Abstract
Venous neointimal hyperplasia (VNH) is responsible for hemodialysis vascular access malfunction. Here we tested whether VNH formation occurs, in part, due to vascular endothelial growth factor-A (VEGF-A) and matrix metalloproteinase (MMP)-9 gene expression causing adventitial fibroblast transdifferentiation to myofibroblasts (α-SMA-positive cells). These cells have increased proliferative and migratory capacity leading to VNH formation. Simvastatin was used to decrease VEGF-A and MMP-9 gene expression in our murine arteriovenous fistula model created by connecting the right carotid artery to the ipsilateral jugular vein. Compared to fistulae of vehicle-treated mice, the fistulae of simvastatin-treated mice had the expected decrease in VEGF-A and MMP-9 but also showed a significant reduction in MMP-2 expression with a significant decrease in VNH and a significant increase in the mean lumen vessel area. There was an increase in terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining, and decreases in α-SMA density, cell proliferation, and HIF-1α and hypoxyprobe staining. This latter result prompted us to determine the effect of simvastatin on fibroblasts subjected to hypoxia in vitro. Simvastatin-treated fibroblasts had a significant decrease in myofibroblast production along with decreased cellular proliferation, migration, and MMP-9 activity but increased caspase 3 activity suggesting increased apoptosis. Thus, simvastatin results in a significant reduction in VNH, with increase in mean lumen vessel area by decreasing VEGF-A/MMP-9 pathway activity.
Keywords: arteriovenous fistula, chronic kidney disease, murine model, restenosis, veins
In the United States, approximately 600,000 patients have end-stage renal disease, with the vast majority of patients requiring chronic hemodialysis for long-term survival, and this population of patients will double in the coming decades.1 Arteriovenous fistula (AVF) is the preferred vascular access; however, venous stenosis formation and lack of maturation are major problems, and thus 1-year patency rates are estimated to be 62%.2 Venous stenosis occurs in AVFs because of neointimal hyperplasia.3, 4, 5 Histologic analysis of AVF specimens reveals that there is angiogenesis located within the neointima and adventitia of the vessel, accompanied by increased proliferation of cells staining positive for α-smooth muscle actin (α-SMA) in the neointima.3, 4, 5 Recent experimental studies have demonstrated a pivotal role for adventitial and medial fibroblasts that convert to myofibroblasts (α-SMA-positive cells) and can subsequently contribute to the formation of venous neointimal hyperplasia.6, 7, 8 As a consequence, over a billion dollars are spent annually to maintain the functioning of hemodialysis AVFs and grafts.1 Effective, noninvasive treatments, which would prevent and/or reduce AVF stenosis, could greatly benefit patients with end-stage renal disease.
Vascular endothelial growth factor-A (VEGF-A) has been shown to be involved in the pathogenesis of arterial stenosis, vein bypass grafts, and venous neointimal hyperplasia associated with hemodialysis vascular access.5, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Previous work from our laboratory and other laboratories has demonstrated increased expression of VEGF-A and other profibrotic genes, including matrix metalloproteinase-2 (MMP-2) and metalloproteinase-9 (MMP-9), at the site of venous stenosis in murine and porcine models of hemodialysis AVF and AV graft failure. However, the mechanism(s) through which VEGF-A and MMPs have a role in venous neointimal hyperplasia formation has not been carefully investigated.17, 19
Our investigations were performed in a murine model of chronic kidney disease in animals with an AVF. Simvastatin has been shown to decrease VEGF-A and MMP expression.20, 21, 22 We tested the hypothesis that the reduction of VEGF-A and MMP-9 gene expression via systemic delivery of simvastatin before the placement of an AVF leads to a reduction in venous neointimal hyperplasia with positive vascular remodeling. Gene and protein expression studies, as well as histomorphometric analyses, were performed at the outflow vein removed from animals treated with either simvastatin or controls. We ascertained whether simvastatin reduces fibroblast to myofibroblast (α-smooth muscle actin–positive cells) differentiation induced by hypoxia, and we determined its effect on several important cellular functions including proliferation and migration, with increased caspase 3 activity and decreased MMP-9 activity.
RESULTS
Surgical outcomes
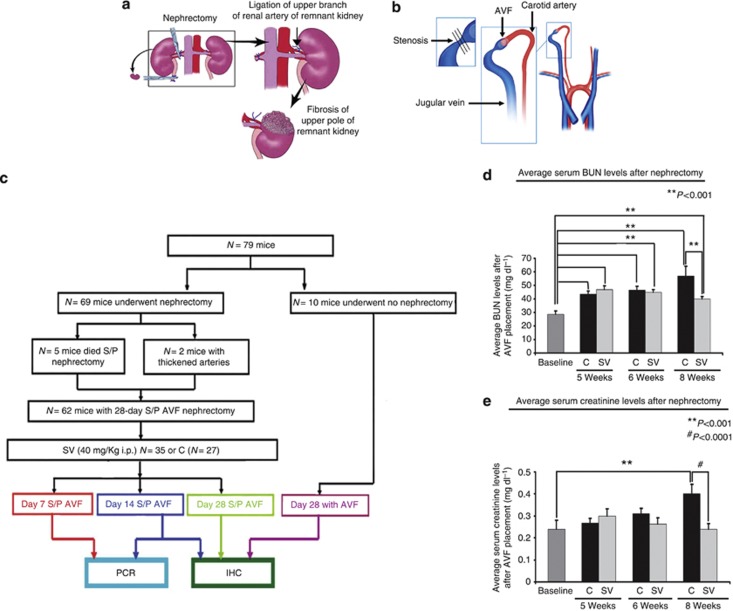
Sixty-nine male C57BL/6 mice weighing 25–30 g were used for the study. Five mice died after nephrectomy, and two mice (control) had thickened arteries at the time of AVF placement and were excluded. Therefore, 62 mice comprise this study18, 19 (Figure 1a and b). Either 40 mg/g of simvastatin (SV, n=35) or phosphate-buffered saline only (control, C, n=27) was given intraperitoneally every other day starting 1 week before fistula placement until the time of killing (Figure 1c).
Figure 1.
Overview of study. (a) The nephrectomy procedure is shown, and (b) the placement of the arteriovenous fistula (AVF) is demonstrated. (c) The study schema. (d) Average serum blood urea nitrogen (BUN) levels after nephrectomy. SV is the simvastatin group and C is the control group. There was a significant increase in the average BUN values after nephrectomy for the C and SV groups (P<0.001). For the SV group, when compared with Cs, at 8 weeks, there was a significant decrease in the mean BUN (P<0.01). (e) Average serum creatinine levels after nephrectomy. There was a significant decrease in the average creatinine values after nephrectomy for the C and SV groups at 8 weeks (P<0.01). For the SV group, when compared with Cs, at 8 weeks, there was a significant decrease in the mean creatinine values (P<0.001). Each bar represents mean±s.e.m. of 4–6 animals. Two-way analysis of variance (ANOVA) followed by Student's t-test with post hoc Bonferroni's correction was performed. Significant differences between the SV-treated group and Cs are indicated by *P<0.01 or **P<0.001. IHC, immunohistochemistry; PCR, polymerase chain reaction; S/P, status post. #P<0.0001.
Serum BUN and creatinine after nephrectomy
The serum blood urea nitrogen (BUN) and creatinine was used to assess the kidney function. After nephrectomy, the average BUN was significantly higher for the simvastatin and the control group at all time points (P<0.001) when compared with baseline (Figure 1d). At 5 to 8 weeks after nephrectomy, the average BUN was significantly higher in the control group when compared with the simvastatin group (Figure 1e). At 8 weeks after nephrectomy only, the average serum BUN was significantly higher in the control group when compared with the simvastatin group (P<0.001). At 8 weeks after nephrectomy, the average serum creatinine was significantly increased in the control group when compared with the simvastatin group (P<0.001).
