Abstract
Background
Mucinous cystic neoplasms of the liver (hepatobiliary cystadenomas) are rare neoplastic lesions. Such cysts are often incorrectly diagnosed and managed, and carry a risk of malignancy. The objective of this study was to review the surgical experience with these lesions over 15 years.
Methods
A retrospective chart review identified consecutive patients undergoing surgery for liver cystadenomas from 1997–2011. Clinical data were collected and summarized.
Results
Thirteen patients (mean age 51 years, 12/13 females) with cysts 4.6–18.1 cm were identified. Most cysts were located in the left lobe/centrally (11/12) and had septations (8/13). Mural nodularity was infrequent (3/13). Nine patients had liver resection/enucleation, whereas four had unroofing. Frozen section analysis had a high false-negative rate (4/6). All patients had cystadenomas, of which two had foci of invasive carcinoma (cystadenocarcinoma) within mural nodules. There was no 90-day mortality. All but one patient (myocardial infarction) were alive at a median follow-up of 23.1 months. No patient with unroofing has developed malignancy to date.
Conclusions
Non-invasive hepatobiliary cystadenomas present as large central/left-sided cysts in young or middle-aged women. Associated malignancy was relatively uncommon and found within mural nodules. Intra-operative frozen section analysis was ineffective at ruling out cystadenomas. Complete excision is recommended, but close follow-up might be considered in patients with a prohibitive technical or medical risk, in the absence of nodularity on high-quality imaging.
Introduction
Liver cysts, once thought to be relatively rare,1 are now understood to be common incidental findings on cross-sectional imaging.2 The differential diagnosis of such cysts is complex, and includes primary hepatobiliary cysts (simple cyst, polycystic disease, mucinous cystic neoplasm, cystic intra-ductal biliary neoplasms and intra-hepatic biliary cysts/Caroli's disease), infectious collections (pyogenic, parasitic and fungal) and other cysts (traumatic, degenerative and post-ablation).3
Mucinous cystic neoplasms (MCN, hepatobiliary cystadenomas), also commonly referred to by its subtype of mucinous cystadenoma, or neoplastic cyst of the liver, are rare lesions, with an estimated incidence of 1 in 20 000–100 000 for non-invasive MCN and 1 in 10 000 000 for cystadenocarcinoma.4 They can present with non-specific abdominal complaints, can be found incidentally on cross-sectional imaging or can be found on a pathological specimen after the unroofing or enucleation of presumed benign cysts. Although the natural history of MCN is uncertain, many authors have recommended complete excision of presumed or confirmed lesions on the basis of potential malignant evolution to cystadenocarcinoma.5,6 While cases of associated malignancy have clearly been documented in the literature,7,8 this risk remains poorly defined, as the total body of published literature on this pathology remains small. In this context, this study sought to review a single tertiary-care centre's experience in patients who underwent liver surgery for MCN and associated cystadenocarcinomas of the liver.
Patients and methods
After obtaining approval from the Ottawa Hospital Research Ethics Board, the charts of eligible patients were obtained and reviewed retrospectively (January 1997 to December 2011). Patients were included in the study after undergoing surgery at our institution for hepatic cysts, and had pathologically confirmed hepatic MCN with or without an associated cystadenocarcinoma. Patients who underwent surgery at outside institutions and were only followed in our clinic were excluded. Patients who underwent surgery for presumed MCN but had simple cysts on final pathology were excluded, as were patients with cystic variants of biliary intra-ductal papillary neoplasm (Bil-IPN). Eligible patients were identified from surgeon records and hospital charts using procedure codes, discharge diagnoses and pathological data. Independent review of selected pathological slides was carried out by the study pathologist (C.M.), where doubt existed regarding the diagnosis of MCN.
