Abstract
Background
The aim of this study was to evaluate the effect of topical fibrin glue applied externally to all anastomoses after a pancreaticoduodenectomy (PD) on drain lipase levels, anastomotic leaks, complication rates and length of hospital stay.
Methods
A standardized non-pylorus preserving PD was performed with or without fibrin glue applied to each anastomosis.
Results
Fifty-seven patients were randomized: 32 with and 25 without TISSEEL. There were no statistical differences in each group with respect to drain lipase levels (high 40% versus 43%, P = 0.794), complications including gastric or biliary leaks (24% versus 28%, P = 1.00), wound infection (16% versus 9%, P = 0.28) and a Clavien score of 3 or more (16% versus 25%, P = 0.757) or hospital stay (12 versus 17 days, P = 0.777). Most patients with elevated drain lipase levels had an unaltered clinical course not predictive of adverse outcomes. However, the operative finding of a soft pancreas (27 out of 57 patients) was associated with post-operative complications (P = 0.002). There were no peri-operative deaths.
Conclusions
Fibrin glue application to all anastomoses does not alter drain lipase levels.
Drain lipase levels are not a significant surrogate marker for clinically significant anastomotic leaks or complications.
Fibrin glue application did not reduce the incidence of an anastomotic leak or complications.
Introduction
Although peri-operative mortality associated with a pancreaticoduodenectomy (PD) has improved significantly over the years (0–3%),1,2 a pancreatic anastomotic leak remains a major complication after a pancreatic resection.3–5 A pancreatic fistula is the sequel to further complications such as wound infection, an intra-abdominal abscess and sepsis. Several manoeuvres have been employed in attempt to reduce the rates of pancreatic fistulae. The three main interventions trialled have been: variations of the pancreatic anastomosis; the use of long-acting somatostatin analogues such as octreotide; and the use of fibrin glue sealants. Numerous randomized controlled trials were conducted with the first two interventions, the majority failing to provide a statistical difference in reducing rates of pancreatic fistulae or mortality.6 In contrast, the use of fibrin glue sealant about the pancreatic anastomosis after PD alone has only been performed in only one randomized trial. Similarly, the authors are not aware of any randomized trials using fibrin glue about all of the anastomoses in PD. The role of drain lipase as opposed to drain amylase in this setting has also not been defined.
TISSEEL (Hemacure Corp., Sarasota, FL, USA; Baxter Health Care Corp., Glendale, CA, USA) is an adhesive fibrin glue sealant that consists mainly of human fibrinogen and thrombin marketed for use in both haemostasis and tissue sealing. Several previous non-randomized studies have suggested that the incidence of a fistula decreases after a pancreatic resection with the application of fibrin glue.7–9 Two recent non-randomized studies have suggested that the combination of fibrin glue and polyglicolic acid may similarly reduce the risk of a pancreatic fistula for patients undergoing a PD.10,11 A prospective randomized multicentre study that applied intraductal fibrin glue to patients after a PD or a distal pancreatectomy showed no difference in the rates or severity of pancreatic fistulae or intra-abdominal complications.
Only one randomized controlled trial that applied topical fibrin glue about the pancreatic anastomosis specifically after PD has been reported.12 This excellent trial in a high-volume centre showed no significant differences in the rates of pancreatic fistulae or complications among 125 patients with ‘high-risk’ pancreatic anastomoses (soft or medium texture pancreas and small pancreatic duct).
Furthermore, the use of TISSEEL to reduce the incidence of a gastrointestinal anastomotic leak in major visceral surgery has been trialled in non-randomized trials, some with positive results.13 No randomized trial of its use in PD about all of the anastomoses in PD has previously been performed.
Lipase may be found in lingual, intestinal, hepatic or pancreatic secretions. Hence a drain lipase elevation may reflect a leak from any of the anastomoses after a PD (pancreatic, biliary or gastric). Pancreatic lipase activity requires the presence of another enzyme colipase. Most commercial lipase assays try to favour specificity for pancreatic lipase by adding bile acids and colipase to partially inhibit other lipases. In spite of this methodology, lipase on commercial testing is still present in sputum, gastric and biliary secretions.