Simvastatin-treated vessels have a significant reduction in average gene expression of VEGF-A at the outflow vein at days 7 and 14
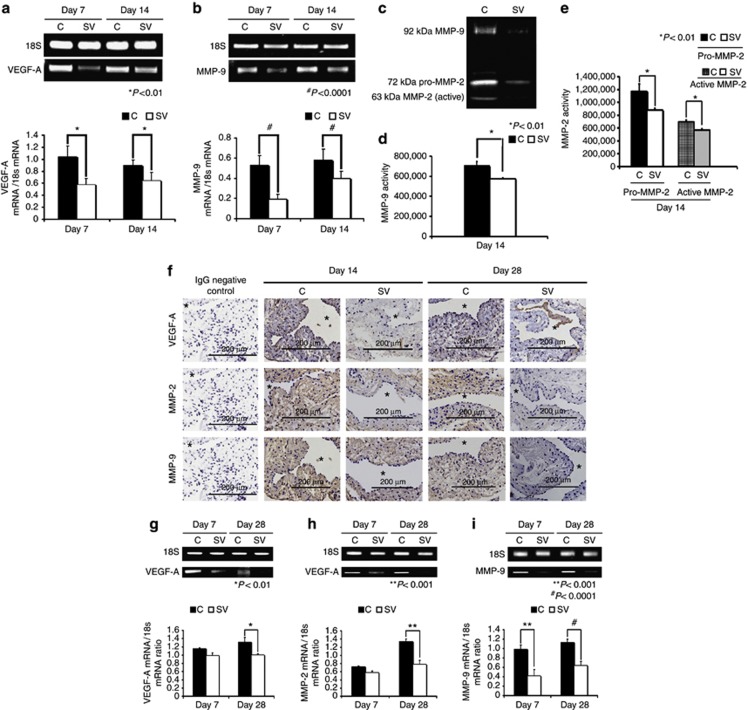
VEGF-A expression is increased in failed hemodialysis vascular accesses (AV fistulas or AV grafts) and in experimental animal models.5, 6, 17, 18 By day 7, the mean gene expression of VEGF-A at the simvastatin-treated vessels was significantly lower than the control vessels (average reduction: 44%, P<0.01 (Figure 2a)), and also at day 14 (average reduction: 49%, P<0.01). Taken collectively, these results indicate that the average gene expression of VEGF-A is reduced at the outflow vein in simvastatin-treated vessels when compared with control vessels.
Figure 2.
Gene expression levels of vascular endothelial growth factor (VEGF)-A and matrix metalloproteinases (MMPs) are reduced in simvastatin (SV)-treated vessels and kidneys when compared with controls (Cs). Real-time polymerase chain reaction (RT-PCR) analysis of (a) VEGF-A and (b) MMP-9 expression after treatment with either C or SV at day 7 or day 14 after arteriovenous fistula (AVF) placement. RT-PCR analysis of (c) VEGF-A, and (d) MMP-2 and MMP-9 expression after treatment with either C or SV at day 7 or day 28 after AVF placement. A typical blot showing the RT-PCR bands from 18S and the genes of interest are shown in a, b, and f–h. (a) The average VEGF-A expression is significantly decreased in the SV-treated vessels when compared with Cs by days 7 and 14 (both P<0.01). (b) The average MMP-9 expression is significantly decreased at days 7 (P<0.0001) and 14 (P<0.0001) in the SV-treated vessels when compared with Cs. (c) The upper panel is a typical zymogram from the outflow vein 14 days after treatment with SV or C. In d, there is significant reduction in the average MMP-9 activity in the SV-treated vessels when compared with C vessels (P<0.01). In e, there is significant reduction in the average pro- and active MMP-2 activity in the SV-treated vessels when compared with C vessels (both P<0.01). (f) The immunostaining of VEGF-A, MMP-2, and MMP-9 with decreased immunoreactivity qualitatively at days 14 and 28 for MMP-2 and MMP-9 and VEGF-A by day 14 in SV-treated vessels when compared with C. (g) The average VEGF-A expression is significantly decreased at day 28 in the SV-treated kidneys when compared with Cs (P<0.01), whereas in h, there is a significant reduction in the average MMP-2 expression in the SV-treated kidneys when compared with Cs (P<0.001). (i) The average MMP-9 is significantly decreased at days 7 (P<0.001) and 28 (P<0.0001) in the SV-treated kidneys when compared with Cs. Each bar represents mean±s.e.m. of 3–5 animals. Two-way analysis of variance (ANOVA) followed by Student's t-test with post hoc Bonferroni's correction was performed. Significant differences between simvastatinSV-treated group and Cs are indicated by *P<0.01, **P<0.001, and #P<0.0001.
Simvastatin-treated vessels have a significant reduction in gene expression of MMP-9 at the outflow vein at days 7 and 14
Studies have shown increased expression of MMP-9 in failed hemodialysis vascular accesses (AV fistulas or AV grafts) and in experimental animal models.6, 17, 23 By day 7, the average gene expression of MMP-9 was significantly lower in the simvastatin-treated vessels when compared with controls (average reduction: 69%, P<0.0001 (Figure 2b)), and also at day 14 (average reduction: 41%, P<0.0001). Overall, these results indicate that simvastatin-treated vessels have a significant reduction in MMP-9 when compared with control vessels.
Because the expression of the protein can lag behind the gene expression, we performed zymography at day 14 to assess MMP-2 and MMP-9 activity in simvastatin-treated vessels as compared with controls (Figure 2c). There was significant reduction in both the MMP-9 and MMP-2 activity in the simvastatin-treated vessels when compared with controls (MMP-9—average reduction: 18%, P<0.01 (Figure 2d); pro-MMP-2—average reduction: 33%, P<0.0001; and active MMP-2—average reduction: 18%, P<0.0001 (Figure 2e)).
Kidneys in simvastatin-treated animals have decreased gene expression of VEGF-A, MMP-2, and MMP-9 at 4 weeks
Because we observed a decrease in average serum BUN and creatinine in the simvastatin-treated animals when compared with controls at 8 weeks, we determined whether the improvement in kidney function was due to a decrease in genes implicated in causing chronic kidney disease, such as VEGF-A (Figure 2f), MMP-2 (Figure 2g), and MMP-9 (Figure 2h). The average gene expression of VEGF-A, MMP-2, and MMP-9 was significantly reduced at day 28 (VEGF-A—average reduction: 24%, P<0.01; MMP-2—average reduction: 42%, P<0.001; and MMP-9—average reduction: 57%, P<0.01) in the simvastatin-treated kidneys when compared with controls.
Simvastatin-treated vessels have positive vascular remodeling at days 14 and 28
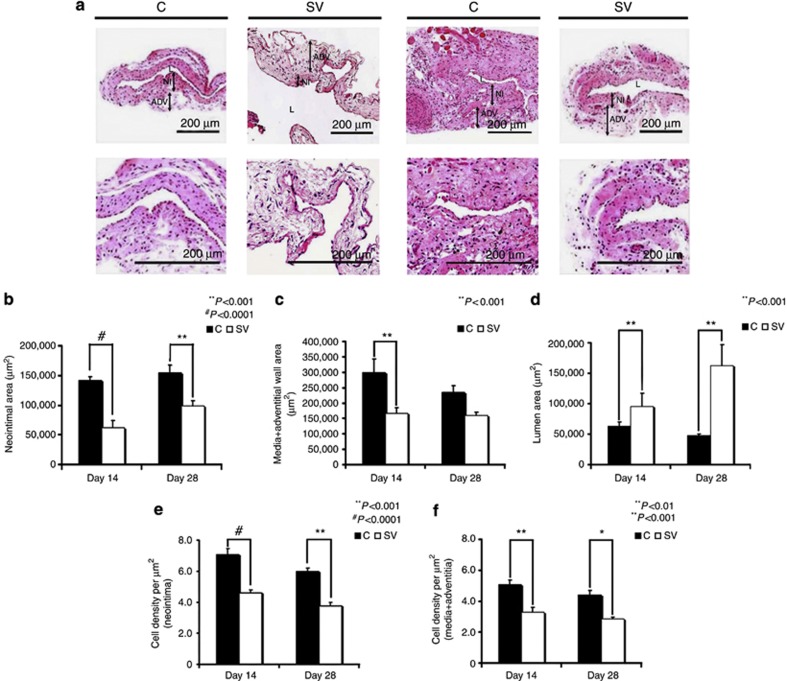
On hematoxylin- and eosin-stained sections, we were able to differentiate between the neointima and media/adventitia (Figure 3a). Semiquantitative histomorphometric analysis was performed on sections removed from the outflow veins of simvastatin-treated vessels and control vessels for the following: the area of the neointima (Figure 3b), media/adventitia (Figure 3c), and lumen vessel (Figure 3d). There was a significant reduction in the average area of the neointima of the simvastatin-treated vessels when compared with the controls by days 14 to 28 (average reduction: 56%, P<0.0001; day 28—average reduction: 45%, P<0.001). By day 14, the average area of the media/adventitia was significantly lower in the simvastatin-treated vessels when compared with the control group (average reduction: 43%, P=0.0028).
Figure 3.
Hematoxylin and eosin (H and E) staining of the simvastatin (SV)-treated vessels showing reduced venous neointimal hyperplasia and positive vascular remodeling. (a) The representative section after H and E staining at the venous stenosis of either control (C) or SV at day 14 or day 28 after arteriovenous fistula (AVF) placement. Upper panels, original magnification: × 10, and lower panels, original magnification: × 40. ADV, adventitia/media; L, lumen; NI, neointima. (b) The semiquantitative analysis that shows a significant decrease in the average area of the neointima of the SV-treated vessels when compared with the C group for days 14 (P<0.0001) and 28 (P<0.001). (c) The semiquantitative analysis that shows a significant decrease in the average area of the media/adventitia of the SV-treated vessels when compared with the C group for day 14. (d) A significant increase in the average lumen vessel area of the SV-treated vessels when compared with the C group for days 14 and 28 (P<0.001). (e) A significant decrease in the average cell density in the neointima of the SV-treated vessels when compared with the C group for days 14 (P<0.0001) and 28 (P<0.001). (f) Similar results in that there is a significant reduction in the average cell density in the media/adventitia of the SV-treated vessels when compared with the C group for days 14 (P<0.001) and 28 (P<0.01). Each bar represents mean±s.e.m. of 3–4 animals. Two-way analysis of variance (ANOVA) followed by Student's t-test with post hoc Bonferroni's correction was performed. Significant differences between the SV-treated group and Cs are indicated by *P<0.01, **P<0.001, or #P<0.0001.
