Abstract
Background
High-intensity focused ultrasound (HIFU) ablation is a non-invasive treatment for hepatocellular carcinoma (HCC). At present, data on the treatment's long-term outcome are limited. This study analysed the survival outcome of HIFU ablation for HCCs smaller than 3 cm.
Patients and methods
Forty-seven patients with HCCs smaller than 3 cm received HIFU treatment between October 2006 and September 2010. Fifty-nine patients who received percutaneous radiofrequency ablation (RFA) were selected for comparison. The two groups of patients were compared in terms of pre-operative variables and survival.
Results
More patients in the HIFU group patients had Child–Pugh B cirrhosis (34% versus 8.5%; P = 0.001). The 1- and 3-year overall survival rates of patients whose tumours were completely ablated in the HIFU group compared with the RFA group were 97.4% versus 94.6% and 81.2% versus 79.8%, respectively (P = 0.530). The corresponding 1- and 3-year disease-free survival rates were 63.6% versus 62.4% and 25.9% versus 34.1% (P = 0.683).
Conclusions
HIFU ablation is a safe and effective method for small HCCs. It can achieve survival outcomes comparable to those of percutaneous RFA and thus serves as a good alternative ablation treatment for patients with cirrhosis.
Introduction
Hepatocellular carcinoma (HCC) is a common cancer and the third most common cause of cancer mortality. The incidence of HCC is particularly high in Asia where hepatitis B virus infection is endemic. Hepatitis-B-related cirrhosis and HCC make a bad combination for patients as treatment options are relatively limited.1 Hepatectomy and liver transplantation are widely accepted as good treatment choices for most patients with small HCCs. However, local ablation therapy is a very good alternative for patients who do not want or cannot tolerate a major surgical procedure.2 Among the different methods of ablation for HCC, radiofrequency ablation (RFA) is relatively popular because of its simplicity and repeatability.
Since 2005, RFA has been recommended as a treatment option in the practice guidelines issued by the American Association for the Study of Liver Disease.3 Percutaneous RFA has been shown to provide complete ablation results and good survival outcomes, especially in treating small HCCs. However, when lesions are located at difficult sites, such as the liver dome or sites near the liver capsule, percutaneous RFA will probably result in a residual tumour. Moreover, patients with gross ascites which is a sign of decompensation of cirrhosis may not be suitable for this treatment modality.
High-intensity focused ultrasound (HIFU) ablation is a relatively new treatment for HCC. Long-term data are still lacking. HIFU treatment utilizes a unique frequency of the ultrasound wave of 0.8 to 3.5 MHz, which can be focused at a distance from the therapeutic transducer. The accumulated energy at the focused region causes oscillation of the particles, inducing necrosis of the target lesion by elevating the tissue temperature to above 60°C.4,5 As very little collateral damage to the peripheral structure is made, this treatment modality has been shown to be a safe procedure in patients even with advanced cirrhosis.6 This study analysed the results of HIFU ablation and percutaneous RFA in the treatment of HCCs smaller than 3 cm.
Patients and methods
From October 2006 to September 2010, 1321 patients at Queen Mary Hospital were diagnosed with HCC, and 457 (34.6%) of them received surgical intervention. The clinical data were recorded prospectively. Patients with primary HCCs smaller than 3 cm and first recurrence were included in this study. Patients who had multiple treatments and patients with extrahepatic disease were excluded from the study.
HCC was diagnosed if (i) the AFP is greater than 200 ng/ml and the radiological appearance of the mass is suggestive of HCC or (ii) there are typical arterial enhancement patterns with portal venous wash out in two imaging modalities.3
All patients with HCCs smaller than 3 cm were evaluated for operability. Their general condition, liver function, tumour status and tumour location were assessed. As a general guideline, a hepatectomy or open RFA was offered as a first treatment if the patient's liver function and physical status were satisfactory and a liver transplantation was offered if the patient had advanced cirrhosis rendering a hepatectomy a high-risk procedure.
