Abstract
Introduction
Patients with incidental pT2-T3 gallbladder cancer (IGC) after a cholecystectomy may benefit from a radical re-resection although their optimal treatment strategy is not well defined. In this Unit, such patients undergo delayed staging at 3 months after a cholecystectomy to assess the evidence of a residual tumour, extra hepatic spread and the biological behaviour of the tumour. The aim of this study was to evaluate the outcome of patients who had delayed staging at 3 months after a cholecystectomy.
Methods
From July 2003 to July 2011, 56 patients with T2-T3 gallbladder cancer were referred to this Unit of which 49 were diagnosed incidentally on histology after a cholecystectomy. All 49 patients underwent delayed pre-operative staging using multi-detector computed tomography (MDCT) followed selectively by laparoscopy at 3 months after a cholecystectomy. Data were collected from a prospectively held database. The peri-operative and long-term outcomes of patients were analysed. SPSS software was used for statistical analysis.
Results
There were 38 pT2 and 11 pT3 tumours. After delayed staging, 24/49 (49%) patients underwent a radical resection, 24/49 (49%) were found to be inoperable on pre-operative assessment and 1/49 (2%) patient underwent an exploratory laparotomy and were found to be unresectable. The overall median survival from referral was 20.7 months (54.8 months for the group who had a radical re-resection versus 9.7 months for the group who had unresectable disease, P < 0.001). These results compare favourably with the reported outcome of fast-track management for incidental pT2-T3 gallbladder cancer from other major series in the literature.
Conclusion
Delayed staging in patients with incidental T2-T3 gallbladder cancer after a cholecystectomy is a useful strategy to select patients who will benefit from a resection and avoid unnecessary major surgery.
Introduction
Incidental gallbladder cancer (IGC) is discovered in 0.3–2% of all cholecystectomies.1 Up to 75% of patients with GC are unresectable at diagnosis and the 5-year survival is very poor (3–13%), with a median survival of 3 to 11 months.2 If IGC is diagnosed at an early enough stage, patients may benefit from a radical resection, with some encouraging results.3–5
T2IGC (a tumour involving the perimuscular connective tissue)6) may have several implications: first, the tumour may have been breached during the operation causing a potential peritoneal dissemination; second, microscopic residual disease might be left behind during the cholecystectomy resulting in a final tumour stage being underestimated; finally, the aggressiveness of the tumour remains unknown. For these reasons, inoperable disease may be found in a large number of patients with IGC at laparotomy7–9 and a high percentage of patients undergoing a radical resection will experience early metastatic diseases and poor long-term survival.10
In order to minimize unnecessary surgery, a protocol of delayed restaging prior to re-resection was adopted: those patients presenting with IGC staged T2 or above after a cholecystectomy were assessed at 3 months by a multi-detector computed tomography (MDCT) scan followed selectively by laparoscopic staging before being offered a radical resection.
The aim of this study was to retrospectively review the impact of delayed restaging on the resectability rate, laparotomies for unresectable disease and long-term survival in T2-T3 IGC patients.
Methods
The Hepato-Pancreato-Biliary (HPB) Surgery Unit at Freeman Hospital is a referral centre in the UK for HPB malignancies. Data of patients with GC referred for further treatment are collected in a prospectively maintained database and they include: demographics, histopathology of the initial cholecystectomy (including completeness of a resection and gallbladder disruption at the initial operation), operation data and follow-up.
All patients referred to our centre with a gallbladder mass from May 2003 to July 2011 were considered. All cases were discussed in a multidisciplinary meeting (MDM) with dedicated specialists. Tumours were staged according to the 6th edition of AJCC/UICC TNM system.11 Inoperability was defined by the presence of metastatic disease (including lymphadenopathy beyond the hepatoduodenal ligament) or local infiltration of the main vascular structures (portal vein and hepatic artery).
Once the diagnosis of resectable gallbladder cancer is confirmed, patients are seen in the outpatient clinic and if they are fit and willing for a radical re-resection, an MDCT scan is organized at 3 months after a cholecystectomy to restage disease prior to re-exploration. The staging CT scan is reviewed again in the MDM by dedicated radiologists before proceeding with a radical resection or laparoscopy. Indications for a laparoscopy include high-risk pathological and imaging findings, or biopsy of undetermined lesions. All laparoscopies are done as separate procedures prior to a laparotomy and include inspection of the peritoneal cavity and intra-operative ultrasound of the liver and porta hepatis.