Patients with hepatic cysts were referred to our institution for surgical consultation after ultrasound imaging of the liver. Patients with symptomatic cysts or with clinical or radiological suspicion for MCN were further imaged with a dedicated liver computed tomography (CT) scan and/or magnetic resonance imaging (MRI). MRIs were systematically obtained when the diagnosis of MCN on CT was inconclusive or to characterize a suspicion of mural nodularity. Patients with a suspicion of MCN underwent definitive surgery, in the form of a formal liver resection and/or complete enucleation. Patients who were found to have MCN on pathology after unroofing of presumed simple liver cysts were assessed by our group and offered definitive surgery, if medically fit and if a resection was technically feasible. All operative frozen sections were done at the surgeon's discretion. Patients with an incomplete resection were followed clinically and radiologically every 6 months, or until the patient declined further follow-up.
Pre-operative data (demographics, comorbidities, symptoms, examination features, imaging findings and blood tests), operative data (type of procedure, frozen section analysis, operative time and blood transfusion) and post-operative data (length of stay, pathology, complications and follow-up) were extracted from the patients' records. Post-operative complications were compiled according to the Dindo–Clavien classification. Follow-up data were obtained from clinic notes and phone records. All follow-up imaging studies were reviewed.
Results
Thirteen patients underwent surgery for liver MCN between 1997 and 2011, of which 8 had surgery in 2008–2011. Demographical detail and presenting findings are documented in Table 1. Ten of 13 patients were under 60 years of age. Almost all patients had normal serum liver function tests. Serum carbohydrate antigen 19-9 (CA 19-9) levels were not routinely assessed. All patients who underwent only ultrasound examination were treated prior to 1999. All but one cyst were located centrally or within the left liver (Fig. 1).
Table 1.
Patient characteristics (n = 13)
| Patient demographics | |
| Gender, female | 12 |
| Age, years, mean ± SD | 51.2 ± 15.9, range 29–87 |
| BMI, kg/m2, mean ± SD | 29.4 ± 6.0 |
| ASA I/II/III/IV | 0/6/6/1 |
| Presenting symptoms | |
| Abdominal discomfort | 11 |
| Early satiety/nausea/vomiting | 5 |
| Weight loss | 2 |
| Asymptomatic | 1 |
| Physical exam findings | |
| None | 6 |
| Epigastric fullness/mass | 5 |
| Abdominal tenderness | 4 |
| Definitive imaging studya | |
| Ultrasound | 2 |
| MRI | 7 |
| CT | 3 |
| Imaging characteristics | |
| Maximal cyst diameter | 12.4 ± 4.6 cm, range 4.8–18.1 |
| Septations | 8 |
| Mural modules | 3 |
| Increasing in size | 4 |
| Reported as possible cystadenomab | 8 |
| Reported as possible cystadenocarcinomab | 2 |
| Cyst locationa | |
| Left hemiliver only | 5 |
| Central | 6 |
| Right hemiliver only | 1 |
Data are missing for one patient.
Interpretation as reported by abdominal/liver radiologists at the authors' institution.
ASA: American society of anesthesiologists score; BMI: body mass index; CT: computed tomography; MRI: magnetic resonance imaging; SD: standard deviation.
Figure 1.