The aim of this study was therefore to evaluate the effect of topical application of fibrin glue applied to all anastomoses after a PD on the drain lipase level, anastomotic leak, complication rates and length of hospital stay in a medium volume centre.
Methods
Surgical technique
All operations were conducted by a single surgeon (I.M.). A standard non-pylorus preserving PD was performed using the same anastomotic technique. An end-to-side pancreatico-jejunostomy was fashioned in two layers using six interrupted 3/0 monocryl transpancreatic U-sutures to the anterior and posterior serosal layers of the jejunum as first described by Blumgart14 with 6–10 interrupted 5/0 monocryl pancreatic duct to mucosa sutures. A hepaticojejunostomy was fashioned using a running 5/0 monocryl, with a loop gastroenterostomy and a side-to-side jejunojejunostomy constructed using an EndoGIA (Covidian, Mansfield, MA, USA) stapling device, 60 × 3.5 mm. No feeding tubes were placed. Peri-operative intravenous (i.v.) pantoperazole 40 mg daily and subcutaneous octreotide 100 μg 8 hourly for 8–10 days was utilized.
Randomization criteria
Pre-operative informed consent was obtained from each patient with randomization occurring during the operation using a coin toss performed by a theatre nurse (‘Tails for TISSEEL’). At the end of the procedure, after a thorough lavage, patients randomized to receive fibrin glue sealant had TISSEEL applied topically through a double-barrel syringe connected to a Y-shaped catheter circumferentially to ensure all four anastomoses received a thin covering layer (4–10 ml in total required). TISSEEL was not applied into the pancreatic duct. The randomisation flow chart is shown in Fig. 1.
Figure 1.
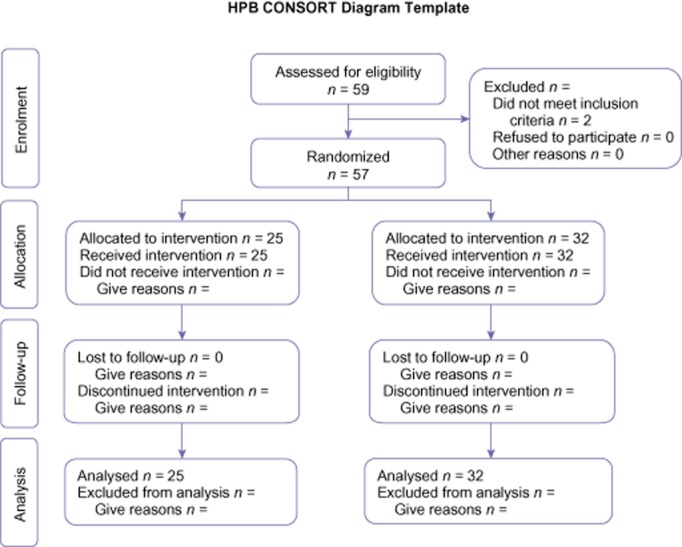
HPB CONSORT diagram template
Post-operative management
Two perianastomotic 19-Fg Blake drains were placed and removed on the third post-operative day regardless of the drain lipase level unless the effluent was bile or enteric stained or turbid.
Nasogastric tubes were removed in the first post-operative day and i.v. erythromycin 50–150 mg 8 hourly commenced on days 3–8 in most patients. Post-operative assessment included measurement of drain and serum lipase from the third day. Creon forte was given to supplement meals on discharge.
Data collection
Patient trial entry data were collected prospectively and outcomes assessed retrospectively. Data were collected by single non-blinded medical researcher.