As the simvastatin-treated vessels had reduced average wall area when compared with controls, we wanted to determine whether the simvastatin-treated vessels had a larger average lumen vessel area (Figure 3d). By days 14 to 28, the average lumen vessel area was significantly higher in the simvastatin-treated vessels when compared with controls (average increase: 150%, P<0.001; day 28—average increase: 343%, P<0.001).
Because VEGF-A is necessary for cellular homeostasis, we determined the cell density in the neointima (Figure 3e) and media/adventitia (Figure 3f). By days 14 and 28, the average cell density of the neointima and media/adventitia in the simvastatin-treated vessels was significantly lower than the control vessels (neointima—day 14: average reduction: 65%, P<0.0001; day 28: average reduction: 70%, P<0.001; media/adventitia—day 14: average reduction: 37%, P<0.001; day 28: average reduction: 35%, P<0.01).
Simvastatin-treated vessels have decreased cellular proliferation at the outflow vein at days 14 and 28
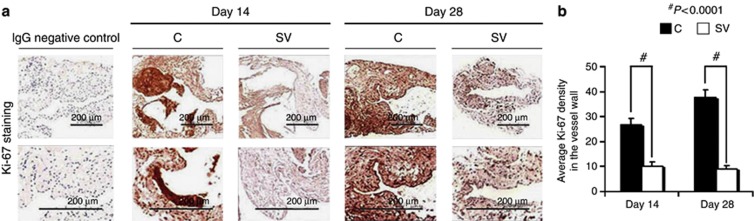
VEGF-A and MMPs are needed for cells to proliferate, and cellular proliferation was assessed using Ki-67 (brown-stained nuclei (Figure 4a)). By days 14 and 28, in the simvastatin-treated vessels, when compared with control vessels, the average Ki-67 density was significantly reduced (day 14: average reduction: 66%, P<0.001; day 28: average reduction: 76%, P<0.0001 (Figure 4b)).
Figure 4.
Ki-67 staining is decreased in simvastatin-treated vessels. Cellular proliferation is decreased in simvastatin-treated vessels. (a) The representative section after Ki-67 staining at the venous stenosis after treatment with either control (C) or simvastatin (SV) at day 14 or day 28 after arteriovenous fistula (AVF) placement. Upper panel: original magnification, × 40, and the lower panel is a magnification view of the box. Brown-staining nuclei are positive for Ki-67. IgG-negative controls are shown. (b) The semiquantitative analysis that shows a significant decrease in the average Ki-67 staining in the SV-treated vessels when compared with the C group for days 14 (P<0.001) and 28 (P<0.0001). Each bar represents mean±s.e.m. of 3–4 animals. Two-way analysis of variance (ANOVA) followed by Student's t-test with post hoc Bonferroni's correction was performed. Significant differences between the SV-treated group and Cs are indicated by *P<0.01 and #P<0.0001.
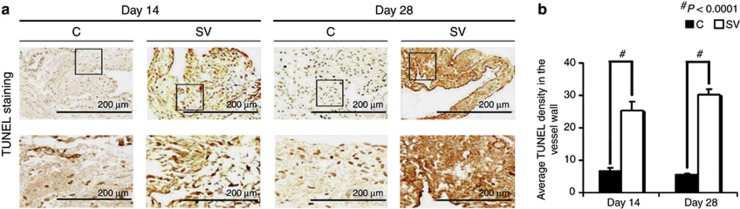
Simvastatin-treated vessels have increased TUNEL staining at days 14 and 28
Current literature suggests that VEGF-A is needed for maintaining cellular homeostasis; therefore, we hypothesized that the decrease in cell density was due to an increase in apoptosis.24 Apoptosis was assessed using terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining (Figure 5a). By days 14 to 28, the average density of cells staining positive for TUNEL (brown) at the outflow vein of the simvastatin group was significantly higher than the control group (day 14: average increase: 366%, P<0.0001; day 28: average increase: 561%, P<0.0001 (Figure 5b)). Overall, these results indicate that simvastatin-treated vessels have increased TUNEL activity implying cellular apoptosis when compared with controls.
Figure 5.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining is significantly increased in simvastatin (SV)-treated vessels. (a) The representative section after TUNEL staining at the venous stenosis after treatment with either control (C) or SV at day 14 or day 28 after arteriovenous fistula (AVF) placement. Cells staining brown are positive for TUNEL. Upper panel: original magnification, × 40, and the lower panel is a magnification view of the box. (b) The semiquantitative analysis that shows a significant increase in the average TUNEL staining in the SV-treated vessels when compared with the C group for days 14 (P<0.0001) and 28 (P<0.0001). Each bar represents mean±s.e.m. of 3–4 animals. Two-way analysis of variance (ANOVA) followed by Student's t-test with post hoc Bonferroni's correction was performed. Significant differences between the SV-treated group and Cs are indicated by *P<0.01 and #P<0.0001.
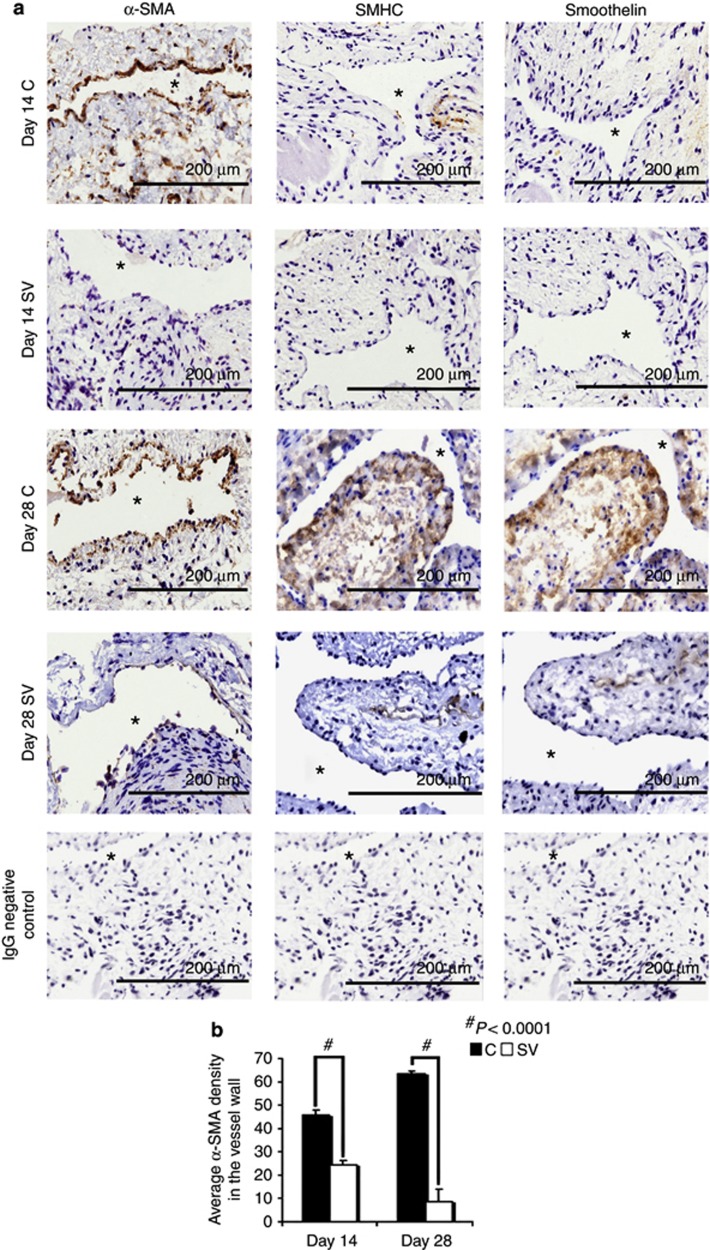
Simvastatin-treated vessels have decreased α-SMA, smoothelin, and smooth muscle myosin heavy-chain expression by days 14 and 28
The majority of cells that comprise the venous neointimal hyperplasia are α-SMA positive (brown-stained cells), and we determined whether the decrease in the cell density was due to a decrease in α-SMA-positive cells (Figure 6a). The average α-SMA density at the outflow vein of simvastatin-treated vessels was significantly lower than the control group by day 14 (average reduction: 46%, P<0.0001 (Figure 6b)). Smooth muscle myosin heavy chain (SMHC) and smoothelin are expressed by contractile smooth muscle cells. Qualitatively, by day 28, there was decreased staining for both smoothelin and SMHC in the simvastatin-treated vessels when compared with controls, which suggests a decrease in contractile smooth muscle cells along with myofibroblasts (Figure 6a).
