Abstract
Mesenchymal stem cells (MSCs) are multipotent adult stem cells that have regenerative capability and exert paracrine actions on damaged tissues. Since peritoneal fibrosis is a serious complication of peritoneal dialysis, we tested whether MSCs suppress this using a chlorhexidine gluconate model in rats. Although MSCs isolated from green fluorescent protein–positive rats were detected for only 3 days following their injection, immunohistochemical staining showed that MSCs suppressed the expression of mesenchymal cells, their effects on the deposition of extracellular matrix proteins, and the infiltration of macrophages for 14 days. Moreover, MSCs reduced the functional impairment of the peritoneal membrane. Cocultures of MSCs and human peritoneal mesothelial cells using a Transwell system indicated that the beneficial effects of MSCs on the glucose-induced upregulation of transforming growth factor-β1(TGF-β1) and fibronectin mRNA expression in the human cells were likely due to paracrine actions. Preincubation in MSC-conditioned medium suppressed TGF-β1-induced epithelial-to-mesenchymal transition, α-smooth muscle actin, and the decrease in zonula occludens-1 in cultured human peritoneal mesothelial cells. Although bone morphogenic protein 7 was not detected, MSCs secreted hepatocyte growth factor and a neutralizing antibody to this inhibited TGF-β1 signaling. Thus, our findings imply that MSCs ameliorate experimental peritoneal fibrosis by suppressing inflammation and TGF-β1 signaling in a paracrine manner.
Keywords: epithelial-to-mesenchymal transition, hepatocyte growth factor, mesenchymal stem cells, peritoneal fibrosis, transforming growth factor-β1
Peritoneal dialysis (PD) has been used as a beneficial treatment for end-stage kidney disease. However, peritoneal fibrosis remains a serious complication of long-term PD that leads to the failure of peritoneal function.1, 2 Peritoneal fibrosis arises in response to a variety of injurious factors, including bioincompatible dialysate components, uremic toxins, refractory or recurrent infectious peritonitis, and chronic inflammation.3, 4, 5 The characteristic histological features of the fibrotic sclerosing peritoneum are loss of peritoneal mesothelial cells, abnormal proliferation of α-smooth muscle actin (α-SMA)-positive myofibroblasts, marked accumulation of collagen, and a progressive increase in the thickness of the submesothelial compact zone.5, 6
As the essential mechanism of peritoneal fibrosis, the epithelial-to-mesenchymal transition (EMT) has been identified in cultured human peritoneal mesothelial cells (HPMCs)7 and in PD patients.8 Many studies have reported that transforming growth factor-β1 (TGF-β1) has a pivotal role in the induction of EMT,7, 9 typically characterized by reduced cell–cell adhesion, loss of epithelial polarity, and de novo expression of α-SMA and other fibrogenic mediators.10, 11 On binding to its receptors (types I and II serine/threonine kinases), TGF-β1 signaling leads to phosphorylation of receptor-regulated Smads (R-Smads) such as Smad2 and Smad3. Subsequently, R-Smads form a heteromultimer with Smad4, translocate to the nucleus, and regulate transcription of target genes.12 The Smad proteins are thought to have an important role in the process of fibrosis. Various studies performed in vitro and in animal models have suggested that drugs, peptides, and gene therapy targeting fibrosis and/or angiogenesis could be useful for treating peritoneal fibrosis,13, 14, 15, 16 whereas clinically available treatment for peritoneal fibrosis in PD patients remains limited at present.
Mesenchymal stem cells (MSCs) are multipotent adult stem cells that can be obtained from the bone marrow and many other tissues.17, 18, 19 MSCs have been used for the regeneration of various tissues because these cells have the capability to differentiate into a variety of lineages, including bone, cartilage, cardiac myocytes, and neurons.20, 21, 22, 23 In addition to their regenerative capability, MSCs display immunomodulatory and antifibrotic activity that could be important in the response to injury.24 The antifibrotic effects of cultured MSCs have been demonstrated in different animal models.25, 26 Although ex vivo-expanded MSCs have been used in many basic studies and clinical trials, few authors have assessed the potential of MSCs to inhibit peritoneal fibrosis. Mesothelial cell transplantation has been studied in animal models and PD patients,27, 28 and this approach may be one of the options for alleviating peritoneal membrane dysfunction. Because infusion of autologous MSCs is considered to be safe and has few adverse effects, it seems to be promising for clinical use.
In this work, we initially examined whether intraperitoneal infusion of MSCs could engraft and ameliorate chlorhexidine gluconate (CG)-induced peritoneal fibrosis in a rat model. To elucidate the paracrine effect of MSCs, we cocultured glucose-stimulated HPMCs with human MSCs using a Transwell system and investigated the expression of TGF-β1 and fibronectin. Lastly, we investigated the effects of MSC-conditioned medium (MSC-CM) on TGF-β1-induced Smad2 activation and the EMT response of HPMCs.
Our findings demonstrated that MSCs were retained in the peritoneum, inhibited inflammation, and ameliorated peritoneal fibrosis, thereby reducing the functional impairments of the peritoneal membrane. Moreover, MSCs inhibited glucose-induced TGF-β1 production and ameliorated the TGF-β1-induced EMT by inhibition of TGF-β1 signaling in HPMCs by exerting paracrine actions, including secretion of hepatocyte growth factor (HGF).
RESULTS
MSCs suppressed the accumulation of peritoneal cells and the thickening induced by CG
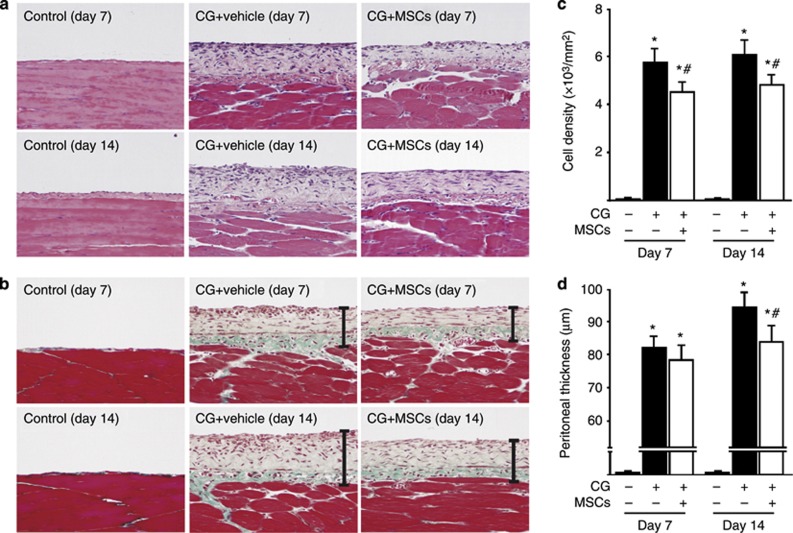
Hematoxylin–eosin staining (Figure 1a) was used to assess changes in cell density, and Masson's trichrome staining (Figure 1b) was used to analyze peritoneal thickening. We found that injection of 2 ml of 0.1% CG induced thickening of the submesothelial compact zone, and that the thickness and cellularity of this zone gradually increased until day 14. On day 7, the thickness of the submesothelial compact zone did not differ markedly between the rats treated with the vehicle and the rats that had been treated with MSCs (1 × 107 cells) injected intraperitoneally 30 min after CG stimulation. In contrast, the cell density of the collagenous compact zone was significantly suppressed in rats treated with MSCs compared with rats given the vehicle. On day 14, both peritoneal cell density and thickness were suppressed in rats treated with MSCs compared with rats given the vehicle (Figure 1c and d).