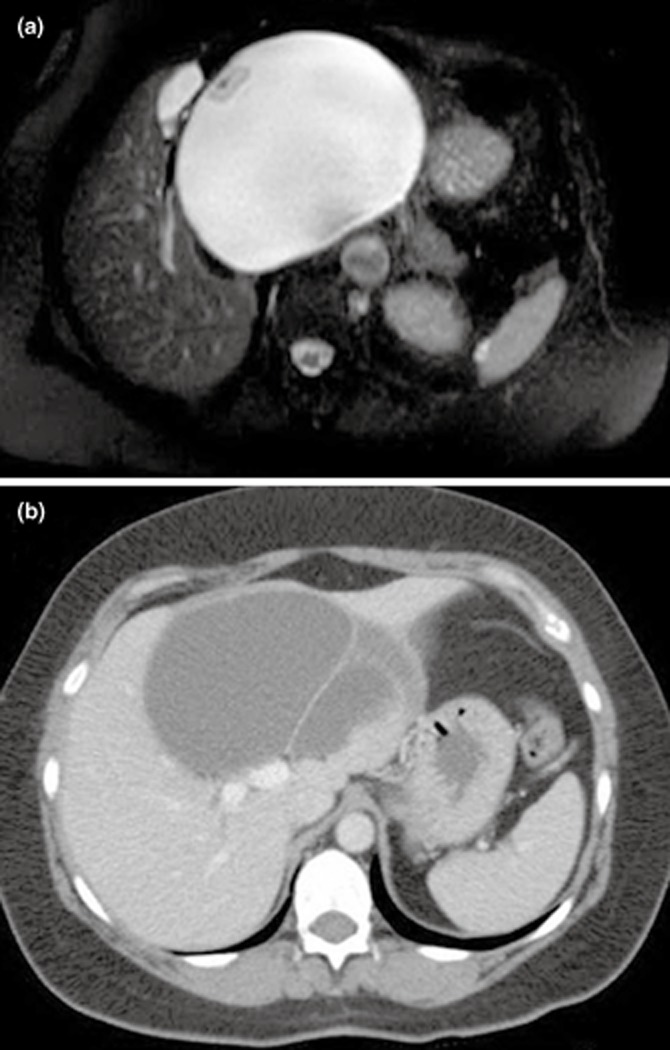
(a) T2-weighted magnetic resonance imaging (MRI) demonstrating a 14 × 15 cm centrally-located liver cystadenoma with mural nodularity and inferior vena cava compression in a 63-year-old female. (b) Computed tomography (CT) scan demonstrating an 11 × 8 cm centrally-located multi-sepatated liver cystadenoma arising above the portal bifurcation in a 56-year-old female
Operative and post-operative characteristics are presented in Table 2. All fully-excised central or right-sided cysts were treated with enucleation (n = 6), whereas fully-excised left-sided cysts (n = 3) underwent formal liver resection. Three patients underwent unroofing as a direct consequence of false-negative frozen sections. An operative complication occurred in one patient who suffered an inferior vena cava (IVC) injury with immediate reconstruction.
Table 2.
Procedures and outcomes (n = 13)
| Item | |
|---|---|
| Operative procedure | |
| Liver resection | 3 |
| Enucleation/pericystectomy | 6 |
| Unroofinga | 4 |
| Intra-operative frozen section analysis | 6 |
| False-negative | 4 |
| Operative time, median, minutes | 182, range 86–493 |
| Hospital stay, median, days | 6, range 4–19 |
| Follow-up, median, months | 23.1, range 0.3–159 |
| Recurrence after resection/enucleation | 0/9 |
| Cystadenocarcinoma recurrence | 0/2 |
| Progression to cystadenocarcinoma after unroofing | 0/4 |
One patient had both a left lateral liver sectionectomy and unroofing of the medial cyst wall (included under unroofing).
Immediate peri-operative complications occurred in six patients. The Dindo–Clavien grading was: grade 4a (myocardial infarction, n = 1), grade 3a (intra-abdominal haematoma, n = 1), grade 2 (peri-operative blood transfusion, n = 5, ileus, n = 1, pulmonary embolism, n = 1) and grade 1 (wound infection, n = 1). In addition, two patients developed delayed complications: grade 3b (incisional hernia, n = 2) and grade 3a (chronic IVC narrowing with temporary lower extremities venous stasis ulceration, n = 1). There was no 90-day mortality.
Final pathology revealed non-invasive MCN in 11 patients. Two patients had foci of invasion within mural nodules with negative surgical margins (cystadenocarcinoma).
