [DOI] [PubMed] [Google Scholar]
- Koschinsky M.L., Tomlinson J.E., Zioncheck T.F., Schwartz K., Eaton D.L., Lawn R.M. Apolipoprotein(a): expression and characterization of a recombinant form of the protein in mammalian cells. Biochemistry. 1991;30:5044–5051. doi: 10.1021/bi00234a029. [DOI] [PubMed] [Google Scholar]
- Kostner G.M., Gavish D., Leopold B., Bolzano K., Weintraub M.S., Breslow J.L. HMG CoA reductase inhibitors lower LDL cholesterol without reducing Lp(a) levels. Circulation. 1989;80:1313–1319. doi: 10.1161/01.cir.80.5.1313. [DOI] [PubMed] [Google Scholar]
- Kraft H.G., Lingenhel A., Raal F.J., Hohenegger M., Utermann G. Lipoprotein(a) in homozygous familial hypercholesterolemia. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:522–528. doi: 10.1161/01.atv.20.2.522. [DOI] [PubMed] [Google Scholar]
- Laufs U., Kilter H., Konkol C., Wassmann S., Bohm M., Nickenig G. Impact of HMG CoA reductase inhibition on small GTPases in the heart. Cardiovascular Research. 2002;53:911–920. doi: 10.1016/s0008-6363(01)00540-5. [DOI] [PubMed] [Google Scholar]
- Liu D., Hou J., Hu X., Wang X., Xiao Y., Mou Y. Neuronal chemorepellent Slit2 inhibits vascular smooth muscle cell migration by suppressing small GTPase Rac1 activation. Circulation Research. 2006;98:480–489. doi: 10.1161/01.RES.0000205764.85931.4b. [DOI] [PubMed] [Google Scholar]
- Liu L., Craig A.W., Meldrum H.D., Marcovina S.M., Elliott B.E., Koschinsky M.L. Apolipoprotein(a) stimulates vascular endothelial cell growth and migration and signals through integrin alphavbeta3. Biochemical Journal. 2009;418:325–336. doi: 10.1042/BJ20080744. [DOI] [PubMed] [Google Scholar]
- Marcovina S.M., Koschinsky M.L., Albers J.J., Skarlatos S. Report of the National Heart, Lung, and Blood Institute workshop on lipoprotein(a) and cardiovascular disease: recent advances and future directions. Clinical Chemistry. 2003;49:1785–1796. doi: 10.1373/clinchem.2003.023689. [DOI] [PubMed] [Google Scholar]
- MBewu A.D., Durrington P.N., Bulleid S., Mackness M.I. The immediate effect of streptokinase on serum lipoprotein(a) concentration and the effect of myocardial infarction on serum lipoprotein(a), apolipoproteins A1 and B, lipids and C-reactive protein. Atherosclerosis. 1993;103:65–71. doi: 10.1016/0021-9150(93)90040-2. [DOI] [PubMed] [Google Scholar]
- Mehta D., George S.J., Jeremy J.Y., Izzat M.B., Southgate K.M., Bryan A.J. External stenting reduces long-term medial and neointimal thickening and platelet derived growth factor expression in a pig model of arteriovenous bypass grafting. Nature Medicine. 1998;4:235–239. doi: 10.1038/nm0298-235. [DOI] [PubMed] [Google Scholar]
- Nasr N., Ruidavets J.B., Farghali A., Guidolin B., Perret B., Larrue V. Lipoprotein (a) and carotid atherosclerosis in young patients with stroke. Stroke. 2011;42:3616–3618. doi: 10.1161/STROKEAHA.111.624684. [DOI] [PubMed] [Google Scholar]
- O’Neil C.H., Boffa M.B., Hancock M.A., Pickering J.G., Koschinsky M.L. Stimulation of vascular smooth muscle cell proliferation and migration by apolipoprotein(a) is dependent on inhibition of transforming growth factor-beta activation and on the presence of kringle IV type 9. Journal of Biological Chemistry. 2004;279:55187–55195. doi: 10.1074/jbc.M409860200. [DOI] [PubMed] [Google Scholar]