Figure 6.
α-Smooth muscle actin (α-SMA), smooth muscle heavy chain (SMHC), and smoothelin staining are decreased in simvastatin-treated vessels. (a) Representative sections after α-SMA (first column), SMHC (second column), and smoothelin (third column) staining at the venous stenosis after treatment with either control (C) or simvastatin (SV) at day 14 or day 28 after arteriovenous fistula (AVF) placement. All are original magnifications × 40. Brown-staining cytoplasm is positive for α-SMA, SMHC, or smoothelin. (b) Semiquantitative analysis that shows a significant decrease in the average α-SMA staining in the SV-treated vessels when compared with the C group for days 14 (P<0.0001) and 28 (P<0.0001). Qualitatively, by day 28, SMHC and smoothelin staining are reduced in the simvastatin-treated vessels when compared with Cs. Each bar represents mean±s.e.m. of 3–4 animals. Two-way analysis of variance (ANOVA) followed by Student's t-test with post hoc Bonferroni's correction was performed. Significant differences between the simvastatin-treated group and controls are indicated by *P<0.01 and #P<0.0001.
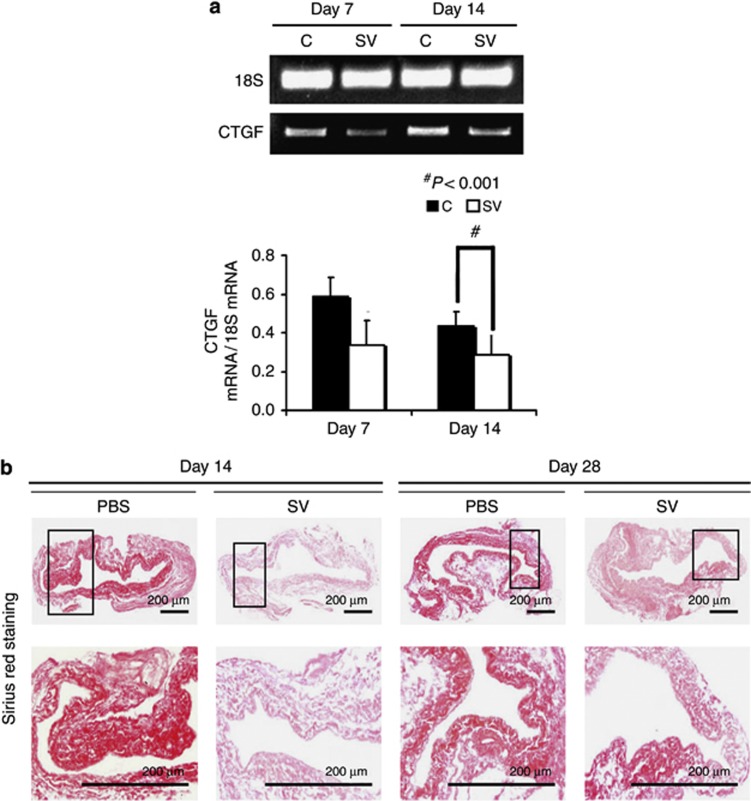
Simvastatin-treated vessels have reduced gene expression of CTGF at day 14
Several genes including connective tissue growth factor (CTGF) control the regulation of extracellular matrix. We assessed the gene expression of CTGF using real-time polymerase chain reaction (RT-PCR) analysis performed at different time points. The mean gene expression of CTGF (Figure 7a) at the simvastatin-treated vessels was significantly lower than the control vessels by day 14 (average reduction: 45%, P<0.001).
Figure 7.
Gene expression of connective tissue growth factor (CTGF) and Picrosirius red staining are reduced in simvastatin (SV)-treated vessels when compared with controls (Cs). (a) The real-time polymerase chain reaction (RT-PCR) analysis of CTGF expression after treatment with either C or SV at day 7 or day 14 after arteriovenous fistula (AVF) placement. A typical blot is shown in the upper panel and the pooled data in the lower panel. (a) The average CTGF expression is significantly decreased at day 14 in the SV-treated vessels when compared with Cs (P<0.001). (b) Representative sections after Picrosirius red staining at the venous stenosis after treatment with either C or SV at day 14 or day 28 after AVF placement. Upper panel: original magnification, × 40, and the lower panel is a magnification view of the box. The more intense red staining is representative of collagen 1 and 3 staining. Qualitatively, there is decreased Sirius red staining in the simvastatin-treated vessels when compared with Cs. Each bar represents mean±s.e.m. of 3–5 animals. Two-way analysis of variance (ANOVA) followed by Student's t-test with post hoc Bonferroni's correction was performed. Significant differences between the SV-treated group and controls are indicated by *P<0.01.
Simvastatin-treated vessels have reduced Sirus red staining
Next, we assessed the changes in extracellular matrix using Sirus red staining, which allows for the evaluation of collagen 1 and 3. Sirus red staining was performed on outflow vein sections removed from simvastatin-treated and control vessels at day 14 and day 28, respectively (Figure 7b). Qualitatively, this demonstrated a reduction in the intensity of Sirus red staining in the simvastatin-treated vessels when compared with control vessels at both days 14 and 28. This implies that there is a decrease in constrictive remodeling in the simvastatin-treated vessels when compared with controls.
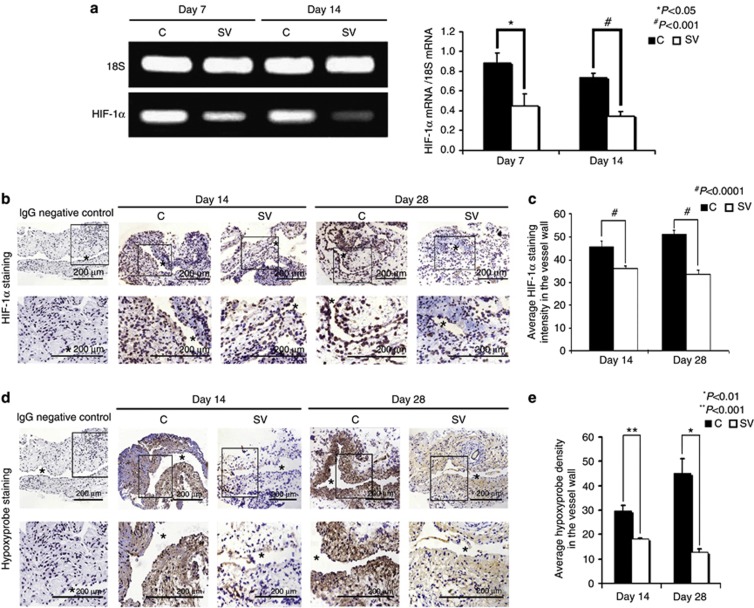
Simvastatin-treated vessels have decreased hypoxyprobe staining and decreased mRNA levels of HIF-1α
Several studies have demonstrated increased hypoxia-inducible factor HIF-1α expression in animal models of hemodialysis graft failure and in clinical specimens from patients with hemodialysis vascular access failure.18, 23 The mean gene expression of HIF-1α (Figure 8a) at the simvastatin-treated vessels was significantly lower than the control vessels by day 7 (average reduction: 54%, P<0.001) and day 14 (average reduction: 54%, P<0.001). We assessed HIF-1α staining as well. Cells staining positive for HIF-1α are brown (Figure 8b). By days 14 to 28, there was a significant reduction in the average density of HIF-1α staining in the simvastatin-treated vessels when compared with controls (day 14: average reduction: 20%, P<0.001; day 28: average reduction: 36%, P<0.001 (Figure 8c)). We next performed hypoxyprobe staining in the outflow vein treated with either simvastatin or controls (Figure 8d). Cells staining positive for hypoxyprobe are brown. By days 14 to 28, there was a significant reduction in the average density of hypoxyprobe staining in the simvastatin-treated vessels when compared with controls (Day 14: average reduction: 40%, P<0.001; day 28: average reduction: 70%, P<0.01 (Figure 8e)). Overall, these results indicate that there is decreased expression of both HIF-1α and hypoxyprobe in simvastatin-treated vessels when compared with controls.
Figure 8.
Hypoxia-inducible factor (HIF-1α) expression and hypoxyprobe staining are reduced in simvastatin (SV)-treated vessels when compared with controls (Cs). (a) The real-time polymerase chain reaction (RT-PCR) analysis of HIF-1α expression after treatment with either SV or C at day 7 or day 14 after arteriovenous fistula (AVF) placement. A typical blot is shown in the upper panel and the pooled data in the lower panel. (a) The average HIF-1α expression is significantly decreased at days 7 (P<0.001) and 14 (P<0.0001) in the SV-treated vessels when compared with Cs. (b) HIF-1α staining at the venous stenosis after treatment with either C or SV at day 14 or day 28 after AVF placement. Cells staining brown are positive for HIF-1α. Immunoglobulin G (IgG)-negative controls are shown. (c) The average staining for HIF-1α is significant in the SV-treated vessels when compared with the Cs at days 14 (P<0.001) and 28 (P<0.001). (d) The hypoxyprobe staining at the venous stenosis after treatment with either C or SV at day 14 or day 28 after AVF placement. Cells staining brown are positive for hypoxyprobe. IgG-negative controls are shown. (e) The average staining for hypoxyprobe is significant in the simvastatin-treated vessels when compared with controls at days 14 (P<0.001) and 28 (P<0.01). Each bar represents mean±s.e.m. of 3–5 animals. Two-way analysis of variance (ANOVA) followed by Student's t-test with post hoc Bonferroni's correction was performed. Significant differences between the SV-treated group and Cs are indicated by *P<0.01 or **P<0.001.