Among the four patients who underwent unroofing, three were incorrectly presumed to have simple cysts. Two have declined further surgery and are followed closely. The third patient, an 87-year-old male, was unfit for definitive surgery and died 13 months later of a second myocardial infarction (grade 4a complication during initial surgery). The fourth, a 44-year-old female, had a massive central cyst compressing the three hepatic veins and was highly symptomatic. She was not considered resectable from a technical standpoint and underwent wide unroofing understanding that recurrence was certain. After 40 months of close follow-up with clear symptoms recurrence, the patient was referred to another high-volume hepatobiliary centre for an attempt at resection. During surgery, a definitive resection was not deemed feasible, and the treating team carried out an internal drainage procedure using a Roux loop. The patient remains well at 2 months of follow-up.
The median study follow-up was 23.1 months. All but four patients were followed for at least 12 months. All but one patient in the study were alive at maximal follow-up. No patient who underwent enucleation or resection has had a recurrence. None of the two patients with foci of cystadenocarcinoma have had local recurrence or metastatic disease. No adjuvant therapy was given. No malignant degeneration was identified among the four patients who underwent unroofing (follow-up 6.8–62.9 months).
Discussion
The present study has addressed the management of intra-hepatic MCN. This series adds to a relatively small body of literature pertaining to this rare pathology. Review of the literature to 1990 would suggest that fewer than 300 cases have been reported.5,6,9–16 Lesions identified in this study were described as MCN (hepatobiliary cystadenomas) according to the most recent WHO classification.17 These were composed of multiple cysts of varying sizes, with no communication with the bile ducts. Using microscopy, they demonstrated a well-defined capsule, a single-layered simple cuboidal, columnar, or flat epithelium, and a basement membrane (Fig. 2a). Mucin-containing vacuoles (intestinal metaplasia) were typically seen. Underneath the basement membrane, a layer of highly-cellular mesenchymal tissue was usually present, resembling normal ovarian stroma.18 The ovarian-like stroma was composed of bland spindle cells (Fig. 2b), which typically stains with vimentin, actin, desmin, oestrogen, progesterone and inhibin.18 In general, MCN are nearly always mucinous, but a serous variety lacking an ovarian-like stroma is recognized. Foci of epithelial dysplasia can occur in the epithelial lining of MCN, from which mural nodules and ductal adenocarcinomas can arise, as was identified in this study. As the areas of invasion are typically focal in nature, extensive sampling of these neoplasms was required.
Figure 2.
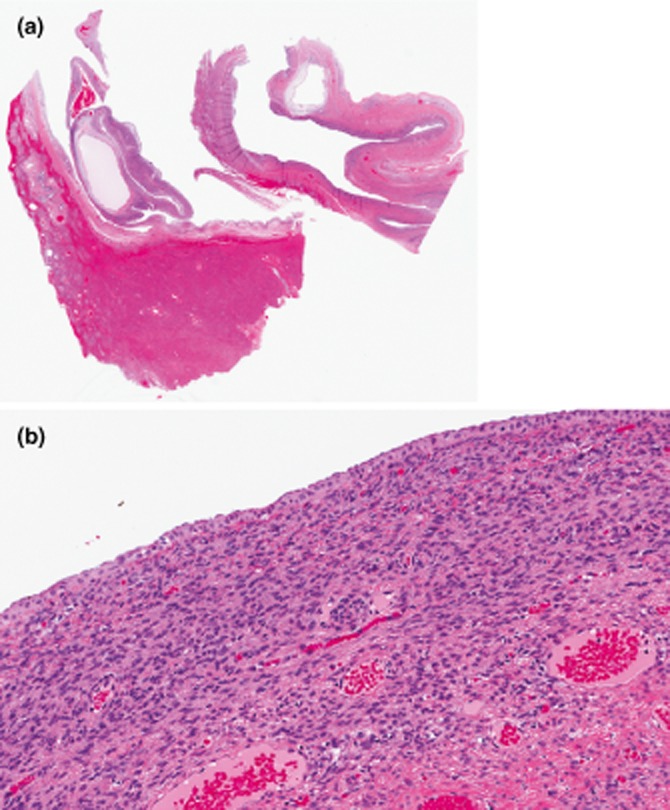
(a) Mucinous cystadenoma (10×, haematoxylin and eosin stain). There is a large cyst lined by a simple biliary-type columnar epithelium. (b) The ovarian-like mesenchymal stroma is seen underneath the simple epithelium (100×, haematoxylin and eosin stain).