For patients who preferred a less invasive approach, percutaneous RFA was offered if technically feasible as assessed by an experienced radiologist. HIFU ablation was offered to patients with poor liver function or decompensated cirrhosis as documented by (i) the presence of gross ascites, (ii) Child–Pugh B or above and (iii) tumours located at sites considered difficult for percutaneous RFA. HIFU ablation was also offered to patients as an alternative ablation treatment for those who want to avoid needle puncture from RFA. Transarterial chemoembolization was reserved for non-ablatable tumours in this series. Patients who received multiple treatments and crossover treatment with RFA were not included in this study.
Percutaneous RFA
Percutaneous RFA was performed by an experienced liver surgeon together with one or two experienced interventional radiologists. The ablation was performed with ultrasound guidance under local anaesthesia with light sedation. All RFA treatments were performed according to a standard protocol, using the cool-tip RF system (Radionics, Burlington, MA, USA). A single electrode with a 2- or 3-cm exposed tip was used for tumours smaller than 3 cm in diameter, whereas a clustered probe consisting of three parallel electrodes was used for tumours around 3 cm in size. Ablation was performed with a curative intention, aiming to achieve an ablation margin of 1 cm. A dose of antibiotic (Augmentin; Beecham Pharmaceuticals, Brentford, London, UK) was given just before the operation and oral antibiotics were given for 5 days after the operation.7,8 to avoid a potential risk of infection.
HIFU ablation
HIFU ablation was performed by the same team of surgeons and radiologists. We used the JC HIFU system (Chongqing Haifu Technology, Chongqing, China), which consists of an ultrasound energy transducer which focuses the ultrasound energy at a 12-cm focal point. A degassed water circulation unit provides a medium for ultrasound transmission outside the body. Patients were usually placed in a right lateral position for right-sided lesions and in a prone position for left-sided lesions. General anaesthesia allowed a more comfortable procedure. In addition, interval cessation of respiratory movement, which was performed by an anaesthesiologist, facilitated better localization of the HCC during energy transfer. In patients with tumours at the dome of the liver, an artificial right pleural effusion was induced before treatment. An artificial pleural effusion is introduced by instilling 600 to 800 ml of warm saline into the patient's right thoracic cavity. The saline displaces the lung parenchyma temporarily and a good acoustic pathway for the ultrasound energy is formed. With respiratory control, the tumour can be displaced to a location where HIFU beams can be properly administered. Grey-scale changes of the ablated sites were observed during the ablation procedure, indicating the temperature change inside the target lesion. A dose of antibiotic (Augmentin; Beecham Pharmaceuticals) was given just before the operation and oral antibiotics were given for 5 days after the operation.5,6
Follow-up
Post-operative blood tests for a complete blood picture, prothrombin time and liver and renal functions were performed routinely on days 1, 3, 7 and 14, or according to specific clinical situations. Contrast computed tomography or magnetic resonance imaging was performed 1 month after ablation. Assessment scans were performed with 3-month intervals during the first 2 years and with 6-month intervals thereafter. Complications were defined as any deviation from the normal post-operative course. This definition also takes into account asymptomatic complications such as arrhythmia and atelectases.9 Complete ablation was defined as the disappearance of the enhancement pattern of the lesion as compared with the pre-operative lesion on imaging performed 1 month after ablation. Mortality was defined as any death during the hospital stay or less than 30 days after the intervention. The location of the liver tumour was described according to the Brisbane 2000 classification.10
Statistical analysis
Baseline characteristics of patients were expressed as medians with range. The Mann-Whitney U-test was used to compare continuous variables, and a chi-squared test was used to compare discrete variables. Survival analysis was performed using the time of disease-free survival versus recurrence of the tumour or death. Survival curves were computed using the Kaplan–Meier method and compared between groups using the log-rank test. A P-value below 0.05 signified statistical significance. All statistical calculations were made with the SPSS/PC+ computer software (SPSS, Chicago, IL, USA).