Statistical analysis
Comparison of demographic details and clinical characteristics between the two groups was performed using Fisher's exact tests and the linear-by-linear chi-square. For means in the case of continuous numeric data, we used the independent samples t-test and the Mann–Whitney U-test, respectively, for data normally and non-normally distributed; the data were previously tested for normality by the Kolmogorov–Smirnov test. Survival was estimated using Kaplan–Meier analysis. A two-tailed P-value less than 0.05 was considered significant. SPSS 19.0 (SPSS, Chicago, IL, USA) was used for statistical analysis.
Results
Two-hundred and seventy patients with presumed GC were referred to this Unit during the study time. IGC was diagnosed in 56 patients. Seven patients were not offered any further treatment: five pT1 and two pT2 patients were unfit for surgery. Forty-nine patients with incidental pT2-T3 GC who were considered for re-exploration were included in this analysis. Patients' characteristics are summarized in Table 1. There were 20 (40.8%) men and 29 (51.2%) women with a median age of 64.4 years (range 48–85). Twenty-four patients (49%) had an incomplete resection at cholecystectomy. Evidence of residual disease was found in 49% of the cholecystectomy specimens, and gallbladder disruption at cholecystectomy was recorded in 59% of the cases. There were no cases of a positive margin on the cystic duct at the initial cholecystectomy.
Table 1.
Patients' characteristics
| Age at referral years, median (range) | 64.3 (48–85) |
| Gender | |
| • M | 20 (40.8%) |
| • F | 29 (51.2%) |
| T at referral | |
| • T2 | 37 |
| • T3 | 12 |
| R + * | 24 (49%) |
| Grading** | |
| • 1 | 19 |
| • 2 | 20 |
| • 3 | 9 |
| Specimen disruption | 29 (59%) |
Residual disease at cholecystectomy;
Adenocarcinoma only.
According to our staging protocol, 24 (49%) patients underwent a radical re-resection and 25 (51%) were deemed to be inoperable at 3-months staging. An algorithm of delayed restaging is shown in Fig. 1.
Figure 1.
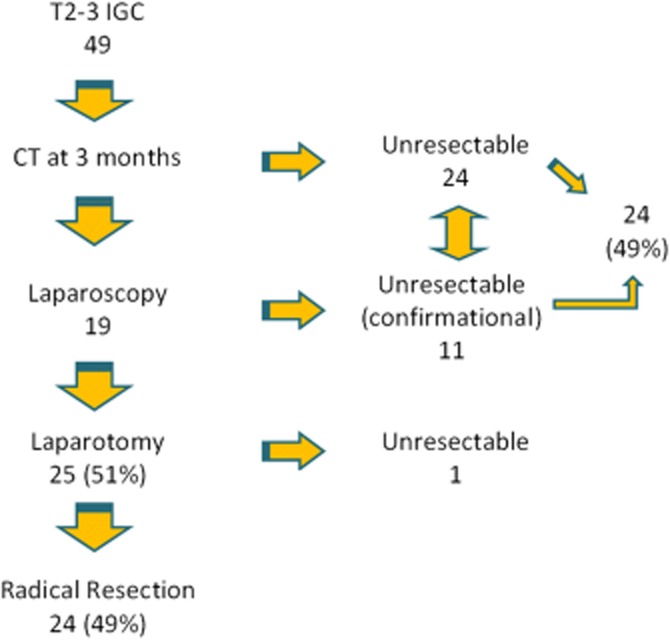
Algorithm of the delayed restaging protocol
No patients refused surgery. The rationale of the delay was explained to all patients and none requested early surgery.
Inoperability findings were peritoneal disease only (n = 7), liver metastases (n = 7), combined liver and peritoneal metastases (n = 1), vascular involvement (n = 2), lymph nodes metastases (3), combined hilar mass and peritoneal metastases (n = 1), combined hilar mass and liver metastases (n = 2), lymph nodes and distant metastases (1) and combined liver and distant metastases (n = 1). One of the 25 inoperable patients was diagnosed with metastatic nodal disease at exploratory laparotomy.