The present study only examined MCN and excluded the recently-described cystic variant of Bil-IPN.15,18 Zen et al. have argued that many older series of MCN may have been contaminated by cystic Bil-IPN, thus warranting careful clinical interpretation of existing series, as had been the case with pancreatic cystic lesions.15 Indeed, cystic Bil-IPN can be associated with clinically-significant cyst formation, as a result of ductal dilation and mucin plugging. It can be differentiated from MCN by the presence of communication with the biliary tract on imaging or pathology, and the absence of ovarian-type stroma on histology. Unfortunately, ovarian-type stroma can sometimes be discontinuous and require considerable sampling for identification.18 This distinction is further complicated by a rare variant of MCN without ovarian-type stroma, which is sometimes seen in men.18
This study has demonstrated that MCN present typically as large cysts almost exclusively in young or middle-aged female patients. This finding is consistent with existing series in the literature where almost all patients were females with an average age in the late forties. Interestingly, Sang et al. identified 11 male patients with such neoplastic cysts, although 9 of those in fact had cystadenocarcinomas.16 While certain authors have suggested that MCN can only be found in women,15 this study and other series in the literature would suggest that males can account for 0–10.5% of patients with this pathology when cystadenocarcinomas are excluded.5,6,9,10,13–16 Similarly, the maximal mean cyst diameter was found to be 12.4 cm in this study, which is comparable to other series where cysts always averaged greater than 10 cm.5,6,9,10,13,14,16 The generally large volume of these neoplastic cysts is probably related to the relative lack of symptoms associated with smaller cyst sizes.
This study has demonstrated that the vast majority of cysts (11/12) were located within the anatomical left lobe of the liver or centrally in close proximity to the portal or hepatic veins confluences. Vogt et al. have reported a similar pattern whereby only 3/22 patients had a right lobe cyst, whereas the others had left-sided (n = 14), central (n = 2) or caudate disease (n = 1).6 Wang et al. reported similarly striking data of only 4/30 right lobe cysts.16 The underlying mechanism for this observation is not readily apparent, but may reflect patterns of location of ectopic embryonic tissue normally found in the adult gallbladder.19
Intra-operative frozen section analysis was not effective at ruling out MCN in this series, with 4/6 false-negative results. This is an important finding in that simple liver cysts could not be reliably differentiated from MCN in the operating room and this in spite of pathological assessment. The reasons behind this finding may pertain to insufficient or non-representative surgical material, insufficient pathological sampling or difficult histological interpretation. At least one other group has reported false-negative results in the context of liver cysts.6 In light of poor results from frozen section analysis, the present study would suggest operative decisions for undifferentiated cysts should be made on pre-operative imaging such as MRI or a triphasic CT scan. Although not examined in this study, other groups have advocated pre-operative cyst fluid analysis for CA 19-9 and carcinoembryonic antigen, with mixed results.11,13
In this series, malignant cystadenocarcinoma was identified in 2/13 pathological specimens (15%). Both patients presented with small foci of malignant cells within mural nodules that were identified pre-operatively. A third patient had an area of low-grade dysplasia within her cyst, while a fourth had an area of nodularity that did not contain carcinoma or dysplasia. While suggestive of the potential for malignant transformation, these data do not allow for a more accurate understanding of the natural history of MCN, as such information would have to be derived from a likely prolonged period of non-operative observation. The rate of malignancy within comparable surgical or pathological series of MCN was highly variable at 3.4–42%.5,6,9,10,12,14,16,20 These data are unfortunately difficult to interpret, as many series examined both de novo cystadenocarcinomas as well as malignant degeneration within existing MCN.6 De novo lesions have been reported more frequently in men, and may harbour a different prognosis.6,9,14 This issue is further confused by data on cystic variants of intra-ductal papillary neoplasms of the bile ducts, which appear to harbour a higher risk of malignancy. Li et al. have indeed reported a malignancy rate of 38.5% with MCN and of 78.9% with B-IPN.20 In contrast, Zen et al. reported rates of 3% and 100%, respectively.15 It is likely that this pathological entity may have contaminated certain series of MCN, and thus overestimated the risk of malignancy.