Figure 1.
Mesenchymal stem cells (MSCs) suppressed peritoneal cell density and thickening in chlorhexidine gluconate (CG)-injected rats. (a, b) Representative light microscopic features of peritoneal tissues on days 7 and 14 (a: hematoxylin–eosin stain; b: Masson's trichrome stain, original magnification × 200) in control rats, CG-injected rats treated with the vehicle, and CG-injected rats treated with MSCs. (c, d) The thickness of the submesothelial compact zone increased along with its cellularity until day 14 in rats treated with the vehicle, whereas cell density and thickening of the zone were suppressed in rats treated with MSCs. Bars show the compact zone area. *P<0.01 versus control group, #P<0.05 versus CG+vehicle group.
MSCs suppressed the expression of mesenchymal markers and extracellular matrix proteins in rats with peritoneal fibrosis
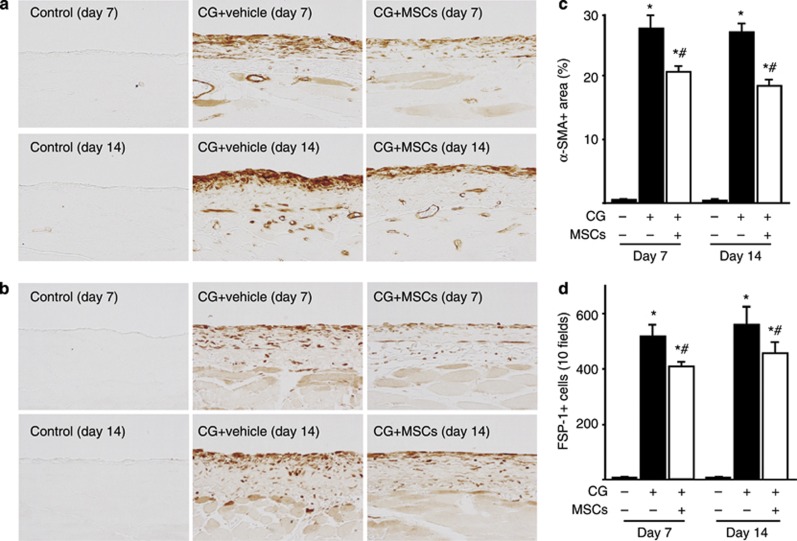
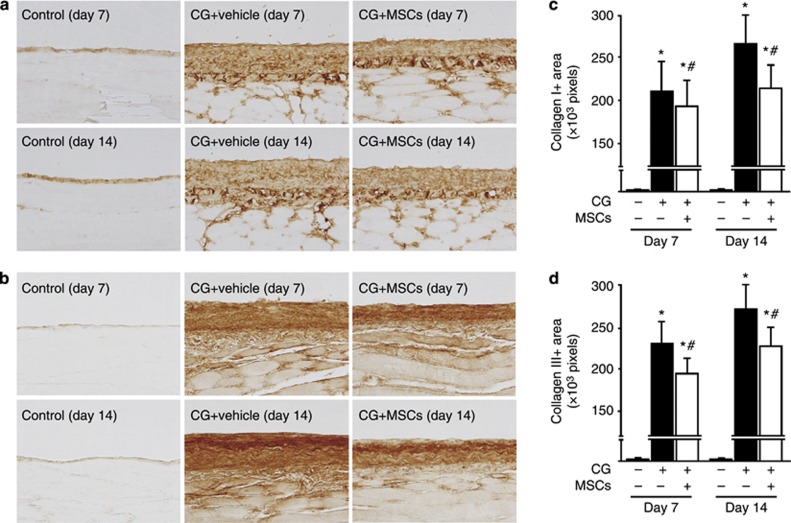
We examined the peritoneal expression of α-SMA and fibroblast-specific protein-1 (FSP-1) as mesenchymal markers. After injection of CG, α-SMA expression was found in myofibroblasts that accumulated in the upper layer of the submesothelial compact zone on days 7 and 14, as well as in vascular smooth muscle cells (Figure 2a). Treatment with MSCs significantly suppressed the extent of the α-SMA-positive area on days 7 and 14 compared with rats receiving the vehicle (Figure 2c). Figure 2b shows that FSP-1+ cells also accumulated in the upper layer of the submesothelial compact zone on days 7 and 14. Treatment with MSCs significantly suppressed the number of FSP-1+ cells on days 7 and 14 compared with that in rats receiving the vehicle (Figure 2d). Collagens I and III are extracellular matrix proteins, and we showed that collagens I and III were expressed in the submesothelial compact zone on day 7 and showed an increase by day 14 (Figure 3a and b). Treatment with MSCs reduced the area in which collagens I and III accumulated on days 7 and 14 compared with the effect of the vehicle alone (Figure 3c and d).
Figure 2.
Mesenchymal stem cells (MSCs) suppressed α-smooth muscle actin (α-SMA) and fibroblast-specific protein-1 (FSP-1) expressions in rats with peritoneal fibrosis. Immunohistochemical analyses of (a) α-SMA and (b) FSP-1 expression in peritoneal tissues on days 7 and 14 (original magnification × 200) in control rats, chlorhexidine gluconate (CG)-injected rats treated with the vehicle alone, and CG-injected rats treated with MSCs. (c, d) Accumulation of α-SMA+ and FSP-1+ cells was observed on days 7 and 14 in CG-injected rats treated with the vehicle, whereas the areas (percentage) of α-SMA expression and the number of FSP-1+ cells were significantly smaller on both days 7 and 14 in CG-injected rats treated with MSCs. *P<0.01 versus control group, #P<0.05 versus CG+vehicle group.
Figure 3.
Mesenchymal stem cells (MSCs) suppressed collagen I and III expressions in rats with peritoneal fibrosis. Immunohistochemical analyses of (a) collagen I and (b) collagen III expression in peritoneal tissues on days 7 and 14 (original magnification × 200) in control rats, chlorhexidine gluconate (CG)-injected rats treated with the vehicle alone, and CG-injected rats treated with MSCs. (c, d) The numbers of collagen I+ and III+ pixels were increased on days 7 and 14 in CG-injected rats treated with the vehicle, whereas the numbers of collagen I+ and III+ pixels were significantly smaller on days 7 and 14 in CG-injected rats treated with MSCs. *P<0.01 versus control group, #P<0.05 versus CG+vehicle group.