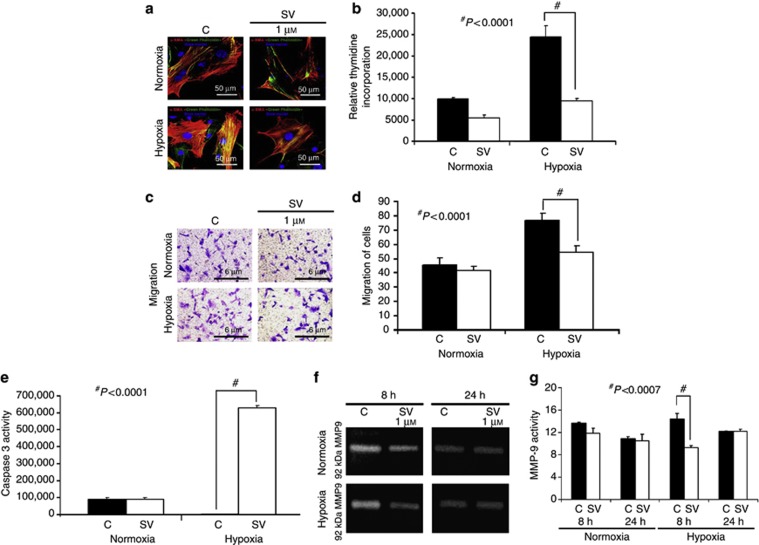
Simvastatin treatment in hypoxic fibroblasts reduces α-SMA production at 24 hours
Studies indicate that hypoxia can cause an increase in fibroblast to myofibroblast differentiation.25, 26 To determine whether simvastatin treatment could decrease the conversion of fibroblasts to α-SMA-positive cells under hypoxic stress, we used NIH 3T3 cells, which were treated with 1 μmol/l of simvastatin (SV) or control (C) and subjected to incubation for 24 h. We first determined the synthetic phenotype of the SMC using confocal imaging for phalloidin and α-SMA (Figure 9a). Cells staining red are positive for α-SMA, and cells staining green are positive for phalloidin, with the nuclei staining blue. As shown, this demonstrated a qualitative reduction in α-SMA plus phalloidin staining for the simvastatin-treated cells when compared with controls for both 24 h of normoxia and hypoxia.
Figure 9.
There is decreased α-smooth muscle cell expression, migration, proliferation, and matrix metalloproteinase (MMP)-9 activity with increased caspase 3 activity in simvastatin-treated cells subjected to hypoxia. (a) Confocal microscopy for α-smooth muscle cell actin staining and phalloidin in NIH 3T3 cells at 24 h of hypoxia. Red-staining cells are positive for α-smooth muscle cell actin; phalloidin is green and the nuclei are blue. This demonstrates qualitatively a reduction in costaining for α-smooth muscle cell actin and phalloidin in the simvastatin-treated cells at 24 h of hypoxia (P<0.001). (b) The pooled proliferation data for three separate experiments for simvastatin (1 μmol/l). There is a significant reduction in the proliferation of NIH 3T3 cells for the 1 μmol/l concentration of simvastatin when compared with controls subjected to 24-hour hypoxia (P<0.0001). (c) A representative picture from a migration experiment. (d) The pooled migration data for three separate experiments for controls and simvastatin (1 μmol/l). There is a significant reduction in the migration of NIH 3T3 cells for the 1-μmol/l concentration of simvastatin when compared with controls subjected to 24-hour hypoxia (P<0.0001). (e) The pooled caspase 3 data for three separate experiments for controls and simvastatin (1 μmol/l). There is a significant increase in the caspase 3 activity of NIH 3T3 cells for the 1-μmol/l concentration of simvastatin compared with controls subjected to 24-hour hypoxia (P<0.0007). (f) A representative zymogram from NIH 3T3 cells treated with controls or a 1-μmol/l concentration of simvastatin, or controls subjected to 8 and 24 hours of normoxia or hypoxia. (g) The pooled MMP-9 data for three separate experiments of controls and simvastatin (1 μmol/l). There is a significant increase in the MMP-9 activity of NIH 3T3 cells treated with a 1-μmol/l concentration of simvastatin subjected to 8 hours of hypoxia when compared with controls (P<0.0007). Each bar represents mean±s.e.m. of three experiments. One-way analysis of variance (ANOVA) followed by Student's t-test with post hoc Bonferroni's correction was performed. Significant differences between the simvastatin-treated group and controls are indicated by #P<0.0001.
Simvastatin treatment reduces proliferation and migration in hypoxic fibroblasts
We determined whether the proliferative capacity of simvastatin-treated NIH 3T3 cells is reduced under hypoxia when compared with controls. This demonstrated that there was significant reduction in the proliferative ability of fibroblasts treated with simvastatin as compared with controls for 24-h hypoxia (average reduction: 70%, P<0.0001 (Figure 9b)). We next determined whether the migratory capacity of simvastatin-treated NIH 3T3 cells is reduced under hypoxia when compared with controls using a Matrigel invasion assay (Figure 9c). This demonstrated that the migratory capacity of simvastatin-treated cells was significantly decreased for simvastatin when compared with controls for 24-h hypoxia (average reduction: 33%, P<0.0001 (Figure 9d)). We next determined the effect of simvastatin on caspase 3 activity on NIH 3T3 cells under hypoxia and normoxia. We observed a significant increase in caspase 3 activity in cells treated with simvastatin when exposed to hypoxia as compared with controls (average increase: 600%, P<0.0007 (Figure 9e)). Finally, we determined the effect of simvastatin on the activity of MMP-9 in NIH 3T3 cells under hypoxia and normoxia at 8 and 24 hours (Figure 9f). We observed a significant decrease in MMP-9 activity at 8 hours of hypoxia (average reduction: 33%, P<0.0001 (Figure 9g)) and at 24 hours of normoxia (average reduction: 33%, P<0.0001 (Figure 9g)).
DISCUSSION
In this study, we demonstrated that the venous stenosis from the AVF of simvastatin-treated vessels have reduced gene expression of VEGF-A and MMP-9, with protein expression of pro- and active MMP-2 and MMP-9 and a significant reduction in the average area of the neointima and media/adventitia, as well as positive vascular remodeling. In simvastatin-treated vessels, we observed a significant decrease in cellular proliferation, cell density (α-SMA), smoothelin, and SMHC, and a significant increase in apoptosis. In addition, a significant decrease in the local vessel hypoxia was observed, which was confirmed using two different approaches: HIF-1α and hypoxyprobe staining with RT-PCR for HIF-1α. Simvastatin-treated vessels had a decrease in extracellular matrix, with a significant reduction in CTGF, a profibrotic gene responsible for regulating extracellular matrix. In vitro experiments showed that NIH 3T3 fibroblasts when exposed to hypoxia and treated with simvastatin had a decrease in α-smooth muscle cell expression, proliferation, migration, and MMP-9 activity with increased caspase 3 activity. These results, taken in aggregate, indicate pretreatment with simvastatin before the placement of AVF, and we hypothesize that this results because of a decrease in fibroblast to myofibroblast conversion mediated through a VEGF-A/MMP-9 pathway associated with a decrease in CTGF, resulting in positive vascular remodeling and decrease in venous neointimal hyperplasia.