Given the association with malignancy, many have advocated complete excision of all intra-hepatic MCN.5,6 That being said, the natural history of this lesion is not well understood, and malignant degeneration is likely to occur over several years.7,8 In the present study, four patients underwent incomplete excision, with no evidence of progression to malignancy after follow-up times of 6.8–62.9 months. Two patients have had follow-up times of at least 3 and 5 years, respectively. In this context, the authors' management strategy has been to offer definitive surgery to all patients with sufficient clinical or radiological suspicion of MCN, with the exception of patients who are unfit for major liver surgery or those who are deemed technically unresectable. Older patients or marginal surgical candidates without evidence of mural nodularity could be considered for surgery or close follow-up on a case-by-case basis after consensus discussion. In the case of technical unresectability, a second opinion would be sought at another hepatobiliary centre.
The current study has clear limitations. Most significantly, it has a small sample size, which limits one's ability to draw broad conclusions. That being said, when compared against the existing literature on this topic, the current series is in fact one of only a handful of publications with at least 10 patients originating from a Western centre.5,6,9–11 In addition to the above concern, this work is limited by its retrospective nature, in that study variables were limited to available reports and pathological specimens. Although this limitation is unavoidable in retrospective series, our group has attempted to mitigate this potential source of bias by seeking additional sources of information, such as surgeon and phone records, and by reviewing selected pathological specimens. Finally, three patients were lost to follow-up after their first post-operative visit owing to a long-distance travel time. Given that all three patients had completely excised cysts it is unlikely, although not impossible, that they would have experienced recurrence without being referred back to our centre by their primary care physicians.
Conclusion
In the current series, non-invasive MCN (hepatobiliary cystadenomas) of the liver presented as large central/left-sided cysts in young or middle-aged women. Associated malignancy was relatively uncommon and found within mural nodules. Intra-operative frozen section analysis was ineffective at ruling out MCN. Complete excision is recommended, but close follow-up might be considered in patients with prohibitive technical or medical risk, in the absence of nodularity on high-quality imaging.
Conflicts of interest
No conflicts of interest.
References
- 1.Sanfelippo PM, Beahrs OH, Weiland LH. Cystic disease of the liver. Ann Surg. 1974;179:922–925. doi: 10.1097/00000658-197406000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol. 2003;58:626–629. doi: 10.1016/s0009-9260(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 3.Reid-Lombardo KM, Khan S, Sclabas G. Hepatic cysts and liver abscesses. Surg Clin N Am. 2010;90:679–697. doi: 10.1016/j.suc.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Manouras A, Markogiannakis H, Lagoudianakis E, Katergiannakis V. Biliary cystadenoma with mesenchymal stroma: report of a case and review of the literature. Word J Gastroenterol. 2006;12:6062–6069. doi: 10.3748/wjg.v12.i37.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas KT, Welch D, Trueblood A, Sulur P, Wise P, Gorden DL, et al. Effective treatment of biliary cystadenoma. Ann Surg. 2005;241:769–775. doi: 10.1097/01.sla.0000161982.57360.