MSCs suppressed infiltration of monocytes/macrophages and TGF-β1/Smad signaling
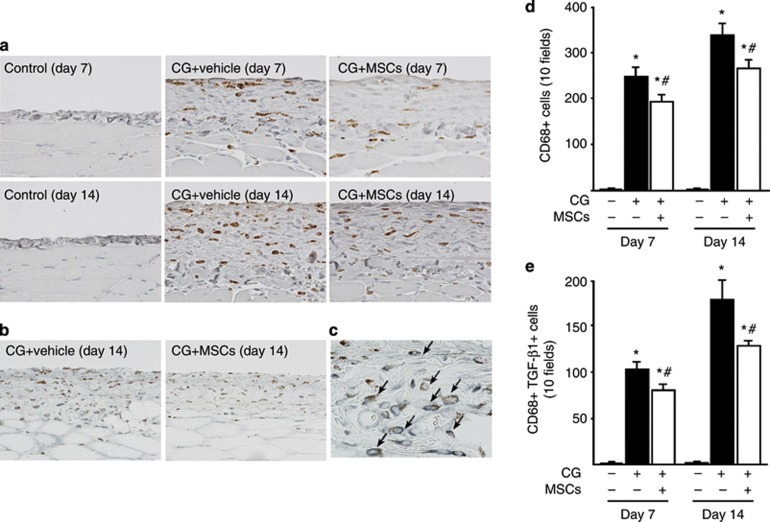
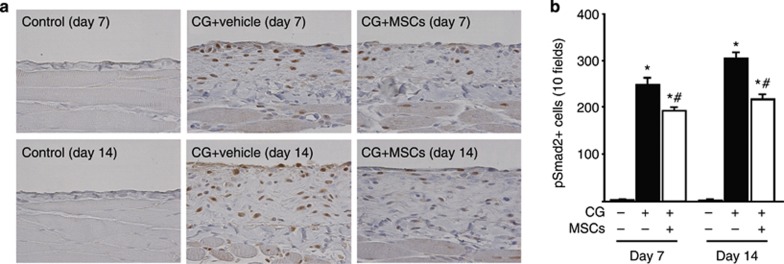
To evaluate monocyte/macrophage infiltration into the peritoneum, we examined CD68 expression. We found an accumulation of CD68+ cells in the submesothelial zone on day 7 and a further increase on day 14 (Figure 4a). Treatment with MSCs significantly inhibited the infiltration of CD68+ cells on days 7 and 14 compared with that in rats receiving the vehicle alone (Figure 4d). The majority of CD68+ cells showed immunoreactivity for TGF-β1 (Figure 4c, arrows), demonstrating colocalization of CD68 and TGF-β1. The number of cells double positive for CD68 and TGF-β1 in the submesothelial zone increased until day 14 (Figure 4b). Administration of MSCs significantly inhibited the infiltration of cells double positive for CD68 and TGF-β1 on days 7 and 14 compared with treatment with the vehicle alone (Figure 4e). Moreover, immunoreactivity of phosphorylated Smad2 (pSmad2) was seen in nuclei. pSmad2+ cells increased in the submesothelial zone on day 7 and showed a further increase on day 14 (Figure 5a). Treatment with MSCs significantly inhibited the number of pSmad2+ cells on days 7 and 14 compared with those in rats receiving the vehicle alone (Figure 5b).
Figure 4.
Mesenchymal stem cells (MSCs) suppressed monocyte/macrophage infiltration and transforming growth factor-β1 (TGF-β1) expression in rats with peritoneal fibrosis. (a) Immunohistochemical analysis of CD68 expression in peritoneal tissues on days 7 and 14 in control rats, chlorhexidine gluconate (CG)-injected rats treated with vehicle alone, and CG-injected rats treated with MSCs. (b) Two-color immunohistochemical analysis of CD68 (brown) and TGF-β1 (blue–gray) expression in peritoneal tissues on day 14 in CG-injected rats treated with the vehicle alone and CG-injected rats treated with MSCs. (c) The majority of CD68+ cells showed immunoreactivity for TGF-β1 (arrows). (d) The number of CD68+ cells increased until day 14 in CG-injected rats treated with the vehicle alone, whereas it was significantly smaller on days 7 and 14 in CG-injected rats treated with MSCs. (e) The number of cells double positive for CD68 and TGF-β1 increased until day 14 in CG-injected rats treated with vehicle alone, whereas it was significantly smaller on days 7 and 14 in CG-injected rats treated with MSCs. *P<0.01 versus control group, #P<0.05 versus CG+vehicle group (original magnification: a and c × 400; b × 200).
Figure 5.
Mesenchymal stem cells (MSCs) suppressed phosphorylated Smad2 (pSmad2) expression in rats with peritoneal fibrosis. (a) Immunohistochemical analysis of pSmad2 expression in peritoneal tissues on days 7 and 14 (original magnification × 200) in control rats, chlorhexidine gluconate (CG)-injected rats treated with vehicle alone, and CG-injected rats treated with MSCs. (b) The number of pSmad2+ cells increased until day 14 in CG-injected rats treated with vehicle alone, whereas it was significantly smaller on days 7 and 14 in CG-injected rats treated with MSCs. *P<0.01 versus control group, #P<0.05 versus CG+vehicle group.
MSCs reduced the functional impairments of the peritoneal membrane in rats with peritoneal fibrosis
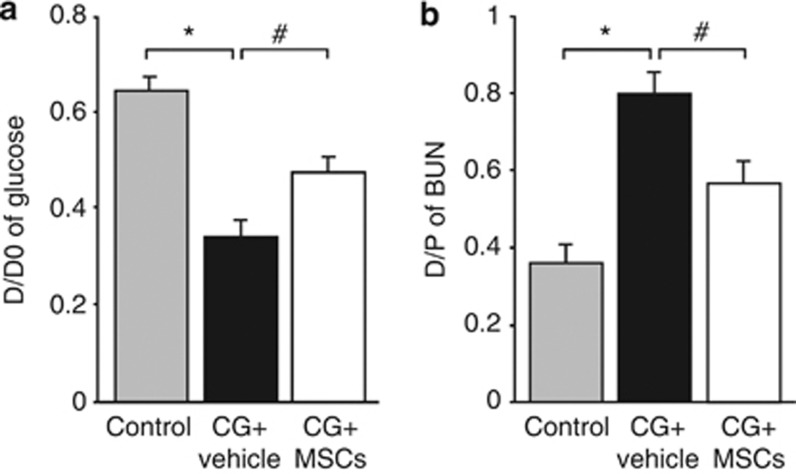
A peritoneal equilibrium test was performed on day 14 to assess the functional alteration of the peritoneal membrane. The absorption rate of glucose from the dialysate and the transport rate of blood urea nitrogen from the plasma were significantly higher in CG-injected rats treated with the vehicle alone than in control rats, whereas it was significantly improved in CG-injected rats treated with MSCs (Figure 6a and b).
Figure 6.