Results
Forty-seven patients received HIFU ablation as a curative treatment and 59 patients received percutaneous RFA. Patients were enrolled to HIFU treatment in this study as a result of the following reasons: (i) 27 (57.4%) patients had failed RFA screening by a radiologist owing to either poor visualization of lesions or technical difficulties; (ii) 6 patients (12.8%) had decompensated liver cirrhosis evidenced by the presence of gross ascites; (iii) 5 patients (10.6%) had a platelet count lower than 50 × 109/l; (iv) 15 patients (31.9%) had tumours located close to the diaphragm; (v) 16 patients (34%) had Child–Pugh B cirrhosis; and (vi) 10 patients (21.3%) preferred a totally non-invasive treatment modality.
In the HIFU group, 47 patients were treated with HIFU ablation and ablated 52 tumours. The details of patients' characteristics are listed in Table 1. Sixteen patients (34%) in the HIFU group and five patients (8.5%) in the RFA group had Child–Pugh B cirrhosis (P = 0.001). The two groups showed no difference in terms of the international normalized ratio, platelet count, and serum levels of total bilirubin, creatinine, albumin and alpha-fetoprotein. In the HIFU group, more lesions (55.6%) were located at sections 7 and 8. In the RFA group, fewer tumours (38.4%) were located at the same sections (P = 0.028). The details of tumours' characteristics are listed in Table 2.
Table 1.
Patient characteristics
| HIFU ablation (n = 47) | Percutaneous RFA (n = 59) | P | |
|---|---|---|---|
| Age (years) | 62 (34–84) | 60 (23–83) | 0.095 |
| Male/female | 36:11 | 43:16 | 0.663 |
| Hepatitis B virus infection | 42 (79.2%) | 46 (80.7%) | 0.849 |
| Hepatitis C virus infection | 9 (17%) | 10 (17.2%) | 0.971 |
| Ascites | |||
| Absent | 41 (87.2%) | 57 (96.6%) | 0.148 |
| Present | 6 (12.8%) | 2 (3.4%) | |
| Child–Pugh class | |||
| A | 31 (66%) | 54 (91.5%) | 0.001 |
| B | 16 (34%) | 5 (8.5%) | |
| Total bilirubin (umol/l) | 14 (7–50) | 13 (4–57) | 0.371 |
| Creatinine (umol/l) | 86 (44–878) | 78 (46–912) | 0.281 |
| Albumin (g/l) | 39 (24–46) | 39 (23–46) | 0.388 |
| International normalized ratio | 1.0 (0.8–1.5) | 1.1 (0.9–1.4) | 0.124 |
| Platelet count (1 × 109) | 104 (17–268) | 98 (34–228) | 0.731 |
| Alpha-fetoprotein (ng/ml) | 18 (2–937) | 16 (2–10050) | 0.694 |
HIFU, high-intensity focused ultrasound; RFA, radiofrequency ablation.
Table 2.
Tumour characteristics and operative outcomes
| HIFU ablation (n = 47) | Percutaneous RFA (n = 59) | P | |
|---|---|---|---|
| Tumour size (cm) | 1.5 (0.8–2.7) | 1.9 (1.0–2.8) | 0.006 |
| Tumour number | |||
| 1 | 42 (89.4%) | 52 (88.1%) | 0.668 |
| 2 | 5 (10.6%) | 6 (10.2%) | |
| 3 | 0 (0%) | 1 (1.7%) | |
| Tumour location | |||
| I | 0 (0%) | 3 (4.8%) | 0.029 |
| II | 1 (1.9%) | 5 (7.7%) | |
| III | 6 (11.5%) | 3 (4.8%) | |
| IV | 3 (5.7%) | 8 (12.3%) | |
| V | 0 (0%) | 8 (12.3%) | |
| VI | 12 (23%) | 13 (20%) | |
| VII | 17 (32.6%) | 6 (9.2%) | |
| VIII | 13 (25%) | 19 (29.2%) | |
| Complete ablation | 41 (87.2%) | 56 (94.9%) | 0.290 |
| Hospital stay (days) | 4 (2–18) | 6 (1–31) | 0.028 |
HIFU, high-intensity focused ultrasound; RFA, radiofrequency ablation.