Twenty-two patients received a segment 4b-5 resection with a lymphadenectomy of the hepatic pedicle, and 16 out of these 22 patients underwent a resection of the extrahepatic biliary tree. A major hepatectomy (>4 segments) with resection of the extrahepatic biliary tree was performed in the remaining two cases.
At final histology, evidence of a tumour on the final specimen was demonstrated in 10 patients. Six patients had liver invasion, three patients had liver invasion and lymph node metastasis and one patient had lymph node metastasis only. None of the patients had final involvement of the bile duct. Amongst the nine patients with liver invasion, four had a positive margin at the time of the initial cholecystectomy. The rationale for bile duct removal was based on an intra-operative suspicion of bile duct involvement. None of the patients had final involvement of the bile duct.
The complication and mortality rate were 21% (5 patients) and 4% (1 patient), respectively. Post-operative histology showed a pT and pN upgrade in three (12.5%) and five (21%) cases. Six patients received an R1 resection (25%). The median survival was 20.7 months (54.8 months for resectable patients versus 9.7 months for unresectable patients, P < 0.001) (Fig. 2).
Figure 2.
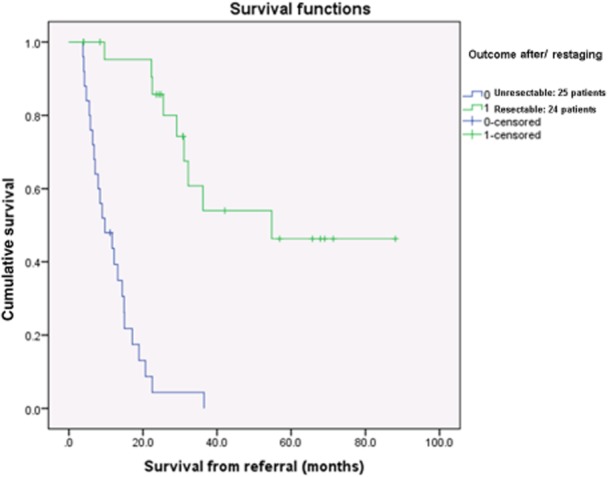
Kaplan–Meier analysis showing a statistically significant difference between resectable and unresectable patients (54.8 versus 9.7 months, P < 0.001)
Discussion
Encouraging results have been reported from tertiary centres performing very aggressive surgery for incidental pT2-T3 GB and acceptable survival can be achieved when the lymph node status is negative.3,12–15
In this study, it was demonstrated that an acceptable 5-year survival can be obtained delaying the staging before a radical resection. This can result in a more accurate patient selection and a reduction in the number of exploratory laparotomies. When comparing our results with other major series (Table 2), a very good median survival for those patients who received a radical surgery and who were selected for having less aggressive disease was observed. This would obviously imply a lower resection rate, considering that we did not have any T4 tumours in the series.
Table 2.
Patterns of respectability and survival after radical surgery for incidental gallbladder cancer (IGC)
| Author | N | T | Resection % | 5-year survival % (resected) | Unresectable at laparotomy, % | Mortality % |
|---|---|---|---|---|---|---|
| This series | 49 | T2-3 | 49 | 50 | 4 | 4 |
| Fong21 | 248 | T2-4 | 31 | 35 | NA | 4 |
| Shih22 | 53 | T2-4 | 62 | 34 | 11 | 4 |
| Fuks12 | 218 | T1-4 | 67 | 42 | 21 | 3 |
NA, not available.