Mesenchymal stem cells (MSCs) reduced the functional impairments of the peritoneal membrane in rats with peritoneal fibrosis. (a) The peritoneal absorption of glucose from the dialysate (D/D0) and the (b) dialysate-to-plasma (D/P) ratio of blood urea nitrogen (BUN) were assessed in control rats, chlorhexidine gluconate (CG)-injected rats treated with vehicle alone, and CG-injected rats treated with MSCs during 30-min dwell of dialysate (4.25% Dianeal) at 100 ml/kg body weight. The peritoneal permeabilities of glucose and BUN were significantly higher in CG-injected rats treated with the vehicle alone than in control rats, whereas they were significantly improved in CG-injected rats treated with MSCs. *P<0.01, #P<0.05.
GFP-labeled MSCs showed temporary retention in the peritoneum following stimulation by CG
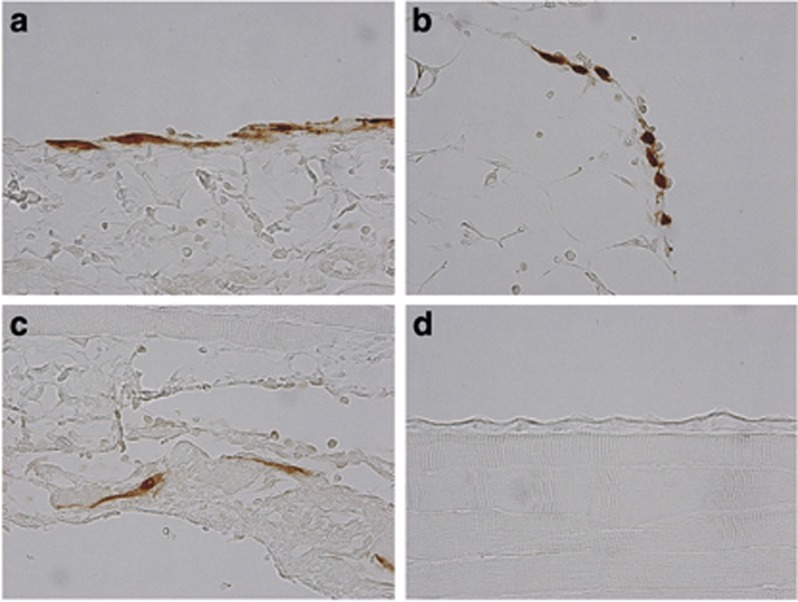
To assess the fate of intraperitoneally injected MSCs, we injected green fluorescent protein (GFP)-labeled MSCs after CG stimulation. As a control, we injected GFP+ MSCs without CG injection (vehicle injection alone). As a result, GFP+ MSCs were observed in the parietal peritoneum, omentum, and diaphragm of MSC-treated rats until day 3 (Figures 7a–c), but these cells were not detected on day 7 or day 14. The number of GFP+ MSCs in the parietal peritoneum was 7.39±2.06 cells/10 fields ( × 100 magnification) on day3. In contrast, no GFP+ MSCs were observed at any time in the parietal peritoneum of MSC-treated rats without intraperitoneal injection of CG (Figure 7d). We also could not detect GFP+ MSCs in other tissues such as the spleen, liver, lungs, or kidneys in either CG-stimulated rats or control rats at any time of assessment. These results indicate that MSCs were retained in the affected tissues for only a few days.
Figure 7.
Green fluorescent protein (GFP)-positive mesenchymal stem cells (MSCs) were briefly retained in the peritoneum after stimulation by chlorhexidine gluconate (CG). At 3 days after the injection of cells, GFP+ MSCs were present in the (a) parietal peritoneum, (b) omentum, and (c) diaphragm of CG-injected rats. (d) GFP-positive MSCs were not found in the parietal peritoneum of control rats treated with vehicle alone (original magnification × 400, all sections).
Human MSCs suppressed glucose-induced TGF-β1 and fibronectin expression in HPMCs
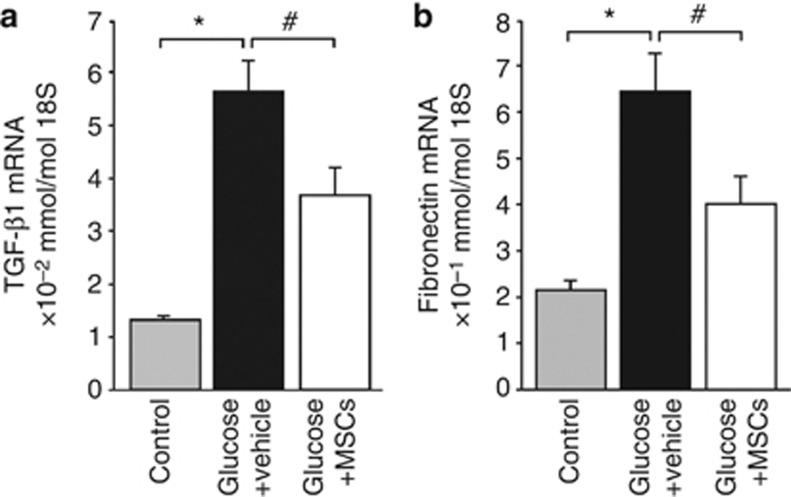
To investigate whether the effect of human MSCs on glucose-induced TGF-β1 and fibronectin gene expression in HPMCs required cell–cell contact, HPMCs were treated with 4% D-glucose for 12 h and then were cocultured with the vehicle alone or with human MSCs using a Transwell system for 24 h. Glucose treatment significantly induced the expression of TGF-β1 and fibronectin mRNAs compared with that in control cells, whereas coculture of HPMCs with human MSCs resulted in a significant reduction of TGF-β1 and fibronectin mRNA expression compared with the levels in vehicle-treated cells (Figure 8a and b). These results suggest that MSCs acted to suppress the fibroproliferative response of HPMCs in a paracrine manner.
Figure 8.
Mesenchymal stem cells (MSCs) suppressed glucose-induced upregulation of transforming growth factor-β1 (TGF-β1) and fibronectin expression in human peritoneal mesothelial cells. Incubation with glucose significantly induced expressions of (a) TGF-β1 mRNA and (b) fibronectin mRNA compared with control cells, whereas coculture with human MSCs led to a significant reduction of TGF-β1 and fibronectin mRNA expression (n=6). *P<0.01, #P<0.05.