Ten patients (21.3%) in the HIFU group developed post-operative complications. Two of them developed a pneumothorax after the introduction of an artificial pleural effusion which required chest tube insertion. One patient had third-degree skin burn and required surgical debridement. The other patients had relatively minor complications. Five patients (8.5%) in the RFA group developed post-operative complications. Two of them developed a pleural effusion requiring tapping. One patient developed liver abscess and required percutaneous drainage. One patient developed oesophageal variceal bleeding owing to decompensation of cirrhosis and required endoscopic haemostasis. The remaining patient had only a mild wound infection. The details of the complications are listed in Table 3. When serious complications (Clavein-Dindo grade IIIA or above) were considered, the two groups showed no difference.
Table 3.
Treatment-related complications
| HIFU ablation | Percutaneous RFA | P | |
|---|---|---|---|
| (n = 47) | (n = 59) | ||
| Patients with surgical complications | 10 (21.3%) | 5 (8.5%) | 0.06 |
| Moderate subcutaneous swelling | 1 | 0 | |
| Pneumothorax | 2 | 0 | |
| Skin burn | 2 | 0 | |
| Myocardiac infarction | 1 | 0 | |
| Ascites without tapping | 2 | 0 | |
| Fever | 1 | 0 | |
| Mild right chest wall swelling and pain | 1 | 0 | |
| Wound infection | 0 | 1 | |
| Pleural effusion requiring tapping | 0 | 2 | |
| Liver abscess | 0 | 1 | |
| Variceal bleeding | 0 | 1 | |
| Clavien–Dindo grade IIIA complication | 2 (4.3%) | 4 (6.8%) | 0.892 |
| Clavien–Dindo grade IVA complication | 1 (2.1%) | 0 (0%) | 0.508 |
| Hospital mortality | 0 (0%) | 0 (0%) | 1 |
HIFU, high-intensity focused ultrasound; RFA, radiofrequency ablation.
In the HIFU group, the 1-year overall survival was 97.4% and the 3-year overall survival was 81.2%. In the RFA group, the corresponding rates were 94.6% and 79.8% (P = 0.53) (Fig. 1). In the HIFU group, the 1-year disease-free survival was 63.6% and the 3-year disease-free survival was 25.9%. In the RFA group, the corresponding rates were 62.4% and 34.1% (P = 0.683) (Fig. 2).
Figure 1.
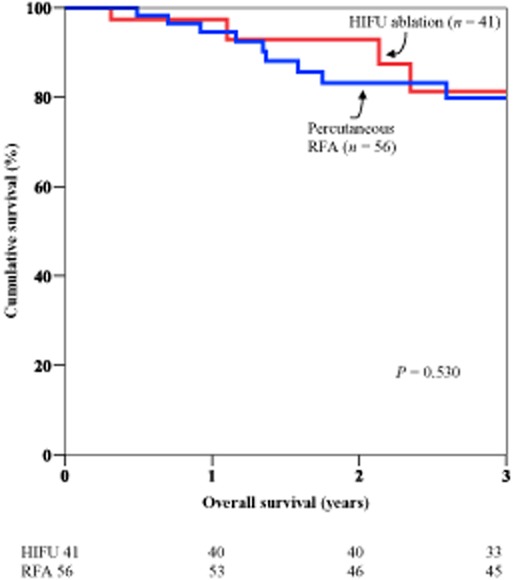
Comparison of overall survival
Figure 2.