Study end points
The primary study end points consisted of drain lipase levels, pancreatic fistulae, anastomotic leak, all complications, length of hospital stay and death. The validated Clavien score was used to grade post-operative complications.15
A post-operative pancreatic fistula (POPF) was defined as drainage fluid of the lipase level three times greater than the normal serum level (>750 u/l), regardless of the volume of drainage fluid from day 3 post-operatively. This is the same as for the international study group definition with the distinction that lipase levels were tested rather than amylase levels.4 Grading into A, B or C was also used as per the international definition.
The effect of fibrin glue on lipase drain levels has not previously been reported. The sample size of this binary outcome superiority trial was based on the premise that an elevated day 3 drain lipase would fall from 40% to 8% in the treatment group at a significance level of 5% and a power of 80%. A total of 54 patients were required with 27 in each group to meet this criteria. Statistical analyses were performed in R [R Development Core Team (2011), R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria]. Comparisons between groups were made using Wilcoxon's rank sum test in the case of continuous variables and Fisher's exact test in the case of categorical variables. All P-values were two-sided and we used a statistical significance level of 0.05.
Statistical analyses
Statistical analyses were performed in R (R Development Core Team). Comparisons between groups were made using Wilcoxon's rank-sum test in the case of numerical variables and Fisher's exact test in the case of categorical variables. All P-values are two sided with a statistical significance level of 0.05.
Results
Patient population
The patients’ demographic data and pre-operative risk factors associated with PD morbidity are shown in Table 1. These patients were randomized with 25 patients in the TISSEEL group and 32 patients in the control group. There were no significant differences between the groups in terms of gender and pre-operative factors except for age. The mean patient age was 67.0 ± 7.9 years in the TISSEEL group and 60.5 ± 9.7 in the control group (P = 0.017).
Table 1.
Patient characteristics and preoperative factors
| Patient characteristics | TISSEEL (N = 25) | No TISSEEL (N = 32) | P-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Age (year), mean ± SD, median | 67.0 ± 7.9, 67 | 60.5 ± 9.7, 62 | 0.017 |
| Male gender | 10 (40.0%) | 19 (59.4%) | 0.186 |
| Smoking history | 5 (20.0%) | 9 (28.1%) | 0.548 |
| Alcohol history | 3 (12.0%) | 8 (25.0%) | 0.315 |
| Pre-operative factors | |||
| Jaundice | 11 (44.0%) | 21 (65.6%) | 0.117 |
| Weight loss | 13 (52.0%) | 16 (50.0%) | 1.000 |
| Diabetes mellitusa | 4 (16.0%) | 6 (18.8%) | 1.000 |
| Peripheral vascular disease | 0 (0) | 4 (12.5%) | 0.123 |
| Pancreatitis | 2 (8.0%) | 4 (12.5%) | 0.686 |
TISSEEL, N = 24.
There were no significant differences between the groups in terms of pancreatic texture, pancreatic duct diameter or operation times (Table 2). The histopathological findings are shown in Table 3. Fifteen patients (60%) in TISSEEL group had a soft pancreas compared with 17 patients (53.1%) in the control group.
Table 2.
Intra-operative parameters
| Variable | TISSEEL | No TISSEEL | P-value |
|---|---|---|---|
| (N = 25) | (N = 32) | ||
| Texture of pancreas | 0.936 | ||
| Soft | 15 (60.0%) | 17 (53.1%) | |
| Intermediate | 4 (16.0%) | 6 (18.8%) | |
| Firm | 6 (24.0%) | 9 (28.1%) | |
| Width of pancreatic duct (mm) | 4.4 ± 1.8, 4.0 | 3.9 ± 1.5, 3.5 | 0.154 |
| Operation | |||
| Surgical time (h:min)a | 3:14 ± 0:56, 3:00 | 3:20 ± 0:45, 3:25 | 0.514 |
| Blood loss (ml)b | 495.0 ± 280.0, 425.0 | 581.7 ± 329.4, 450 | 0.584 |
| Blood transfusion (ml)b | 0 ± 0, 0 | 0 ± 0, 0 | NA |
TISSEEL group, N = 21; No TISSEEL, N = 30, mean and median.