In patients with malfunctioning hemodialysis vascular access and in experimental animal models of hemodialysis AVF or graft failure, increased expression of MMP-2 and MMP-9 has been observed.6, 17, 18, 23, 27 Previous studies in experimental animal models of atherosclerosis,28 arterial stenosis after angioplasty,29 and saphenous vein bypass grafting20, 21, 22, 30 have demonstrated that simvastatin therapy reduces intimal hyperplasia mediated through an MMP pathway. Porter et al.20 demonstrated in a saphenous vein–based organ culture model that MMP-9 expression is decreased after statin use and is mediated via a Rho A/ROCK signaling pathway. In this study, we demonstrate that simvastatin therapy in a murine model of AVF with chronic kidney disease causes a significant reduction in MMP-9, which in turn is accompanied by a significant reduction in the average area of the neointima and media/adventitia and a concomitant increase in lumen vessel area. These findings are consistent with experimental studies using a porcine model of arteriovenous hemodialysis graft failure, in which the MMP inhibition reduces venous neointimal hyperplasia formation.31
In addition to MMPs, increased expression of VEGF-A has been localized to the adventitia and neointima in specimens removed from patients with failed hemodialysis vascular access.5 Previous studies from our laboratory in experimental animal models of venous neointimal hyperplasia formation in hemodialysis AVF or graft failure have demonstrated that there is increased expression of VEGF-A at the venous stenosis when compared with the control vein.18, 27 Simvastatin has been shown to decrease VEGF expression in vascular smooth muscle cells and macrophages, as it relates to atherosclerosis.20, 21, 22, 30, 32, 33, 34 At low doses, simvastatin has angiogenic properties, and at higher doses, simvastatin has antiangiogenic properties mediated by decreasing VEGF-A.35, 36 Interestingly, in endothelial cells, simvastatin activates Akt, which is a protein kinase responsible for angiogenesis.37 Indeed, it is well known that Akt signaling is downstream to VEGF-A activation.38 This protective endothelial effect of simvastatin is thought to be responsible for the clinical efficacy observed in patients with vascular disease.39 In this study, we used a high dose of simvastatin and observed that simvastatin-treated vessels have decreased expression of VEGF-A with a decrease in neointimal hyperplasia.40 These results are consistent with reports in animal models of arterial stenosis, which have demonstrated that blockade of VEGF-A using soluble VEGF receptor 1 reduces intimal hyperplasia.16
On histologic analysis, it was found that venous neointimal hyperplasia is characterized by an increase in α-smooth muscle cell staining, cellular proliferation, and increased extracellular matrix composed primarily of collagen 1 and 3.3, 4, 5 Using Ki-67 staining, we observed a significant decrease in cellular proliferation in simvastatin-treated vessels when compared with controls. These findings are consistent with several studies conducted in different vascular injury models that have demonstrated that simvastatin-treated vessels have decreased cellular proliferation.20, 21, 22, 28, 29, 30, 41
Cellular proliferation can be often accompanied by changes in cell death as demonstrated by TUNEL staining. In the simvastatin-treated vessels, when compared with controls, there was a significant increase in the average TUNEL staining. These findings are consistent with previous studies that have demonstrated that there is increased TUNEL staining.29, 41 Furthermore, the increase in TUNEL staining was accompanied by a decrease in the average cellular density. Finally, a significant decrease in α-smooth muscle cell actin staining was demonstrated in the simvastatin-treated vessels, consistent with prior studies utilizing different animal models of vascular injury.21, 22, 28, 30, 42 In addition, we observed a decrease in the contractile smooth muscle cell phenotype as indicated by a decrease in staining for smoothelin and SMHC staining.
Picrosirius red staining was performed to assess the changes in extracellular matrix in vessels treated with simvastatin, and controls. This demonstrated that there was a decrease in the intensity of the staining, demonstrating a decrease in extracellular matrix deposition in the outflow vein in the simvastatin-treated vessels when compared with controls. This is consistent with an animal model of atherosclerosis that demonstrated similar results with simvastatin treatment.28, 43 Furthermore, we investigated the expression of CTGF, which was found to be decreased in simvastatin-treated vessels when compared with controls. A recent study demonstrated that simvastatin causes a decrease in vascular fibrosis mediated through a angiotensin II/Smad pathway, resulting in a decrease in the expression of CTGF.44 A second study showed that the decrease in CTGF is also mediated through a Rho pathway in fibroblasts treated with simvastatin.45
One unanticipated result of systemic simvastatin therapy was an improvement in kidney function at 5 weeks after initiation of simvastatin. To determine a potential cause, we determined the gene expression of VEGF-A, MMP-2, and MMP-9 in the kidneys of simvastatin-treated animals compared with controls. Each of these genes have been implicated in the progression of chronic kidney disease.46 We observed that by day 28, in the simvastatin-treated kidneys when compared with controls, there was a significant reduction in the expression of VEGF-A, MMP-2, and MMP-9. We postulate that the reduction of these genes resulted in the improvement in kidney function, which was consistent with other studies.47
Hypoxic injury is known to accelerate the conversion of fibroblasts to myofibroblasts, and increased HIF-1α has been observed in animal models and in clinical specimens of AVF or graft failure.18, 23, 25 VEGF-A-mediated activation of fibroblasts to the myofibroblast phenotype can be accompanied by activation of matrix regulatory signaling moieties including MMP-2 and MMP-9.26, 48 We used 1 μmol/l concentration of simvastatin, as previous data indicate that the dose of simvastatin used in our in vivo experiments is in the range of 1–2 μmol/l.49 Fibroblasts treated with either simvastatin or controls when subjected to hypoxia had a decrease in α-SMA expression with a significant decrease in migration and proliferation, as well as decreased MMP-9 activity and increased caspase 3 activity. The role of statin therapy has been studied in fibroblasts and myofibroblasts but not on hypoxia and its role on conversion of fibroblast to myofibroblasts.20, 30, 34, 50
There are some limitations of this study. First, there was an improvement in the kidney function at 28 days, which could explain the decrease in VNH and improvement in the lumen vessel area.51, 52 Second, there are multiple mechanisms that are affected by simvastatin, and we demonstrate a change in one of these pathways only. Finally, a recent study has demonstrated that statin use was not associated with an improvement in hemodialysis vascular access.53 This study had several limitations, including the fact that there was no protocol for the dosing of statins used. In addition, there were differences in the group of patients who received statins, and it was not noted which statin was used, as there are differences in the efficacy of different statins.21
In conclusion, we demonstrate that systemic delivery of simvastatin is associated with a decrease in the expression of several important matrix-regulating genes such as VEGF-A, MMP-2, MMP-9, and CTGF. The net result is an overall decrease in the venous neointimal hyperplasia with a decrease in α-SMA-positive cells, migration, proliferation, and an increased apoptosis with positive vascular remodeling. The clinical significance of this study is that it provides a rationale for using simvastatin before the placement of AVF in reducing venous neointimal hyperplasia formation.
METHODS AND MATERIALS
Experimental animals
Appropriate Institutional Animal Care and Use Committee approval was obtained before performing any procedures. Sixty-nine male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) weighing 25–30 g were used for this study as depicted (Figure 1). Chronic kidney disease was created by surgical removal of the right kidney, accompanied by ligation of the arterial blood supply to the upper pole of the left kidney as described previously (Figure 1a).18 Three weeks after nephrectomy, the animals were started on simvastatin (40 mg/kg administered intraperitoneally three times per week) or phosphate-buffered saline (equal amount of volume used for simvastatin intraperitoneal controls). Simvastatin was prepared as described elsewhere.54 A week later, an AVF was created by connecting the right carotid artery to the ipsilateral jugular vein, as described previously, and the mice were then killed (Figure 1b).18, 19 Please see Supplementary Methods online for full details.
Tissue processing and immunohistochemistry
Each outflow vein from each animal was embedded in paraffin lengthwise, so that the sections would be orthogonal to the long axis of the vessel (Figure 1b). Typically, 80 to 120, 4-mm sections were obtained, and the cuff used to make the anastomosis could be visualized. —Two to four, 40 micrometer sections were stained with hematoxylin and eosin, Ki-67, α-SMA, hypoxyprobe, HIF-1 α, smoothelin, SMHC, picrosirius red, or TUNEL. Cellular proliferation was determined by staining for Ki-67 on sections removed from the outflow vein by performing quantification at different time points. Smooth muscle density was determined by staining for α-SMA on sections removed from the outflow vein by performing quantification at the different time points. Immunohistochemistry for Ki-67 and α-SMA was performed on paraffin-embedded sections from the outflow vein after transfection with either simvastatin or control groups using the EnVision (Dako, Carpinteria, CA) method with a heat-induced antigen-retrieval step.6, 17 The following antibodies were used: mouse monoclonal antibody Ki-67 (DAKO, Carpentaria, CA; 1:400), mouse monoclonal HIF-1α (Novus Biologicals, Littleton, CO; 1:500), rabbit polyclonal antibody to mouse for α-SMA (Abcam, Cambridge, MA; 1:400), mouse monoclonal to SMHC (Novus Biologicals; 1:500), or smoothelin (Santa Cruz Biotechnology, Santa Cruz; 73042; 1:500). IgG antibody staining was performed to serve as controls.
The outflow vein with the cuff anastomosis was collected as shown (Figure 1b) and then embedded. In this model, the stenosis forms at the outflow vein, and this can be identified easily, as the cuff used to create the anastomosis is a landmark. An average of 12 multiple contiguous serial 4-μm sections were stained with hematoxylin and eosin and analyzed for histomorphometric analyses (see later).
Hypoxyprobe staining at days 14 and 28
We assessed hypoxic changes in the outflow vein after treatment with either simvastatin or controls using HypoxyprobeTM-1 (a substituted derivative of pimonidazole hydrochloride). HypoxyprobeTM-1 upon activation forms stable covalent adducts with thiol groups of proteins, peptides, and amino acids of hypoxic tissue. Please see Supplementary Methods online for full details.
TUNEL staining at days 14 and 28
TUNEL staining was performed on paraffin-embedded sections from the outflow vein after transfection with either simvastatin or controls, as specified by the manufacturer (DeadEnd Colorimetric tunnel assay system, G7360; Promega, Madison, WI).