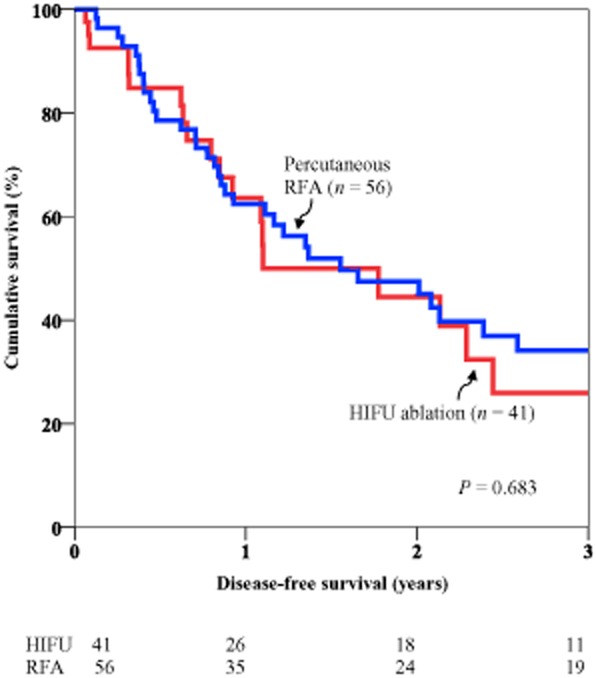
Comparison of disease-free survival
Discussion
In recent years, there has been much development in HCC-treating technology and devices, and numerous trials on targeted therapy have been carried out.11–17 Nevertheless, the general guidelines for the treatment algorithm for HCC have been formulated in actual clinical practice. For HCC patients who do not have cirrhosis, surgical resection is undoubtedly the treatment of choice with best survival benefits. On the other hand, the treatment of HCC in patients with cirrhosis is an entirely different story. Patient with HCCs even smaller than 3 cm may not tolerate a hepatectomy if they have advanced cirrhosis.2,18,19 A large tumour size, difficult tumour location, poor underlying liver function, comorbidity etc. can also render a hepatectomy impossible. A liver transplantation may be the only ultimate solution for patients unsuitable for a hepatectomy.20 The post-transplant survival rate of patients within the Milan criteria can be higher than 90% in 5 years at experienced centres.21,22 Although the results of liver transplantations are generally good, the scarcity of liver grafts makes it impossible to transplant everyone in need. Many patients on the transplant waiting list end up delisted as their disease progresses rendering them unsuitable for transplantation. So, in an ‘intention-to-treat’ analysis, survival of patients receiving transplantation may not be better than that of patients carefully selected for other surgical means.23
The technique of local ablation provides a bright hope for patients who have cirrhosis in addition to small HCCs.24 RFA is now widely practiced for its simplicity and repeatability. The complete ablation rate achieved by RFA ranges from 75% to 96%.25,26 RFA can be performed percutaneously under ultrasound or computed tomography guidance. Patients need only local anaesthesia with sedation. Although percutaneous RFA has been performed safely in many patients, patients with advanced cirrhosis may not tolerate it satisfactorily. Many interventional radiologists opine that patients with gross ascites should avoid percutaneous RFA treatment. As documented in one of the studies on RFA treatment, patients with slightly a raised bilirubin level and hypoalbuminaemia are prone to complications after RFA treatment for HCC.7
Initial studies on HIFU were mainly carried out in Mainland China, but now many other centres have started using HIFU for the ablation of solid tumours.4,27–30 HIFU is a totally extracorporeal tool that has been shown to be effective in treating various tumours. HIFU treatment makes use of the unique frequency of the ultrasound wave of 0.8 MHz, which makes penetration in soft tissue up to 12 cm deep possible. Ascites inside the peritoneal cavity not only provides a clear image for the diagnostic ultrasound unit, it also serves as a good medium for energy transfer. The presence of ascites also protects subcutaneous tissue from being damaged by the focused ultrasound energy.6,31 With HIFU, poor liver function with ascites is no longer a contraindication to HCC treatment.6 In the present study, there were more patients with Child–Pugh B cirrhosis in the HIFU group. This was because patients with ascites were not considered suitable for other treatments and were included for HIFU therapy. Liver function test results of patients having received HIFU ablation are not markedly different, which is unlike the case with patients having received RFA. Patients having received HIFU treatment also have a relatively shorter hospital stay as frequent liver function monitoring is not required as collateral damage to the normal liver tissue is minimal.