MSC-CM inhibited TGF-β1-induced phosphorylation of Smad2 and counteracted TGF-β1-induced EMT of HPMCs
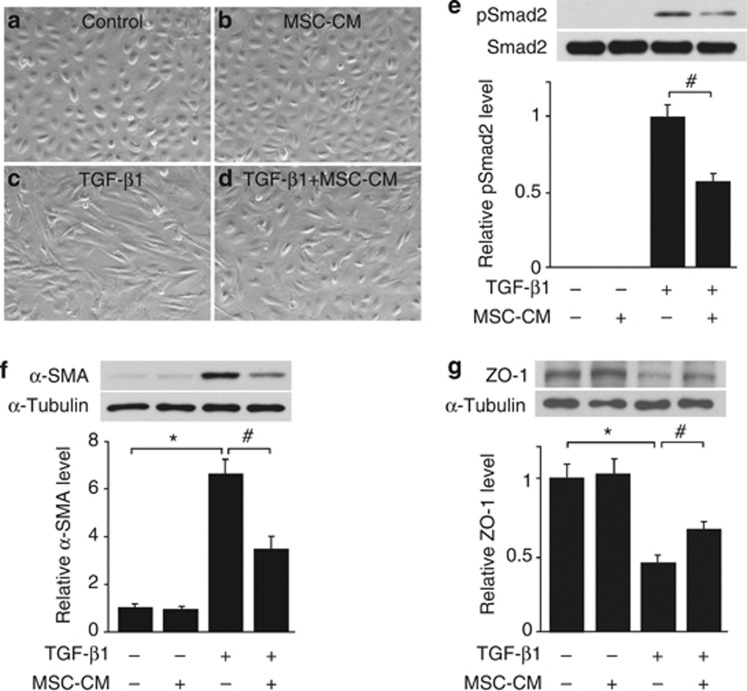
TGF-β1 is the most potent growth factor that can induce EMT in various types of cells.29 To investigate whether MSCs suppressed fibrotic change through paracrine effects, we prepared MSC-CM from 2-day cultures of MSCs and examined its effects on TGF-β1-induced EMT of HPMCs. HPMCs were incubated with MSC-CM for 12 h and then stimulated with TGF-β1 at 2.5 ng/ml. Morphologic changes of cultured HPMCs 48 h after the stimulation are shown in Figure 9a–d. There were no significant changes in the characteristic cobblestone-like appearance of control HPMCs and HPMCs incubated with MSC-CM without TGF-β1 stimulation. Fibroblastoid change was observed following TGF-β1 stimulation, whereas the change was milder in HPMCs incubated with MSC-CM than that in HPMCs incubated with normal medium. Western blot analysis shows that MSC-CM inhibited TGF-β1-induced phosphorylation of Smad2 in HPMCs (Figure 9e). TGF-β1 treatment for 48 h increased α-SMA (mesenchymal marker) protein expression and decreased zonula occludens-1 (epithelial marker) protein expression. MSC-CM attenuated these TGF-β1-induced EMT responses (Figure 9f and g).
Figure 9.
Mesenchymal stem cell (MSC)-conditioned media (CM) blocked transforming growth factor-β1 (TGF-β1)-induced epithelial-to-mesenchymal transition (EMT). Representative photomicrograph shows characteristic cobblestone-like appearances of (a) control human peritoneal mesothelial cells (HPMCs) and (b) HPMCs incubated with MSC-CM without TGF-β1 stimulation. Fibroblast-like change was observed following TGF-β1 stimulation for 48 h, but the change was reduced in (d) HPMCs incubated with MSC-CM than in (c) HPMCs incubated with normal medium. HPMCs were observed by an independent investigator who was blinded to the experimental conditions. (e) Western blot analysis shows that MSC-CM inhibited phosphorylation of Smad2 in HPMCs after 30 min of TGF-β1 stimulation. (f, g) TGF-β1 treatment for 48 h caused increased α-smooth muscle actin (α-SMA) protein expression and decreased zonula occludens-1 (ZO-1) protein expression. MSC-CM attenuated these TGF-β1-induced EMT responses (n=6). *P<0.01, #P<0.05.
MSC secreted HGF and inhibited TGF-β1 signaling in HPMCs
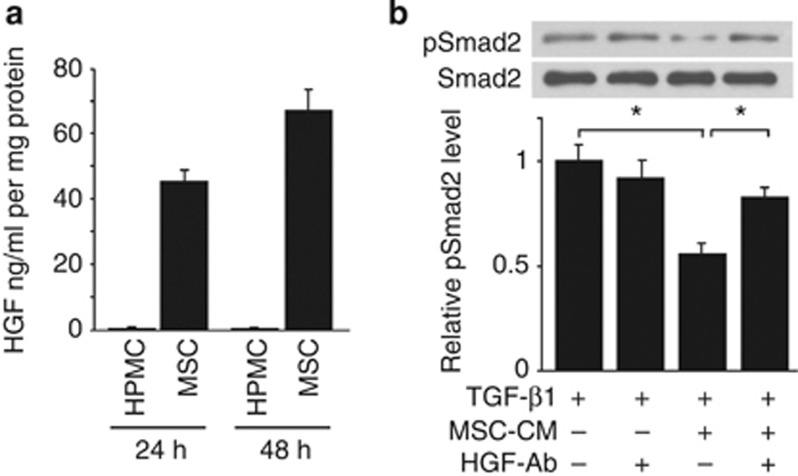
It is well recognized that HGF and bone morphogenic protein 7 (BMP-7) have inhibitory effects on TGF-β1 signaling and suppress EMT.30, 31, 32 We cultured HPMCs and human MSCs in 0.1% fetal bovine serum (FBS) containing Dulbecco's modified Eagle's medium for 2 days, and the concentrations of HGF and BMP-7 in conditioned medium were measured by enzyme-linked immunosorbent assays. HGF concentration in the MSC-CM increased in a time-dependent manner, whereas it was below the level of detection in HPMC-CM (Figure 10a). On the other hand, the concentrations of BMP-7 in MSC-CM and HPMC-CM were below the level of detection (data not shown). To assess the effect of HGF on TGF-β signaling, MSC-CM and normal media were preincubated with or without HGF-neutralizing antibody (10 μg/ml) for 1 h before its addition to HPMC cultures. Western blot analysis shows that MSC-CM inhibited phosphorylation of Smad2 in HPMCs after 30 min of TGF-β1 stimulation and HGF-neutralizing antibody blocked the inhibitory effect of MSC-CM. These results suggest the possibility that HGF secreted by MSCs inhibits TGF-β1-induced EMT responses (Figure 10b).
Figure 10.
Mesenchymal stem cells (MSCs) secreted hepatocyte growth factor (HGF) and inhibited transforming growth factor-β1 (TGF-β1) signaling. (a) Human peritoneal mesothelial cells (HPMCs) and human MSCs were cultured in 0.1% fetal bovine serum (FBS) containing Dulbecco's modified Eagle's medium (DMEM) for 48 h and HGF concentration in the conditioned media (CM) was measured in triplicate by enzyme-linked immunosorbent assay (ELISA) and the mean data were used. HGF concentration in the MSC-CM was increased in a time-dependent manner, whereas it was below the detection level in the HPMC-CM. (b) MSC-CM and normal medium were preincubated with or without HGF-neutralizing antibody (HGF-Ab; 10 μg/ml) for 1 h before its addition to HPMC cultures. Western blot analysis shows that MSC-CM inhibited phosphorylation of Smad2 (pSmad2) in HPMCs after 30 min of TGF-β1 stimulation and HGF-neutralizing antibody blocked the inhibitory effect of MSC-CM (n=6). *P<0.05.