Centres that use a more aggressive approach might consider our protocol too selective. However, even in these studies it is clear that many post-cholecystectomy pT2-T3 patients will not have a good outcome. Fong et al. in their series of pT2-T4 IGB patients showed that:
nearly half of the initial group of patients did not receive radical surgery because of inoperability at restaging or because of intra-operative findings;
some more patients were unfit for radical surgery and were therefore not referred or not considered;
the mortality rate was approximately 4%;
the percentage of lymph node metastasis was very high, and the 5-year survival rate for AJCC stage III (N1) is lower than 10%; and
some patients had R1 resections
Ultimately, patient selection is very important to achieve a good outcome. In spite of rigorous staging with cross-sectional imaging and laparoscopy, disseminated disease is diagnosed at re-exploration in 24–60.3% of cases and an exploratory laparotomy should be carefully avoided in these patients as it has a negative impact on survival.15,16
In this institution, a radical re-resection is offered to all patients with pT2-T3 GC diagnosed after a cholecystectomy who are medically fit. An agreed delayed restaging approach prior to re-exploration allows us to consider the likelihood of tumour violation at cholecystectomy, a planed ‘test of time’, avoiding surgery on patients with microscopic peritoneal dissemination and aggressive disease. This last issue seems to be increasingly important and in fact some authors are now questioning the utility of aggressive surgery in GC17–19
The choice of a 3-months' time frame was essentially based on an estimation that a tumour would require to show up on a follow-up scan. This time frame is what Radiologists usually recommend to follow-up undetermined nodules or discover tumour recurrence at intensive follow-up and it has been previously validated in other studies.20 In addition, the two senior authors based the 3 months on a personal experience of re-operating within 6 weeks and found an unacceptable incidence of ‘open and close’ as a result of peritoneal metastases.
The interpretation of a delayed CT scan by a specialist radiologist and the discussion of every single case in a multidisciplinary meeting with dedicated specialists, have of course contributed to increase the accuracy of our patients selection. A laparoscopy was done selectively in the present patients and proved a useful means to obtain a tissue diagnosis in inoperable patients (n = 11) with the view of palliative treatment but did not alter the management in any of the patients with a provisional diagnosis of operable disease on CT (n = 9).
This study has several limitations: first, it is a retrospective study and although the data have been collected from a prospectively held database, many patients have been referred from other centres. Second, the lack of a control group makes it very difficult to compare this protocol with a more aggressive approach. On the other hand, given the small sample size even for national referral centres, it would be very difficult to design a prospective study. Unfortunately historical data are not available for comparison as this study backdates to the beginning of HPB centralization in the UK, and that is why we can only compare our data with other authors' experiences. Given the small number of patients presenting with this disease, a control arm would require a much longer recruitment time for a single-centre study.
In conclusion, this study attempts to address the impact of timing in the selection process for a radical re-resection in patients with a pT2-T3 gallbladder diagnosed after a cholecystectomy. The present results show that delayed restaging minimizes exploratory laparotomies without apparently affecting the outcome. Further multicentre studies are needed to confirm our findings.
Conflicts of interest
None declared.
References
- 1.Goetze TO, Paolucci V. Adequate extent in radical re-resection of incidental gallbladder carcinoma: analysis of the German Registry. Surg Endosc. 2010;24:2156–2164. doi: 10.1007/s00464-010-0914-4. [DOI] [PubMed] [Google Scholar]
- 2. American College of Surgeons/American Cancer Society National Cancer Data Base, based on more than 10,000 patients diagnosed with gallbladder cancer from 1989 to 1996. (Apr 2012.) http://www.cancer.org/acs/groups/cid/documents/webcontent/003101-pdf.pdf (last accessed 12 December 2012)