- Owens C.D. Adaptive changes in autogenous vein grafts for arterial reconstruction: clinical implications. Journal of Vascular Surgery. 2010;51:736–746. doi: 10.1016/j.jvs.2009.07.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G.K., Kumar M.S., Wamhoff B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological Reviews. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Pellegrino M., Furmaniak-Kazmierczak E., LeBlanc J.C., Cho T., Cao K., Marcovina S.M. The apolipoprotein(a) component of lipoprotein(a) stimulates actin stress fiber formation and loss of cell–cell contact in cultured endothelial cells. Journal of Biological Chemistry. 2004;279:6526–6533. doi: 10.1074/jbc.M309705200. [DOI] [PubMed] [Google Scholar]
- Pena-Diaz A., Izaguirre-Avila R., Angles-Cano E. Lipoprotein Lp(a) and atherothrombotic disease. Archives of Medical Research. 2000;31:353–359. doi: 10.1016/s0188-4409(00)00084-9. [DOI] [PubMed] [Google Scholar]
- Pokrovsky S.N., Ezhov M.V., Il’ina L.N., Afanasieva O.I., Sinitsyn V.Y., Shiriaev A.A. Association of lipoprotein(a) excess with early vein graft occlusions in middle-aged men undergoing coronary artery bypass surgery. Journal of Thoracic and Cardiovascular Surgery. 2003;126:1071–1075. doi: 10.1016/s0022-5223(03)00365-9. [DOI] [PubMed] [Google Scholar]
- Porter K.E., Turner N.A. Statins and myocardial remodelling: cell and molecular pathways. Expert Reviews in Molecular Medicine. 2011;13:e22. doi: 10.1017/S1462399411001931. [DOI] [PubMed] [Google Scholar]
- Porter K.E., Naik J., Turner N.A., Dickinson T., Thompson M.M., London N.J. Simvastatin inhibits human saphenous vein neointima formation via inhibition of smooth muscle cell proliferation and migration. Journal of Vascular Surgery. 2002;36:150–157. doi: 10.1067/mva.2002.122029. [DOI] [PubMed] [Google Scholar]
- Riches K., Porter K.E. Lipoprotein(a): cellular effects and molecular mechanisms. Cholesterol. 2012;2012:923289. doi: 10.1155/2012/923289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y., Takuwa N., Sugimoto N., Sakurada S., Usui S., Okamoto H. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circulation Research. 2002;90:325–332. doi: 10.1161/hh0302.104455. [DOI] [PubMed] [Google Scholar]
- Sawada N., Itoh H., Ueyama K., Yamashita J., Doi K., Chun T.H. Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation. 2000;101:2030–2033. doi: 10.1161/01.cir.101.17.2030. [DOI] [PubMed] [Google Scholar]
- Seed M., Hoppichler F., Reaveley D., McCarthy S., Thompson G.R., Boerwinkle E. Relation of serum lipoprotein(a) concentration and apolipoprotein(a) phenotype to coronary heart disease in patients with familial hypercholesterolemia. New England Journal of Medicine. 1990;322:1494–1499. doi: 10.1056/NEJM199005243222104. [DOI] [PubMed] [Google Scholar]
- Shi Y., Patel S., Davenpeck K.L., Niculescu R., Rodriguez E., Magno M.G. Oxidative stress and lipid retention in vascular grafts: comparison between venous and arterial conduits. Circulation. 2001;103:2408–2413. doi: 10.1161/01.cir.103.19.2408. [DOI] [PubMed] [Google Scholar]
- Sotiriou S.N., Orlova V.V., Al-Fakhri N., Ihanus E., Economopoulou M., Isermann B. Lipoprotein(a) in atherosclerotic plaques recruits inflammatory cells through interaction with Mac-1 integrin. FASEB Journal. 2006;20:559–561. doi: 10.1096/fj.05-4857fje. [DOI] [PubMed] [Google Scholar]
- Syrovets T., Thillet J., Chapman M.J., Simmet T. Lipoprotein(a) is a potent chemoattractant for human peripheral monocytes. Blood. 1997;90:2027–2036. [PubMed] [Google Scholar]