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogt DP, Henderson JM, Chmielewski E. Cystadenoma and cystadenocarcinoma of the liver: a single center experience. J Am Coll Surg. 2005;200:727–733. doi: 10.1016/j.jamcollsurg.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Woods GL. Biliary cystadenocarcinoma: case report of hepatic malignancy origination in benign cystadenoma. Cancer. 1981;47:2936–2940. doi: 10.1002/1097-0142(19810615)47:12<2936::aid-cncr2820471234>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Matsuoka Y, Hayashi K, Yano M. Case report: malignant transformation of biliary cystadenoma with mesenchymal stroma: documentation by CT. Clin Radiol. 1997;52:318–321. doi: 10.1016/s0009-9260(97)80066-9. [DOI] [PubMed] [Google Scholar]
- 9.Devaney K, Goodman ZD, Ishak JG. Hepatobiliary cystadenoma and cystadenocarcinoma: a light microscopic and immunohistochemical study of 70 patients. Am J Surg Pathol. 1994;18:1078–1091. [PubMed] [Google Scholar]
- 10.Buetow PC, Buck JL, Pantongrag-Brown L, Ros PR, Devaney K, Goodman ZD, et al. Biliary cystadenoma and cystadenocarcinoma: clinical-imaging-pathologic correlation with emphasis on the importance of the ovarian stroma. Radiology. 1995;196:805–810. doi: 10.1148/radiology.196.3.7644647. [DOI] [PubMed] [Google Scholar]
- 11.Koffron A, Rao S, Ferrario M, Abecassis M. Intrahepatic biliary cystadenoma: role of cyst fluid analysis and surgical management in the laparoscopic era. Surgery. 2004;136:926–936. doi: 10.1016/j.surg.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Lim JH, Jang KT, Rhim H, Kim YS, Lee KT, Choi SH. Biliary cystic intraductal papillary mucinous tumor and cystadenoma/cystadenocarcinoma: differentiation by CT. Abdom Imaging. 2007;32:644–651. doi: 10.1007/s00261-006-9161-5. [DOI] [PubMed] [Google Scholar]
- 13.Choi HK, Lee JK, Lee KH, Lee KT, Rhee JC, Kim KH, et al. Differential diagnosis for intrahepatic biliary cystadenoma and hepatic simple cyst: significance of cystic fluid analysis and radiologic findings. J Clin Gastroenterol. 2010;44:289–293. doi: 10.1097/MCG.0b013e3181b5c789. [DOI] [PubMed] [Google Scholar]
- 14.Sang X, Sun Y, Mao Y, Yang Z, Lu X, Yang H, et al. Hepatobiliary cystadenomas and cystadenocarcinomas: a report of 33 cases. Liver Int. 2011;31:1337–1344. doi: 10.1111/j.1478-3231.2011.02560.x. [DOI] [PubMed] [Google Scholar]
- 15.Zen Y, Pedica F, Patcha VR, Capelli P, Zamboni G, Casaril A, et al. Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Mod Pathol. 2011;24:1079–1089. doi: 10.1038/modpathol.2011.71. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Miao R, Liu H, Du X, Liu L, Lu X, et al. Intrahepatic biliary cystadenoma and cystadenocarcinoma: an experience of 30 cases. Dig Liver Dis. 2012;44:426–431. doi: 10.1016/j.dld.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Tsui WMS, Adsay NV, Crawford JM, Hruban R, Klöppel G, Wee A. Mucinous cystic neoplasms of the liver. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2010. pp. 236–238. [Google Scholar]
- 18.Guettier C. Intrahepatic biliary cystic lesions. Ann Pathol. 2010;30:448–454. doi: 10.1016/j.annpat.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Subramony C, Herrera GA, Turbat-Herrera EA. Hepatobiliary cystadenoma: a study of five cases with reference to histogenesis. Arch Pathol Lab Med. 1993;117:1036–1042. [PubMed] [Google Scholar]
- 20.Li T, Ji Y, Zhi XT, Wang L, Yang XR, Shi GM, et al. A comparison of hepatic mucinous cystic neoplasms with biliary intraductal papillary neoplasms. Clin Gastroenterol Hepatol. 2009;7:586–593. doi: 10.1016/j.cgh.2009.02.019. [DOI] [PubMed] [Google Scholar]