- 3.Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557–569. doi: 10.1097/00000658-200010000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hueman MT, Vollmer CM, Jr, Pawlik TM. Evolving treatment strategies for gallbladder cancer. Ann Surg Oncol. 2009;16:2101–2115. doi: 10.1245/s10434-009-0538-x. [DOI] [PubMed] [Google Scholar]
- 5.Glauser PM, Strub D, Kaser SA, Mattiello D, Rieben F, Maurer CA. Incidence, management, and outcome of incidental gallbladder carcinoma: analysis of the database of the Swiss association of laparoscopic and thoracoscopic surgery. Surg Endosc. 2010;24:2281–2286. doi: 10.1007/s00464-010-0952-y. [DOI] [PubMed] [Google Scholar]
- 6.Edge SB, Byrd DR, Compton CC. AJCC Cancer Staging Manual. 7th edn. New York, NY: Springer; 2010. pp. 211–217. [Google Scholar]
- 7.Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg. 2007;11:1478–1486. doi: 10.1007/s11605-007-0309-6. discussion 86-7. [DOI] [PubMed] [Google Scholar]
- 8.Butte JM, Waugh E, Meneses M, Parada H, De La Fuente HA. Incidental gallbladder cancer: analysis of surgical findings and survival. J Surg Oncol. 2010;102:620–625. doi: 10.1002/jso.21681. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett DL, Fong Y, Fortner JG, Brennan MF, Blumgart LH. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1996;224:639–646. doi: 10.1097/00000658-199611000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiran RP, Pokala N, Dudrick SJ. Incidence pattern and survival for gallbladder cancer over three decades–an analysis of 10301 patients. Ann Surg Oncol. 2007;14:827–832. doi: 10.1245/s10434-006-9224-4. [DOI] [PubMed] [Google Scholar]
- 11.Sobin LH, Wittekind C. In: Gallbladder Cancer. TNM Classification of Malignant Tumours. 6th edn. Sobin LH, Wittekind C, editors. New York: Wiley-Liss; 2002. [Google Scholar]
- 12.Fuks D, Regimbeau JM, Le Treut YP, Bachellier P, Raventos A, Pruvot FR, et al. Incidental gallbladder cancer by the AFC-GBC-2009 Study Group. World J Surg. 2011;35:1887–1897. doi: 10.1007/s00268-011-1134-3. [DOI] [PubMed] [Google Scholar]
- 13.Smith GC, Parks RW, Madhavan KK, Garden OJ. A 10-year experience in the management of gallbladder cancer. HPB. 2003;5:159–166. doi: 10.1080/13651820310000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shukla PJ, Barreto G, Kakade A, Shrikhande SV. Revision surgery for incidental gallbladder cancer: factors influencing operability and further evidence for T1b tumours. HPB. 2008;10:43–47. doi: 10.1080/13651820701867794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong SL, Garcea G, Thomasset SC, Neal CP, Lloyd DM, Berry DP, et al. Ten-year experience in the management of gallbladder cancer from a single hepatobiliary and pancreatic centre with review of the literature. HPB. 2008;10:446–458. doi: 10.1080/13651820802392346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg. 1994;219:275–280. doi: 10.1097/00000658-199403000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawlik TM, Choti MA. Biology dictates prognosis following resection of gallbladder carcinoma: sometimes less is more. Ann Surg Oncol. 2009;16:787–788. doi: 10.1245/s10434-009-0319-6. [DOI] [PubMed] [Google Scholar]
- 18.Maker AV, Butte JM, Oxenberg J, Kuk D, Gonen M, Fong Y, et al. Is port site resection necessary in the surgical management of gallbladder cancer? Ann Surg Oncol. 2012;19:409–417. doi: 10.1245/s10434-011-1850-9. [DOI] [PubMed] [Google Scholar]
- 19.Sikora SS, Singh RK. Surgical strategies in patients with gallbladder cancer: nihilism to optimism. J Surg Oncol. 2006;93:670–681. doi: 10.1002/jso.20535. [DOI] [PubMed] [Google Scholar]
- 20.Gomez D, Sangha VK, Morris-Stiff G, Malik HZ, Guthrie AJ, Toogood GJ, et al. Outcomes of intensive surveillance after resection of hepatic colorectal metastases. Br J Surg. 2010;97:1552–1560. doi: 10.1002/bjs.7136. [DOI] [PubMed] [Google Scholar]
- 21.Fong Y, Brennan MF, Turnbull A, Colt DG, Blumgart LH. Gallbladder cancer discovered during laparoscopic surgery. Potential for iatrogenic tumor dissemination. Arch Surg. 1993;128:1054–1056. doi: 10.1001/archsurg.1993.01420210118016. [DOI] [PubMed] [Google Scholar]
- 22.Shih SP, Schulick RD, Cameron JL, Lillemoe KD, Pitt HA, Choti MA, et al. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg. 2007;245:893–901. doi: 10.1097/SLA.0b013e31806beec2. [DOI] [PMC free article] [PubMed] [Google Scholar]