- Tsimikas S., Bergmark C., Beyer R.W., Patel R., Pattison J., Miller E. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. Journal of the American College of Cardiology. 2003;41:360–370. doi: 10.1016/s0735-1097(02)02769-9. [DOI] [PubMed] [Google Scholar]
- Tsimikas S., Hall J.L. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease: a rationale for increased efforts to understand its pathophysiology and develop targeted therapies. Journal of the American College of Cardiology. 2012;60:716–721. doi: 10.1016/j.jacc.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Tsimikas S., Lau H.K., Han K.R., Shortal B., Miller E.R., Segev A. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 2004;109:3164–3170. doi: 10.1161/01.CIR.0000130844.01174.55. [DOI] [PubMed] [Google Scholar]
- Turner N.A., Ball S.G., Balmforth A.J. The mechanism of angiotensin II-induced extracellular signal-regulated kinase-1/2 activation is independent of angiotensin AT1A receptor internalisation. Cellular Signalling. 2001;13:269–277. doi: 10.1016/s0898-6568(01)00135-8. [DOI] [PubMed] [Google Scholar]
- Turner N.A., Hall K.T., Ball S.G., Porter K.E. Selective gene silencing of either MMP-2 or MMP-9 inhibits invasion of human saphenous vein smooth muscle cells. Atherosclerosis. 2007;193:36–43. doi: 10.1016/j.atherosclerosis.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Wong A.P., Nili N., Jackson Z.S., Qiang B., Leong-Poi H., Jaffe R. Expansive remodeling in venous bypass grafts: novel implications for vein graft disease. Atherosclerosis. 2008;196:580–589. doi: 10.1016/j.atherosclerosis.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Yu H., Hong J., Wang B., Peng F., Li X., He C. Effects of wild-type (Trp72) and mutant (Arg72) apolipoprotein(a) kringle IV-10 on the proliferation of human arterial smooth muscle cells. Chinese Medical Journal (England) 2003;116:721–726. [PubMed] [Google Scholar]
- Zhang Q.J., Goddard M., Shanahan C., Shapiro L., Bennett M. Differential gene expression in vascular smooth muscle cells in primary atherosclerosis and in stent stenosis in humans. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22:2030–2036. doi: 10.1161/01.atv.0000042206.98651.15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of passage number on SV-SMC migration. (A) Basal migration rates were collated for SV-SMC from all patient donors used in the study and separated according to passage number. (B) Fold change in migration in response to apo(a) in the upper chamber or (C) PDGF in the lower chamber according to passage number. All comparisons were non-significant (one-way ANOVA).
Effect of apo(a) on PDGF-induced ERK phosphorylation. Cells were treated with increasing concentrations of apo(a) either alone or in combination with 10 ng/mL PDGF for 20 min, and lysates blotted for phospho-ERK-1/2 and total ERK-1/2 (upper panel). Densitometry data were normalised to the lane containing PDGF alone, and analysed using ratio repeated measures one-way ANOVA with Newman–Keuls post-hoc test (lower panel n = 4, **P < 0.01).
Effect of RhoA siRNA and ROCK inhibition on apo(a) migration in the presence of PDGF. (A) Number of migrated cells per HP field in response to PDGF and apo(a) following RhoA silencing (closed bars) compared to mock transfected cells (open bars, n = 7). (B) Migration in response to PDGF and apo(a) following pre-treatment with vehicle control (0.002% dH2O; open bars) or 10 μM ROCK inhibitor, Y-27632 (closed bars) for 1 h (n = 5). **P < 0.01, NS = non-significant.