TISSEEL group, N = 20; No TISSEEL, N = 30, mean and median.
NA, not applicable.
Data are presented as n (%) or mean ± SD, median.
Table 3.
Histopathological findings
| Patient characteristics | TISSEEL (n = 25) | No TISSEEL (n = 32) |
|---|---|---|
| Pancreas | ||
| Pancreatic adenocarcinoma | 8 (32%) | 15 (46.8%) |
| Intraductal papillary mucinous neoplasm | 1 (4%) | 2 (6.3%) |
| Mucinous cystic adenocarcinoma | 0 | 1 (3.1%) |
| Neuroendocrine carcinoma | 1 (4%) | 1 (3.1%) |
| Serous cystadenoma | 2 (8%) | 1 (3.1%) |
| Chronic pancreatitis | 2 (8%) | 0 |
| Autoimmune pancreatitis | 1 (4%) | 1 (3.1%) |
| Ampullary adenocarcinoma/adenoma | 4 (16%) | 7 (21.8%) |
| Bile duct | ||
| Cholangiocarcinoma | 2 (8%) | 1 (3.1%) |
| Fibroinflammatory | 1 (4%) | 0 |
| Duodenum | ||
| Duodenal adenocarcinoma | 1 (4%) | 2 (6.3%) |
| Tubulovillous adenoma | 1 (4%) | 1 (3.1%) |
| Others | ||
| Colonic adenocarcinoma invading pancreas | 1 (4%) | 0 |
Complications
Post-operative complications and course are shown in Table 4. There were no deaths with 44% of all patients experiencing one or more post-operative complication (48% in the TISSEEL and 41% the control group). The most common post-operative complication was POPF, followed by delayed gastric emptying and wound infection. There were no significant differences in post-operative complications and length of hospital stay (TISSEEL group: mean = 14.1 ± 12.7 days, median = 11.0 days; control group: mean = 17.4 ± 17.5 days, median = 10.0 days). TISSEEL application did not alter morbidity rates using a Clavien score of 3 or 4 (P = 0.757).
Table 4.
Post-operative complications and course
| Post-operative complications and course | TISSEEL (n = 25) | No TISSEEL (n = 32) | P-value |
|---|---|---|---|
| Death | 0 | 0 | |
| Re-operation | 5 (20%) | 8 (25.0%) | 0.757 |
| Percutaneous radiological intervention | 1 (4%) | 3 (9.4%) | 0.623 |
| Any complication | |||
| Pancreatic fistulaa | 10 (40%) | 14 (43.8%) | 0.794 |
| Grade A, B, C | 10,0,0 | 12,1,1 | |
| Bile leak | 2 (8%) | 3 (9.4%) | 1.000 |
| Gastric anastomotic leak | 2 (8%) | 2 (6.2%) | 1.000 |
| Early delayed gastric emptying | 3 (12%) | 5 (15.6%) | 1.000 |
| Wound infection | 5 (20%) | 3 (9.4%) | 0.280 |
| No significant complications | 13 (52%) | 18 (56.2%) | 0.794 |
| Clavien score | 0.668 | ||
| I | 13 (52%) | 19 (59.4%) | |
| II | 7 (28%) | 5 (15.6%) | |
| IIIa | 1 (4%) | 3 (9.4%) | |
| IIIb | 4 (16%) | 5 (15.6%) | |
| IV | 0 | 0 | |
| V | 0 | 0 | |
| Post-operative hospital stay (days) | 14.1 ± 12.7, 11.0 | 17.4 ± 17.5, 10.0 | 0.777 |
Defined as the drain lipase level > 750 μ/l, regardless of volume of drainage fluid as on or after day 3, graded as per International POPF classification.