The needleless feature of HIFU treatment makes it unique among all ablation therapies. As HCC is a vascular tumour, RFA needle placement can cause torrential bleeding.32 Although minor tumour bleeding or rupture can be controlled by the thermal ablation at the end of the treatment, patients may suffer transient hypotension or develop complications; closer monitoring at a hospital is required. In addition, the insertion of an ablation needle into a tumour can produce a theoretic threat of tumour dissemination, particularly if the lesion is near major vessels.32,33 The use of HIFU may reduce these potential problems.
This study included patients with primary HCC because outcome analysis of HIFU and percutaneous RFA provided important clinical information. In addition, patients with first recurrent HCC were included because RFA was considered a popular and effective treatment option amongst surgeons for recurrent HCC.34 Haemostasis could be a problematic issue for open procedures such as adhesiolysis and hepatectomy particularly in patients with cirrhosis and a low platelet count. Percutaneous RFA and HIFU treatments were considered less invasive measures for this targeted group of patients. Although HCC patients with multiple recurrence and cross-over treatment were not uncommon, they were not included in this study for comparison. The outcome would be difficult to interpret if the analysis included patients receiving different treatments modalities.
The feasibility of percutaneous RFA is sometimes limited by tumour location. A location near the liver dome at section 7 or section 8 is a contraindication to percutaneous RFA because of potential damage to the diaphragm. On the other hand, HIFU energy can be targeted at lesions even in difficult positions, like those close to the diaphragm or the heart.35 After introduction of an artificial pleural effusion, the saline inside the thoracic cavity also acts as a protection cushion preventing damage to the diaphragm and surrounding soft tissue. Unlike RFA energy which is usually transferred to the lesion in continuous cycles of 12 min each, HIFU energy is transferred to the tumour in cycles of approximately 10 s each with 1-min breaks in between. The operator has full control on the location, the power of energy transmitted and the duration of break between cycles of energy dissipation. This makes HIFU treatment more operator-dependent; nonetheless, each treatment is tailor-made for the patient.
There was a case of third-degree burn to the dermis which required surgical debridement in the early study period. This occurred in a patients with a lesion located near the subcapsular region of section 5. When the ultrasound energy was focused near the rib cage, reflection of the energy might cause accidental soft tissue oedema and damage to the overlying structure. In addition, the complication was also contributed to by the early learning curve effect where the operator was unaware of the soft tissue oedema demonstrated by the diagnostic ultrasound unit. A high index of clinical suspicious could prevent a serious complication during the procedure. Otherwise, complications arising from HIFU are relatively minor. They are mostly related to soft tissue oedema or discomfort after the procedure. Symptoms may be more obvious if the tumour is located close to the subcapsular region.36 These complications are usually self-limiting. In the present study, the two groups of patients had similar rates of serious complications.
There was no survival difference between the two groups. The complete ablation rate was slightly better in the RFA group but no significant difference was observed. However, patients with advanced cirrhosis and considered not suitable for other treatments were included in the HIFU group. More patients were cured in spite of their background cirrhosis. Long-term survival data on HIFU treatment will be collected as the follow-up period lengthens.
In the management of small HCC, we suggest a hepatectomy for primary HCC with preserved liver function as the first treatment option. RFA should be performed in patients with small but unresectable HCC where liver transplantation is not an option. A percutaneous approach should be adopted if technically feasible. Patients with gross ascites or a tumour located closed to bile duct, major vessels, gallbladder and diaphragm should not be considered suitable for percutaneous RFA. HIFU should be considered as an alternative ablation option for this group of patients. In the case of recurrent disease, only patients with Child–Pugh class A cirrhosis and selected patients with Child–Pugh class B cirrhosis should be considered for re-resection or RFA. The usual indication for re-resection was a solitary or oligonodular tumour within a single section of the liver in the presence of a sufficient future liver remnant. A re-resection should be avoided in the presence of gross ascites, an indocyanine green retention rate more than 15% at 15 min and a platelet count lower than 100 × 109/l. Percutaneous RFA should be considered when the recurrent tumour is in a deep-seated location where an anatomical resection will sacrifice a large amount of functional liver parenchyma. RFA should generally be avoided in the presence of gross ascites, a platelet count lower than 50 × 109/l in spite of a platelet transfusion and a tumour located at difficult position as described above. HIFU should be considered as an alternative ablation option for this group of patients.