DISCUSSION
This study has provided the first evidence that MSCs have a therapeutic potential for the treatment of peritoneal fibrosis by directly inhibiting TGF-β1 signaling in a paracrine manner. Intraperitoneal administration of MSCs ameliorated CG-induced peritoneal fibrosis in rats, as evidenced by a significant reduction of peritoneal thickening and collagen deposition, as well as a significant suppression of myofibroblast and macrophage accumulation. In addition, MSCs reduced the functional impairments of the peritoneal membrane. These results indicate that the beneficial effects of MSCs on peritoneal damage include reductions in local pathology, as well as improvements in physiological function. The injected MSCs were detected on the peritoneal surface until day 3 in rats following CG-induced peritoneal fibrosis, but were not seen in rats without peritoneal fibrosis. These findings suggest that intraperitoneally injected MSCs migrated to the sites of peritoneal injury and displayed both immunomodulatory and antifibrotic effects in the absence of long-term engraftment of the administered cells.
MSCs are known to migrate preferentially to the sites of inflammation following tissue injury when infused into animals.33 Although the underlying molecular mechanism of homing and engraftment by these cells is not yet fully understood, expression of specific growth factors, chemokines, and extracellular matrix receptors on the surfaces of MSCs may facilitate their trafficking, adhesion, and infiltration into sites of injury.34, 35 In this study, short-term retention of intraperitoneally administered GFP+ MSCs into the peritoneal tissues was demonstrated in rats with CG-induced peritoneal fibrosis, whereas GFP+ MSCs were not detected in control rats lacking fibrosis. These findings suggest that the MSCs migrated to the sites of CG-induced peritoneal damage.
Recently, Sekiguchi et al.36 reported that bone marrow–derived cells accumulated and were maintained in the submesothelium for 3 weeks after discontinuation of CG in a mouse model of CG-induced peritoneal fibrosis. Their results suggest that bone marrow–derived cells have an important role in the process of peritoneal repair and mesothelial remodeling. In this study, retention of bone marrow–derived MSCs expanded ex vivo was only observed until day3. However, the anti-inflammatory and antifibrotic effects in the peritoneum were prolonged until day 14. Such effects may be independent of transdifferentiation of MSCs into functional peritoneal mesothelial cells and are more likely to be due to paracrine activities of MSCs. Previous in vivo and in vitro studies have suggested that paracrine mechanisms involving the production of growth factors and anti-inflammatory cytokines by MSCs are associated with these properties.37, 38 To identify an anti-inflammatory factor, we performed enzyme-linked immunosorbent assay to detect anti-inflammatory interleukin-10 in MSC-CM, but the interleukin-10 concentration of MSC-CM was below the level of detection (data not shown). In addition, we could not detect inhibitory effects of MSCs on inflammatory cytokine signaling such as interleukin-6-induced phosphorylation of signal transducer and activator of transcription 3 (data not shown). Although the mechanism of the anti-inflammatory effect of MSCs in our rat model of peritoneal fibrosis remains unclear, it has raised the possibility that the anti-inflammatory paracrine action may affect host macrophages and not peritoneal mesothelial cells. In fact, Németh et al.39 reported that MSCs attenuate sepsis through prostaglandin E2–dependent production of interleukin-10, which was not directly produced by injected MSCs but by macrophages.
As part of the immune response to peritoneal injury or infection in PD patients, various cytokines and growth factors from migrating macrophages or mesothelial cells were suggested to contribute to the progression of peritoneal fibrosis. Among them, TGF-β1 has been identified as the most potent growth factor inducing EMT in peritoneal mesothelial cells.7, 8, 9, 40 Moodley et al.41 reported that systemically administered human umbilical cord MSCs suppressed fibrosis of bleomycin-induced lung injury and inhibited TGF-β1 expression that led to reduction in Smad2 phosphorylation. In this study, we demonstrated that intraperitoneal injection of MSCs suppressed TGF-β1 expression by migrating macrophages in CG-stimulated rat peritoneum. Moreover, coculture with MSCs suppressed glucose-induced gene expression of TGF-β1 by HPMCs in vitro. Furthermore, we showed that MSC-CM inhibited TGF-β1-induced phosphorylation of Smad2 and ameliorated TGF-β1-induced changes in the protein levels of α-SMA and zonula occludens-1. These results suggest that MSCs acted to suppress TGF-β1 signaling and TGF-β1-induced EMT responses in a paracrine manner.
Many investigators have shown that MSCs actively secrete several essential growth factors.42 HGF and BMP-7 are potent mediators of antifibrotic cytokines that display therapeutic potential in preventing tissue fibrosis and disturb TGF-β1 signaling.30, 31, 32 A number of studies suggest that HGF and BMP-7 have a protective role in peritoneal fibrosis.43, 44 We showed that the concentration of HGF in MSC-CM increased in a time-dependent manner, whereas BMP-7 was undetectable (data not shown). In addition, we demonstrated that HGF secreted by MSCs is implicated in the inhibition of the TGF-β1 signaling.
In this study, most of the injected MSCs were presumably lost because of acute toxicity of CG. However, immunomodulatory and antifibrotic effects of MSCs on peritoneal fibrosis were observed despite the short retention of administered cells, and no adverse effects were detected. In order to improve the outcome, further studies are warranted to confirm the effect of MSCs in other models of peritoneal fibrosis and optimal dosage and timing of MSC administration. In conclusion, our results suggest that MSCs ameliorate experimental peritoneal fibrosis by suppressing inflammation and inhibiting TGF-β1 signaling in a paracrine manner.
MATERIALS AND METHODS
Isolation and culture of MSCs
Bone marrow was collected from rats by flushing the femurs and cultured as described previously.45 Fourth-passage cells were used for transplantation. It was confirmed that these cells were MSCs by promoting their differentiation into osteocytes and adipocytes with specific differentiation media.45
Injection of MSCs
We used male Fisher 344 rats (CLEA Japan, Tokyo, Japan) at 8 weeks of age. An injection of 0.1% CG in 15% ethanol dissolved in 2 ml of saline was given intraperitoneally. After 30 min, bone marrow MSCs (1 × 107 cells) suspended in 1 ml of phosphate-buffered saline were injected intraperitoneally or vehicle was injected (n=10 in each group at each time point). At 7 and 14 days after injection, the rats were killed and the parietal peritoneum was carefully dissected for examination. A peritoneal equilibrium test was performed on day 14. Rats were instilled with PD solution (4.25% Dianeal; Baxter HealthCare, Deerfield, IL) at 100 ml/kg body weight before being killed. After 30 min, the peritoneal fluid was removed and blood samples were obtained by cardiac puncture. The peritoneal permeabilities of glucose and blood urea nitrogen were expressed as the peritoneal absorption of glucose from the dialysate and the dialysate-to-plasma ratio of blood urea nitrogen.