Pancreatic fistula
The overall incidence of a pancreatic fistula was 42% (40% TISSEEL and 44% the control group). The mean post-operative hospital stay for patients with a pancreatic fistula was 14.1 ± 6.9 days (median = 13.0), whereas the mean post-operative hospital stay for patients without a pancreatic fistula was 19.5 ± 14.6 days (median = 12.0). The reason was that of the twenty-four patients with POPF only two had an altered clinical course, one requiring percutaneous drainage and the other requiring reoperation (Grades A 22, B 1, C 1). Pancreatic texture was associated with a pancreatic fistula (P = 0.002) but was not associated with elevated morbidity as per the Clavien scores of 3 or 4. The results suggest that a Clavien score of 3 or 4 is more likely for patients with a high drain lipase. However, the difference in percentages with a high Clavien score failed to achieve statistical significance (P = 0.052). This may be because of the relatively small sample size.
Discussion
POPF has previously been associated with patients found to have a soft texture of the pancreas.12 This finding was significantly supported in this study. We defined POPF as per the international study group definition4 (drain fluid of any volume with an elevated amylase level three times the serum level from the third postoperative day) with the exception that lipase was used instead of amylase. The role of lipase has previously been reported as having a sensitivity of 93% and specificity of 77% for grades B or C POPF at a threshold level in the drain of 1000 units/l or four times the normal serum level.16 Their study recommends that a drain lipase level of four times the serum level might be a more appropriate cut-off to define POPF. Our study supported this finding as we found a high proportion of patients with lipase-rich drain fluid more than three times the serum level (42%, 24 of 59 patients) had an unaltered clinical course (grade A POPF 22 of 24 patients).
In the present series only two patients with POPF required some form of intervention: one grade B requiring percutaneous drainage only and the other grade C requiring re-operation at day 7 with a normal drain lipase level on the third post-operative day.
The weaknesses of this study were the small sample size and the method of randomization. As we did not find any trend suggesting a reduction in lipase-rich drain effluent in the TISSEEL group it appears unlikely that a larger study would have altered the final results. Using a coin toss for randomization may result in imbalanced sample sizes, as occurred in this study. Furthermore, baseline characteristics may be imbalanced. We found a difference in age in the two groups (Table 1); however, all other covariates were similar. Age was not predictive of adverse outcome or POPF rates in this study.
This study was unique in its methodology as the fibrin glue was applied to all the reconstructive PD anastomoses. Theoretically gastric, biliary and pancreatic lipase will follow the intestinal lumen and appear in the drain effluent as a surrogate marker to aid in the diagnosis of any leaking anastomosis. However, this was not born out in this study as 9 out of 13 reoperations were in fact for leaking biliary or gastric anastomoses. The biliary leaks were early (first 3 days) and self evident with bile in the drain effluent whereas the gastric leaks were delayed (days 6–16) and as a consequence of gastroparesis or incomplete intestinal obstruction. Thus it appears that drain lipase was not a useful or practical surrogate marker for either intestinal or pancreatic anastomotic leak in this series.
In summary, fibrin glue application to all anastomoses did not alter drain lipase levels. It was found that drain lipase levels were not a useful surrogate marker for clinically significant anastomotic leaks or complications. It appears that high drain lipase levels as per the international classification of three times the serum level for POPF as stated for amylase, is not uncommon post PD and in the majority of cases the clinical outcome was unaltered (Grade A POPF).
Conflicts of interest
None declared.