Picrosirius red staining at days 14 and 28
Picrosirius staining was performed to assess for collagen 1 and 3 depsotion. Please see Supplementary Methods online for full details.
SDS-PAGE zymography for MMP-2 and MMP-9
MMP-2 and MMP-9 protein activities were determined using zymographic analysis. This was performed on homogenates from cultured cells or outflow veins treated with simvastatin or control as described previously.6, 17
RT-PCR analysis
Expression for the gene of interest was determined using RT-PCR analysis.19 Briefly, first-strand cDNA was synthesized using SuperScript III First Strand (Invitrogen, Carlsbad, CA) according to the manufacturer's guidelines. cDNAs specific for the genes analyzed were amplified using commercial primers purchased from SA Biosciences (Frederick, MD). Please see Supplementary Methods online for full details.
Hypoxia chamber
One hundred thousand NIH 3T3 cells were treated with either simvastatin or controls and then made hypoxic for 8 or 24 hours as described previously.26
Proliferation assay
One hundred thousand NIH 3T3 cells were treated with either simvastatin or controls and then seeded in 24-well plates and cultured for 24 h in Dulbecco's modified Eagle's medium. After 20 h, 1 mCi of 3H-thymidine was added to each well; 4 h later, cells were washed with chilled phosphate-buffered saline, fixed with 100% cold methanol, and collected for measurement of trichloroacetic acid precipitable for radioactivity. Experiments were repeated at least three times for each time point.
Cell migration assay
One hundred thousand NIH 3T3 cells were treated with either simvastatin or controls and were seeded in 8 micrometer trans-wells precoated with low growth factor Matrigel in a serum-free media. The complete medium was supplemented under the trans-well and incubated for 6 h at 37 °C. After 6 h, trans-wells were washed with phosphate-buffered saline and fixed with paraformaldehye (4% (v/v)). Finally trans-wells were stained with bromophenol (0.1%) solution. The cells from the upper side were removed with cotton-tip applicators. The cells at the bottom side were counted for analysis.
Morphometry and image analysis
Sections immunostained for hematoxylin and eosin stains were viewed with an Axioplan 2 Microscope (Zeiss, Oberkochen, Germany) equipped with a Neo-Fluor × 20/0.50 objective and digitized to capture a minimum of 3090 × 3900 pixels using a Axiocam camera (Zeiss).6, 17 Images covering one entire cross-section from each section of the outflow vein treated with simvastatin or controls were acquired and analyzed using the KS 400 Image Analysis software (Zeiss). Ki-67 (brown), α-SMA positive (brown), SMHC (brown), smoothelin (brown), TUNEL positive (brown), HIF-1α (brown), or hypoxyprobe (brown) were highlighted, in turn, by selecting the appropriate red–green–blue color intensity range and then counted. The color intensity was adjusted for each section to account for the decreasing intensity of positive staining over time. This was repeated two times to ensure that the intraobserver variability was <10%. Sections were subsequently viewed with an Axioplan 2 Microscope (Zeiss) equipped with a Neo-Fluor × 20/0.50 objective and digitized to capture at least 1030 × 1300 pixels, and cell density determined along with the vessel wall and luminal vessel areas was performed on 12 contiguous sections. The area was measured by tracing the vessel wall using an automated program.17
Statistical methods
Data are expressed as mean±s.e.m. Multiple comparisons were performed with two-way analysis of variance, followed by Student's t-test with post hoc Bonferroni's correction. Because of the Bonferroni correction, significant difference from control value was indicated by *P<0.01, **P<0.001, or #P<0.0001. SAS version 9 (SAS Institute, Cary, NC) was used for statistical analyses.
Acknowledgments
This work was funded by an R01HL098967 (SM) from the National Heart, Lung, and Blood Institute.
All the authors declared no competing interests.
Footnotes
SUPPLEMENTARY MATERIAL
Figure S1. Hematoxylin and eosin (H and E) staining of the simvastatin-treated vessels showing reduced venous neointimal hyperplasia and positive vascular remodeling in animals with AVF and normal kidney function at day 28.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
Supplementary Material
References
- Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States Renal Data System 2003 Annual Data Report: Atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2003;42:A5–A7. [PubMed] [Google Scholar]
- Rooijens PPGM, Tordoir JHM, Stijnen T, et al. Radiocephalic wrist arteriovenous fistula for hemodialysis: meta-analysis indicates a high primary failure rate. Eur J Vasc Endovasc Surg. 2004;28:583–589. doi: 10.1016/j.ejvs.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Rekhter M, Nicholls S, Ferguson M, et al. Cell proliferation in human arteriovenous fistulas used for hemodialysis. Arterioscler Thromb. 1993;13:609–617. doi: 10.1161/01.atv.13.4.609. [DOI] [PubMed] [Google Scholar]
- Swedberg SH, Brown BG, Sigley R, et al. Intimal fibromuscular hyperplasia at the venous anastomosis of PTFE grafts in hemodialysis patients. Clinical, immunocytochemical, light and electron microscopic assessment. Circulation. 1989;80:1726–1736. doi: 10.1161/01.cir.80.6.1726. [DOI] [PubMed] [Google Scholar]
- Roy-Chaudhury P, Kelly BS, Miller MA, et al. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001;59:2325–2334. doi: 10.1046/j.1523-1755.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- Misra S, Doherty MG, Woodrum D, et al. Adventitial remodeling with increased matrix metalloproteinase-2 activity in a porcine arteriovenous polytetrafluoroethylene grafts. Kidney Int. 2005;68:2890–2900. doi: 10.1111/j.1523-1755.2005.00763.x. [DOI] [PubMed] [Google Scholar]
- Li L, Terry CM, Blumenthal DK, et al. Cellular and morphological changes during neointimal hyperplasia development in a porcine arteriovenous graft model. Nephrol Dial Transplant. 2007;22:3139–3146. doi: 10.1093/ndt/gfm415. [DOI] [PubMed] [Google Scholar]
- Wang Y, Krishnamoorthy M, Banerjee R, et al. Venous stenosis in a pig arteriovenous fistula model anatomy, mechanisms and cellular phenotypes. Nephrol Dial Transplant. 2007;22:3139–3146. doi: 10.1093/ndt/gfm547. [DOI] [PubMed] [Google Scholar]
- Bhardwaj S, Roy H, Heikura T, et al. VEGF-A, VEGF-D and VEGF-D(DeltaNDeltaC) induced intimal hyperplasia in carotid arteries. Eur J Clin Invest. 2005;35:669–676. doi: 10.1111/j.1365-2362.2005.01555.x. [DOI] [PubMed] [Google Scholar]
- Di Marco GS, Reuter S, Hillebrand U, et al. The soluble VEGF receptor sFlt1 contributes to endothelial dysfunction in CKD. J Am Soc Nephrol. 2009;20:2235–2245. doi: 10.1681/ASN.2009010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter R, Carrick FE, Valdiviezo C, et al. Vascular endothelial growth factor regulates reendothelialization and neointima formation in a mouse model of arterial injury. Circulation. 2004;110:2430–2435. doi: 10.1161/01.CIR.0000145120.37891.8A. [DOI] [PubMed] [Google Scholar]
- Inoue M, Itoh H, Ueda M, et al. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: possible pathophysiological significance of vegf in progression of atherosclerosis. Circulation. 1998;98:2108–2116. doi: 10.1161/01.cir.98.20.2108. [DOI] [PubMed] [Google Scholar]
- Simons M. VEGF and restenosis: the rest of the story. Arterioscler Thromb Vasc Biol. 2009;29:439–440. doi: 10.1161/ATVBAHA.109.183970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojima I, Walsh K. The role of vascular endothelial growth factor in restenosis: the controversy continues. Circulation. 2004;110:2283–2286. doi: 10.1161/01.CIR.0000146723.23523.47. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Egashira K, Hiasa K, et al. Essential role of vascular endothelial growth factor and Flt-1 signals in neointimal formation after periadventitial injury. Arterioscler Thromb Vasc Biol. 2004;24:2284–2289. doi: 10.1161/01.ATV.0000147161.42956.80. [DOI] [PubMed] [Google Scholar]
- Ohtani K, Egashira K, Hiasa K-i, et al. Blockade of vascular endothelial growth factor suppresses experimental restenosis after intraluminal injury by inhibiting recruitment of monocyte lineage cells. Circulation. 2004;110:2444–2452. doi: 10.1161/01.CIR.0000145123.85083.66. [DOI] [PubMed] [Google Scholar]