Next, we used male Sprague–Dawley rats (CLEA Japan) at 8 weeks of age. An injection of 0.1% CG in 15% ethanol dissolved in 2 ml of saline was given intraperitoneally, or 15% ethanol in 2 ml of saline was injected as a control (n=5 in each group at each time point). After 30 min, MSCs (1 × 107 cells) obtained from the bone marrow of GFP-transgenic Sprague–Dawley rats (Japan SLC, Shizuoka, Japan) and suspended in 1 ml of phosphate-buffered saline were injected intraperitoneally. At 1, 3, 7, and 14 days after injection, rats were killed and the parietal peritoneum, omentum, and diaphragm were carefully dissected to immunohistochemically examine the retention of GFP-positive MSCs.
All of the animal experiments were approved by the institutional animal care and use committee of Hiroshima University (Hiroshima, Japan) and were performed in accordance with the National Institutes of Health Guidelines on the Use of Laboratory Animals.
Histology and immunohistochemistry
Histology and immunohistochemical staining of 4-μm-thick tissue sections were performed as described previously.46 The following primary antibodies were used: mouse monoclonal anti-α-SMA antibody (Sigma, St Louis, MO); rabbit polyclonal anti-FSP-1 antibody (Abcam, Cambridge, UK); rabbit polyclonal anti-collagen I antibody (Abcam); rabbit polyclonal anti-collagen III antibody (Chemicon International, Temecula, CA); mouse monoclonal anti-rat CD68 antibody (Serotec, Oxford, UK); rabbit polyclonal anti-TGF-β1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit polyclonal anti-pSmad2 antibody (Chemicon); and rabbit polyclonal anti-GFP antibody (Clontech, Palo Alto, CA).
The areas of α-SMA, collagen I, and collagen III were assessed in predetermined fields ( × 200) of the submesothelial compact zone captured by a digital camera and the area stained was determined using a Lumina Vision 2.20 (Mitani, Osaka, Japan) in 10 fields. The numbers of cells positive for FSP-1, CD68, pSmad2 ( × 400), GFP ( × 100), and CD68/TGF-β1 (double positive, × 200) of the submesothelial compact zone were counted in 10 fields.
Cell culture
Specimens of human omentum were obtained from patients undergoing elective abdominal surgery without peritonitis or peritoneal carcinosis. The HPMCs were isolated from human omentum as described previously.47 Chemicals and plastic tissue culture dishes were as described in our earlier studies,48 and cells from the second to third passages were used. Harvesting of the omentum was permitted by the Medical Ethics Committee of Hiroshima Graduate School of Biomedical Science, and informed consent was obtained from each patient. We performed individual experiments using HPMCs from different isolates at least three times. Human bone marrow MSCs were purchased from Bio Whittaker (Walkersville, MD).
HPMCs (1 × 105 cells/well) were seeded into six-well plates and grown to subconfluence. The culture medium was then replaced with Dulbecco's modified Eagle's medium plus 2% FBS alone (control) or 2% FBS plus 4% D-glucose for 12 h. To test the effect of coculture of glucose-stimulated HPMCs with human MSCs while avoiding direct cell contact, human MSCs (1 × 105 cells/well) were grown on the bottom of upper well inserts (0.4 μm pore size; Becton Dickinson Labware, Franklin Lakes, NJ). Inserts with cultured MSCs or medium alone were then dipped into the basal plate of cultured glucose-stimulated HPMCs for 24 h. Expression of TGF-β1 and fibronectin mRNAs by HPMCs was assessed by reverse transcription–PCR.
MSC-CM was prepared by exposing ∼80% confluent human MSC cultures to 0.1% FBS containing Dulbecco's modified Eagle's medium for 2 days. HPMCs (1 × 105 cells/well) were seeded into six-well plates and grown until subconfluence. The culture medium was then replaced with Dulbecco's modified Eagle's medium plus 0.1% FBS alone or MSC-CM for 12 h and HPMCs were incubated in the absence or presence of 2.5 ng/ml recombinant human TGF-β1 (R&D Systems, Minneapolis, MN). Antibody neutralization of MSC-CM was performed with 10 μg/ml anti-human HGF antibody (AF-294-NA; R&D Systems) at 37 °C for 1 h before its addition to HPMC cultures. Whole-cell lysates were prepared and subjected to western blot analysis.
RNA extraction and quantitative real-time reverse transcription–PCR
RNA extraction and reverse transcription–PCR were performed as previously described.49 Specific oligonucleotide primers and probes for TGF-β1 (assay ID: Hs99999918_m1), fibronectin (assay ID: Hs00365058_m1), and 18S rRNA (endogenous control) were obtained as TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA). The mRNA levels were normalized for the level of 18S rRNA.
Western blot analysis and enzyme-linked immunosorbent assay
Sample collection and immunoblotting were performed as previously described.50 Primary antibodies used in this study were anti-phosphorylated Smad2 (Cell Signaling Technology, Beverley, CA), anti-Smad2 (Cell Signaling), mouse monoclonal anti-α-SMA antibody (Sigma), rabbit polyclonal anti-zonula occludens-1 (Zymed, San Francisco, CA), and mouse monoclonal anti-α-tubulin antibody (Santa Cruz Biotechnology). Secondary antibodies used in this study were horseradish peroxidase–conjugated goat anti-rabbit immunoglobulin G antibody (Dako, Glostrup, Denmark) or goat anti-mouse immunoglobulin G antibody (Dako). Signals were detected using the SuperSignal West Dura system (Thermo Fisher, Rockford, IL). The intensity of each band was determined using ImageJ software (version 1.44p; National Institutes of Health, Bethesda, MD). The enzyme-linked immunosorbent assay analyses for HGF and BMP-7 (R&D Systems) were performed following the manufacturer's instructions. The results were normalized to the total protein content.
Statistical analysis
Results are expressed as the mean±s.e. Statistical analysis was performed using analysis of variance followed by Tukey's post hoc test, and P<0.05 was considered to be statistically significant.
Acknowledgments
This work was supported by grants-in-aid for science from the Ministry of Education, Culture, Sport, Science, and Technology of Japan, by a scientific grant from Baxter, and also by grants-in-aid for kidney failure and hemodialysis research from the Japanese Association of Dialysis Physicians.
All the authors declared no competing interests.