References
- 1.Farnell MB, Nagorney DM, Sarr MG. The Mayo clinic approach to the surgical treatment of adenocarcinoma of the pancreas. Surg Clin North Am. 2001;81:611–623. doi: 10.1016/s0039-6109(05)70147-x. [DOI] [PubMed] [Google Scholar]
- 2.Reid-Lombardo KM, Farnell MB, Crippa S, Barnett M, Maupin G, Bassi C, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1,507 patients: a report from the Pancreatic Anastomotic Leak Study Group. J Gastrointest Surg. 2007;11:1451–1458. doi: 10.1007/s11605-007-0270-4. discussion 9. [DOI] [PubMed] [Google Scholar]
- 3.Balzano G, Zerbi A, Cristallo M, Di Carlo V. The unsolved problem of fistula after left pancreatectomy: the benefit of cautious drain management. J Gastrointest Surg. 2005;9:837–842. doi: 10.1016/j.gassur.2005.01.287. [DOI] [PubMed] [Google Scholar]
- 4.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Bilimoria MM, Cormier JN, Mun Y, Lee JE, Evans DB, Pisters PW. Pancreatic leak after left pancreatectomy is reduced following main pancreatic duct ligation. Br J Surg. 2003;90:190–196. doi: 10.1002/bjs.4032. [DOI] [PubMed] [Google Scholar]
- 6.Schulick RD, Yoshimura K. Stents, glue, etc.: is anything proven to help prevent pancreatic leaks/fistulae? J Gastrointest Surg. 2009;13:1184–1186. doi: 10.1007/s11605-009-0866-y. [DOI] [PubMed] [Google Scholar]
- 7.Kram HB, Clark SR, Ocampo HP, Yamaguchi MA, Shoemaker WC. Fibrin glue sealing of pancreatic injuries, resections, and anastomoses. Am J Surg. 1991;161:479–481. doi: 10.1016/0002-9610(91)91116-z. discussion 82. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y, Kuroda Y, Morita A, Fujino Y, Tanioka Y, Kawamura T, et al. Fibrin glue sealing for the prevention of pancreatic fistulas following distal pancreatectomy. Arch Surg. 1995;130:952–955. doi: 10.1001/archsurg.1995.01430090038015. [DOI] [PubMed] [Google Scholar]
- 9.Tashiro S, Murata E, Hiraoka T, Nakakuma K, Watanabe E, Miyauchi Y. New technique for pancreaticojejunostomy using a biological adhesive. Br J Surg. 1987;74:392–394. doi: 10.1002/bjs.1800740523. [DOI] [PubMed] [Google Scholar]
- 10.Uemura K, Murakami Y, Hayashidani Y, Sudo T, Hashimoto Y, Ohge H, et al. Combination of polyglicolic acid felt and fibrin glue for prevention of pancreatic fistula following pancreaticoduodenectomy. Hepatogastroenterology. 2009;56:1538–1541. [PubMed] [Google Scholar]
- 11.Ochiai T, Sonoyama T, Soga K, Inoue K, Ikoma H, Shiozaki A, et al. Application of polyethylene glycolic acid felt with fibrin sealant to prevent postoperative pancreatic fistula in pancreatic surgery. J Gastrointest Surg. 2010;14:884–890. doi: 10.1007/s11605-009-1149-3. [DOI] [PubMed] [Google Scholar]
- 12.Lillemoe KD, Cameron JL, Kim MP, Campbell KA, Sauter PK, Coleman JA, et al. Does fibrin glue sealant decrease the rate of pancreatic fistula after pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2004;8:766–772. doi: 10.1016/j.gassur.2004.06.011. discussion 72–4. [DOI] [PubMed] [Google Scholar]
- 13.Upadhyaya VD, Gopal SC, Gangopadhyaya AN, Gupta DK, Sharma S, Upadyaya A, et al. Role of fibrin glue as a sealant to esophageal anastomosis in cases of congenital esophageal atresia with tracheoesophageal fistula. World J Surg. 2007;31:2412–2415. doi: 10.1007/s00268-007-9244-7. [DOI] [PubMed] [Google Scholar]
- 14.Grobmyer SR, Kooby D, Blumgart LH, Hochwald SN. Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J Am Coll Surg. 2010;210:54–59. doi: 10.1016/j.jamcollsurg.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Facy O, Chalumeau C, Poussier M, Binquet C, Rat P, Ortega-Deballon P. Diagnosis of postoperative pancreatic fistula. Br J Surg. 2012;99:1072–1075. doi: 10.1002/bjs.8774. [DOI] [PubMed] [Google Scholar]