To conclude, in the treatment of HCCs smaller than 3 cm, HIFU ablation provides a good oncological outcome, which is comparable to that of percutaneous RFA. This relatively new treatment modality can be offered to patients who have a poor liver function and advanced cirrhosis which render other therapies suboptimal.
Conflicts of interest
None declared.
References
- 1.Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827–841. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 2.Fan ST. Surgical therapy of hepatocellular carcinoma in the cirrhotic liver. Swiss Surg. 1999;5:107–110. doi: 10.1024/1023-9332.5.3.107. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 4.Wu F, Wang ZB, Chen WZ, Wang W, Gui YZ, Zhang M, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem. 2004;11:149–154. doi: 10.1016/j.ultsonch.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Ng KK, Poon RT, Chan SC, Chok KS, Cheung TT, Tung H, et al. High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience. Ann Surg. 2011;253:981–987. doi: 10.1097/SLA.0b013e3182128a8b. [DOI] [PubMed] [Google Scholar]
- 6.Cheung TT, Chu FS, Jenkins CR, Tsang DS, Chok KS, Chan AC, et al. Tolerance of high-intensity focused ultrasound ablation in patients with hepatocellular carcinoma. World J Surg. 2012;36:2420–2427. doi: 10.1007/s00268-012-1660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung TT, Ng KK, Poon RT, Fan ST. Tolerance of radiofrequency ablation by patients of hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:655–660. doi: 10.1007/s00534-009-0103-9. [DOI] [PubMed] [Google Scholar]
- 8.Ng KK, Poon RT, Lo CM, Yuen J, Tso WK, Fan ST. Analysis of recurrence pattern and its influence on survival outcome after radiofrequency ablation of hepatocellular carcinoma. Ann Surg. 2007;253:981–987. doi: 10.1007/s11605-007-0276-y. [DOI] [PubMed] [Google Scholar]
- 9.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB. 2000;2:333–339. doi: 10.1080/136518202760378489. HPB (Oxford) 2002; 4: 99-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noterdaeme O, Leslie TA, Kennedy JE, Phillips RR, Brady M. The use of time to maximum enhancement to indicate areas of ablation following the treatment of liver tumours with high-intensity focused ultrasound. Br J Radiol. 2009;82:412–420. doi: 10.1259/bjr/18470679. [DOI] [PubMed] [Google Scholar]
- 12.Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST. Radiofrequency ablation for subcapsular hepatocellular carcinoma. Ann Surg Oncol. 2004;11:281–289. doi: 10.1245/aso.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Zhu H, Jin CB, Zhou K, Li KQ, Su HB, et al. High-intensity focused ultrasound (HIFU): effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol. 2009;19:437–445. doi: 10.1007/s00330-008-1137-0. [DOI] [PubMed] [Google Scholar]
- 14.Chapman WC, Debelak JP, Wright PC, Washington MK, Atkinson JB, Venkatakrishnan A, et al. Hepatic cryoablation, but not radiofrequency ablation, results in lung inflammation. Ann Surg. 2000;231:752–761. doi: 10.1097/00000658-200005000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong H, Tang YF, Yao TJ, Chiu J, Leung R, Chan P, et al. The outcomes and safety of single-agent sorafenib in the treatment of elderly patients with advanced hepatocellular carcinoma (HCC) Oncologist. 2011;16:1721–1728. doi: 10.1634/theoncologist.2011-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yau T, Wong H, Chan P, Yao TJ, Pang R, Cheung TT, et al. Phase II study of bevacizumab and erlotinib in the treatment of advanced hepatocellular carcinoma patients with sorafenib-refractory disease. Invest New Drugs. 2012;30:2384–2390. doi: 10.1007/s10637-012-9808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 18.Fan ST, Ng IO, Poon RT, Lo CM, Liu CL, Wong J. Hepatectomy for hepatocellular carcinoma: the surgeon's role in long-term survival. Arch Surg. 1999;134:1124–1130. doi: 10.1001/archsurg.134.10.1124. [DOI] [PubMed] [Google Scholar]