References
- Dobbie J. Morphology of the peritoneum in CAPD. Blood Purif. 1989;7:74–85. doi: 10.1159/000169580. [DOI] [PubMed] [Google Scholar]
- Nakamoto H, Kawaguchi Y, Suzuki H. Encapsulating peritoneal sclerosis in patients undergoing continuous ambulatory peritoneal dialysis in Japan. Adv Perit Dial. 2002;18:119–123. [PubMed] [Google Scholar]
- Margetts P, Bonniaud P. Basic mechanisms and clinical implications of peritoneal fibrosis. Perit Dial Int. 2003;23:530–541. [PubMed] [Google Scholar]
- Mortier S, De Vriese A, Van de Voorde J, et al. Hemodynamic effects of peritoneal dialysis solutions on the rat peritoneal membrane: role of acidity, buffer choice, glucose concentration, and glucose degradation products. J Am Soc Nephrol. 2002;13:480–489. doi: 10.1681/ASN.V132480. [DOI] [PubMed] [Google Scholar]
- Williams J, Craig K, Topley N, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13:470–479. doi: 10.1681/ASN.V132470. [DOI] [PubMed] [Google Scholar]
- Mateijsen M, van der Wal A, Hendriks P, et al. Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit Dial Int. 1999;19:517–525. [PubMed] [Google Scholar]
- Yang A, Chen J, Lin J. Myofibroblastic conversion of mesothelial cells. Kidney Int. 2003;63:1530–1539. doi: 10.1046/j.1523-1755.2003.00861.x. [DOI] [PubMed] [Google Scholar]
- Yáñez-Mó M, Lara-Pezzi E, Selgas R, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003;348:403–413. doi: 10.1056/NEJMoa020809. [DOI] [PubMed] [Google Scholar]
- Margetts P, Bonniaud P, Liu L, et al. Transient overexpression of TGF-{beta}1 induces epithelial mesenchymal transition in the rodent peritoneum. J Am Soc Nephrol. 2005;16:425–436. doi: 10.1681/ASN.2004060436. [DOI] [PubMed] [Google Scholar]
- Aroeira L, Aguilera A, Sánchez-Tomero J, et al. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol. 2007;18:2004–2013. doi: 10.1681/ASN.2006111292. [DOI] [PubMed] [Google Scholar]
- Zavadil J, Böttinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Aroeira L, Lara-Pezzi E, Loureiro J, et al. Cyclooxygenase-2 mediates dialysate-induced alterations of the peritoneal membrane. J Am Soc Nephrol. 2009;20:582–592. doi: 10.1681/ASN.2008020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K, Maeshima Y, Ichinose K, et al. Endostatin peptide, an inhibitor of angiogenesis, prevents the progression of peritoneal sclerosis in a mouse experimental model. Kidney Int. 2007;71:227–238. doi: 10.1038/sj.ki.5002040. [DOI] [PubMed] [Google Scholar]
- Nishino T, Miyazaki M, Abe K, et al. Antisense oligonucleotides against collagen-binding stress protein HSP47 suppress peritoneal fibrosis in rats. Kidney Int. 2003;64:887–896. doi: 10.1046/j.1523-1755.2003.00169.x. [DOI] [PubMed] [Google Scholar]
- Guo H, Leung J, Lam M, et al. Smad7 transgene attenuates peritoneal fibrosis in uremic rats treated with peritoneal dialysis. J Am Soc Nephrol. 2007;18:2689–2703. doi: 10.1681/ASN.2007010121. [DOI] [PubMed] [Google Scholar]
- Prockop D. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Lee R, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- Wang H, Hung S, Peng S, et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- Horwitz E, Prockop D, Fitzpatrick L, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Yoo J, Barthel T, Nishimura K, et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Toma C, Pittenger M, Cahill K, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- Kopen G, Prockop D, Phinney D. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A, Dennis J. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Suga H, Eto H, Shigeura T, et al. IFATS collection: fibroblast growth factor-2-induced hepatocyte growth factor secretion by adipose-derived stromal cells inhibits postinjury fibrogenesis through a c-Jun N-terminal kinase-dependent mechanism. Stem Cells. 2009;27:238–249. doi: 10.1634/stemcells.2008-0261. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang S, Zhang Y, et al. Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Mol Biol Rep. 2009;36:725–731. doi: 10.1007/s11033-008-9235-2. [DOI] [PubMed] [Google Scholar]
- Hekking L, Zweers M, Keuning E, et al. Apparent successful mesothelial cell transplantation hampered by peritoneal activation. Kidney Int. 2005;68:2362–2367. doi: 10.1111/j.1523-1755.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- Di Paolo N, Sacchi G, Vanni L, et al. Autologous peritoneal mesothelial cell implant in rabbits and peritoneal dialysis patients. Nephron. 1991;57:323–331. doi: 10.1159/000186283. [DOI] [PubMed] [Google Scholar]
- Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- Shukla MN, Rose JL, Ray R, et al. Hepatocyte growth factor inhibits epithelial to myofibroblast transition in lung cells via Smad7. Am J Respir Cell Mol Biol. 2009;40:643–653. doi: 10.1165/rcmb.2008-0217OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Dai C, Liu Y. A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J Am Soc Nephrol. 2005;16:68–78. doi: 10.1681/ASN.2003090795. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Hanai J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Abe R, Fujita Y, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- Ponte A, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- Salem H, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi Y, Hamada C, Ro Y, et al. Differentiation of bone marrow-derived cells into regenerated mesothelial cells in peritoneal remodeling using a peritoneal fibrosis mouse model. J Artif Organs. 2012;15:272–282. doi: 10.1007/s10047-012-0648-2. [DOI] [PubMed] [Google Scholar]
- Kim Y, Park H, Lee G, et al. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009;57:13–23. doi: 10.1002/glia.20731. [DOI] [PubMed] [Google Scholar]
- Imberti B, Morigi M, Tomasoni S, et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro J, Aguilera A, Selgas R, et al. Blocking TGF-β1 protects the peritoneal membrane from dialysate-induced damage. J Am Soc Nephrol. 2011;22:1682–1695. doi: 10.1681/ASN.2010111197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley Y, Atienza D, Manuelpillai U, et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;175:303–313. doi: 10.2353/ajpath.2009.080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Niwa T. Pyridoxal phosphate and hepatocyte growth factor prevent dialysate-induced peritoneal damage. J Am Soc Nephrol. 2005;16:144–150. doi: 10.1681/ASN.2004020120. [DOI] [PubMed] [Google Scholar]
- Yu MA, Shin KS, Kim JH, et al. HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium. J Am Soc Nephrol. 2009;20:567–581. doi: 10.1681/ASN.2008040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi S, Shimazu A, Miyazaki K, et al. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288:413–419. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Taniguchi Y, Nakashima A, et al. Mizoribine suppresses the progression of experimental peritoneal fibrosis in a rat model. Nephron Exp Nephrol. 2009;112:e59–e69. doi: 10.1159/000213896. [DOI] [PubMed] [Google Scholar]
- Stylianou E, Jenner L, Davies M, et al. Isolation, culture and characterization of human peritoneal mesothelial cells. Kidney Int. 1990;37:1563–1570. doi: 10.1038/ki.1990.150. [DOI] [PubMed] [Google Scholar]
- Ito T, Yorioka N, Yamamoto M, et al. Effect of glucose on intercellular junctions of cultured human peritoneal mesothelial cells. J Am Soc Nephrol. 2000;11:1969–1979. doi: 10.1681/ASN.V11111969. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Masaki T, Kiribayashi K, et al. 15-Deoxy-Delta12,14-prostaglandin J2 inhibits angiotensin II-induced fibronectin expression via hepatocyte growth factor induction in human peritoneal mesothelial cells. Ther Apher Dial. 2010;14:43–51. doi: 10.1111/j.1744-9987.2009.00702.x. [DOI] [PubMed] [Google Scholar]
- Doi S, Zou Y, Togao O, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–8665. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]