- Misra S, Fu AA, Puggioni A, et al. Increased shear stress with up regulation of VEGF-A and its receptors and MMP-2, MMP-9, and TIMP-1 in venous stenosis of hemodialysis grafts. Am J Physiol Heart Circ Physiol. 2008;294:H2219–H2230. doi: 10.1152/ajpheart.00650.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Shergill U, Yang B, et al. Increased expression of HIF-1alpha, VEGF-A and its receptors, MMP-2, TIMP-1, and ADAMTS-1 at the venous stenosis of arteriovenous fistula in a mouse model with renal insufficiency. J Vasc Interv Radiol. 2010;21:1255–1261. doi: 10.1016/j.jvir.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Shergill U, Fu AA, et al. The mouse arteriovenous fistula model. J Vasc Interv Radiol. 2009;20:946–950. doi: 10.1016/j.jvir.2009.03.044. [DOI] [PubMed] [Google Scholar]
- Porter KE, Naik J, Turner NA, et al. Simvastatin inhibits human saphenous vein neointima formation via inhibition of smooth muscle cell proliferation and migration. J Vasc Surg. 2002;36:150–157. doi: 10.1067/mva.2002.122029. [DOI] [PubMed] [Google Scholar]
- Turner NA, Midgley L, O'Regan DJ, et al. Comparison of the efficacies of five different statins on inhibition of human saphenous vein smooth muscle cell proliferation and invasion. J Cardiovasc Pharmacol. 2007;50:458–461. doi: 10.1097/FJC.0b013e318123767f. [DOI] [PubMed] [Google Scholar]
- Turner NA, O'Regan DJ, Ball SG, et al. Simvastatin inhibits MMP-9 secretion from human saphenous vein smooth muscle cells by inhibiting the RhoA/ROCK pathway and reducing MMP-9 mRNA levels. FASEB J. 2005;19:804–806. doi: 10.1096/fj.04-2852fje. [DOI] [PubMed] [Google Scholar]
- Misra S, Fu AA, Rajan DK, et al. Expression of hypoxia inducible factor-1 alpha, macrophage migration inhibition factor, matrix metalloproteinase-2 and -9, and their inhibitors in hemodialysis grafts and arteriovenous fistulas. J Vasc Interv Radiol. 2008;19:252–259. doi: 10.1016/j.jvir.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Shay-Salit A, Shushy M, Wolfovitz E, et al. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci USA. 2002;99:9462–9467. doi: 10.1073/pnas.142224299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Burns N, Wilson SJ, et al. Hypoxia exposure induces the emergence of fibroblasts lacking replication repressor signals of PKC{zeta} in the pulmonary artery adventitia. Cardiovasc Res. 2008;78:440–448. doi: 10.1093/cvr/cvn014. [DOI] [PubMed] [Google Scholar]
- Misra S, Fu AA, Misra KD, et al. Hypoxia-induced phenotypic switch of fibroblasts to myofibroblasts through a matrix metalloproteinase 2/tissue inhibitor of metalloproteinase-mediated pathway: implications for venous neointimal hyperplasia in hemodialysis access. J Vasc Interv Radiol. 2010;21:896–902. doi: 10.1016/j.jvir.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Fu A, Anderson J, et al. The rat femoral arteriovenous fistula model: Increased expression of MMP-2 and MMP-9 at the venous stenosis. J Vasc Interv Radiol. 2008;19:587–594. doi: 10.1016/j.jvir.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y, Libby P, Rabkin E, et al. Statins alter smooth muscle cell accumulation and collagen content in established atheroma of watanabe heritable hyperlipidemic rabbits. Circulation. 2001;103:993–999. doi: 10.1161/01.cir.103.7.993. [DOI] [PubMed] [Google Scholar]
- Chen Z, Fukutomi T, Zago AC, et al. Simvastatin reduces neointimal thickening in low-density lipoprotein receptor-deficient mice after experimental angioplasty without changing plasma lipids. Circulation. 2002;106:20–23. doi: 10.1161/01.cir.0000022843.76104.01. [DOI] [PubMed] [Google Scholar]
- Porter KE, Turner NA, O'Regan DJ, et al. Simvastatin reduces human atrial myofibroblast proliferation independently of cholesterol lowering via inhibition of RhoA. Cardiovasc Res. 2004;61:745–755. doi: 10.1016/j.cardiores.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Rotmans JI, Velema E, Verhagen HJ, et al. Matrix metalloproteinase inhibition reduces intimal hyperplasia in a porcine arteriovenous-graft model. J Vasc Surg. 2004;39:432–439. doi: 10.1016/j.jvs.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Bellosta S, Via D, Canavesi M, et al. HMG-CoA reductase inhibitors reduce MMP-9 secretion by macrophages. Arterioscler Thromb Vasc Biol. 1998;18:1671–1678. doi: 10.1161/01.atv.18.11.1671. [DOI] [PubMed] [Google Scholar]
- Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol. 2003;23:769–775. doi: 10.1161/01.ATV.0000068646.76823.AE. [DOI] [PubMed] [Google Scholar]
- Porter KE, Turner NA, O'Regan DJ, et al. Tumor necrosis factor alpha induces human atrial myofibroblast proliferation, invasion and MMP-9 secretion: inhibition by simvastatin. Cardiovasc Res. 2004;64:507–515. doi: 10.1016/j.cardiores.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Urbich C, Dernbach E, Zeiher AM, et al. Double-edged role of statins in angiogenesis signaling. Circ Res. 2002;90:737–744. doi: 10.1161/01.res.0000014081.30867.f8. [DOI] [PubMed] [Google Scholar]
- Weis M, Heeschen C, Glassford AJ, et al. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- Kureishi Y, Luo Z, Shiojima I, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- Campbell MJ, Esserman LJ, Zhou Y, et al. Breast cancer growth prevention by statins. Cancer Res. 2006;66:8707–8714. doi: 10.1158/0008-5472.CAN-05-4061. [DOI] [PubMed] [Google Scholar]
- Baetta R, Donetti E, Comparato C, et al. Proapoptotic effect of atorvastatin on stimulated rabbit smooth muscle cells. Pharmacol Res. 1997;36:115–121. doi: 10.1006/phrs.1997.0211. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lu H, Huang J, et al. Simvastatin exerts favourable effects on neointimal formation in a mouse model of vein graft. Eur J Vasc Endovasc Surg. 2011;42:393–399. doi: 10.1016/j.ejvs.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Sukhova GK, Williams JK, Libby P. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arterioscler Thromb Vasc Biol. 2002;22:1452–1458. doi: 10.1161/01.atv.0000030360.72503.56. [DOI] [PubMed] [Google Scholar]
- Rodrigues Diez R, Rodrigues-Diez R, Lavoz C, et al. Statins inhibit angiotensin II/Smad pathway and related vascular fibrosis, by a TGF-beta-independent process. PLoS One. 2010;5:e14145. doi: 10.1371/journal.pone.0014145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts KL, Spiteri MA. Connective tissue growth factor expression and induction by transforming growth factor-beta is abrogated by simvastatin via a Rho signaling mechanism. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1323–L1332. doi: 10.1152/ajplung.00447.2003. [DOI] [PubMed] [Google Scholar]
- Ahmed AK, Haylor JL, El Nahas AM, et al. Localization of matrix metalloproteinases and their inhibitors in experimental progressive kidney scarring. Kidney Int. 2007;71:755–763. doi: 10.1038/sj.ki.5002108. [DOI] [PubMed] [Google Scholar]
- Chade AR, Zhu XY, Grande JP, et al. Simvastatin abates development of renal fibrosis in experimental renovascular disease. J Hypertens. 2008;26:1651–1660. doi: 10.1097/HJH.0b013e328302833a. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang M, Bianchi M, et al. Fetuin (alpha 2-HS-glycoprotein) opsonizes cationic macrophagedeactivating molecules. Proc Natl Acad Sci USA. 1998;95:14429–14434. doi: 10.1073/pnas.95.24.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina RJ, O'Neill CL, Devine AB, et al. The pleiotropic effects of simvastatin on retinal microvascular endothelium has important implications for ischaemic retinopathies. PLoS One. 2008;3:e2584. doi: 10.1371/journal.pone.0002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copaja M, Venegas D, Aranguiz P, et al. Simvastatin induces apoptosis by a Rho-dependent mechanism in cultured cardiac fibroblasts and myofibroblasts. Toxicol Appl Pharmacol. 2011;255:57–64. doi: 10.1016/j.taap.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Langer S, Kokozidou M, Heiss C, et al. Chronic kidney disease aggravates arteriovenous fistula damage in rats. Kidney Int. 2010;78:1312–1321. doi: 10.1038/ki.2010.353. [DOI] [PubMed] [Google Scholar]
- Kokubo T, Ishikawa N, Uchida H, et al. CKD accelerates development of neointimal hyperplasia in arteriovenous fistulas. J Am Soc Nephrol. 2009;20:1236–1245. doi: 10.1681/ASN.2007121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni R, Barker-Finkel J, Allo M. Statin therapy is not associated with improved vascular access outcomes. Clin J Am Soc Nephrol. 2010;5:1447–1450. doi: 10.2215/CJN.02740310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SH, Simari RD, Best PJ, et al. Simvastatin preserves coronary endothelial function in hypercholesterolemia in the absence of lipid lowering. Arterioscler Thromb Vasc Biol. 2001;21:122–128. doi: 10.1161/01.atv.21.1.122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.