- 19.Belghiti J, Kianmanesh R. Surgical treatment of hepatocellular carcinoma. HPB. 2005;7:42–49. doi: 10.1080/13651820410024067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belghiti J, Carr BI, Greig PD, Lencioni R, Poon RT. Treatment before liver transplantation for HCC. Ann Surg Oncol. 2008;15:993–1000. doi: 10.1245/s10434-007-9787-8. [DOI] [PubMed] [Google Scholar]
- 22.Lo CM, Fan ST, Liu CL, Chan SC, Ng IOL, Wong J. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg. 2007;94:78–86. doi: 10.1002/bjs.5528. [DOI] [PubMed] [Google Scholar]
- 23.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 24.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 25.Francica G, Marone G. Ultrasound-guided percutaneous treatment of hepatocellular carcinoma by radiofrequency hyperthermia with a ‘cooled-tip needle’. A preliminary clinical experience. Eur J Ultrasound. 1999;9:145–153. doi: 10.1016/s0929-8266(99)00022-1. [DOI] [PubMed] [Google Scholar]
- 26.Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759–768. doi: 10.2214/ajr.167.3.8751696. [DOI] [PubMed] [Google Scholar]
- 27.Dubinsky TJ, Cuevas C, Dighe MK, Kolokythas O, Hwang JH. High-intensity focused ultrasound: current potential and oncologic applications. Am J Roentgenol. 2008;190:191–199. doi: 10.2214/AJR.07.2671. [DOI] [PubMed] [Google Scholar]
- 28.Feng W, Wang ZB, Chen WZ, Hui Z, Jin B, Zou JZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann Surg Oncol. 2004;11:1061–1069. doi: 10.1245/ASO.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Leslie TA, Kennedy JE, Illing RO, Ter Haar GR, Wili F, Phillips RR, et al. High-intensity focused ultrasound ablation of liver tumours: can radiological assessment predict the histological response? Br J Radiol. 2008;81:564–571. doi: 10.1259/bjr/27118953. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy JE, Wu F, Ter Haar GR, Gleeson FV, Phillips RR, Middleton MR, et al. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics. 2004;42:931–935. doi: 10.1016/j.ultras.2004.01.089. [DOI] [PubMed] [Google Scholar]
- 31.Wu CC, Chen WS, Ho MC, Huang KW, Chen CN, Yen JY, et al. Minimizing abdominal wall damage during high-intensity focused ultrasound ablation by inducing artificial ascites. J Acoust Soc Am. 2008;124:674–679. doi: 10.1121/1.2839907. [DOI] [PubMed] [Google Scholar]
- 32.Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206–1222. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 33.Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450–458. doi: 10.1097/01.sla.0000118373.31781.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan AC, Poon RT, Cheung TT, Chok KS, Chan SC, Fan ST, et al. Survival analysis of re-resection versus radiofrequency ablation for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. World J Surg. 2012;36:151–156. doi: 10.1007/s00268-011-1323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung TT, Poon RT, Yau T, Tsang DS, Lo CM, Fan ST. High-intensity focused ultrasound as a treatment for colorectal liver metastasis in difficult position. Int J Colorectal Dis. 2011;27:987–988. doi: 10.1007/s00384-011-1304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li JJ, Gu MF, Luo GY, Liu LZ, Zhang R, Xu GL. Complications of High Intensity Focused Ultrasound for Patients with Hepatocellular Carcinoma. Technol Cancer Res Treat. 2009;8:217–224. doi: 10.1177/153303460900800306. [DOI] [PubMed] [Google Scholar]


